Abstract
Exposure to alcohol during embryonic development leads to changes in the hypothalamic-pituitary-adrenal (HPA) axis such that adult offspring release more adrenocorticotrophic hormone (ACTH) than controls when exposed to stress. In the present work, we tested the hypothesis that changes in the activity of the catecholaminergic system modulate, at least in part, this upregulation of the HPA axis. Pregnant Sprague Dawley rats were exposed to alcohol 6 hours daily during gestation days 7–18 using the vapour chamber model, which generated mean blood alcohol levels of 188.6 ± 10 mg/dl. All experiments were performed on 2–3 month-old offspring. We measured the ACTH response to intracerebroventricular injection of adrenergic receptor agonists. In rats exposed to footshocks, we investigated the activity of corticotrophin-releasing factor (CRF) as well as indexes of catecholamine immunoreactivity, namely tyrosine hydroxylase (TH) immunopositive neurons in the paraventricular nucleus (PVN), TH immunopositive neurons in the locus coeruleus, and phenylethanolamine N-methyltransferase (PNMT) immunopositive neurons in the brain stem. While adult females exposed to alcohol during fetal development (FAE) displayed the expected enhanced ACTH response to stress, there were no significant differences in response to adrenergic receptor agonists or in shock-induced CRF/TH immunoreactivity (ir) and neuronal activity, as determined by c-fos colocalization. In contrast, FAE female offspring exposed to footshocks showed a significant increase in the activity of adrenergic neurons in the C1 region of the brain stem, a population of cells that project to the PVN. Collectively, these results suggest that while FAE-induced hyperactivity of the HPA axis is not accompanied by significant changes in CRF or TH-ir neurons, it is characterized by an upregulation of C1 adrenergic neurons of the brain stem. This novel finding should lead to the functional characterization of this brain region in the FAE model.
Keywords: paraventricular nucleus, locus coeruleus, catecholamine, CRF, ACTH, medulla oblongata
Introduction
Alcohol exposure during gestation leads to many changes that persist into adulthood classified as fetal alcohol spectrum disorder and, in more extreme cases, fetal alcohol syndrome (FAS). Of the varying effects of fetal alcohol exposure (FAE), studies conducted in recent decades have identified less pronounced changes that occur in the brain without presenting the classic dismorphology that occurs with FAS. These changes range from alterations in neuroendocrine, immune and behavioural functions (Taylor et al., 1984; Angelogianni and Gianoulakis, 1989; Halasz et al., 1993; Nagahara and Handa, 1995; Yirmiya et al., 1996; Taylor et al., 2002) to impairment of the hippocampus affecting adult neurogenesis (Choi et al., 2005) and learning and memory (Girard et al., 2000; Dursun et al., 2006). For many years, our laboratory has focused on one of the most prevalent neuroendocrine dysfunctions caused by prenatal alcohol exposure, namely the hyperresponsiveness of the hypothalamic-pituitary-adrenal (HPA) axis, which results in exaggerated adrenocorticotrophic hormone (ACTH) release in response to a variety of stressors {see for example (Taylor et al., 1982; Nelson et al., 1986; Weinberg, 1988; Lee et al., 1990; Weinberg, 1992a, b; Ogilvie and Rivier, 1997; Yirmiya et al., 1998)}.
The HPA axis consists of neurons in the paraventricular nucleus of the hypothalamus (PVN) that produce corticotrophin-releasing factor (CRF) and vasopressin (VP), specialized cells in the pituitary (corticotrophs) where CRF receptors type 1 respond to CRF and VP by releasing ACTH into the general circulation, and cells in the adrenal cortex whose stimulation by ACTH results in increased production of glucocorticoids (GC) such as corticosterone in rats. The upregulated activity of the HPA axis that is observed in adult rodents exposed to alcohol prenatally could therefore result from changes at the level of the PVN, pituitary and/or adrenal. While still controversial, at least in adrenal-intact animals (Lee et al., 2000a), possible alterations in pituitary sensitivity to its trophic factors remain (Redei et al., 1993; Halasz et al., 1997; Osborn et al., 2000; Glavas et al., 2001) but probably only play a modest role. Indeed, at present, the most convincing evidence suggests that at least in rats, prenatal alcohol primarily increases CRF gene expression in PVN neurons (Gabriel et al., 2000; Lee et al., 2000a). It also alters the activity of CRF neurons, which may be achieved by changes in PVN neuron responsiveness to stimuli, the neuronal input to the PVN and/or PVN responsiveness to secretagogues. Indeed, work by Dr. Weinberg and colleagues has suggested a possible deficit in GC feedback (Osborn et al., 1996; Kim et al., 1999) and a role of the serotonin system (Hofmann et al., 2007), while studies performed in our own laboratory have reported increased PVN responsiveness to the gaseous neurotransmitter nitric oxide (Lee et al., 2003).
A variety of neurotransmitters are considered to exert key roles in regulating CRF PVN neurons (Sawchenko et al., 1992; Watts, 1996; Ziegler and Herman, 2002; Herman et al., 2003), among which catecholamines (adrenaline and noradrenaline) occupy a central position to play a role in stress-induced activation of the HPA axis. Of the catecholaminergic neurons (adrenergic and noradrenergic) that innervate the PVN and respond to stress, most reside in the brain stem. Indeed, one of the main innervating pathways to the PVN is the brainstem noradrenergic system, in particular, the locus coeruleus {see for example (Valentino et al., 1998; Ziegler et al., 1999; Sved et al., 2002)}, which is activated by stressors and modulates the HPA axis response to various stimuli (Sawchenko, 2000; Douglas, 2005). Likewise, adrenergic neurons of the brain stem (C1, C2, and C3) also innervate the PVN, where the most prominent projections (70%) are from the C1 region (Cunningham et al., 1990). They primarily innervate the parvocellular region of the PVN, where CRF perikarya reside. These neurons respond to stress (Dayas et al., 2001b) and can influence ACTH in circulation via arterial pressure (Baertschi et al., 1976; Reis, 1986). Examples of the influence of catecholamines on the HPA axis include the finding that noradrenaline (NA) administration into the PVN activates the HPA axis (Leibowitz et al., 1989; Cole and Sawchenko, 2002), a response probably mediated by alpha 1 adrenergic receptors (Kiss and Aguilera, 2000; Seo et al., 2003). Blockade of adrenergic receptors are known to interfere with this neuroendocrine function (Stone and Zhang, 1995). Also, stress enhances NA release (Pacak et al., 1992) while lesions of catecholamineregic neurons impair HPA axis activity (Li et al., 1996). Lastly, CRF-containing neurons in the bed nucleus of the stria terminalis are surrounded by cells that express tyrosine hydroxylase (TH) (Meloni et al., 2006), the rate-limiting step in the biosynthesis of the catecholamines dopamine, noradrenaline and adrenaline that is used as a general marker of this synthesis {ref. in (McArthur et al., 2005)}. Likewise, neurons of the PVN are surrounded by projections from neurons that express phenylethanolamine N-methyltransferase (PNMT), an enzyme that converts noradrenaline to adrenaline (Cunningham et al., 1990). In view of these findings, we hypothesized that exposure to alcohol during embryonic development might alter HPA axis activity via changes in catecholamine-dependent mechanisms. Here we report work aimed at investigating the possible influence of prenatal alcohol treatment to the HPA axis responsiveness to adrenergic agonists, on TH expressing neurons in the PVN and locus coeruleus, and on PNMT-expressing neurons in the brain stem.
Experimental Procedures
Animals and alcohol exposure
Female Sprague Dawley and proven breeder male rats were purchased from Charles River Laboratories (Wilmington, MA) and kept under controlled lighting conditions (12L:12D) with food and water ad libitum. The females were injected with the GnRH agonist [D-Trp6, Pro9, NEtNH2]GnRH to synchronize the estrous cycle (Rivier and Vale, 1990). This agonist, synthesized by solid phase methodology (Kornreich et al., 1992) and generously provided by Dr. Jean Rivier (The Salk Institute, La Jolla, CA), was administered at 2 μg, s.c., at 0900 and 1400 hours. Two days later, the females were introduced to the male cages and remained paired until vaginal plugs were found. The day on which a plug was found was designated as day 0 of gestation. Females assumed to be pregnant were divided into two groups: one control group and one group exposed to vapour alcohol for 6 consecutive hours (0700–1300) per day on gestational days 7–18 (Lee et al., 2000a). Vapour alcohol levels were increased daily in the chambers until maternal blood alcohol levels (BALs) reached 180–200 mg/dl, and were then maintained at these levels. BALs were measured in tail blood samples at the end of the sessions, and care was taken that no animal was sampled more then twice over the course of the treatment. The rats were returned to their home cages after each alcohol treatment and had free access to food and water. Control dams were placed in control vapour chambers for the same duration as the alcohol dams, with only air circulating at the same flow rate as the alcohol chambers. To control for litter differences, the pups were randomized at postnatal day 2/3 across litters within each treatment group (control or vapour alcohol). Each dam was placed with five female and five male pups (totalling 10 pups/litter, n = 108–110/group). All pup weights were obtained by weighing individual pups and were recorded at postnatal day (PND) 7, 14, and 21. Pups were weaned at 21 days of age and remained group housed (n = 3/cage) until testing. All protocols were approved by the Salk Institute IACUC.
Alcohol treatment
We used individual alcohol vapour chambers provided by La Jolla Alcohol Research, Inc. (La Jolla, CA, http://www.ljari.com), which we have validated for use in both adult male rats (Lee et al., 2000b) and pregnant animals (Lee et al., 2000a).
Adult testing
I.c.v
All tests were conducted on rats between 2–3 months of age. All adult female offspring received the GnRH agonist mentioned above to synchronize their estrous cycles. They were tested during diestrous II (7th day following GnRH injection; injection day is day 0) in order to avoid cycle-induced changes in HPA axis responsiveness (Rivier, 1999). I.c.v. cannulae were inserted according to previously published methodology (Lee et al., 1999). All cannulae were placed in the right lateral ventricle with the coordinates measured from bregma (0.4 mm posterior, 1.4 mm lateral, and 3.8 mm ventral). Correct cannulae placement was verified at the end of the experiments by injecting Dextran Blue i.c.v. and only rats with correct placement were used for the statistical analysis of the data. After one week of recovery, flexible cannulae were placed in the left carotid vein to permit repeated blood draws with minimal stress (Lee et al., 1999). Rats were allowed to recover for another two days before experiments began. Prior to agonist infusion, all rats were allowed to acclimate to the experiment room overnight. On the morning of the experiment, all rats received connections to both cannulae and were placed in containers and left undisturbed for two hours prior to the first blood draw. After the first blood draw, the vehicle (sterile H2O) or an adrenergic receptor agonist phenylephrine HCl (Sigma, St. Louis, MO) or isoproterenol HCl (Sigma, St. Louis, MO) in sterile H2O was infused into the right lateral ventricle at a rate of 1 μl/8 sec, totalling 5 μl per infusion. The second, third, and fourth blood samples were taken at 15, 30, and 60 min post-infusion, respectively. Blood (0.4 ml/draw) was collected in tubes with 10 μl 0.5 M EDTA and kept on ice until spun at 3,000 rpm for 10 min to separate and collect plasma. Doses of the adrenergic agonists and time of blood sampling were based on our prior studies (Seo and Rivier, 2001) as well as unpublished experiments conducted in our laboratory in both genders. All agonists were dissolved in sterile H2O and delivered at the following doses: Males received phenylephrine at 3 μg, 22 μg, or 30 μg or isoproterenol at 4 μg or 14 μg per infusion. Females received phenylephrine at 2 μg or 7.5 μg, or isoproterenol at 2.5 μg or 5 μg per infusion. Animals that did not receive the complete infusion of the agonist were excluded from the data analyses.
Inescapable footshocks for ACTH analysis
Control and FAE rats were tested with inescapable footshocks (Coulbourn HO2–08 grid floor shockers controlled by a Macintosh computer; Coulbourn Instruments, Allentown, PA) to verify the effect of FAE in the HPA axis. The use of this shocks model has been abundantly characterized in our laboratory {see for example (Rivier et al., 2003)}. Intravenous cannulae were placed in the left carotid vein as described above. Following a one-week recovery, rats were placed in individual shock chambers and exposed to shocks (0.3 mA for males and 0.25 mA for females, 1 sec duration, average of 2 shocks/min) for 45 minutes. Blood samples (0.4 ml) were taken prior to the first shock (t = 0) as well as 10, 25, and 45 min later. Blood was collected in tubes with 10 μl 0.5 M EDTA and kept on ice until centrifuged at 3,000 rpm for 10 min.
Inescapable footshocks for immunohistological analysis
A second group of control and FAE female rats were tested with inescapable footshock stress. Rats were exposed to shocks (0.35 mA, 1 sec duration, 2 shocks/min) for 60 minutes. The rats were then allowed to rest in individual containers for one hour prior to receiving a lethal injection of chloral hydrate in the intraperitoneal cavity followed by transcardial perfusion with 4% paraformaldehyde.
Assays
ACTH
ACTH was measured in plasma with a commercially available immunoradiometric assay (IRMA) kit (DiaSorin, Stillwater, MN). Plasma was diluted 1:4 with the Zero Calibrator (provided in the kit) totalling 200 μl per sample. Each sample was incubated overnight with the tracer and a biotin coated bead, after which the beads were washed with a buffer and counted in a gamma counter for 1 min. Resulting counts were analyzed according to the standard controls provided with the kit. The lower detection limit of this assay is 15 pg/ml and samples in which ACTH levels were less than 15 pg/ml were assigned that value for statistical analysis. The intra- and inter-assay coefficients of variation are 7 and <15%, respectively.
BALs
BALs, taken from tail vein samples, were measured with 5 μl plasma using an Analox AM 1 analyzer available from Analox Instruments LTD (Lunenberg, MA). The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol + O2 → acetaldehyde + H2O2) (Lee et al., 2000b). All rats were bled regardless of whether they were exposed to alcohol or not, and each animal was not bled more than twice.
Immunohistochemistry
Brains were placed in 4% paraformaldehyde for 4 hours, at which time they were transferred to 10% sucrose as a cryoprotectant for 2 days before being frozen on dry ice. All brains were maintained at −80° C until sectioned on the microtome. Perfused brains were cut in the coronal plane at a thickness of 30 μm. All sections were kept in an anti-freeze solution (at −20° C) until analyzed. Each analysis consisted of sampling every fourth section throughout the rostral-caudal extent of the PVN, the locus coeruleus, and the brain stem. Staining for each antibody followed the general method where free-floating sections were used. Briefly, the sections were rinsed with 0.01 M phosphate buffered saline (PBS) between each incubation step. After an initial rinse in PBS, sections were incubated with 0.1% H2O2 to reduce background, then 0.1% NaBH4 to permeabilize the cell membranes. For single DAB immunohistochemistry, the sections were treated with a blocking solution containing 5% normal serum before an overnight incubation with the primary antibody (mouse anti-tyrosine hydroxylase 1:5000 for the PVN and 1:20,000 for the locus coeruleus, Novus, Littletom, CO; rabbit anti-corticotrophin releasing factor 1:13,000, gift from W. Vale, The Salk Institute, La Jolla, CA) at 25° C. The sections were then incubated with the secondary antibody (biotinylated goat anti-mouse 1:500; biotinylated goat anti-rabbit 1:500, Vector labs, Burlingame, CA) followed by a one-hour incubation with avidin-biotin complex (ABC kit, Vectastain, Burlingame, CA). Following several rinses with PBS and 0.1 M NaOAc, sections were developed with a Ni-DAB solution for 45 sec then rinsed with 0.1 M NaOAc and PBS. Double DAB immunohistochemistry consisted of using one of the following antibodies as the first primary antibody [mouse anti-GAD65 antibody (1:2000, Chemicon, Temecula, CA) or rabbit anti-c-fos antibody (1:10,000, Calbiochem, San Diego, CA)] and one of the following antibodies as the second primary antibody [rabbit anti-TH antibody (1:1000, Novus, Littleton, CO), mouse anti-TH primary antibody (1:5000), rabbit anti-CRF (1:13,000), or sheep anti-PNMT (1:10,000, Chemicon, Temecula, CA)]. General labelling protocol proceeded as described above with the first primary antibody, omitting the incubation with 0.1% H2O2 and using the appropriate biotinylated secondary antibody (goat anti-mouse or goat-anti-rabbit 1:500, Vector labs, Burlingame, CA). After the final rinse with PBS, upon visualization of the first primary antibody, the sections were incubated with the second primary antibody overnight and then with the appropriate biotinylated secondary antibody (goat anti-mouse, goat-anti-rabbit, or rabbit-anti-sheep 1:500, Vector labs, Burlingame, CA). Following the ABC incubation, sections were rinsed with PBS and 0.05 M Tris buffered saline (TBS) before visualizing the antibody labelling with DAB-H2O2 solution for approximately 30 – 90 sec (times varied depending on the antibody used). The sections were rinsed with TBS and then PBS before being mounted onto subbed slides, dried and coverslipped with DPX mounting media (Fluka Biochemika, Ronkonkoma, NY). This technique resulted in the first antibody to stain black and the second antibody to stain brown. All antibody staining labelled either the cell body or nuclei. However, GAD65 labelled as punctate dots, since it is only expressed in the terminals. Hence, we determined GAD65/TH positive count as a TH positive cell body with a GAD65 positive puncta overlapping the TH stain. All cell counts were performed using a 40x dry objective.
Bright-field Images
All bright-field images were taken with the Leitz orthoplan 2 microscope. The Optronics MicroFIRE camera and Optronics PictureFrame software were used to collect the picture files. All pictures were edited using Image J 1.34s (NIH, Bethesda, MD), Adobe Photoshop CS 8.0 (Adobe, San Jose, CA), and Canvas 8.0 (Deneba Systems, Miami, Fl).
Statistical Analysis
The ACTH data were analyzed with the use of a three-way ANOVA with pre-treatment (control vs. FAE), agonist (vehicle, phenylephrine and isoproterenol), and time (repeated measures: 0 min, 15 min, 30 min, and 60 min) as variables with the Statistica 7 (Stat Soft, Tulsa, OK) software. The ACTH data for footshock stress were analyzed with a two-way ANOVA, with pre-treatment and time (repeated measures) as variables with SPSS 12.0.1 (SPSS Inc., Chicago, IL). Experiments for each gender were conducted and analyzed separately and therefore were not included as a variable for the statistical analyses. Cumulative data was generated by collapsing ACTH values over time. Raw data were surveyed for outliers, where a value residing greater than two standard deviations away from the mean were excluded from the data analysis. All other data were analyzed by a two-way ANOVA with pre-treatment and stress (no shock vs. footshock) as variables, using SPSS 12.0.1 (SPSS Inc., Chicago, IL). A p value less than or equal to 0.05 was considered significant. All graphs were generated using GraphPad Prism 4 (Graphpad Software, San Diego, CA).
Results
Maternal BALs and pups’ weights
Maternal BALs measured on gestational days 9, 13, and 16 were 214.2 ± 26.5 mg/dl, 175.9 ± 7.9 mg/dl, and 176.8 ± 10.5 mg/dl, respectively (n = 6–7). All dams appeared intoxicated at the end of each ethanol exposure session. Pups were weighed on postnatal days (PND) 7, 14 and 21. The only significant difference observed was a main effect of FAE (PND 7 [F(1, 438) = 12.995, p < 0.001], PND 14 [(F(1, 438) = 24.063, p < 0.001], and PND 21 [F(1, 438) = 12.068, p = 0.001]) where pups exposed to ethanol were lighter than controls (n = 108–110/group/gender). This difference disappeared in adulthood, when experiments were conducted (Table 1). The litter size, gender distribution per litter or the number of gestation days were not significantly different between groups.
Table 1. Litter Demographics.
(A) Duration of gestation, number of pups per litter and gender distribution per litter are presented as mean ± SEM with n denoting the number of dams. There were no significant differences between controls and FAE. (B) Weights are presented in grams as mean ± SEM. There was a significant main effect of FAE for each PND (p ≤ 0.001) that was eliminated by adulthood. Likewise, a two-way ANOVA did not reveal any significant differences when accounting for both alcohol treatment and gender.
| A. Litter Demographics | ||||
|---|---|---|---|---|
| Control | FAE | |||
| # gestation days | 21.4 ± 0.2 | n = 24 | 21.8 ± 0.1 | n = 24 |
| # pups per litter | 14.0 ± 0.5 | n = 16 | 14.2 ± 0.5 | n = 19 |
| # male pups | 7.0 ± 0.7 | n = 16 | 7.5 ± 0.5 | n = 19 |
| # female pups | 6.9 ± 0.6 | n = 16 | 6.5 ± 0.5 | n = 19 |
| B. Pup Weight | ||||
|---|---|---|---|---|
| Control | FAE | |||
| male (n = 108) | female (n = 110) | male (n = 110) | female (n = 110) | |
| PND 7 | 16.7 ± 0.2 | 16.6 ± 0.2 | 16.1 ± 0.2 | 15.5 ± 0.2 |
| PND 14 | 34.1 ± 0.3 | 33.4 ± 0.3 | 32.9 ± 0.3 | 31.9 ± 0.3 |
| PND 21 | 54.6 ± 0.5 | 53.5 ± 0.5 | 53.3 ± 0.5 | 51.6 ± 0.5 |
Effect of FAE on shock-induced ACTH release
The effect of FAE was verified with inescapable footshock stress. Previous work carried out in our laboratory had demonstrated that FAE mice (Kang et al., 2004) and rats (Lee et al., 2000a) released more ACTH in response to footshocks. In the present work, we only observed a significant main effect of FAE in female rats [F(1, 48) = 4.591, p = 0.037] (n = 6–7/group) (Figure 1B). While differences did not reach statistical significance for individual points because of large individual variations (a common feature of plasma ACTH levels measured in females even when they are studied at comparable stages of the estrous cycle), the difference measured for cumulative data (ACTH levels collapsed over time) was significant (P<0.05) (Figure 1C). As these cumulative values represent the overall amount of ACTH that reaches the adrenals, they are usually considered more physiologically relevant than individual time points. On the other hand, there were no significant differences observed in male rats [F(1, 30) = 0.156, p = 0.701] (n = 6–7/group) (Figure 1A). While our laboratory previously observed FAE-induced differences in both genders, we used significantly lower overall doses of alcohol in the current study (188.6 ± 10 mg/dl vs. 273 ± 11 mg/dl). In addition, these current findings are consistent with data reported by Weinberg and colleagues (Weinberg, 1992a) where there were gender differences on the HPA axis response to stress in FAE rats.
Figure 1.
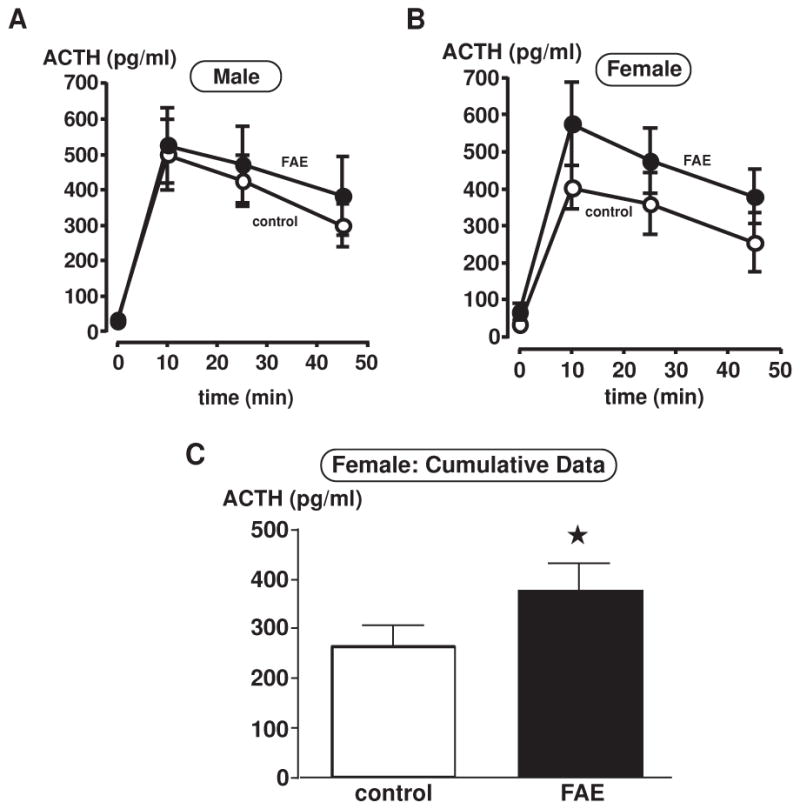
Plasma ACTH measurements in control and FAE male (A) and female (B–C) rats with inescapable footshock stress. Plasma was taken at 0 min (prior to footshocks), 10, 25 and 45 min upon footshock initiation. ACTH levels are presented in pg/ml. Each data point displays mean ± SEM (n = 6–7/group). (C) Bar graph displays cumulative data, where ACTH levels are collapsed over time. There is only a significant main effect of FAE in female rats (*, p = 0.037).
Effect of FAE on shock-induced CRF expression
Since FAE generated a significant main effect on ACTH response to stress in females, we evaluated CRF immunoreactivity (ir) in female rats to determine its involvement in the PVN in relation to FAE. Overall, there were no significant differences attributed to the effects of FAE. The total number of CRF positive cells in the PVN was not different between control and FAE rats under basal conditions (control: 118.6 ± 14.1, FAE: 126.7 ± 13.9) or upon exposure to footshocks (control: 119.2 ± 9.5, FAE: 116.7 ± 6.7) (n = 6–7/group). As discussed later, while most stressors are known to activate PVN CRF neurons (Kang et al., 2004), changes in signals measured by in situ hybridization do not necessarily correspond with changes detected using immunohistochemistry. In the present work, shock-induced changes in PVN CRF neuronal activity was readily apparent when we measured localization of c-fos protein within CRF-ir neurons in the PVN. Using double immunohistochemical labelling techniques, we counted the number of CRF and c-fos colocalized cells in the PVN. While there was a significant main effect of footshocks on the activity of CRF neurons [F(1,20) = 29.47, p < 0.0001] (Figure 2), the number of c-fos activated CRF cells was not altered by FAE.
Figure 2.
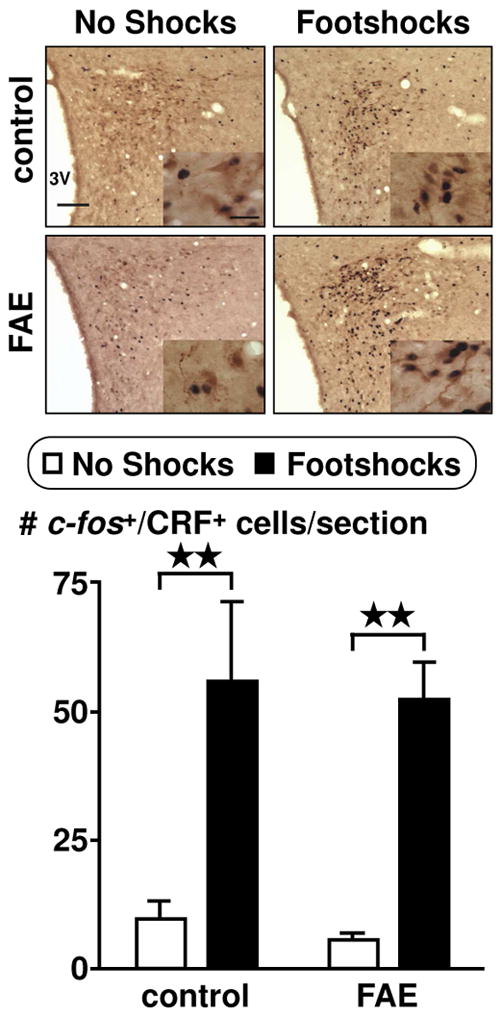
Colocalization of c-fos in CRF immunoreactive cells in the PVN of female rats. Pictures display PVN at 10X objective (scale bar = 100 μm) and insets at 60X oil objective (scale bar = 20 μm). Double immunohistochemistry techniques labelled c-fos as black and CRF as brown. Cell counts were obtained throughout the rostral-caudal extent of the PVN. Results are displayed as the number of CRF and c-fos positive cells per section. Rats were either undisturbed (no shocks) or exposed to inescapable footshock stress (footshocks) for one hour prior to transcardial perfusion. There was a significant main effect of footshock stress (p < 0.0001, n = 12). Bonferroni post-hoc test reveals a significance between no shocks and footshocks within each group (control and FAE) **, p < 0.01.
Effect of FAE on adrenergic receptor agonists-induced ACTH secretion
Preliminary experiments using i.c.v. infusion of phenylephrine or isoproterenol were conducted to determine the dose of agonist that would induce significant, but not maximum ACTH responses. Both agonists caused the expected (Seo et al., 2003) rise in ACTH levels. The low and high doses used were previously shown to generate significantly different levels of ACTH at 15 minutes post-injection (unpublished data). In general, there was no significant effect of FAE on ACTH levels in response to either agonist infusion for both males [F(1, 58) = 2.76), p = 0.102] (n = 4–9/group, n = 4 only applies to the FAE rats infused with 22 μg phenylephrine) (Figure 3) and females [F(1, 45) = 0.27, p = 0.609] (n = 6–8/group) (Figure 4).
Figure 3.
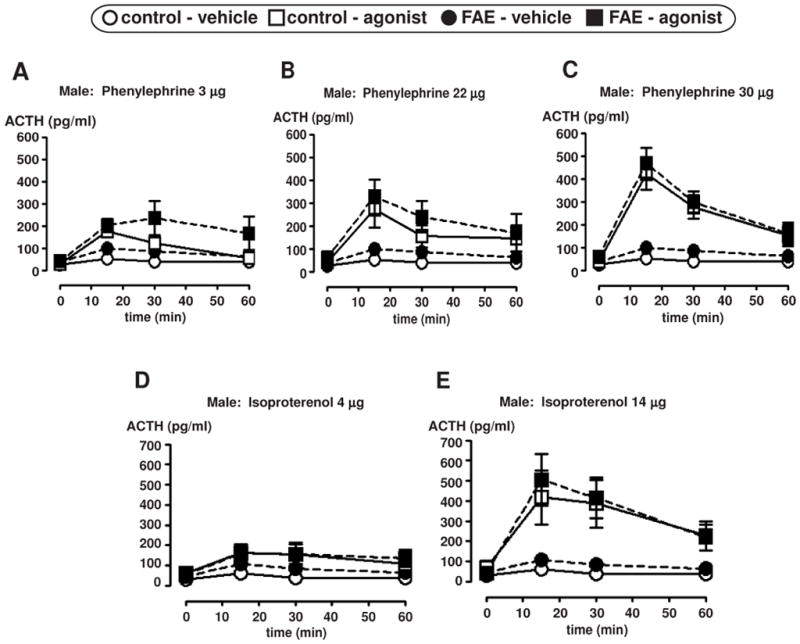
Plasma ACTH measurements in male control and FAE rats after i.c.v. infusion of an adrenergic receptor agonist. ACTH levels are presented in pg/ml at four time points (0, 15, 30, and 60 min) relative to the agonist infusion, with 0 min time point conducted just prior to agonist infusion. Graphs display mean ± SEM (n = 4–9/group, n = 4 only applies to the FAE rats infused with 22 μg of phenylephrine). Alpha 1 agonist (phenylephrine HCl) infusion results are displayed in figures A–C. Beta agonist (isoproterenol HCl) infusion results are displayed in figures D and E.
Figure 4.
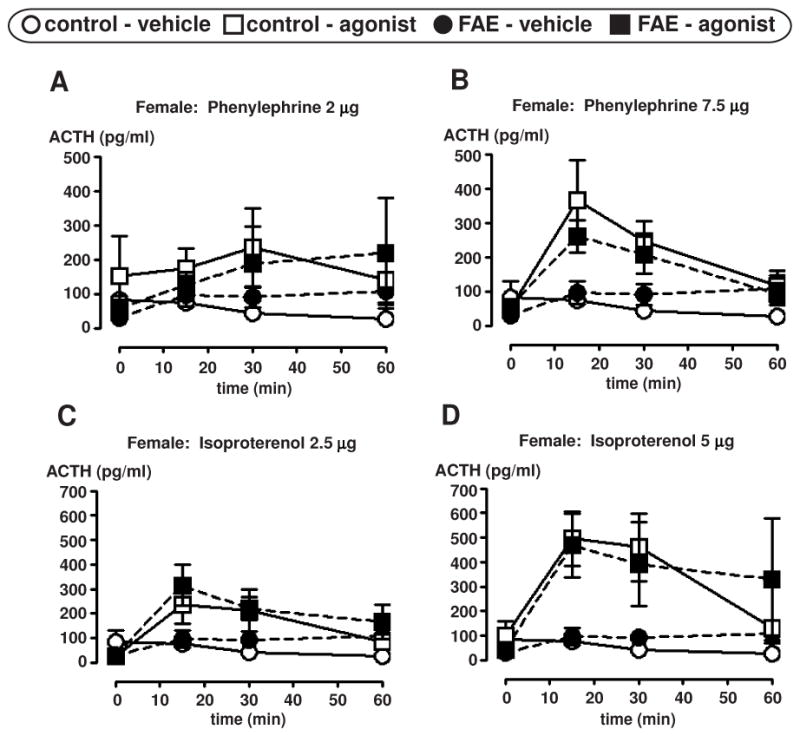
Plasma ACTH measurements in female control and FAE rats after i.c.v. infusion of an adrenergic receptor agonist. ACTH levels are presented in pg/ml at four time points (0, 15, 30, and 60 min) relative to the agonist infusion, with 0 min time point conducted just prior to agonist infusion. Graphs display ± SEM (n = 6–8/group). Alpha 1 agonist (phenylephrine HCl) infusion results are displayed in figures A and B. Beta agonist (isoproterenol HCl) infusion results are displayed in figures C and D.
Effect of FAE on shock-induced TH, GAD, and PNMT expression
To further identify possible differences between control and FAE offspring, we counted the number of TH-positive neurons in the PVN and locus coeruleus of female rats, a group chosen because it displayed enhanced ACTH release when exposed to footshocks. Since TH is the rate-limiting enzyme in the synthesis of NA, and dopamine β hydroxylase is only expressed in nerve terminals at the PVN and locus coeruleus, we used TH as a marker to label catecholaminergic neurons. Overall, there was no significant difference in the total number of TH positive cells in the PVN and locus coeruleus between controls or animals exposed to alcohol prenatally (n = 6–7/group) (Table 2). We then measured TH activity by c-fos and TH colocalization. In both control and FAE rats, shocks induced significant increases in the number of TH-ir cells that expressed c-fos [F(1, 20) = 11.203, p = 0.003] (n = 12/group) (Figure 5). However, prenatal alcohol did not exert a detectable influence on these changes.
Table 2. Tyrosine hydroxylase (TH) immunoreactivity in the PVN and locus coeruleus.
The number of TH positive cells per section is presented as the mean ± SEM (n = 6–7). There were no significant differences with either FAE or footshock stress.
| Control | FAE | |||
|---|---|---|---|---|
| no shocks | footshocks | no shocks | footshocks | |
| PVN | 128.4 ± 3.8 | 121.2 ± 11.6 | 116.0 ± 7.0 | 127.6 ± 9.4 |
| locus coeruleus | 303.8 ± 28.2 | 270.4 ± 16.0 | 297.5 ± 13.8 | 270.8 ± 11.1 |
Figure 5.
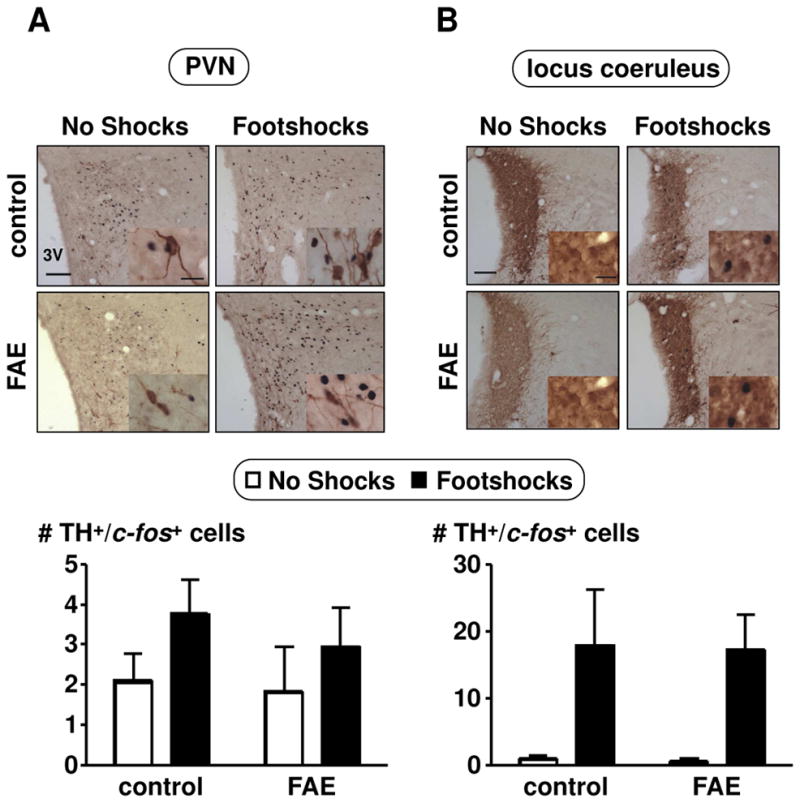
Colocalization of c-fos in TH immunoreactive cells in the PVN (A) and locus coeruleus (B) of female rats. Pictures were taken with a 10X objective (scale bar = 100μm) and insets with a 60X oil objective (scale bar = 20 μm). Double immunohistochemical techniques stained c-fos in black and TH in brown. Cell counts were obtained throughout the rostral-caudal extent of the PVN and locus coeruleus. Results are displayed as the number of TH and c-fos positive cells per section. No shocks and footshocks conditions are described in Figure 2. There was only a significant main effect of footshock stress in the locus coeruleus (p = 0.003, n = 12).
In view of this lack of an FAE effect, we then investigated possible GABA interactions with the dopaminergic system. As GABA is not expressed within the PVN, we labelled cells for glutamic acid decarboxylase (GAD) 65, which is expressed in the processes of GABAergic neurons that synapse onto TH-ir neurons in the PVN and locus coeruleus. Double immunohistochemical labelling techniques indicated no significant differences for GAD65 positive terminals synapsing with TH-ir neurons in the PVN of FAE rats [F(1,49) = 3.324, p = 0.075] (n = 12–13/group) (Figure 6). To further investigate the influence on catecholaminergic neurons, we quantified the activity of adrenergic neurons in the brain stem, counting c-fos and PNMT colocalization (Figure 7). While TH limited us to labelling all catecholaminergic neurons, PNMT allowed to the specific labeling of adrenergic neurons. Both C1 and C2 areas displayed a significant main effect of footshock stress with an increased number of c-fos and PNMT colocalized neurons (C1: [F (1, 20) = 20.028, p = 0.0002]; C2: [F (1, 20) = 13.927, p = 0.001]). There was also a significant main effect of FAE in the C1 area [F (1, 20) = 5.569, p = 0.029] and a significant interaction of FAE and shock stress [F (1, 20) = 7.185, p = 0.014] (n = 12/group). Moreover, FAE rats displayed increased c-fos and PNMT colocalization when stressed compared to controls (p < 0.01) (Figure 8). However, there were no significant differences with c-fos and PNMT colocalization in the C3 area (Figure 7) as well as overall PNMT positive cells for all C1, C2, and C3 areas.
Figure 6.
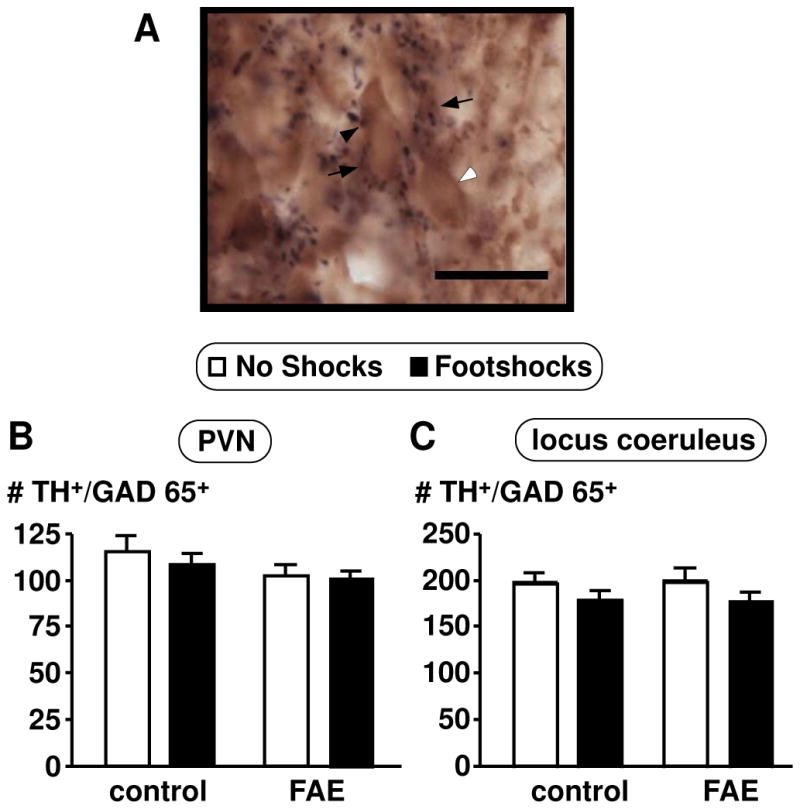
Number of TH positive neurons with GAD65 positive terminals contacting the cell body in female rats. (A) Picture was taken with a 60X oil objective (scale bar = 20 μm). Image shows a representation of the double immunohistochemical technique which stained GAD65 in black and TH in brown. Black arrowhead indicates GAD65 positive stain, white arrowhead indicates TH positive stain, and black arrows indicate double labelled cells. Cell counts were obtained for TH and GAD65 throughout the rostral-caudal extent of the PVN (B) and locus coeruleus (C). Results are displayed as the number of TH and GAD65 positive cells per section. No shocks and footshocks conditions are described in Figure 2. There were no significant differences across any groups (p = 0.075, n = 12–13).
Figure 7.
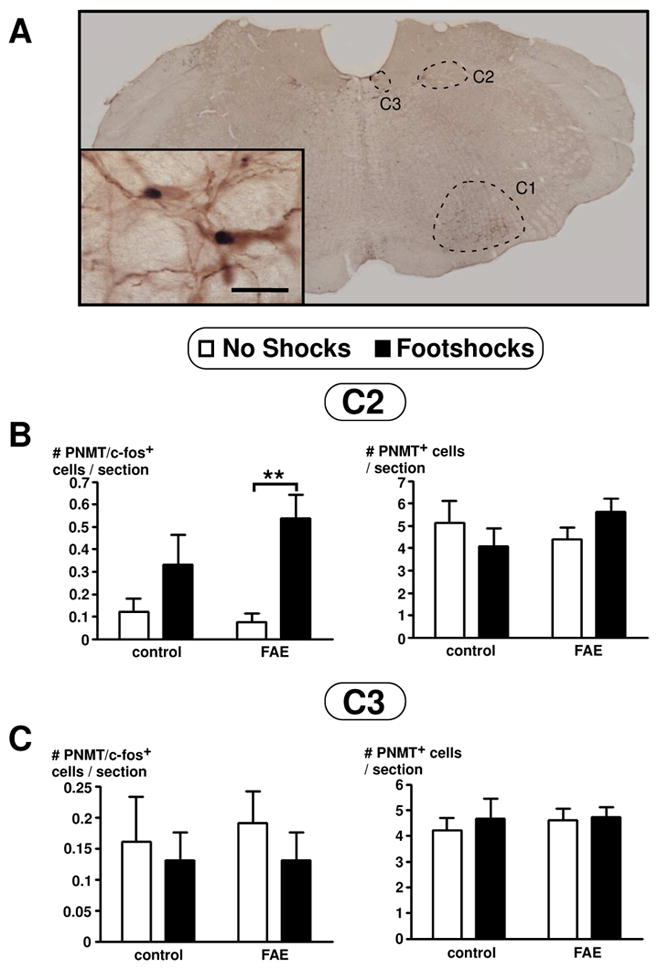
PNMT and c-fos colocalization visualized using double immunohistochemical technique in the brain stem of female rats (A). The three adrenergic regions (C1, C2, and C3) are outlined as indicated. Inset picture display colocalization of PNMT in brown and c-fos in black. Picture was taken with a 2X dry objective and inset picture using a 60X oil objective (inset scale bar = 20 μm). (B–C) Number of PNMT and colocalization of c-fos in PNMT immunoreactive cells in the C2 (B) and C3 (C) regions of the brain stem of female rats. Results are displayed as the number of PNMT and c-fos positive cells per section (on left panel) or number of PNMT positive cells per section (on right panel), as indicated. Cell counts were obtained throughout the rostral-caudal extent of the C2 and C3 areas of the brain stem. There was only a significant main effect of stress in the C2 area (p = 0.001, n = 12). Bonferroni post-hoc test revealed significance as indicated by asterisks (**, p < 0.01). There were no significant effects observed in the C3 area.
Figure 8.
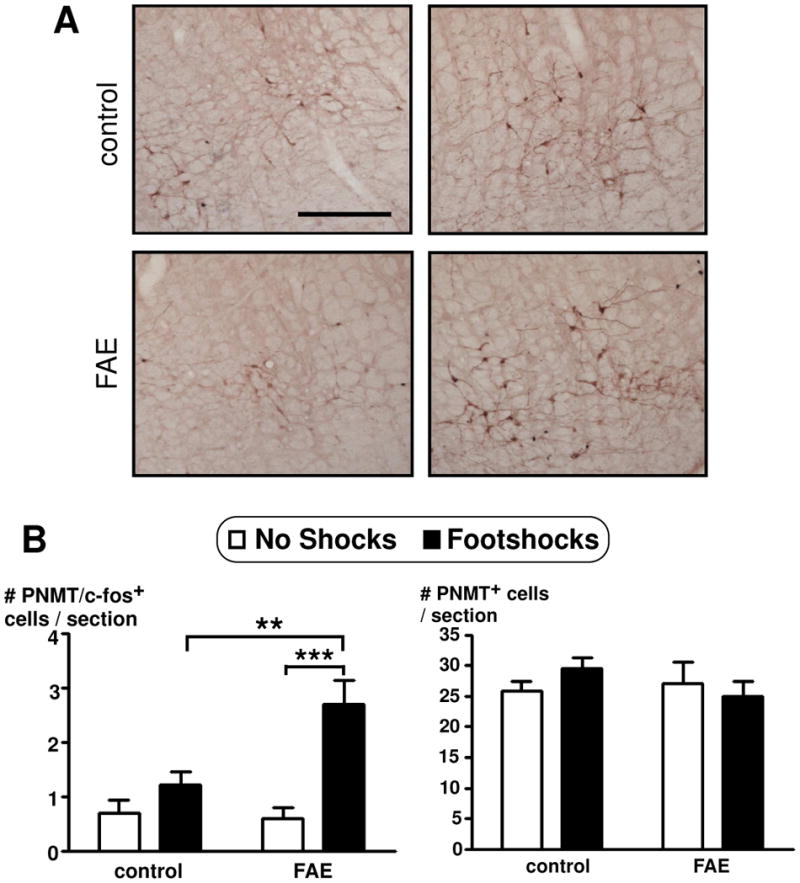
Number of PNMT and colocalization of c-fos in PNMT immunoreactive cells in the C1 region of the brain stem of female rats. (A) Double immunohistochemical techniques stained c-fos in black and PNMT in brown. Picture was taken with a 10X dry objective and display panels showing comparison of the C1 are of the brain stem for each of the four conditions, as identified (scale bar = 200 μm). (B) Results are displayed as the number of PNMT and c-fos positive cells per section (on left panel) or number of PNMT positive cells per section (on right panel), as indicated. Cell counts were obtained throughout the rostral-caudal extent of the C1 area of the brain stem. No shocks and footshocks condition are described in Figure 2. There was a significant main effect of stress (p < 0.001, n = 12) and a significant main effect of FAE (p = 0.029). There was also a significant interaction of FAE pre-treatment and stress (p = 0.014). Bonferroni post-hoc test revealed significance as indicated by asterisks (**, p < 0.01; ***, p < 0.0005).
Discussion
We report here that prenatal exposure to alcohol did not lead to an upregulation of this response following the i.c.v. injection of adrenergic receptor agonists. Moreover, there were no alterations in the TH-ir neuronal population, overall activity of the PVN and locus coeruleus, or the number of GABAeric terminals in the PVN. On the other hand, female FAE offspring showed a significant increase in the activity of C1 adrenergic neurons in the brain stem.
Although CRF expression during development has been shown to be affected by FAE (Aird et al., 1997), we did not observe significant differences in either the total number of CRF positive cells or PVN neuronal activity. In fact, c-fos signals in CRF-ir cells only increased in response to footshock stress as previously shown (Rivest and Rivier, 1994). Likewise, even though our laboratory has previously demonstrated footshock-induced increases of CRF heteronuclear (hn) RNA and mRNA levels in the PVN (Lee et al., 1990; Lee et al., 2000a), our current study did not show any changes in the overall number of CRF-ir neurons. These results may seem conflicting, but it is important to note that changes in hnRNA or mRNA levels do not necessarily translate to observable changes in immunoreactive cell counts. Although in situ hybridization is used to detect mRNA levels and Western Blotting to measure protein expression, immunohistochemistry gives the advantage of identifying cells expressing CRF with respect to its location, whereas Western Blotting can only refer to general dissected areas. In addition, using c-fos, immediate early gene antibodies in conjunction with CRF antibodies give the advantage of quantifying the number of CRF-ir neurons that are activated upon a given condition (footshock or FAE). This data allows us to analyze not only the location of CRF-ir neurons, but also permits the quantification of the level of overall activity with c-fos. However, the methodology we used for the immunohistochemical detection of CRF was not sensitive enough to measure direct dynamic changes in protein levels, only the number of CRF-expressing neurons. Thus, we cannot compare our findings with those available in the current literature for stress-induced increases of CRF mRNA levels in the PVN. In this respect, it should be noted that morphine-induced increases of CRF mRNA expression in the amygdala was similarly not accompanied by an increase in CRF-ir neurons (Maj et al., 2003). Thus, there is evidence that stimulation of the HPA axis can sometime occur without significant increases in CRF gene expression, or the CRF-ir neuronal population size.
Another issue that probably had a significant impact on our ability to detect FAE-induced differences in PVN CRF neurons, pertains to the time course we used to collect brains. As indicated under Results, we did not observe significant differences between c-fos signals in the CRF-ir cells of control and FAE rats, which can appear to contradict our previous report that prenatal alcohol increases the neuronal activity of PVN CRF cells (Lee et al., 2000a). As we previously reported (Lee et al., 2000a), FAE-induced differences in PVN CRF hnRNA levels are only observed 15 min after the initiation of shocks, and this difference had disappeared by the 30 min time point. In the present work, we focused on the overall activity CRF-ir cells, and thus studied brains collected 60 minutes after the last footshock because our previous results showed that c-fos protein levels rise within 30–60 minutes post stress (Augustine, 2008). In doing so, we knew that we would most probably miss measurable differences in PVN CRF neuronal activation, as measured by c-fos protein signals. Nevertheless, the fact that FAE female offspring displayed increased shock-induced ACTH response (see Fig. 1) indicated that the activity of their PVN CRF perikarya had indeed been upregulated at an earlier time point. This provided the basis for the search for mechanisms that might mediate this hypothalamic hyperactivity, which in the present work focused on the influence of the noradrenergic system.
The stimulatory influence exerted by catecholamines on the HPA axis is well recognized {see (Li et al., 1996; Cole and Sawchenko, 2002)} and these neurotransmitters thus represent logical modulators for altered CRF and ACTH responses shown by adult stressed rats that had been exposed to alcohol during gestation. At present, there is scant information regarding the influence of prenatal alcohol on rat catecholamine-related systems. To our knowledge, the only available data pertain to the effect of repeated gavage of pregnant dams throughout the entire gestation period, coupled with intubation of the pups for the first 10 days of post-natal development (Tran and Kelly, 1999). These experiments showed an increase in hypothalamic NA levels of adult female, but not male offspring. As the protocol of alcohol treatment used by these investigators differs significantly from ours in terms of the overall length of drug exposure, it is difficult to compare results. Likewise, we believe there is no work currently investigating changes in adrenergic neuronal activity of the brain stem relative to fetal alcohol or other prenatal exposure paradigms. Our first series of experiments therefore investigated potential alterations in HPA axis responses to adrenergic agonists. We report here that there were no significant differences in the HPA axis responsiveness to the i.c.v. injection of the adrenergic receptor agonists phenylephrine and isoproterenol in controls and FAE offspring. On the other hand, it is interesting to note however, that these differences reached significance when the data were transformed and normalized to vehicle treatment (data not shown), though the physiological relevance of this finding is not clear. In view of these results, we then used immunohistochemical techniques to quantify catecholaminergic neurons in the PVN and locus coeruleus. Since dopamine β hydroxylase, the enzyme that converts dopamine to NA, is only expressed in nerve terminals at the PVN, it would be difficult to count cell bodies. Thus, we used TH, which is expressed throughout the cell body and processes, as a marker for catecholaminergic (adrenergic and noradrenergic) neurons. Our results show that despite a significant effect of FAE on shock-induced increases in ACTH of our female rats, there were no quantifiable changes in the number of TH-ir cells in the PVN or locus coeruleus. Likewise, the activity of TH-ir positive cells (measured with c-fos colocalization) in the PVN or locus coeruleus remained unaltered with FAE. In fact, we only observed increased TH and c-fos colocalization with footshock stress in the locus coeruleus. As previously mentioned, others reported changes in hypothalamic NA levels in fetal alcohol exposed female rats (Tran and Kelly, 1999). This suggests that although there may be changes caused by FAE on NA production in the PVN, our current findings show that such potential changes in our rats are not observed with alterations in the number or activity of TH-expressing neurons in the PVN.
As prenatal alcohol exposure did not alter the number or activity of TH-ir neurons in the PVN and locus coeruleus, we then considered the possibility that other systems might influence the catecholaminergic neurons that regulate the HPA axis. The GABAergic system is known to exert a strong influence on the PVN (Decavel and Van den Pol, 1990) and locus coeruleus (Olpe et al., 1988; Passerin et al., 2000; Ishida et al., 2002), and synapses on catecholaminergic neurons to regulate their activity. In fact, the PVN is under constant inhibition with the GABAergic neurons in the surrounding tissue that influence the activity of the HPA axis to stress (Herman et al., 2004). We show here that prenatal alcohol does not significantly alter the number of TH positive neurons with GAD65 positive synapses in the PVN. Since GAD65 is only expressed in nerve terminals, we determined colocalization of GAD65-ir and TH-ir as a TH positive cell body with a GAD65 positive puncta overlapping the TH stain. We understand that absolute synapse is difficult to determine without the use of electron microscopy, however, we are confident that the GAD65 positive puncta overlapping a TH cell body stain likely indicates a GABAergic synapse onto a catecholaminergic neuron, since GAD65 is only expressed in nerve terminals (Erlander et al., 1991). Regardless of our technique, there were no discernable changes with GAD65 terminals “touching” TH-ir neurons. Although there was no significant effect of FAE, it may be of future interest to further elucidate the GABAergic system relative to the catecholaminergic neurons in the PVN. Previous studies had shown an effect of FAE on the GABAergic system in relation to the HPG axis (Blaine et al., 1999) and the GABAA receptor (Allan et al., 1998), where there was decreased sensitivity of GABAA receptor stimulation in FAE males but not females. Also, after moderate exposure to alcohol during gestation, adult FAE rats displayed altered sensitivity to the GABAA receptors in the prefrontal cortex, hippocampus, and cerebellum. These studies did not report any changes in the hypothalamus or the locus coeruleus; however, it would be of interest to study GABAA receptors in the context of FAE-induced hyperresponsiveness of the HPA axis.
In light of the projections of adrenergic neurons in the brain stem that heavily innervate the PVN (Cunningham et al., 1990) and influence ACTH release (Baertschi et al., 1976; Reis, 1986), we quantified the number of PNMT expressing neurons in three areas of the brain stem: C1, C2, and C3. We did not observe any alteration in the number of PNMT-ir neurons in these three regions by FAE. Similarly, developmental studies have shown that during normal development, there is no change in the number of medullary catecholamine neurons (C1, C2, and C3) between newborns and adults (Rinaman, 2001). However, we observed increased activity of c-fos in PNMT-ir cells in both C1 and C2 adrenergic areas in offspring exposed to footshocks, as well as FAE-induced hyperactivity in the C1 adrenergic neurons. This area which, together with the LC, represents the origin of most of the catecholaminergic innervation to the PVN, shows increased neuronal activity following exposure of adult rodents to a variety of stressors (ex. haemorrhage, pro-inflammatory cytokines, noise, forced swimming, restraint or shocks) (Li et al., 1996; Li and Sawchenko, 1998; Dayas et al., 2001b) and plays an important functional role in conveying the occurrence of various stressors to the hypothalamus {see (Szafarczyk et al., 1985; Lachuer et al., 1994; Dayas et al., 2001a; Amelita et al., 2004; Hollis et al., 2005)}. These observations suggest that changes in the neuronal activity of the C1/C2 regions such as those observed in our FAE rats, which to our knowledge represent the first report of this phenomenon, are likely to play a role in the enhanced HPA axis function that is the hallmark of this model. Finally, it is of interest to note that the influence exerted by the brain stem appears more robust as it relates to the HPA axis, as we observed no changes with CRF or TH-expressing populations in the PVN or TH-ir in the locus coeruleus. In fact, if the projections from the medulla oblongata to the PVN are transected, footshock-induced c-fos and dopamine β hydroxylase co-expression in the medulla oblongata is ablated (Li et al., 1996), emphasising the importance of the connection to the PVN to elicit the brain stem response to stress. This emphasizes the concept that in our model, increased c-fos expression in the medulla oblongata contributed to, rather than was the result of, increased ACTH release.
A final point pertains to the gender difference in the ACTH response to footshock stress. Several other investigators have reported that in the FAE model, one gender can show an upregulation of the HPA axis while the other does not. For example, Tran and colleagues found FAE-induced increases in hypothalamic NA release of female but not male rats (Tran and Kelly, 1999). Likewise, Osborn and colleagues demonstrate significant increases in CORT release in females but not males upon exposure to the elevated plus maze, with prior experience to an open field (Osborn et al., 1998). Although previous studies conducted in our laboratory found increased ACTH response to footshocks in both females and males (Ogilvie and Rivier, 1997), the amount of alcohol used in the current study was significantly lower than the one we previously used. The work reported by others, and quoted above, suggests that male offspring may be more resistant to the influence of prenatal alcohol. If this is the case, exposing pregnant dams to a smaller dose of alcohol may have allowed effects to manifest themselves in females but not males.
In conclusion, we show here that prenatal alcohol exposure does not produce an alteration in the responsiveness of the HPA axis to adrenergic receptor agonists. Likewise, there were no changes in the number of TH-ir neurons or overall activity in the PVN and locus coeruleus. These negative findings suggest that FAE-induced changes in HPA axis activity are not due to alterations in the cyto-architecture, but are likely from more finite changes that occur within the various neuronal populations that comprise of the HPA axis. On the other hand, prenatal alcohol exposure significantly increased the stress-induced activity of adrenergic neurons in the C1 area of the brain stem, which represents a very novel finding. It is thus tempting to propose that this change contributes, either directly through the hypothalamus or indirectly through the arterial adrenergic receptors, to the well known hyperactivity of the HPA axis of FAE offspring. Nevertheless, these claims of causation will need to be tested experimentally. It is also important to note that other systems may also influence the FAE-induced hyperactivity of the HPA axis. Alterations to GABAA receptor sensitivity in the PVN, as others have shown with FAE in the prefrontal cortex, hippocampus, and cerebellum as well as changes in other downstream receptors all represent potential candidates that mediate FAE-induced hyperactivity of the HPA axis. In future studies, it will be of great interest to determine what role, if any, the changes we observed in the C1 adrenergic neurons of the brain stem play of rats exposed to alcohol prenatally, in mediating the HPA axis hyperactivity that characterizes this model.
Acknowledgments
The authors are grateful to Cristin Roach and Brian Baridon for their excellent technical help, to Dr. Jean Rivier (The Salk Institute, La Jolla, CA) for the generous gift of the GnRH agonist used to synchronize the females’ estrous cycles, to Dr. Paul Sawchenko and Casey Peto for their assistance in capturing the slide images, and to Dr. John Polich (The Scripps Institute, La Jolla, CA) for his valuable help with the statistical analysis of the data. Research supported by NIH grant AA08924.
Abbreviations
- ACTH
adrenocorticotropic homone
- ANOVA
analysis of variance
- BALs
blood alcohol levels
- CRF
corticotrophin-releasing factor
- FAE
fetal alcohol exposure
- FAS
fetal alcohol syndrome
- GAD
glutamic acid decarboxylase
- GC
glucocorticoids
- HPA
hypothalamic-pituitary-adrenal
- ir
immunoreactive
- NA
noradrenaline
- PBS
phosphate buffered saline
- PND
postnatal day
- PNMT
phenylethanolamine N-methyltransferase
- PVN
paraventricular nucleus
- TH
tyrosine hydroxylase
- VP
vasopressin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aird F, Halasz I, Redei E. Ontogeny of hypothalamic corticotropin-releasing factor and anterior pituitary pro-opiomelanocortin expression in male and female offspring of alcohol-exposed and adrenalectomized dams. Alcohol Clin Exp Res. 1997;21:1560–1566. [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- Amelita M, Estacio C, Tukamura H, Reyes B, Uenoyama Y, I’Anson H, Maeda K. Involvement of brainstem catecholaminergic inputs to the hypothalamic paraventricular nucleus in estrogen receptor a expression in thie nucleus during different stress conditions in female rats. Endocrinology. 2004;145:4917–4926. doi: 10.1210/en.2004-0469. [DOI] [PubMed] [Google Scholar]
- Angelogianni P, Gianoulakis C. Prenatal exposure to ethanol alters the ontogeny of the β-endorphin response to stress. Alcoholism: Clin Exper Res. 1989;13:564–571. doi: 10.1111/j.1530-0277.1989.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. Neural Signaling: Molecular signaling within neurons. In: Purves D, Augustine GJ, Fitzpatrick D, Halle W, LaMantia A-S, McNamara JO, White L, editors. Neuroscience. Sunderland: Sinauer Associates Inc; 2008. pp. 153–176. [Google Scholar]
- Baertschi AJ, Ward DG, Gann DS. Role of atrial receptors in the control of ACTH. Am J Physiol. 1976;231:692–699. doi: 10.1152/ajplegacy.1976.231.3.692. [DOI] [PubMed] [Google Scholar]
- Blaine K, Gasser K, Conway S. Influence of fetal alcohol exposure on the GABAergic regulation of growth hormone release in postnatal rats. Alcohol Clin Exp Res. 1999;23:1681–1690. [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcoholism: Clin Exper Res. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001a;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001b;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Douglas AJ. Central noradrenergic mechanisms underlying acute stress responses of the Hypothalamo-pituitary-adrenal axis: adaptations through pregnancy and lactation. Stress. 2005;8:5–18. doi: 10.1080/10253890500044380. [DOI] [PubMed] [Google Scholar]
- Dursun I, Jakubowska-Dogru E, Uzbay T. Effects of prenatal exposure to alcohol on activity, anxiety, motor coordination, and memory in young adult Wistar rats. Pharmacol Biochem Behav. 2006;85:345–355. doi: 10.1016/j.pbb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Yu W, Ellis L, Weinberg J. Postnatal handling does not attenuate hypothalamic-pituitary-adrenal hyperresponsiveness after prenatal ethanol exposure. Alcoholism: Clin Exper Res. 2000;24:1566–1574. [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcoholism: Clin Exper Res. 2000;24:300–306. [PubMed] [Google Scholar]
- Glavas MM, Hofmann CE, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on hypothalamic-pituitary-adrenal regulation after adrenalectomy and corticosterone replacement. Alcoholism: Clin Exper Res. 2001;25:890–897. [PubMed] [Google Scholar]
- Halasz I, Rittenhouse PA, Zorrilla EP, Redei E. Sexually dimorphic effects of maternal adrenalectomy on hypothalamic corticotrophin-releasing factor, glucocorticoid receptor and anterior pituitary POMC mRNA levels in rat neonates. Develop Brain Res. 1997;100:198–204. doi: 10.1016/s0165-3806(97)00033-3. [DOI] [PubMed] [Google Scholar]
- Halasz I, Aird F, Li L, Prystowsky MB, Redei E. Sexually dimorphic effects of alcohol exposure in utero on neuroendocrine and immune functions in chronic alcohol-exposed adult rats. Mol Cell Neurosci. 1993;4:343–353. doi: 10.1006/mcne.1993.1044. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hofmann CE, Ellis L, Yu WK, Weinberg J. Hypothalamic-Pituitary-Adrenal Responses to 5-HT(1A) and 5-HT(2A/C)Agonists Are Differentially Altered in Female and Male Rats Prenatally Exposed to Ethanol. Alcoholism: Clin Exper Res. 2007;31:345–355. doi: 10.1111/j.1530-0277.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- Hollis JH, Lightman SL, Lowry CA. Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic-pituitary-adrenal axis and inhibition of prolactin secretion. J Endocrinol. 2005;184:393–406. doi: 10.1677/joe.1.05839. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Hashiguchi H, Takeda R, Ishizuka Y, Mitsuyama Y, Kannan H, Nishimori T, Nakahara D. Conditioned-fear stress increases Fos expression in monoaminergic and GABAergic neurons of the locus coeruleus and dorsal raphe nuclei. Synapse. 2002;45:46–51. doi: 10.1002/syn.10086. [DOI] [PubMed] [Google Scholar]
- Kang SS, Cole M, Lee S, Rivier C. Development of individual alcohol inhalation chambers for mice: validation in a model of prenatal alcohol. Alcohol Clin Exp Res. 2004;28:1549–1556. doi: 10.1097/01.alc.0000141639.79278.5e. [DOI] [PubMed] [Google Scholar]
- Kim C, Yu W, Edin G, Ellis L, Osborn J, Weinberg J. Chronic intermittent stress does not differentially alter brain corticosteroid receptor densities in rats prenatally exposed to ethanol. Psychoneuroendocrinology. 1999;24:585–611. doi: 10.1016/s0306-4530(99)00015-3. [DOI] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Role of alpha-1-adrenergic receptors in the regulation of corticotropin-releasing hormone mRNA in the paraventricular nucleus of the hypothalamus during stress. Cell Mol Neurobiol. 2000;20:683–694. doi: 10.1023/a:1007098724683. [DOI] [PubMed] [Google Scholar]
- Kornreich WD, Galyean R, Hernandez J-F, Craig AG, Donaldson CJ, Yamamoto G, Rivier C, Vale W, Rivier J. Alanine series of ovine corticotropin releasing factor (oCRF): A structure-activity relationship study. J Med Chem. 1992;35:1870–1876. doi: 10.1021/jm00088a024. [DOI] [PubMed] [Google Scholar]
- Lachuer J, Delton I, Buda M, Tappaz M. The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain Res. 1994;638:196–202. doi: 10.1016/0006-8993(94)90650-5. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim C, Rivier C. Nitric oxide stimulates ACTH secretion and the transcription of the genes encoding for NGFI-B, corticotropin-releasing factor, corticotropin-releasing factor receptor type 1 and vasopressin in the hypothalamus of the intact rat. J Neurosci. 1999;19:7640–7647. doi: 10.1523/JNEUROSCI.19-17-07640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Blanton C, Rivier C. Prenatal ethanol exposure alters the responsiveness of the rat hypothalamic-pituitary-adrenal axis to nitric oxide. Alcoholism: Clin Exper Res. 2003;27:962–969. doi: 10.1097/01.ALC.0000076120.67014.ED. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Rivier C. Increased activity of the hypothalamic-pituitary-adrenal axis in rats exposed to alcohol in utero: Role of altered pituitary and hypothalamic function. Mol Cell Neurosci. 2000a;16:515–528. doi: 10.1006/mcne.2000.0890. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcoholism: Clin Exper Res. 2000b;24:110–122. [PubMed] [Google Scholar]
- Lee SY, Imaki T, Vale W, Rivier CL. Effect of prenatal exposure to ethanol on the activity of the hypothalamic-pituitary-adrenal axis of the offspring: importance of the time of exposure to ethanol and possible modulating mechanisms. Mol Cell Neurosci. 1990;1:168–177. doi: 10.1016/1044-7431(90)90022-v. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Diaz S, Tempel D. Norepinephrine in the paraventricular nucleus stimulates corticosterone release. Brain Res. 1989;496:219–227. doi: 10.1016/0006-8993(89)91069-x. [DOI] [PubMed] [Google Scholar]
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393:244–266. [PubMed] [Google Scholar]
- Li HY, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, Turchan J, Smialowska M, Przewlocka B. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003;37:105–110. doi: 10.1016/s0143-4179(03)00021-0. [DOI] [PubMed] [Google Scholar]
- McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE. Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol. 2005;17:475–482. doi: 10.1111/j.1365-2826.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Fetal alcohol exposure alters the induction of immediate early gene mRNA in the rat prefrontal cortex after an alternation task. Alcoholism: Clin Exper Res. 1995;19:1389–1397. doi: 10.1111/j.1530-0277.1995.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcoholism: Clin Exper Res. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Prenatal alcohol exposure results in hyperactivity of the hypothalamic-pituitary-adrenal axis of the offspring: modulation by fostering at birth and postnatal handling. Alcohol Clin Exp Res. 1997;21:424–429. doi: 10.1111/j.1530-0277.1997.tb03786.x. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Steinmann MW, Hall RG, Brugger F, Pozza MF. GABAA and GABAB receptors in locus coeruleus: effects of blockers. Eur J Pharmacol. 1988;149:183–185. doi: 10.1016/0014-2999(88)90061-1. [DOI] [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal ethanol exposure differentially alters behavior in males and females on the elevated plus maze. Alcohol Clin Exp Res. 1998;22:685–696. [PubMed] [Google Scholar]
- Osborn JA, Yu C, Stelzl GE, Weinberg J. Effects of fetal ethanol exposure on pituitary-adrenal sensitivity to secretagogues. Alcoholism: Clin Exper Res. 2000;24:1110–1119. [PubMed] [Google Scholar]
- Osborn JA, Kim CK, Yu W, Herbert L, Weinberg J. Fetal ethanol exposure alters pituitary-adrenal sensitivity to dexamethasone suppression. Psychoneuroendocrinology. 1996;21:127–143. doi: 10.1016/0306-4530(95)00037-2. [DOI] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Res. 1992;589:91–96. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Cano G, Rabin BS, Delano BA, Napier JL, Sved AF. Role of locus coeruleus in foot shock-evoked Fos expression in rat brain. Neuroscience. 2000;101:1071–1082. doi: 10.1016/s0306-4522(00)00372-9. [DOI] [PubMed] [Google Scholar]
- Redei E, Halasz I, Li L-F, Prystowsky MB, Aird F. Maternal adrenalectomy alters the immune and endocrine functions of fetal alcohol-exposed male offspring. Endocrinology. 1993;133:452–460. doi: 10.1210/endo.133.2.8344191. [DOI] [PubMed] [Google Scholar]
- Reis DJ. The C1 area of rostra1 ventrolateral medulla: Role in tonic and reflex regulation of arterial pressure. In: Magro A, Osswald W, Reis D, Vanhoutte P, editors. Central and Peripheral Mechanisms of Cardiovascular Regulation. New York: Kluwer Academic Pub; 1986. pp. 487–502. [Google Scholar]
- Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- Rivest S, Rivier C. Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol. 1994;6:101–117. doi: 10.1111/j.1365-2826.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide and the HPA response to stress. Pharmacol Biochem Behav. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cytokines act within the brain to inhibit LH secretion and ovulation in the rat. Endocrinology. 1990;127:849–856. doi: 10.1210/endo-127-2-849. [DOI] [PubMed] [Google Scholar]
- Rivier C, Grigoriadis D, Rivier J. Role of corticotropin releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology. 2003;144:2396–2403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. In: Mayer E, Saper C, editors. Progress in Brain Research. Elsevier Sciences; 2000. pp. 61–78. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Cunningham ET, Bittencourt JC. Aminergic and peptidergic pathways subserving the stress response. In: Kvetnansky R, McCarty R, Axelrod J, editors. Stress: Neuroendocrine and Molecular Approaches. New York: Gordon and Breach; 1992. pp. 15–27. [Google Scholar]
- Seo D, Lee S, Rivier C. Role of specific adrenergic receptors in mediating the ACTH response to increased nitric oxide levels. J Neuroendocrinol. 2003;15:530–537. doi: 10.1046/j.1365-2826.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- Seo DO, Rivier C. Microinfusion of a nitric oxide donor in discrete brain regions activates the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13:925–933. doi: 10.1046/j.1365-2826.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y. Adrenoceptor antagonists block c-fos response to stress in the mouse brain. Brain Res. 1995;694:279–286. doi: 10.1016/0006-8993(95)00882-q. [DOI] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiol Behav. 2002;77:737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Alonso G, Ixart G, Malaval F, Assanmacher I. Diurnal-stimulated and stress-induced ACTH release in rats is mediated by ventral noradrenergic. Am J Physiol. 1985;249:E219–E226. doi: 10.1152/ajpendo.1985.249.2.E219. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacol Biochem Behav. 1982;16:585–589. doi: 10.1016/0091-3057(82)90420-8. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Nelson LR, Branch BJ, Kokka N, Poland RE. Altered stress responsiveness in adult rats exposed to ethanol in utero: neuroendocrine mechanisms. Ciba Found Symp. 1984;105:47–65. doi: 10.1002/9780470720868.ch4. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tritt SH, Tio DL, Romeo HE, Yirmiya R. Maternal adrenalectomy abrogates the effect of fetal alcohol exposure on the interleukin-1beta-induced febrile response: gender differences. Neuroendocrinology. 2002;76:185–192. doi: 10.1159/000064524. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Alterations in hippocampal and hypothalamic monoaminergic neurotransmitter systems after alcohol exposure during all three trimester equivalents in adult rats. J Neural Transm. 1999;106:773–786. doi: 10.1007/s007020050198. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM. Activation of the locus ceruleus brain noradrenergic system during stress: circuitry, consequences, and regulation. Adv Pharmacol. 1998;42:781–784. doi: 10.1016/s1054-3589(08)60863-7. [DOI] [PubMed] [Google Scholar]
- Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Hyperresponsiveness to stress: Differential effects of prenatal ethanol on males and females. Alcoholism: Clin Exper Res. 1988;12:647–652. doi: 10.1111/j.1530-0277.1988.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol effects: Sex differences in offspring stress responsiveness. Alcohol. 1992a;8:219–223. doi: 10.1016/0741-8329(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Prenatal ethanol exposure alters adrenocortical response to predictable and unpredictable stressors. Alcohol. 1992b;9:427–432. doi: 10.1016/0741-8329(92)90043-a. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Tio DL, Taylor AN. Effects of fetal alcohol exposure on fever, sickness behavior, and pituitary-adrenal activation induced by interleukin-1β in young adult rats. Brain, Behav Immun. 1996;10:205–220. doi: 10.1006/brbi.1996.0019. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Chiappelli F, Tio DL, Tritt SH, Taylor AN. Effects of prenatal alcohol and pair feeding on lipopolysaccharide-induced secretion of TNF-α and corticosterone. Alcohol. 1998;15:327–335. doi: 10.1016/s0741-8329(97)00153-5. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Neurocircuitry of stress integration: Anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integ Comp Biol. 2002;42:541–551. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]


