Abstract
To appropriately respond to an affective stimulus, we must be able to track its value across changes in both the external and internal environment. The nucleus accumbens (NAc) is a critical component of reward circuitry, but recent work suggests that the NAc encodes aversion as well as reward. It remains unknown whether differential NAc activity reflects flexible changes in stimulus value when it is altered due to a change in physiological state. We measured the activity of individual NAc neurons when rats were given intraoral infusions of a hypertonic salt solution (0.45 M NaCl) across multiple sessions in which motivational state was manipulated. This normally nonpreferred taste was made rewarding via sodium depletion, which resulted in a strong motivation to seek out and consume salt. Recordings were made in three conditions: while sodium replete (REP), during acute sodium depletion (DEP), and following replenishment of salt to normal sodium balance (POST). We found that NAc neurons in the shell and core subregions responded differently across the three conditions. In the shell, we observed overall increases in NAc activity when the salt solution was nonpreferred (REP) but decreases when the salt solution was preferred (DEP). In the core, overall activity was significantly altered only after sodium balance was restored (POST). The results lend further support to the selective encoding of affective stimuli by the NAc and suggest that NAc shell is particularly involved in flexibly encoding stimulus value based on motivational state.
Keywords: reward, aversion, taste, motivation, electrophysiology
proper affective encoding of stimuli is necessary to guide appropriate behavioral responses for survival. The nucleus accumbens (NAc) integrates sensory, affective, and contextual information to direct behavior (Mogenson et al. 1980). Furthermore, altered signaling within the NAc underlies, in part, inappropriate affective responses in nonclinical (Wacker et al. 2009) and clinical populations (Keedwell et al. 2005). Although the NAc has been primarily linked to reward and positive affective stimuli (Carelli 2002; Day et al. 2006; Nicola et al. 2004a, 2004b), recent evidence suggests that it also plays a role in the full spectrum of affective responses, from reward to aversion. Human imaging studies (Cooper and Knutson 2008) as well as electrophysiological recordings in rats (Roitman et al. 2005; Wheeler et al. 2008) demonstrate that the NAc not only processes both rewarding and aversive stimuli but encodes them in qualitatively different ways. For example, individual NAc neurons respond primarily with decreases in activity in response to the rewarding taste of sucrose (Roitman et al. 2005). These decreases appear to be related to approach and consummatory behavior (Krause et al. 2010) and have been proposed to permit the execution of reward-guided behavior (Taha and Fields 2006). In contrast, the taste of quinine, which is aversive, elicits primarily increases in NAc activity (Roitman et al. 2005). These decreases and increases in NAc activity have been postulated to encode positive and negative affect, respectively (Carlezon and Thomas 2009).
Stimuli are rarely exclusively rewarding or aversive but generate positive or negative biases toward them, which may shift with changing circumstances. To test how the encoding of affective responses in NAc to a single stimulus may be altered by changes in physiological state, we employed the motivated behavior of salt appetite. Although animals, including humans, show a preference to ingest salty foods, the taste of highly concentrated salt solutions (such as seawater) is typically nonpreferred by animals. However, when faced with a need for sodium due to dietary deficiency or diuretic treatment, animals actively seek out and ingest concentrated salt solutions that they would otherwise avoid (Geerling and Loewy 2008). This behavior relies on the rapid identification of sodium salt by its taste (Breslin et al. 1993), the palatability of which is enhanced under sodium deprivation (Berridge et al. 1984). Thus experimenter-induced sodium depletion and the resultant salt appetite provide a model system to study shifts in adaptive affective encoding.
We measured the responses of individual neurons in the rat NAc to brief intraoral infusions of a concentrated (0.45 M) NaCl solution when the taste was either avoided (positive sodium balance) or avidly consumed (negative sodium balance). Taste infusions were not dependent on any operant behavior, nor were they predicted by cues. Therefore, we measured NAc responses to the motivational value of the same primary taste stimulus. We expect the taste of a concentrated salt solution to drive overall increased activity in the NAc when animals do not prefer its taste under positive sodium balance. If NAc encoding of affective stimuli is updated by a change in internal state, then following sodium depletion, when the taste of hypertonic salt solution is more rewarding, an overall decrease in the NAc response should be elicited.
METHODS AND MATERIALS
Subjects.
Sixteen male Sprague-Dawley rats (350–400 g) were housed individually and maintained on a 12:12-h light-dark cycle (on at 7:00 AM). Experiments were conducted between 10:00 AM and 5:00 PM. Laboratory chow (LabDiet 5012; Richmond, IN) and water were provided ad libitum except where noted. Animals were treated in accordance with the guidelines put forth by the National Institutes of Health and under the approval of the Animal Care Committee of the University of Illinois at Chicago.
Surgical procedures.
Under ketamine (100 mg/kg ip) and xylazine (20 mg/kg ip) anesthesia, rats were implanted with intraoral (IO) cannulas and two microwire electrode arrays. IO cannulas were inserted just lateral to the first maxillary molar and exteriorized through an incision at the top of the head. Two electrode arrays were implanted bilaterally at AP +1.5, ML ±1.2 to ±0.9 relative to bregma, and −6.5 relative to brain surface. Each array was organized into two columns of four microwires (diameter 50 μm, tip separation 0.25 mm; MicroProbes for Life Science, Gaithersburg, MD). Ground wires from each array were wrapped around a skull screw and implanted at a remote location ∼1 mm into the brain. Electrode arrays and IO cannulas were held in place with dental acrylic adhered to skull screws. All rats were given a 10-day recovery period.
Apparatus.
Recording sessions took place in a 32 × 32 × 32-cm chamber with a house light and a white noise generator (Med Associates, St. Albans, VT) housed within a sound-attenuated box. An infusion line from a 60-ml syringe containing 45 ml of 0.45 M NaCl was attached to a solenoid valve (The Lee Company, Westbrook, CT) placed above the chamber. The infusion line passed through a commutator (Crist Instruments, Hagerstown, MD) and was connected to the IO cannula of the rat.
Experimental design.
To measure NAc activity in response to the taste of salt, we recorded from single neurons during three separate sessions: repletion (REP), with rats having been maintained on normal chow; depletion (DEP), 24 h following sodium depletion via the diuretic furosemide (detailed below), and postreplenishment (POST), defined as 3 days following restoration of positive sodium balance. Each recording session consisted of 30 trials in which a solenoid valve opened for 4 s to deliver an IO infusion of 200 μl of 0.45 M NaCl. After the valve closed, a 6-s postinfusion epoch concluded the trial. A variable intertrial interval (60-s average) followed each trial to prevent anticipation of NaCl solution delivery. Before the three experimental sessions, rats were given one session to acclimate them to the testing apparatus and procedure. In this session, rats were placed in the recording chamber, attached to the IO infusion line, and administered 30 infusions of distilled water according to the parameters of the test sessions.
Two-bottle intake test.
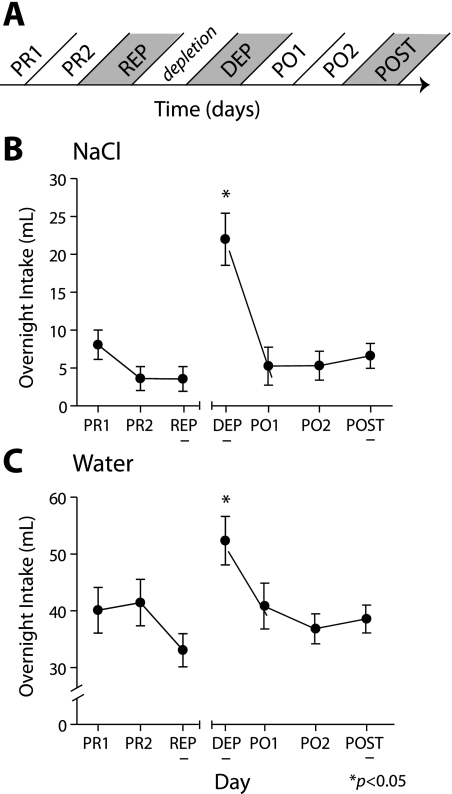
Two-bottle intake tests were administered to measure rats' free consumption of distilled water and 0.45 M NaCl overnight from about 5:00 PM to about 10:00 AM. Intake tests were administered for 2 days before the first recording session (PR1 and PR2), the evenings after the REP and DEP recording sessions, and for the 3 following nights (PO1, PO2, and POST). On the evening of sodium depletion, the test was not administered. Figure 1A illustrates the experimental timeline, detailing the schedule for two-bottle intake tests and recording sessions. For the 3 days corresponding to the recording sessions, intake data from two animals were lost. Both of these animals satisfied the weight loss criterion for sodium depletion (below); hence, their neural data were included for analysis. For days PO1 and PO2, intake data were collected from 10 and 13 rats, respectively.
Fig. 1.
Time course of experimental procedures and behavioral results. A: over the course of the experiment, 2-bottle preference tests were conducted to measure free consumption of 0.45 M NaCl and distilled water daily from 5:00 PM to 10:00 AM. Tests were administered on the 2 nights before (PR1 and PR2) the first recording session; the night after the first, sodium-replete (REP) recording session; the night after the second, sodium-deplete (DEP) recording session; and for 3 days after the DEP session (PO1, PO2, and POST). Recording sessions were run in the afternoons (∼12:00 PM) on the REP, DEP, and POST days (shaded). B: intake of 0.45 M NaCl increased following diuretic treatment (all days n = 14, except PO1, n = 10, and PO2, n = 13). C: intake of water was also elevated following diuretic treatment. Asterisks indicate significantly higher intake on the DEP day than any other day for both NaCl and water.
Sodium depletion.
Rats were depleted of sodium by administration of two injections of furosemide (10 mg/kg) spaced 1 h apart. Sodium depletion was verified by weight loss of 20 g 1 h following the second injection. Rats were maintained on a sodium-deficient diet (Teklad Sodium Deficient Diet; Harlan, Madison, WI) and distilled water for 24 h. After the ensuing recording session (DEP), rats were given free access to normal chow and overnight access to 0.45 M NaCl to restore lost sodium.
Electrophysiology.
Electrophysiological recordings were conducted as previously described (Roitman and Roitman 2010; Roitman et al. 2005). Briefly, each animal was connected to a flexible recording cable (Plexon, Dallas, TX) attached to a commutator to allow for relatively free movement. Signals were amplified and transduced to a multichannel acquisition processor (MAP System; Plexon). The MAP system permitted discrimination of neuronal output by principal component analysis. A second computer recorded the time of solenoid valve openings and permitted time-stamping of this event onto the neural spike activity.
During recording, principal component analysis was used to identify waveforms belonging to individual neurons (SortClient; Plexon), which were subsequently sorted offline (Offline Sorter; Plexon). The timing of action potentials relative to solenoid opening was calculated for each cell from −10 to +10 s in 100-ms bins (NeuroExplorer; Nex Technologies, Littleton, MA), and data were exported to Statistica 8 (StatSoft, Tulsa, OK) for analysis.
Data analysis.
To determine whether the activity of a neuron was modulated in response to IO infusions of salt solution, we computed the firing rate on each trial during three epochs: baseline (10 s preceding onset of infusion), infusion (4 s of infusion), and postinfusion (6 s following offset of infusion). Our primary interest was to determine which neurons showed a change in firing rate during the infusion epoch. In addition, we included analysis of the postinfusion epoch to determine whether there were differences in the persistence of neural modulation following the infusion across sessions. We compared firing rate across trial epochs (baseline × infusion × postinfusion) using one-way ANOVAs. Units with a significant ANOVA were analyzed using Dunnett's tests to identify neurons with significant differences in firing rate between baseline and infusion epochs. These neurons were classified as “phasic,” and the direction of the change (increase or decrease) determined whether they were categorized as decreasing (DEC) or increasing (INC). In these neurons, we measured whether the modulation of response persisted beyond the infusion epoch by using Dunnett's test to identify differences between baseline and postinfusion epochs. Neurons without a significant change in firing rate were classified as nonphasic. The proportions of INC and DEC cells were compared across sessions (REP, DEP, and POST) using χ2 tests.
To compare activity across populations of phasic neurons, we standardized firing rate in 1-s bins from −10 to +10 s relative to infusion onset as a z-score based on the firing rate during the 10-s baseline period. Standardized firing rates during the infusion period were compared across sessions (REP, DEP, and POST) using one-way ANOVA for INC and DEC cells separately and across all phasic cells together. Neurons recorded from the shell and core were analyzed separately. Comparisons of core and shell responses were also made using the average standardized firing rates during the 4-s infusion period in a 3 (REP, DEP, POST) × 2 (shell, core) ANOVA. Main effects were further tested with Tukey's honestly significant difference (HSD) post hoc analysis.
Behavioral data were analyzed to determine the effect of sodium depletion on the intake of 0.45 M NaCl and distilled water using two separate one-way ANOVAs comparing intake across test days.
Histological verification of electrode placements.
At the conclusion of testing, rats were injected with a sublethal dose of pentobarbital sodium (100 mg/kg). Current (100 μA) was passed for 4 s through each electrode with a lesion-making device (UGO Basile S.R.I.; Comerio, Varse, Italy) to mark recording sites. Rats were then transcardially perfused with physiological saline followed by 10% paraformaldehyde mixed with 3% potassium ferrocyanide. Brains were extracted and stored in paraformaldehyde-potassium ferrocyanide for at least 24 h before being sectioned (50-μm coronal slices through the NAc) in a cryostat (−20°C). Potassium ferrocyanide reacts with iron deposited after lesions and causes a Prussian blue reaction product, which was used to help visualize electrode placements. Sections were mounted on gelatin-subbed slides and viewed under a light microscope to verify electrode placement according to visual landmarks in a stereotaxic atlas (Paxinos and Watson 2007) (Supplemental Fig. S1). (Supplemental material for this article is available online at the Journal of Neurophysiology website.)
RESULTS
Sodium depletion increases intake of concentrated NaCl solution.
Rats exhibited a robust increase in voluntary intake of concentrated salt solution following sodium depletion. Across days in which rats were sodium replete (PR1, PR2, REP, PO1, PO2, and POST; Fig. 1A), average intake was 5.41 ± 0.75 ml of salt solution and 38.39 ± 1.38 ml of distilled water (Fig. 1, B and C). Intake of salt solution varied significantly across days (Fig. 1B, F6,86 = 9.25, P < 0.05) and was significantly higher (22.0 ± 3.43 ml) on the DEP day than all other days (post hoc Tukey's HSD, P < 0.05). Water intake also varied across days (Fig. 1C, F6,86 = 2.95, P < 0.05), with greater intake on the DEP day (52.36 ± 4.27 ml) than on both REP and PO2 (Tukey's HSD, P < 0.05). The level of consumption following the DEP session represents a 407% increase in salt solution and a 36% increase in water compared with days in which rats were sodium replete, confirming that sodium depletion effectively altered rats' motivation for, and behavior toward, concentrated salt solution.
Brief oral infusion of hypertonic saline elicits responses in individual neurons.
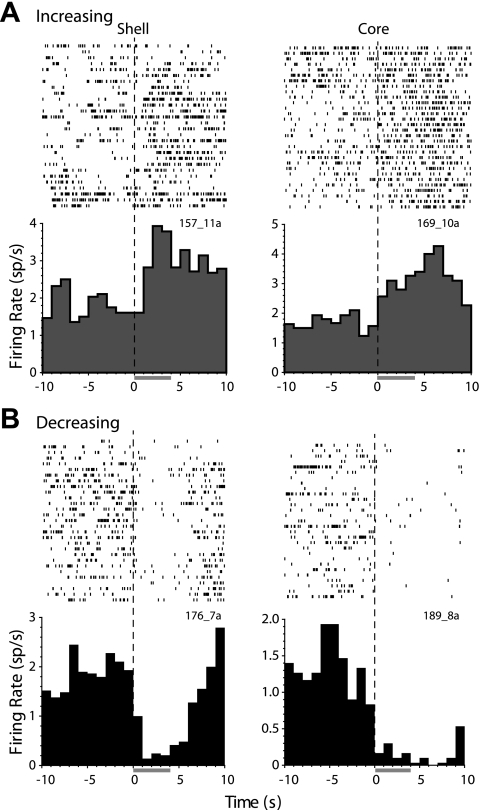
Brief IO infusions of salt solution elicited phasic changes in the activity of individual NAc neurons in both the shell and core subregions. We observed individual neurons with increased (INC; Fig. 2A) or decreased (DEC; Fig. 2B) firing rate during the 4-s infusion. Both INC and DEC responses are similar to those found previously to IO infusions of other taste stimuli (Roitman et al. 2005). In previous studies, we have observed that NAc neurons are more likely to respond with increasing activity to taste stimuli that are avoided and with decreasing activity to stimuli that are rewarding (Roitman et al. 2005, 2010). We analyzed whether the proportions of INC and DEC neurons in the shell and core of the NAc would track rats' preferences for the taste of concentrated salt, leading to shifts in the population output.
Fig. 2.
Examples of individual nucleus accumbens (NAc) shell and core neurons with phasic responses to taste infusions of 0.45 M NaCl. A: phasic increasing (INC) responses. Top: plots of the time of each action potential relative to the onset of the intraoral (IO) infusion (dashed vertical line, time 0) for each trial. Activity is shown for the baseline (−10 to 0 s), infusion (0–4 s, shaded horizontal bar), and postinfusion epochs (4–10 s). Bottom: peri-event histograms show the average response across trials in 1-s bins. B: phasic decreasing (DEC) responses (same conventions as in A).
NAc shell tracks motivational value of hypertonic salt solution.
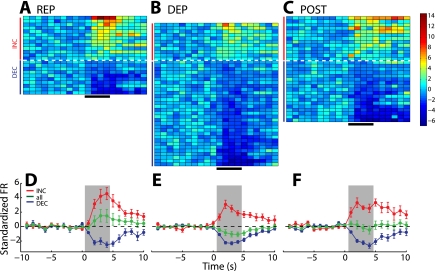
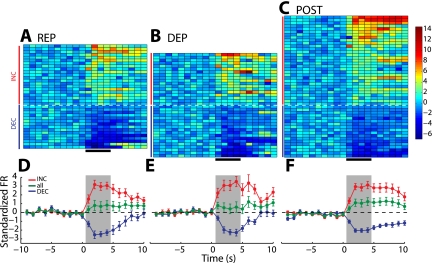
From 77 electrode placements in the NAc shell, we recorded 78 individual neurons on the REP day, 76 on the DEP day, and 70 on the POST day. To determine how the preference for the taste of hypertonic salt solution was encoded in neural activity, we first compared the proportions of INC and DEC neurons across test days (Table 1). Each row in Fig. 3, A–C, shows the average standardized response for one phasic neuron recorded in each session, sorted by response magnitude during the infusion period. On the REP day, when rats were not motivated to consume concentrated salt solution, the majority of phasic neurons (13/23, 57%) responded with INC activity. On the DEP day, following depletion but preceding the opportunity to restore sodium loss, IO infusions of salt solution led to a different pattern of neural responses. Of the phasic neurons recorded, the majority (31/42, 74%) responded with DEC activity [Fig. 3, A vs. B: χ2(1, n = 42) = 15.72, P < 0.0001]. On the POST day, when rats had returned to positive sodium balance, the proportions of INC and DEC neurons did not differ from the those on the REP day [Fig. 3, A vs. C: χ2(1, n = 31) = 2.68, P = 0.10]. Although the total number of INC cells was similar across the three sessions (REP, n = 13; DEP, n = 11, POST, n = 13), the difference results from a greater number of DEC neurons on the DEP day (REP, n = 10; DEP, n = 31; POST, n = 18).
Table 1.
Number of cells by response type for shell and core for each recording session
| Response |
|||||
|---|---|---|---|---|---|
| Region | NP | INC | DEC | Total | No. of Rats |
| Shell | |||||
| REP | 55 | 13 | 10 | 78 | 9 |
| DEP | 34 | 11 | 31 | 76 | 13 |
| POST | 39 | 13 | 18 | 70 | 9 |
| Core | |||||
| REP | 71 | 21 | 15 | 107 | 12 |
| DEP | 55 | 18 | 18 | 91 | 12 |
| POST | 39 | 31 | 18 | 88 | 13 |
Data are numbers of cells with nonphasic (NP), increasing (INC), or decreasing (DEC) response in the shell and core of the nucleus accumbens during the sodium repleted (REP), sodium depleted (DEP), and postreplenishment (POST) recording sessions.
Fig. 3.
NAc shell population response tracks the hedonic value of NaCl taste across different physiological states. Each row of the color plots represents the average standardized response of 1 phasic neuron across 30 infusion trials in the REP (A), DEP (B), and POST (C) conditions. For each plot, activity is shown from 10 s before to 10 s after the onset of the infusion (in 1-s bins; black horizontal bar at bottom marks 4-s infusion period). Color indicates standardized firing rate for each neuron relative to 10-s baseline, as indicated by the legend at right. INC neurons are plotted above the dashed white line (red vertical bars) and DEC below (blue vertical bars). On the DEP day, there was a greater proportion of DEC neurons (31/42) compared with the REP (10/23) and POST (18/31) sessions. The graphs plot the average standardized firing rate across neurons in the REP (D), DEP (E), and POST (F) conditions. For each day, average standardized firing rate is shown for INC neurons (red, corresponding to those marked by red vertical bars in A–C) and DEC neurons (blue, corresponding to those marked by blue vertical bars) separately. The overall response average across all phasic neurons (INC and DEC together) is shown in green. Time scale is the same as in A–C, with the 4-s infusion period indicated by the gray shaded background.
We considered the possibility that the act of sodium depletion itself could cause an increase in the overall basal level of activity in the NAc and as a consequence would make it more likely to observe reductions in activity from this elevated baseline. However, there is no evidence to support a change in overall basal activity on the DEP day. Activity during the baseline epoch did not differ between cell types (INC vs. DEC, F1,90 = 0.90, P = 0.35) or across sessions (F2,90 = 0.36, P = 0.70), nor was there an interaction (F2,90 = 1.05, P = 0.35; Supplemental Fig. S2A). We also failed to detect an increase in the proportion of phasic neurons with changes in activity that persisted into the postinfusion interval (Supplemental Table S1). Thus sodium depletion did not result in a generalized change in the basal level of activity.
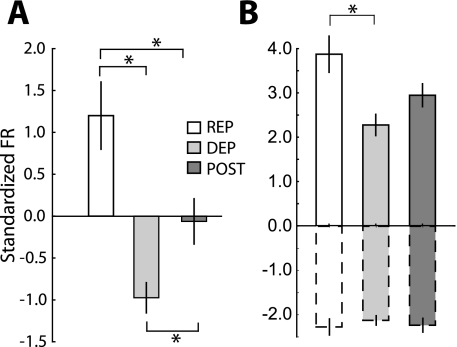
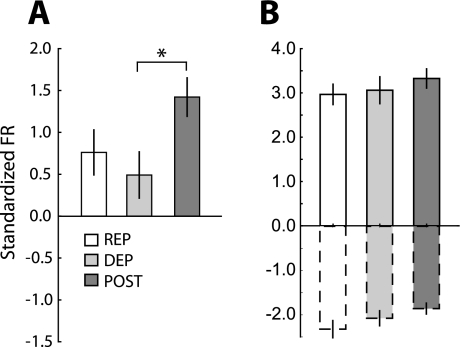
In the NAc shell, the shift in predominant response type from INC to DEC on the DEP day was reflected with a change in the average firing rate across all phasic neurons. Figure 3, D–F, shows the average standardized activity of all neurons together (green), as well as INC (red) and DEC (blue) neurons separately. We compared responses during the 4 s of infusions (gray shading) to determine differences across conditions. Neural responses across all phasic neurons significantly differed across the three conditions (Fig. 4A, F 2,381 = 15.96, P < 0.00001). During this epoch, the overall response increased above baseline on the REP day, decreased below baseline on the DEP day, and did not deviate from baseline on the POST day. Responses on the DEP day differed significantly from both the REP (post hoc, P < 0.001) and POST days (P < 0.05). Responses also differed between the REP and POST days (P < 0.01).
Fig. 4.
Magnitude of NAc shell responses during the 4-s infusion period in each condition. A: average standardized firing rate across all phasic neurons (INC and DEC). Values are averages of the firing rates shown by the green lines in Fig. 4, D–F (gray shading). Across all phasic neurons, the overall average response shifted from an increase in activity on the REP day (n = 23) to a decrease on the DEP day (n = 42) and an intermediate response on the POST day (n = 31). Asterisks indicate significant differences between conditions (P < 0.05). B: across the 3 sessions, the magnitude of the INC neurons (bars with solid outline) and DEC neurons (bars with dashed outline) separately. Values are averages of the firing rates shown by the red and blue lines (INC and DEC, respectively) in Fig. 4. D–F (gray shading). Asterisk indicates a higher magnitude in the INC response on the REP day compared with DEP. There were no differences in the magnitude in DEC responses.
The change from an overall increase in activity on the REP day to an overall decrease on the DEP day could be due to a change in the proportions of the phasic cell types, a change in response magnitude for either phasic cell type, or both. For INC neurons, there was a significant difference in the magnitude of response during the taste infusion across recording sessions (Fig. 4B, bars with solid outline, F2,145 = 6.162, P < 0.01), with larger magnitude increases in firing rate on the REP compared with DEP day (post hoc, P < 0.01). However, there were no differences in the magnitude of reductions in firing rate in DEC neurons across conditions (Fig. 4B, bars with dashed outline, F2,233 = 0.31, P = 0.73). We also tested whether response of each neuron changed within the time course of each test session. We did not find any systematic differences in firing rate of neurons from the beginning of the session and the end, in either INC or DEC neurons (Supplemental Fig. S3, A and B). On the REP day, the overall increase in activity may result from both the greater proportion of INC neurons as well as the larger magnitude of INC response. On the DEP day, the change in the overall response from positive to negative results from the greater proportion of DEC neurons contributing to the average response.
Responses in NAc core are altered following reinstatement of sodium balance.
From 110 electrode placements in NAc core, we recorded the activity of 107 neurons on the REP day, 91 on the DEP day, and 88 on the POST day. Individual neurons were classified as INC, DEC, and nonphasic as described above (Table 1). Each row in Fig. 5, A–C, shows the average standardized firing rate for one phasic neuron recoded from NAc core, sorted by magnitude of the response during the taste infusion. In the core we recorded similar numbers of DEC neurons across conditions (REP, n = 15; DEP, n = 18; POST, n = 18). There was an increase in the number of INC neurons recorded during the POST condition compared with the previous two recording sessions (REP, n = 21; DEP, n = 18; POST, n = 31). However, unlike the shell, there were no significant differences in the proportions of INC and DEC cells on the DEP and POST days compared with the REP day [Fig. 5, A vs. B: χ2(1, n = 36) = 1.03, P = 0.31; Fig. 5, A vs. C: χ2(1, n = 49) = 0.49, P = 0.41]. As in the shell, there was no difference in baseline firing rate between the types of neurons (INC vs. DEC: F1,115 = 0.59, P = 0.45) or across test conditions (F2,115 = 1.24, P = 0.29), nor was there an interaction between type and condition (F2,115 = 2.71, P = 0.07, Supplemental Fig. S2B). There was also no increase in the proportion of neurons with phasic responses that persisted into the postinfusion period (Supplemental Table S1).
Fig. 5.
NAc core population response to NaCl taste shows different pattern from NAc shell across physiological states. Neurons in NAc core show INC responses to the taste of NaCl in 21/37 neurons on the REP day (A), 18/36 neurons on the DEP day (B), and 31/49 neurons on the POST day (C). The graphs plot the average standardized firing rate across neurons in the REP (D), DEP (E), and POST (F) conditions (same conventions as in Fig. 3).
Across sessions, we did observe elevated responses in NAc core on the POST day. Figure 5, D–F, shows the overall average standardized firing rate for each session (green), as well as the averages of INC (red) and DEC (blue) neurons separately. During the 4 s on IO infusions, overall average activity differed across conditions (Fig. 6A, F 2,481 = 3.755, P < 0.05), with significantly higher activity during the POST session than the DEP session (post hoc, P = 0.024). For both INC and DEC neurons, there were no differences in the magnitude of response across sessions (Fig. 6B: INC, bars with solid outline: F2,227 = 0.652, P = 0.52; DEC, bars with dashed outline: F2,201 = 1.998, P = 0.14). We also measured whether there was any modulation of the strength of the each neuron's response from the beginning to the end of each session but did not find evidence of such differences (Supplemental Fig. S3, C and D). Because there were no differences in the magnitude of increasing and decreasing neural responses, the change in overall population response on the POST day is likely due to the increase in the proportion of INC neurons.
Fig. 6.
Magnitude of NAc core responses during the 4-s infusion period in each condition. A: average standardized firing rate across all phasic neurons (INC and DEC). Asterisk indicates a larger increase in the overall response on the POST day (n = 49) compared with the DEP day (n = 36). Average response on the REP day (n = 36) did not differ from the other sessions. B: there were no differences in the magnitudes of INC or DEC responses separately across the 3 sessions (same conventions as in Fig. 4).
A comparison of the average overall neural activity across sessions in the two subregions, shell (Fig. 4A) and core (Fig. 6A), revealed very different patterns of responding. We found a significant interaction between region (shell, core) and condition (DEP, REP, POST) on the level of activity during sodium infusions (F2,862 = 7.687, P < 0.001). The average activity in the shell on the DEP day was significantly different from the level of activity in the core during all three test sessions (post hoc, DEP/shell vs. REP/core, P < 0.001; vs. DEP/core, P < 0.001; vs. POST/core, P < 0.0001). In addition, the average response in the core on the POST day was significantly higher than the response in the shell on the POST day (post hoc, P < 0.001).
We further investigated whether there was a relationship between the neural response and behavior of individual subjects. If stimuli with a positive motivational value are encoded by an overall decrease in NAc activity, then we should be able to positively correlate decreases in NAc activity to the taste of salt and salt consumption. To test this, we correlated the percentage of DEC neurons recorded during the session with the amount of salt solution consumed during the two-bottle intake test that followed the session for each subject. We found a weak positive correlation between the percentage of DEC neurons in the shell and intake [Supplemental Fig. S4A: r2(38) = 0.09, P = 0.07]. No such relationship was observed in the core [Supplemental Fig. S4B: r2(42) = 0.04, P = 0.22]. The correlation may not have achieved significance in the shell due to the fact that our measure of NaCl palatability was not concurrent with our measure of neuronal activity. Intakes were measured for the 12-h dark phase following the test sessions. However, the trend was stronger in the shell, consistent with previous evidence from pharmacological studies suggesting a role for the shell in processing the affective value of primary taste stimuli (Kelley et al. 2005; Pecina and Berridge 2005).
DISCUSSION
The NAc is most widely considered as a critical cog in the brain's reward system. A growing literature using functional MRI and human subjects suggests that the NAc plays an even wider role in affect by, in part, encoding the hedonic valence of stimuli (Cooper and Knutson 2008; Levita et al. 2009). Our data support a systems-level mechanism by which the NAc encodes hedonic valence. In the present study, we used a single stimulus and altered its motivational value by manipulating physiological state. We enhanced the reward value of a normally nonpreferred hypertonic salt solution by acutely depleting rats of sodium. In two-bottle intake tests, rats showed an enhanced preference for hypertonic salt solution following sodium depletion, as shown previously (Berridge et al. 1984; Sakai et al. 1987). Thus we measured the responses of NAc neurons to the taste of salt under conditions in which it is nonpreferred (REP, positive sodium balance) or preferred (DEP, negative sodium balance). We observed an overall decrease in NAc shell activity in response to the taste of sodium only under the DEP condition. Changes in NAc core responses were only observed after the animal had returned to positive sodium balance following depletion. We speculate that these regional differences may reflect different processes in the shell and core, consistent with the idea that the shell plays a more immediate role in encoding the primary affective value of taste stimuli.
Reduced activity in NAc shell to salty taste under sodium depletion is consistent with the idea that decreases in activity to rewarding stimuli may promote positive hedonic reactivity. Pharmacological evidence supports the rostrodorsal region of the NAc shell as exquisitely tuned to the primary hedonic properties of stimuli (Kelley 2004; Pecina and Berridge 2005). Pecina and Berridge (2000, 2005) identified this region as a hedonic “hotspot” into which infusions of the μ-opioid agonist d-Ala2-N-Me-Phe4-Gly5-ol-enkephalin (DAMGO) led to increased appetitive oral-facial reactions to the taste of sucrose. μ-Opioid agonists have been shown to reduce neural activity within the NAc (Hakan et al. 1989). Notably, our recording sites within the shell included the rostrodorsal region of the medial shell, within the identified hedonic hotspot (Pecina and Berridge 2005). Additional neurochemical signals that result in reductions in NAc firing rate also have been shown to initiate reward-directed behaviors. GABA agonists and glutamatergic antagonists strongly promote feeding behavior in the NAc shell (Kelley 2004). Endocannabinoids injected into the NAc shell also enhance hedonic responses to palatable stimuli (Mahler et al. 2007). Finally, dopamine release in the NAc modulates the activity of NAc neurons (Nicola et al. 2000). We have recently shown that dopamine signaling in NAc shell changes rapidly in response to IO infusions of rewarding and aversive stimuli, increasing in response to the taste of sucrose but decreasing in response to quinine (Roitman et al. 2008). Furthermore, antagonism of dopamine receptors reduces consumption of salt solution in sodium-depleted rats (Roitman et al. 1997). The mechanisms underlying taste-evoked decreases in NAc neural activity are of considerable interest and must be determined in future studies.
Responses in NAc core were qualitatively different from those in the shell. We did not observe changes in the proportion of INC and DEC neurons, or in the overall population response, between the REP and DEP conditions, suggesting no effect of depletion-enhanced palatability in this region. The core has been implicated in learned associations between cues and rewarding events (Cardinal et al. 2002; Parkinson et al. 2000; Schoenbaum and Setlow 2003; Smith-Roe and Kelley 2000). In the present study, IO delivery of salt solution was not paired with cues, nor did rats have to perform any operant behavior to obtain them. Thus the type of associative learning thought to rely on NAc core was not required, and as such, the lack of effect on the DEP may not be surprising.
Unexpectedly, we found a greater increase in the NAc core population firing rate 2 days after animals had replenished their need for sodium via intake of salt solution and standard food. Similarly, responses in the NAc shell did not fully return to REP levels on the POST day but were rather intermediate between the REP and DEP responses. These persistent changes on the POST day may suggest a more long-term effect of sodium depletion on NAc processing. A single sodium depletion episode, as used presently, has been shown to sensitize rats to hypertonic NaCl. Compared with a single, acute depletion, rats show increased consumption of (Sakai et al. 1987) and more effort expended for hypertonic sodium solution (Clark and Bernstein 2006) upon subsequent depletions. Notably, a history of sodium depletion can cross-sensitize the locomotor stimulating effects of drugs such as amphetamine (Clark and Bernstein 2004; Roitman et al. 2002). Sensitization to amphetamine, in turn, has been associated with morphological changes in NAc core (Robinson and Kolb 1997). Therefore, our observations of enhanced increasing responses of NAc core neurons under the POST condition may reflect sensitization or learning processes.
Several studies have suggested that decreasing activity in the NAc ultimately leads to consummatory behavior via disinhibition of downstream structures such as the ventral pallidum (VP) (Carlezon and Thomas 2009; Krause et al. 2010; Tindell et al. 2006). Medium spiny NAc neurons are GABAergic and project, in part, to the VP and thus may tonically inhibit premotor neurons there. Blockade of GABAA receptors within the VP with bicuculline has been shown to increase feeding (Shimura et al. 2006; Stratford et al. 1999). In addition, pharmacological manipulations in the VP can enhance hedonic reactivity to taste stimuli (Smith and Berridge 2007). Thus, when rewarding stimuli are encountered, reduced NAc activity may promote consummatory behavior via the VP. Tindell et al. (2006) showed that VP neural activity decreased during consumption of a salt solution before sodium depletion and increased after, which would be predicted if the NAc neurons recorded presently projected to the VP. Recent work has shown that preventing decreases in the NAc via microstimulation interferes with approach and consumption (Krause et al. 2010). In addition, NAc projections to the hypothalamus (Kelley et al. 2005) may be critical in regulating autonomic and regulatory functions, including those underlying sodium appetite (Shelat et al. 1999).
The data presented advance our previous findings that individual NAc neurons responded to a rewarding taste stimulus primarily with decreasing responses and to an aversive taste stimulus primarily with increasing responses (Roitman et al. 2005). A subset of these neurons responded to both stimuli, with most responding to reward with decreases and to aversion with increases in firing rate. In the present study, on the REP day, when rats avoided voluntary consumption of salt solution compared with the DEP day, a rather slim majority of neurons responded to hypertonic salt with increasing responses. This may reflect the fact that rats do not respond to hypertonic salt solutions in a qualitatively or quantitatively similar manner to a purely aversive stimulus such as quinine (Berridge et al. 1984). Another departure from previous reports (Roitman et al. 2005, 2010; Taha and Fields 2006; Wheeler et al. 2008) is the finding of a clear difference between the shell and the core. The present finding may have resulted from a targeted investigation of the shell vs. the core, whereas previous work failed to obtain a large enough sample in either subregion to identify differences between the two. Alternatively, the shell subregion may be particularly sensitive to sodium depletion and the taste of salt solutions. For example, fos immunoreactivity increases specifically in the shell region following multiple sodium depletions (Na et al. 2007). Comparisons between solutions that evoke purely positive affective responses (e.g., sucrose) vs. purely negative (e.g., quinine) or mixed (e.g., hypertonic sodium) may give further insight into taste-based processing in the NAc as it relates to regional differences.
We have shown that the activity of NAc shell neurons tracks the affective value of a typically nonpreferred taste stimulus as it becomes preferred. The decreases in activity observed are thought to be associated with appetitive and consummatory behaviors (Roitman et al. 2005; Taha and Fields 2005). It remains to be determined whether NAc responses simply encode valence or direct motor output in a valence-sensitive manner. Neurons in the core showed modulations that may correlate with learning or longer term adaptations in behavior. Overall, the present results demonstrate that the NAc can track changes in the motivational properties of a stimulus across different physiological states and, in part, enable animals to respond to a given stimulus appropriately based on changes in internal environment.
GRANTS
This work was supported by National Institute of Drug Abuse Grants DA025634 (to M. F. Roitman) and DA027127 (to J. D. Roitman) and the University of Illinois at Chicago Chancellor's Supplemental Graduate Research Fellowship (to A. L. Loriaux).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci 98: 652–660, 1984 [DOI] [PubMed] [Google Scholar]
- Breslin PA, Kaplan JM, Spector AC, Zambito CM, Grill HJ. Lick rate analysis of sodium taste-state combinations. Am J Physiol Regul Integr Comp Physiol 264: R312–R318, 1993 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci 116: 553–567, 2002 [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav 76: 379–387, 2002 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56, Suppl 1: 122–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Bernstein IL. Reciprocal cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav 78: 691–698, 2004 [DOI] [PubMed] [Google Scholar]
- Clark JJ, Bernstein IL. Sensitization of salt appetite is associated with increased “wanting” but not “liking” of a salt reward in the sodium-deplete rat. Behav Neurosci 120: 206–210, 2006 [DOI] [PubMed] [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage 39: 538–547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci 23: 1341–1351, 2006 [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol 93: 177–209, 2008 [DOI] [PubMed] [Google Scholar]
- Hakan RL, Callaway C, Henriksen SJ. Electrophysiological analysis of the neural circuitry underlying opiate effects in the nucleus accumbens septi. Neurosci Lett 101: 163–168, 1989 [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 58: 843–853, 2005 [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27: 765–776, 2004 [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493: 72–85, 2005 [DOI] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30: 4746–4756, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage 44: 1178–1187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32: 2267–2278, 2007 [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97, 1980 [DOI] [PubMed] [Google Scholar]
- Na ES, Morris MJ, Johnson RF, Beltz TG, Johnson AK. The neural substrates of enhanced salt appetite after repeated sodium depletions. Brain Res 1171: 104–110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185–215, 2000 [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol 91: 1840–1865, 2004a [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J Neurophysiol 91: 1866–1882, 2004b [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res 126: 263–285, 2000 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Boston, MA: Academic/Elsevier, 2007, p. 1v [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25: 11777–11786, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 17: 8491–8497, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Roitman MF. Risk-preference differentiates orbitofrontal cortex responses to freely chosen reward outcomes. Eur J Neurosci 31: 1492–1500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci 22: RC225, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Schafe GE, Thiele TE, Bernstein IL. Dopamine and sodium appetite: antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci 111: 606–611, 1997 [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45: 587–597, 2005 [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem 17: 539–546, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11: 1376–1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci 101: 724–731, 1987 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci 23: 9833–9841, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelat SG, King JL, Flanagan-Cato LM, Fluharty SJ. Mineralocorticoids and glucocorticoids cooperatively increase salt intake and angiotensin II receptor binding in rat brain. Neuroendocrinology 69: 339–351, 1999 [DOI] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci 23: 1596–1604, 2006 [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 20: 7737–7742, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27: 1594–1605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res 825: 199–203, 1999 [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25: 1193–1202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci 26: 217–222, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol 96: 2399–2409, 2006 [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46: 327–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron 57: 774–785, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.