Abstract
In the present study, we investigated whether intradermal cheek injection of pruritogens or algogens differentially elicits hindlimb scratches or forelimb wipes in Sprague-Dawley rats, as recently reported in mice. We also investigated responses of primary sensory trigeminal ganglion (TG) and dorsal root ganglion (DRG) cells, as well as second-order neurons in trigeminal subnucleus caudalis (Vc), to pruritic and algesic stimuli. 5-HT was the most effective chemical to elicit dose-dependent bouts of hindlimb scratches directed to the cheek, with significantly less forelimb wiping, consistent with itch. Chloroquine also elicited significant scratching but not wiping. Allyl isothiocyanate (AITC; mustard oil) elicited dose-dependent wiping with no significant scratching. Capsaicin elicited equivalent numbers of scratch bouts and wipes, suggesting a mixed itch and pain sensation. By calcium imaging, ∼6% of cultured TG and DRG cells responded to 5-HT. The majority of 5-HT-sensitive cells also responded to chloroquine, AITC, and/or capsaicin, and one-third responded to histamine. Using a chemical search strategy, we identified single units in Vc that responded to intradermal cheek injection of 5-HT. Most were wide dynamic range (WDR) or nociceptive specific (NS), and a few were mechanically insensitive. The large majority additionally responded to AITC and/or capsaicin and thus were not pruritogen selective. These results suggest that primary and second-order neurons responsive to pruritogens and algogens may utilize a population coding mechanism to distinguish between itch and pain, sensations that are behaviorally manifested by distinct hindlimb scratching and forelimb wiping responses.
Keywords: itch, pain, scratching, calcium imaging
itch is often defined as an unpleasant sensation associated with the desire to scratch. Itch is a symptom common to many skin and systemic disorders and is usually resistant to treatment, thus representing a significant health issue (Carstens 2009). It is therefore important to better understand itch mechanisms under normal and pathophysiological conditions, an effort that benefits greatly from animal models. Scratching behavior is commonly used as a surrogate of itch sensation in rodents (Carstens and Kuraishi 2004). In mice, a variety of pruritogens elicit bouts of hindlimb scratches directed to the site of injection, which is usually the rostral back/nape of the neck. In rats, 5-hydroxytryptamine (5-HT) is the most effective scratch inducer (Jinks and Carstens 2002; Thomsen et al. 2001). However, a drawback of the rostral back model is that it does not distinguish between pruritogens and algogens (Kuraishi et al. 1995). A new mouse model has recently been introduced that appears to distinguish between itch and pain (Shimada and LaMotte 2008). Pruritogens such as histamine elicit hindlimb scratches directed to the injection site in the cheek, whereas algogens such as capsaicin elicit facial wipes by the ipsilateral forelimb, with little or no hindlimb scratching (Shimada and LaMotte 2008; Akiyama et al. 2010a, 2010b). The first aim of the present study was to determine whether cheek injection of pruritogens or algogens similarly elicits distinct behavioral responses in the Sprague-Dawley rat, a species that has been used extensively in pain research.
It has long been debated whether itch and pain are signaled by a common population of neurons based on some parameter of activity such as rate or pattern of firing, or if there are distinct labeled lines for itch and pain (specificity theory). Mechanically insensitive primary afferent C fibers (Schmelz et al. 1997) and cat spinothalamic tract neurons (Andrew and Craig 2001) responded to histamine over a time course consistent with itch sensation. Moreover, ablation of spinal neurons that express the gastrin-releasing peptide (GRP) receptor attenuated or abolished pruritogen-evoked scratching in mice without affecting sensitivity to pain (Sun et al. 2009). These data support the concept of a labeled line for itch. However, the vast majority of spinal neurons that respond to cutaneous application of pruritogens such as histamine, 5-HT, or cowhage spicules (containing itch-inducing proteases; Reddy et al. 2008) also responded to capsaicin and/or allyl isothiocyanate (AITC) (Akiyama et al. 2009a, 2009b; Davidson et al. 2007; Jinks and Carstens 2002; Simone et al. 2004). Behavioral-genetic studies also indicate that nociceptive neurons may be capable of signaling itch (Imamachi et al. 2009; Shim et al. 2007), arguing against labeled-line coding for itch versus pain. In light of the distinct behavioral responses, we wished to investigate whether puritic and algogenic facial stimuli excite overlapping or distinct populations of primary sensory neurons and second-order neurons in rat trigeminal subnucleus caudalis (Vc). To this end, we used the method of calcium imaging of cultured trigeminal ganglion (TG) cells to investigate responses of primary sensory neurons to pruritogens and algogens. We also tested dorsal root ganglion (DRG) cells for comparison. Moreover, thermosensitive transient receptor potential (TRP) channels including TRPV1 (Imamachi et al. 2009; Shim et al. 2007) and TRPA1 (Liang et al. 2011; Wilson et al. 2011) have been implicated in cell signaling by pruriceptors, prompting us to additionally test whether TG and DRG cells respond to agonists of thermosensitive TRP channels. Finally, we additionally employed in vivo single-unit recording to identify 5-HT-responsive Vc neurons and test whether they additionally respond to algesic stimuli.
MATERIALS AND METHODS
Behavioral studies.
All procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats (Harlan, Oxnard, CA, and Simonsen, Gilroy, CA; 290–434 g) were individually housed with rodent chow and water available ad libitum. Behavioral studies were conducted at approximately the same time each day to reduce circadian effects.
Rats were first habituated in three daily 1-h sessions to the recording arena, which consisted of a Plexiglas box placed on a clear glass surface through which the animals were videotaped from below. Each rat's cheek was shaved with clippers at least 24 h before testing. For testing, each rat was briefly restrained in a plastic cone with the cheek area exposed and received a 10-μl intradermal (id) injection in the cheek unilaterally with a Hamilton microsyringe attached via PE-50 tubing to a 30-gauge hypodermic needle. The injection was verified visually as a small wheal. The rat was then immediately placed onto the recording arena and videotaped for 60 min. Experimenters left the room immediately after starting the videotape session. Each videotape was analyzed off-line by two independent observers who were blinded as to treatment. The following behavioral responses were counted in 5-min intervals over the 60-min period: number of hindpaw scratches directed to the injected cheek (off-site scratches, such as ears, were excluded) and number of ipsilateral forelimb wipes directed to the injected cheek (Akiyama et al. 2010a, 2010b; Shimada and LaMotte 2008). The scores provided by the two observers were observed to be in good concordance and were averaged. We also measured the duration of individual hindlimb scratch bouts and forelimb wipes, as well as the within-bout scratch frequency, at early (0–10 min), middle (10–20 min), and late (20–30 min) time intervals following 5-HT and AITC injections.
Chemicals tested were as follows. As potential pruritogens, we used 5-HT (5-hydroxytryptamine HCl; 0.1–1%, 4.7–47 mM, in sterile 0.9% saline; Sigma, St. Louis, MO), chloroquine (5–10%, 97–194 mM, in saline; Sigma), protease-activated receptor (PAR)-2 agonist SLIGRL-NH2 (1%, 15.2 mM, in saline; Quality Controlled Biochemicals, Hopkinton, MA), PAR-4 agonist AYPGKF-NH2 (1%, 14.7 mM; Quality Controlled Biochemicals), and platelet-activating factor (PAF; 95–191 mM in saline; Cayman Chemical, Ann Arbor, MI), based on previous studies reporting that these agents elicit scratching behavior in rodents (e.g., Akiyama et al. 2009c, 2010b; Jinks and Carstens 2002; Kuraishi et al. 1995; Thomsen et al. 2001; Woodward et al. 1995). We also tested cowhage, which was reported to elicit scratching in mice (Akiyama et al. 2010c). Five (±1) native cowhage spicules, or inactivated (autoclaved) spicules, were held with forceps, inserted into the cheek skin for 5 s, and then removed. We believe that the 5-s duration of cowhage spicule application was sufficient, since the same means of cowhage spicule application elicited significant scratching in mice (Akiyama et al. 2010c) and evoked itch when self-tested. As algogens, we used capsaicin [0.1–0.3%, 3.3–9.9 mM, in 7% Tween 80 (Fisher Scientific, Fair Lawn, NJ) in saline (Sigma)], AITC (= mustard oil; 5–10%, 504–1,008 mM, in 7% Tween 80/saline; Sigma), formalin (2–4%, 667–1,333 mM, in saline; Sigma), and histamine 0.1–10% (9–900 mM, in 7% Tween 80/saline; Sigma). In addition, we tested the tingly hydroxy-α-sanshool analog [2E,4E,8Z]-N-isobutylundeca-2,4,8-trienamide (IBA; 21 mM, in propylene glycol; Givaudan Flavors, Cincinnati, OH), to determine whether the distinct sensory quality of tingle elicits behavioral responses suggestive of itch, pain, both, or neither. Each chemical was tested in groups of eight rats, except for saline, which was tested in six. Most rats were tested with different chemicals, with at least 7 days between successive tests of active chemicals to avoid carryover effects.
For comparison, we also made intradermal injections of some agents into the nape of the neck, using methods described previously (Jinks and Carstens 2002), and counted hindlimb scratch bouts directed to the injection site. Briefly, a 10-μl volume was injected in the same manner as described above into the previously shaved nape of the neck at midline, and rats were then placed in the arena and videotaped for 60 min. Chemicals were PAR-2 agonist SLIGRL-NH2 (as above; 0.5–4%, 7.6–61 mM), chloroquine (as above; 5–10%, 97–194 mM), and compound 48/80 (2–5%, 131–327 mM; in saline; Sigma).
The numbers of scratch bouts and wipes elicited by each chemical at a given concentration were compared with those recorded in groups receiving vehicle by unpaired t-tests, with P < 0.05 set as significant. Numbers of scratch bouts and wipes elicited by a given chemical were compared by paired t-tests. Comparisons of numbers of scratch bouts and wipes, as well as bout and wipe duration, elicited by a given chemical at different concentrations were made by multiple analysis of variance (MANOVA) followed by post hoc least significant difference (LSD) tests, with P < 0.05 set as significant. Comparisons of scratch bout duration, wipe duration, and within-bout scratch frequency, with time and with 5-HT and AITC concentration, were subjected to repeated-measures ANOVA. Statistical tests were performed with SPSS software (IBM, Somers, NY).
Calcium imaging.
Under a protocol approved by the University of California, Davis Institutional Animal Care and Use Committee, 64 juvenile (∼3 wk, ∼100 g) male Sprague-Dawley rats (Simonsen) were euthanized under pentobarbital sodium anesthesia and TG and lumbar DRG were extracted, treated, and plated onto poly-d-lysine-coated glass coverslips as previously described (Klein et al. 2011a).
For calcium imaging, TG and DRG cells were loaded with fura-2 AM and coverslips were placed in a perfusion chamber mounted on a Nikon inverted fluorescence microscope. Fluorescence images were obtained at 340- and 380-nm wavelengths with a CoolSnap camera. Ratiometric measurements were recorded at 3-s intervals with Simple PCI software (Klein et al. 2011a). Solutions were administered to the perfusion chamber by a gravity-fed eight-channel solenoid-operated perfusion system (ValveLink 8.2, AutoMate Scientific, San Francisco, CA) and removed via a vacuum line at the other end. The chemicals used were 50–400 μM 5-HT, 100 μM histamine, and 100 μM chloroquine (all from Sigma), 250 μM l-menthol (in 0.015% ethanol; Givaudan), 200 μM cinnamic aldehyde (CA; in 0.015% ethanol; Sigma), 100 μM AITC (in 0.015% ethanol; Sigma), and 1 μM capsaicin (in 0.015% ethanol; Sigma). IBA (100 μM; Givaudan) was dissolved in 0.5% propylene glycol. Each chemical was superfused for a period of 30 s except capsaicin, which was delivered for 10 s. Vehicles were applied separately as controls and had no effect (data not shown).
Each TG or DRG cell's maximum response to a given stimulus was taken as the highest ratio change during the 2-min poststimulus period, relative to the baseline 1 min prior to chemical application. Peak responses were corrected by subtracting the prestimulus baseline ratio. A positive response to a chemical was defined as at least a 20% change from baseline. A pivot table was created to calculate percentages of cells that responded to various combinations of the applied chemicals.
Vc electrophysiology.
Methods were similar to those as described previously (Zanotto et al. 2007, 2008). Briefly, 20 male Sprague-Dawley rats (365–580 g; Simonsen) were anesthetized with thiopental sodium (induction: 85 mg/kg ip; maintenance: 10 mg·kg−1·h−1 iv via a tail vein). The caudal medulla was exposed surgically to allow extracellular single-unit recordings from second-order neurons in the dorsolateral Vc with tungsten microelectrodes. Unit activity was amplified, digitized, and displayed with a Powerlab interface (A–D Instruments, Boulder, CO) and Spike 2 (Cambridge Electronic Design, Cambridge, UK). Recorded action potentials were sorted by spike size and waveform, and responses were quantified as number of action potentials per second or per minute.
Intradermal cheek injection of 5-HT (<0.5 μl) at a concentration of either 0.1% or 1% was used as a search stimulus to identify pruritogen-sensitive units. The injection was made in the same area of the cheek as in the behavioral studies. After the intradermal search stimulus, the microelectrode was positioned to record a spontaneously firing action potential. When the firing waned, 5-HT was reinjected in a 1-μl volume at the identical location through the same injection cannula that remained in place. A unit was considered to respond to the 5-HT stimulus if its firing rate increased 30% or more compared with preinjection baseline measured over a 60-s period. Activity was recorded for 40 min, after which a second identical intradermal injection of 5-HT at the same concentration was made via the same needle. In experiments with 0.1% 5-HT, a third injection was made 40 min later. After this, the unit's mechanical sensitivity was tested with an array of stimuli (calibrated von Frey hairs 0.7–117 mN, cotton wisp, touch, and pinch with forceps), each of which was delivered successively 3 times/5 s. Sensitivity to heating and cooling was then tested with a feedback-controlled Peltier thermode (NTE-2A; Physitemp, Clifton, NJ) that contacted the cheek surface. The circular thermode had a diameter of 12.7 mm and heated or cooled the skin at a rate of ∼1°C/s. Temperature at the thermode-skin interface was recorded online with a fast thermocouple (IT-23; Physitemp) connected to a digital thermometer (BAT-12; Physitemp). After this, units were tested for responses to a variety of additional chemicals. Each chemical stimulus was delivered via a separate intradermal injection needle placed within the facial receptive field in a 1-μl volume or by topical application in a 5-μl volume. For injections, the injection needle was placed intradermally at least 2 min prior to the injection, allowing sufficient time for any activity elicited by needle placement to return to the baseline level. The 5-μl topical volume covered an area smaller than that of the thermode. Chemicals tested were l-menthol (1% in 10% ethanol + 1% Tween 80 id; Givaudan), CA (10% in 0.5% Tween 80 id; Sigma), histamine (1% in saline id; Sigma), capsaicin (0.01% in 10% ethanol id; Sigma), and topical application of 75% AITC (75%), eugenol (75%), or carvacrol (75%) (all in mineral oil; Sigma). Saline (0.9% id) was tested in most experiments as a vehicle control; other vehicles (ethanol, Tween, mineral oil) did not affect firing of those units tested (data not shown). At least 20 min elapsed between successive intradermal injections of chemicals that were administered in a randomized order. At the conclusion of recording experiments, an electrolytic lesion was made at the recording site to allow histological verification as previously described (Zanotto et al. 2007).
Neuronal responses to a given stimulus were summed (usually over 60 s) and compared with baseline firing over the equivalent prestimulus period by paired t-tests, with P < 0.05 considered significant. Responses to repeated application of the same stimulus were also compared by paired t-test to assess tachyphylaxis (self-desensitization).
RESULTS
Behavioral studies.
Intradermal cheek injection of 5-HT primarily elicited bouts of hindlimb scratches directed to the injection site, peaking 10–15 min after injection and persisting up to 40 min (Fig. 1A, filled squares). 5-HT elicited little wiping (Fig. 1A, open circles). In contrast, AITC mainly elicited singular wiping movements of the ipsilateral inner forelimb that were directed from caudal to rostral across the injected cheek, peaking at 15–20 min after injection and persisting out to 40 min (Fig. 1B, open circles). AITC elicited little scratching (Fig. 1B, filled squares). Figure 1C plots the mean number of scratch bouts versus wipes elicited by different concentrations of 5-HT and AITC and shows a clear separation between the respective dose-dependent scratch- or wipe-inducing effects of these agents (filled triangles vs. filled circles). The numbers of scratch bouts or wipes increased significantly with concentration of 5-HT or AITC, respectively (Fig. 1, E and F; Table 1). Capsaicin elicited equivalent numbers of scratch bouts and wipes (Fig. 1C, filled squares), both of which increased significantly with capsaicin concentration (Table 1).
Fig. 1.
Time course of scratching and wiping. A: plot of mean no. of scratch bouts and wipes after intradermal injection of 5-hydroxytryptamine (5-HT, 10 μl). *Significant difference between mean no. of scratch bouts vs. wipes (P < 0.001, repeated-measures ANOVA). B: as in A for intradermal cheek injection of allyl isothiocyanate (AITC). *Significant difference between mean no. of scratch bouts vs. wipes (P < 0.01, repeated-measures ANOVA). C: scatterplot of mean no. of scratch bouts vs. wipes/60 min after injections of 5-HT (0.1–1%), capsaicin (0.01–0.3%) and AITC (5–10%). Error bars are SE (n = 8/group). Dot, 0.9% saline (n = 6); star, 7% Tween in saline (n = 8). Diagonal line indicates an equal no. of scratches and wipes. D: durations (left-hand y-axis) and within-bout frequencies (right-hand y-axis) for scratch bouts from each animal were measured, with averages plotted at the indicated time windows after 5-HT injection. Neither bout duration nor within-bout frequency varied over time (P > 0.1, repeated-measures ANOVA). E: mean no. of scratch bouts (left-hand y-axis) and mean scratch bout duration (right-hand y-axis) vs. 5-HT concentration. *5-HT 1% elicited significantly more scratch bouts than 0.1% and 0.5%, and 0.5% elicited significantly more than 0.1% [P < 0.05, multiple analysis of variance (MANOVA) with post hoc least significant difference (LSD) tests]. Scratch bout duration did not vary significantly with 5-HT concentration. F: as in E plotting mean no. of ipsilateral forelimb wipes (left-hand y-axis) and mean wipe duration (right-hand y-axis) vs. AITC concentration. *AITC at 10% elicited significantly more wipes compared with 5% and 7.5% (P < 0.05, MANOVA with post hoc LSD test). Mean wipe duration did not vary significantly with AITC concentration (P > 0.05, MANOVA with post hoc LSD test).
Table 1.
Average counts of total number of hindlimb scratch bouts and ipsilateral forelimb wipes directed to the cheek after intradermal cheek injection of each chemical
| Chemical | Concentration, mM | Scratch Bouts | Wipes |
|---|---|---|---|
| 5-HT | 4.7 | 8.9 ± 1.7* | 2.8 ± 1.1 |
| 5-HT | 23.5 | 28.4 ± 4.9* | 9 ± 1.5* |
| 5-HT | 47 | 60.1 ± 6.3* | 4.1 ± 2 |
| Formalin | 666.67 | 3.3 ± 0.9 | 7.1 ± 4.4 |
| Formalin | 1,333.34 | 97.3 ± 15.3* | 21 ± 6.4* |
| Chloroquine | 96.92 | 26.3 ± 4.3* | 4.8 ± 1.6 |
| Chloroquine | 193.84 | 22.3 ± 8.7* | 1.8 ± 1.7 |
| SLIGRL-NH2 | 15.22 | 11.8 ± 3.8 | 12.9 ± 2.8* |
| PAR-4 agonist | 14.68 | 0.8 ± 0.2 | 2.7 ± 0.8 |
| PAF | 95.47 | 3.1 ± 0.8 | 4.3 ± 0.9 |
| PAF | 190.94 | 5.9 ± 1.2 | 2.3 ± 0.9 |
| Cowhage spicules | NA | 2.5 ± 0.9 | 1.8 ± 0.8 |
| Autoclaved spicules | NA | 1.1 ± 0.3 | 1.3 ± 0.5 |
| Saline | Vehicle control | 3.3 ± 1.1 | 3.8 ± 1.5 |
| 7% Tween | Vehicle control | 3.4 ± 0.5 | 2.2 ± 0.6 |
| IBA | 21 | 12.1 ± 2.4* | 10.1 ± 2.0* |
| Capsaicin | 0.3 | 4.6 ± 1.2 | 5.4 ± 2.5 |
| Capsaicin | 3.3 | 13.6 ± 3* | 8.5 ± 1.3* |
| Capsaicin | 9.9 | 23.6 ± 3.5* | 23.5 ± 2.9* |
| Histamine | 9 | 2.6 ± 0.7 | 9.5 ± 1.2* |
| Histamine | 90 | 5.5 ± 1.1 | 13.4 ± 3.9* |
| Histamine | 270 | 4.4 ± 0.9 | 11.8 ± 2.1* |
| Histamine | 900 | 13.3 ± 2.5* | 21.2 ± 6.3* |
| AITC | 503.8 | 5.3 ± 1.9 | 8.6 ± 3.7 |
| AITC | 755.6 | 5.6 ± 1 | 13.6 ± 3.4* |
| AITC | 1,007.5 | 4 ± 1.4 | 36.4 ± 9.4* |
Data are average ± SE counts of total number of hindlimb scratch bouts and ipsilateral forelimb wipes/60 min directed to the cheek after intradermal (id) cheek injection of each chemical; n = 8/group. 5-HT, 5-hydroxytryptamine; PAR, protease-activated receptor; PAF, platelet-activating factor; IBA, [2E,4E,8Z]-N-isobutylundeca-2,4,8-trienamide; AITC, allyl isothiocyanate; NA, not applicable.
Significantly different from vehicle (saline or 7% Tween; P < 0.05, unpaired t-test).
The duration of individual scratch bouts ranged from 1 to 7.1 s with a mean duration of ∼3 s; the duration of scratch bouts did not vary significantly with time (Fig. 1D) or concentration of 5-HT (Fig. 1E). The within-bout scratch frequency was ∼10 Hz and did not vary significantly with time (Fig. 1D) or 5-HT concentration (data not shown). Individual wiping movements had a shorter duration of ∼0.29 s (range: 0.13–0.37 s) and did not vary with AITC concentration (Fig. 1F) or time (data not shown).
Table 1 presents mean numbers of hindlimb scratch bouts and forelimb wipes elicited by each chemical tested. 5-HT, formalin, chloroquine, capsaicin, and histamine at the highest concentration (900 mM) elicited a significantly greater number of hindlimb scratch bouts compared with vehicle control animals. Scratching elicited by 5-HT, capsaicin, and formalin, but not chloroquine, was dose dependent. 5-HT, formalin, and chloroquine elicited significantly more scratch bouts than forelimb wipes. Chloroquine elicited no significant wiping, indicating it to be a selective, albeit weak, pruritogen in rats. In contrast, the high dose of formalin and the intermediate dose of 5-HT elicited a significant increase in wipes, but this was significantly less than the number of scratch bouts. Capsaicin and IBA elicited approximately equal numbers of scratch bouts and wipes. The highest dose of histamine elicited a significant increase in scratches, although it evoked significantly more wipes. Lower histamine doses elicited significant wiping but not scratching. AITC dose-dependently elicited forelimb wiping with no significant scratching. PAR-2 and PAR-4 agonists and PAF did not elicit significant scratching, and only the PAR-2 agonist elicited a significant amount of wiping. Intradermal cheek insertion of cowhage spicules did not elicit any significant amount of scratching or wiping.
For comparison, the mean numbers of hindlimb scratch bouts evoked by intradermal injection of agents into the rostral back of rats are shown in Table 2. The only agents that elicited a significant increase in scratching compared with vehicle were 5-HT and formalin, as previously reported (Jinks and Carstens 2002). None of the other agents tested presently (PAR-2 agonist SLIGRL-NH2, chloroquine, compound 48/80) elicited significant scratching behavior, nor did capsaicin or histamine (Jinks and Carstens 2002).
Table 2.
Number of scratch bouts elicited by intradermal injection into rostral back
| Chemical | Concentration, mM | Scratch Bouts |
|---|---|---|
| 5-HT* | 4.7 | 18.4 ± 7.3 |
| 5-HT* | 23.5 | 25.3 ± 3.1 |
| 5-HT* | 47 | 41.6 ± 9.1 |
| Formalin* | 667 | 15.6 ± 5.6 |
| Formalin* | 1,668 | 18.1 ± 5.8 |
| Compound 48/80 | 19.4 | 1.3 ± 0.5 |
| Compound 48/80 | 38.8 | 2.8 ± 1.1 |
| Chloroquine | 96.9 | 2.8 ± 1.5 |
| Chloroquine | 193.8 | 3.8 ± 2.1 |
| SLIGRL-NH2 | 7.6 | 0.7 ± 0.6 |
| SLIGRL-NH2 | 15.2 | 1.5 ± 1.1 |
| SLIGRL-NH2 | 30.4 | 2.5 ± 1.2 |
| SLIGRL-NH2 | 60.8 | 1.8 ± 2.1 |
| Saline | Vehicle | 0.5 ± 0.5 |
Data are mean ± SE numbers of scratch bouts elicited by id injection into rostral back.
Data from Jinks and Carstens 2002.
Calcium imaging of TG and DRG neurons.
A total of 2,293 TG and DRG cells were imaged for responsiveness to 5-HT. Of all cells tested initially with 100 μm 5-HT, 6.4% (28/436) of TG cells and 6.8% (99/1,440 cells) of DRG cells responded. The percentage of responsive TG and DRG cells did not increase at higher (200–400 μM) concentrations of 5-HT. The average diameter of all TG cells tested was 26.1 ± 0.3 μm and that of DRG cells was 25.5 ± 0.2 μm. The average diameter for 5-HT-sensitive TG and DRG cells was 25.4 ± 1.7 μm and 23.4 ± 0.6 μm, respectively.
After the initial application of 5-HT, TG and DRG cells were tested with other chemicals including histamine, chloroquine, the TRPV1 agonist capsaicin, the TRPA1 agonists AITC and CA, the TRPM8 agonist menthol, and the tingly alkylamide IBA, delivered in various combinations in a randomized order with capsaicin and the high-potassium solution always tested last. Examples of TG cell responses to 5-HT and other agents are shown in Fig. 2. All three cells responded to 5-HT (100 μM), AITC (100 μM), and high-potassium solution. One cell (red trace in Fig. 2) also responded to chloroquine (100 μM), one (blue trace) to capsaicin (1 μM), and one (blue trace) to histamine (100 μM).
Fig. 2.
Examples of trigeminal ganglion (TG) cell responses to 5-HT (100 μM) and other agents. A: photomicrographs of 3 TG cells (encircled) before (Pre) and after (Post) application of 5-HT, histamine, and capsaicin. Calibration bar (50 μm) applies to all photomicrographs. B: 340-to-380 nm ratios vs. time for the 3 TG cells shown in A. All 3 cells responded to 5-HT (100 μM), AITC (200 μM), and high potassium solution. One cell (red trace) also responded to chloroquine (CQ; 100 μM), 1 (blue trace) to capsaicin (1 μM), and 1 (blue trace) to histamine (His; 100 μM). Timing of each chemical application is indicated by black bars above traces.
Of all 5-HT-sensitive cells, 22.8% (35.7% of TG and 19.2% of DRG cells) did not respond to any other agonists tested and thus were potentially 5-HT selective. However, the majority of 5-HT-sensitive neurons responded to one or more additional agents, as shown in Tables 3–5. Table 3 shows the incidence of TG and DRG cell responses to the putative itch mediators 5-HT, chloroquine, and histamine. Twenty percent or fewer of the TG and DRG cells responded to any of these agents. There was substantial overlap in the sensitivity of cells to 5-HT and chloroquine. Chloroquine activated the majority of 5-HT-sensitive TG and DRG cells (53% and 70%, respectively), and, conversely, 5-HT activated ∼50% of the chloroquine-responsive cells. There was also some overlap in 5-HT and histamine sensitivity. Histamine activated ∼20% of 5-HT-sensitive TG or DRG cells, while, conversely, 5-HT activated 50% and 80% of histamine-sensitive TG and DRG cells, respectively. There was very little overlap in histamine and chloroquine sensitivity; only one TG and one DRG cell responded to both.
Table 3.
Incidence of response of 5-HT-sensitive and -insensitive TG and DRG cells to chloroquine and histamine
| 5-HT | Chloroquine | Histamine | n | % | |
|---|---|---|---|---|---|
| TG cells | |||||
| + | 0 | 0 | 1 | 1 | |
| + | + | 0 | 7 | 6.6 | |
| + | + | + | 0 | 0 | |
| + | 0 | + | 2 | 1.9 | |
| 0 | + | + | 1 | 1 | |
| 0 | 0 | + | 1 | 1 | |
| 0 | + | 0 | 7 | 6.6 | |
| 0 | 0 | 0 | 86 | 81.9 | |
| n | 10 | 15 | 4 | ||
| % | 9.5 | 14.3 | 3.8 | ||
| DRG cells | |||||
| + | 0 | 0 | 6 | 3.9 | |
| + | + | 0 | 9 | 5.9 | |
| + | + | + | 1 | 0.7 | |
| + | 0 | + | 3 | 1.9 | |
| 0 | + | + | 0 | 0 | |
| 0 | 0 | + | 1 | 0.7 | |
| 0 | + | 0 | 10 | 6.5 | |
| 0 | 0 | 0 | 123 | 80.4 | |
| n | 19 | 20 | 5 | ||
| % | 12.4 | 13.1 | 3.3 |
Incidence of response of 5-HT-sensitive and -insensitive trigeminal ganglion (TG) and dorsal root ganglion (DRG) cells to chloroquine and histamine is shown. +, Responded; 0, did not respond.
Table 4.
Incidence of response of 5-HT-sensitive and -insensitive TG and DRG cells to AITC and capsaicin
| 5-HT | AITC | Capsaicin | n | % | |
|---|---|---|---|---|---|
| TG cells | |||||
| + | 0 | 0 | 1 | 0.6 | |
| + | + | 0 | 7 | 4.4 | |
| + | + | + | 5 | 3.1 | |
| + | 0 | + | 1 | 0.6 | |
| 0 | + | + | 10 | 6.2 | |
| 0 | 0 | + | 75 | 46.6 | |
| 0 | + | 0 | 10 | 6.2 | |
| 0 | 0 | 0 | 52 | 32.3 | |
| n | 14 | 32 | 91 | ||
| % | 8.7 | 19.9 | 56.5 | ||
| DRG cells | |||||
| + | 0 | 0 | 11 | 3 | |
| + | + | 0 | 9 | 2.4 | |
| + | + | + | 10 | 2.7 | |
| + | 0 | + | 24 | 6.5 | |
| 0 | + | + | 23 | 6.2 | |
| 0 | 0 | + | 148 | 39.7 | |
| 0 | + | 0 | 19 | 5.1 | |
| 0 | 0 | 0 | 128 | 34.4 | |
| n | 54 | 51 | 205 | ||
| % | 22.1 | 20.9 | 84 |
Incidence of response of 5-HT-sensitive and -insensitive TG and DRG cells to AITC and capsaicin is shown. +, Responded; 0, did not respond.
Table 5.
Incidence of response of 5-HT-sensitive and -insensitive TG and DRG cells to menthol and cinnamic aldehyde
| 5-HT | Menthol | CA | n | % | |
|---|---|---|---|---|---|
| TG cells | |||||
| + | 0 | 0 | 7 | 2.5 | |
| + | + | 0 | 1 | 0.4 | |
| + | + | + | 0 | 0 | |
| + | 0 | + | 0 | 0 | |
| 0 | + | + | 16 | 5.8 | |
| 0 | 0 | + | 20 | 7.3 | |
| 0 | + | 0 | 34 | 12.4 | |
| 0 | v | 0 | 197 | 71.6 | |
| n | 8 | 51 | 36 | ||
| % | 2.9 | 18.5 | 13.1 | ||
| DRG cells | |||||
| + | 0 | 0 | 17 | 2.7 | |
| + | + | 0 | 6 | 1 | |
| + | + | + | 17 | 2.7 | |
| + | 0 | + | 6 | 1 | |
| 0 | + | + | 48 | 7.8 | |
| 0 | 0 | + | 39 | 6.3 | |
| 0 | + | v | 35 | 5.6 | |
| 0 | 0 | 0 | 451 | 72.9 | |
| n | 46 | 106 | 110 | ||
| % | 7.4 | 17.1 | 17.8 |
Incidence of response of 5-HT-sensitive and -insensitive TG and DRG cells to menthol and cinnamic aldehyde (CA) is shown. +, Responded; 0, did not respond.
Table 4 shows the coincidence of cell responses to 5-HT and the algogens AITC and capsaicin. Substantial fractions of 5-HT-sensitive TG and DRG cells (35% and 86%, respectively) also responded to AITC. Conversely, 5-HT activated 37% of AITC-sensitive TG and DRG cells. Capsaicin activated 43% and 63% of 5-HT-sensitive TG and DRG cells, respectively. Conversely, 5-HT activated 14% and 17% of capsaicin-sensitive TG and DRG cells, respectively. In addition, there was overlap in chloroquine and algogen sensitivity. AITC activated ∼86% of TG or DRG cells that responded to chloroquine. Capsaicin activated 27% of TG cells and 43% of DRG cells that were chloroquine sensitive. Capsaicin also activated 82% of TG and 53.5% of DRG cells that responded to histamine.
Table 5 presents the coincidence of cell responses to 5-HT and the TRP channel agonists menthol and CA. Only 13% of 5-HT-sensitive TG cells responded to menthol, and none responded to CA. Conversely, <3% of menthol- or CA-sensitive TG cells responded to 5-HT. DRG cells exhibited a much higher incidence of coactivation, with 50% of 5-HT-sensitive cells also responding to menthol and CA. Conversely, lower percentages of menthol- or CA-sensitive DRG cells also responded to 5-HT (22% and 21%, respectively).
The tingly alkylamide IBA activated 28% and 26% of all TG and DRG cells tested, respectively, confirming our recent report (Klein et al. 2011b). IBA activated 50% and 64% of 5-HT-sensitive TG and DRG cells, respectively. Conversely, 5-HT activated 7% and 8% of IBA-sensitive TG and DRG cells, respectively.
Vc neuron recordings.
Units were identified by intradermal injections (<0.5 μl) of 0.1% (n = 9) or 1.0% (n = 11) 5-HT. Recording sites were located in superficial laminae of dorsolateral Vc (Fig. 3 and Fig. 4, A and B, insets) at a mean depth of 154.8 μm ± 36.9 (SE). Neurons that responded differentially to innocuous and noxious mechanical stimuli were classified as wide dynamic range (WDR) type (63%, 12/19 units), while those responsive to noxious but not innocuous mechanical stimuli were classified as nociceptive specific (NS) (26%, 5/19) Two units (11%) were unresponsive to mechanical stimuli; one of these responded to menthol and the other to cooling. Some units responded to noxious heat (28%, 5/18) at a mean threshold of 45.4 ± 2.4°C (SE), and many responded to cooling (63%, 12/19) at a mean threshold of 19.9 ± 1.8°C. Examples are shown in Fig. 3, and averaged responses to mechanical and thermal stimuli are shown in Fig. 4.
Fig. 3.
Example of individual superficial trigeminal subnucleus caudalis (Vc) unit responses. Peristimulus time histograms (bin = 1 s) show, from left to right, responses to initial 1-μl 5-HT injection (0.1%), a second 1-μl 5-HT injection 40 min later, graded von Frey (VF) mechanical stimuli, a cotton wisp, pinch, vehicle (0.9% saline), topical AITC (75%), and intradermal injection of 1 μl of capsaicin (Cap; 0.01%). All stimuli were delivered within facial receptive field, shown in left inset (black region). Right inset shows unit recording site in caudal medulla. NTS, nucleus of solitary tract.
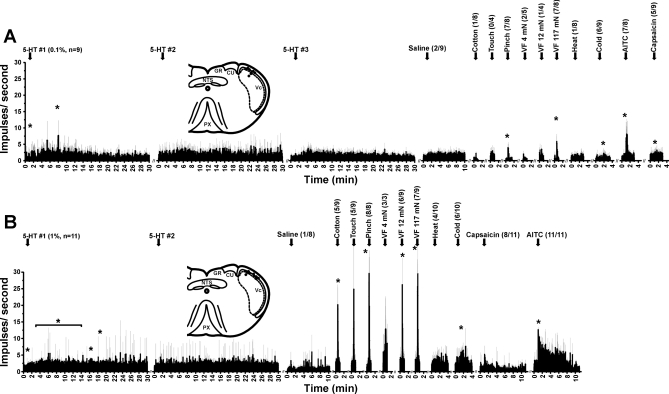
Fig. 4.
Averaged responses of superficial Vc units to 5-HT and other stimuli. A: units responsive to 0.1% (47 mM) 5-HT. Averaged peristimulus time histograms (gray error bars: SE) show, from left to right, responses to 3 successive intradermal injections (arrows) of 5-HT at >40-min intervals, followed by vehicle (saline), mechanical stimuli [cotton wisp, touch, pinch with blunt forceps, von Frey filaments (VF) at indicated bending forces], noxious heat, cooling, topical AITC, and capsaicin (0.01%). Fractions in parentheses indicate no. of units that responded/no. tested. Note absence of significant response to 2nd and 3rd applications of 5-HT, indicating tachyphylaxis. *Significantly greater than prestimulus baseline (P < 0.05, paired t-test). Inset shows unit recording sites (dots) in dorsolateral Vc, compiled on a section of the caudal medulla. GR, gracile nucleus; CU, cuneate nucleus; PX, pyramidal decussation. B: averaged responses of a separate population of superficial Vc units to 1% (47 mM) 5-HT and other stimuli (format as in A). Note tachyphylaxis to 2nd injection of 5-HT, as well as robust response to AITC. Insets in A and B are modified from Paxinos and Watson 1998, Copyright Elsevier.
Neuronal responses to repeated intradermal injection of 5-HT exhibited significant tachyphylaxis. In the example of Fig. 3, the first intradermal injection of 0.1% 5-HT elicited a robust response, while the second injection elicited a much weaker response. This unit responded to graded mechanical stimuli and AITC, weakly to capsaicin, and not to saline. Figure 4A shows averaged responses. The first intradermal injection of 0.1% 5-HT elicited significant increases in firing at minutes 1 and 8, while the second and third injections did not significantly affect firing rate, indicating tachyphylaxis. Figure 4B shows responses of a separate population of units to the higher 1% 5-HT stimulus, which significantly increased firing over a 14-min period. The second injection of 5-HT did not affect firing rate, again indicating tachyphylaxis.
Most of the 5-HT-sensitive units also responded to other chemical stimuli. Figure 4 shows averaged unit responses (as peristimulus time histograms) to these chemicals, and Table 6 lists the percentages of 5-HT-sensitive Vc neurons that also responded to other chemical stimuli. Importantly, high percentages of 5-HT-sensitive Vc units responded to AITC and capsaicin, while lower percentages also responded to menthol, CA (Table 6), and the TRPV3 agonists carvacrol (57%, 4/7) and eugenol (36%, 4/11). Table 6 also lists percentages of 5-HT-sensitive TG and DRG cells that responded to other chemicals for comparison with Vc units. The percentages of Vc and TG/DRG cells that responded to the various chemicals were generally comparable, implying that the sequential stimulus application in the Vc recording experiments did not induce any major cross-sensitizing (or desensitizing) effects.
Table 6.
Percentages of 5-HT-sensitive Vc, TG, and DRG cells that were activated by additional chemical stimuli
| Vc | TG Cells | DRG Cells | |
|---|---|---|---|
| Capsaicin | 68.4 | 35.7 | 60.2 |
| AITC | 94.4 | 85.7 | 35.2 |
| Histamine | 20 | 33.3 | 29.6 |
| Menthol | 26.3 | 36.4 | 40.4 |
| CA | 21.1 | 0 | 50 |
Percentages of 5-HT-sensitive trigeminal subnucleus caudalis (Vc), TG, and DRG cells that were activated by additional chemical stimuli are shown.
DISCUSSION
Behavioral studies.
Cheek injection of 5-HT was presently found to elicit dose-dependent hindlimb scratching, consistent with previous reports that 5-HT evoked hindlimb scratch bouts directed to the injection site in the nape of the neck in rats (Hachisuka et al. 2010; Jinks and Carstens 2002; Nojima and Carstens 2003a, 2003b; Nojima et al. 2003; Thomsen et al. 2001, 2002) and mice (Inagaki et al. 2001; Maekawa et al. 2000; Yamaguchi et al. 1999). Rat mast cells contain higher concentrations of 5-HT than histamine (Purcell et al. 1989), possibly related to the scratch-inducing capacity of 5-HT in this species. 5-HT elicited relatively few forelimb wipes, with only one concentration of 5-HT evoking significantly more wipes compared with vehicle control animals (Table 1). 5-HT has also been reported to induce hyperalgesia (Lin et al. 2011; Oliveira et al. 2007; Taiwo and Leving 1992); however, the present results indicate that 5-HT evokes behavioral responses that are more closely associated with itch rather than pain. Chloroquine, which induces itch in humans (Ajayi et al. 1989), also elicited facial scratching but not wiping. Curiously, chloroquine injected in the nape of the neck did not elicit significant hindlimb scratching (Tables 1 and 2), a finding for which we currently have no adequate explanation beyond possible differences in the composition or innervation of facial versus back skin. In contrast, AITC, which induces burning pain in human skin (Namer et al. 2005), elicited dose-dependent ipsilateral forelimb wiping but not hindlimb scratching. These results support the idea that hindlimb scratching reflects itch while forelimb wiping reflects pain in rats, similar to previous recent studies using mice (Akiyama et al. 2010a, 2010b; Shimada and LaMotte 2008).
Other suspected pruritogens including PAF, PAR-2 and PAR-4 agonists, cowhage, and compound 48/80 did not elicit significant scratching in either the cheek or nape models in rats (Tables 1 and 2; Thomsen et al. 2001). In marked contrast, the identical application of cowhage spicules, as well as intradermal injection of PAR-2 and -4 agonists, elicited robust scratching in mice (Akiyama et al. 2009c, 2010b; Shimada et al. 2006; Tsujii et al. 2009). Cowhage spicules contain mucunain, which acts via PAR-2 and PAR-4 to elicit itch in humans (Reddy et al. 2008). The failure of even high concentrations of PAR-2 and PAR-4 agonists, as well as cowhage spicules, to elicit scratching in Sprague-Dawley rats most likely reflects a species difference.
Histamine elicited significant dose-dependent wiping but did not elicit any significant scratching (Table 1). Injection of histamine in the nape of the neck failed to elicit any significant degree of hindlimb scratching (Jinks and Carstens 2002; Thomsen et al. 2001). These findings support the view that histamine is an algogen rather than a pruritogen in Sprague-Dawley rats. The failure of histamine as well as cowhage and PAR-2 and -4 agonists to elicit scratching in rats stands in marked contrast to the ability of these agents to elicit scratching in mice, and indicates that the Sprague-Dawley rat is inferior to the mouse as a model for histamine- and protease-evoked itch in humans.
Cheek microinjection of capsaicin elicited equivalent scratching and wiping (Fig. 1C, Table 1), suggesting that it simultaneously elicits itch and pain. This differs from the mouse, in which cheek microinjection of capsaicin elicited significant wiping but not scratching (Akiyama et al. 2010c). When injected intradermally in human skin, capsaicin elicits burning pain but not itch; however, intradermal insertion of capsaicin-loaded cowhage spicules elicited both itch and nociceptive qualities (burn, sting, prick) simultaneously (LaMotte et al. 2009; Sikand et al. 2009). Moreover, topical skin application of capsaicin elicited a dominant sensation of itch in a majority of subjects (Green and Shaffer 1993). These observations suggest that capsaicin is capable of activating pruriceptors and that the presently observed facial scratching and wiping behavior reflects a mixed sensation of itch and pain in rats.
IBA, a derivative of hydroxy-α-sanshool that elicits tingle (Albin and Simons 2010), also elicited equivalent scratching and wiping suggesting a mixed sensation. Intraplantar IBA in rats elicited nocifensive behaviors (flinching, guarding, licking) (Klein et al. 2011b) and excited both nociceptive and nonnociceptive spinal dorsal horn neurons (Sawyer et al. 2009). We speculate that the tingle sensation elicited by IBA is novel and hence aversive, but not painful, resulting in the observed range of nocifensive responses that are available to the animal to respond to unusual sensations.
Formalin elicited significant scratching and wiping, consistent with previous data in the mouse cheek model (Akiyama et al. 2010b) and the mouse and rat rostral back model (Jinks and Carstens 2002; Imamachi et al. 2009; Sun and Chen 2007). Nocifensive behavior elicited by a low (0.5%), but not high (2%), concentration of formalin was abolished in mice with neurotoxic destruction of TRPV1-expressing spinal afferents (Shields et al. 2010). The latter authors suggested that nociceptors are not required for formalin-evoked nocifensive scratching and wiping and that high-dose formalin may elicit a mixed sensation of itch and pain via nonspecific activation of multiple afferent fiber types.
Facial scratching was characterized by discrete bouts of hindpaw movements at a constant within-bout frequency of 10 Hz. Neither bout duration nor within-bout frequency varied with time or concentration of 5-HT, consistent with our previous study (Nojima and Carstens 2003b). Facial scratch bouts in rats exhibited lower frequency and longer duration compared with facial scratching in mice (12.6 Hz, 0.4 s) (Akiyama et al. 2010b), with the difference in frequency likely explained by allometric scaling. The duration of ipsilateral forelimb wipes was brief in rats and mice (0.29 and 0.19 s, respectively). These observations suggest that hindlimb scratch bouts and forelimb wipes are discrete, stereotyped movements, and that the intensity of itch or pain is reflected by the number of scratch bouts or wipes over time, rather than parameters of the individual movements.
Primary sensory neurons.
5-HT (100 μM) activated 6.4% of TG and 6.8% of DRG cells. These values are similar to or lower than those reported previously, where ∼20% of rat DRG cells (Nicolson et al. 2002), 11% of mouse TG cells (Akiyama et al. 2010a), and 6.4% of mouse DRG cells (Akiyama et al. 2010c) responded to 100 μM 5-HT. A minority of 5-HT-sensitive TG and DRG cells did not respond to any other tested agents, making them candidates for 5-HT-selective pruriceptors. However, the majority of 5-HT-responsive cells responded to other agents, with >50% also responding to chloroquine, AITC, and capsaicin, and about one-fifth also responding to histamine. These proportions are roughly similar to the proportions of 5-HT-sensitive Vc neurons that also responded to other agents (Table 6), suggesting that input from 5-HT-sensitive primary afferents largely accounts for the chemical response tuning of Vc neurons.
The coactivation of TG and DRG cells by 5-HT and capsaicin is consistent with a role for TRPV1-expressing primary afferent fibers in mediating 5-HT-evoked scratching. This is supported by recent evidence that scratching elicited by intradermal 5-HT or α-methyl-5-HT was nearly completely abolished in mice lacking intraspinal TRPV1-expressing terminals and in mice lacking PLCβ3 (which is coexpressed in ∼35% of TRPV1-expressing sensory neurons), although curiously not in mice lacking TRPV1 per se (Imamachi et al. 2009). Conceivably, 5-HT activation of PLCβ3 may lead to downstream activation of some other currently unidentified ion channel that is coexpressed with TRPV1 in sensory neurons in order to signal itch.
Fourteen percent of TG and DRG cells responded to chloroquine, a pruritogen that specifically binds to MrgprA3 (Liu et al. 2009). The vast majority of chloroquine-sensitive TG and DRG cells were also activated by the TRPA1 agonist AITC, consistent with a role for TRPA1 in chloroquine-evoked scratching behavior (Wilson et al. 2011), and 27–43% were also activated by capsaicin. However, relatively few chloroquine-sensitive TG and DRG cells were activated by histamine (Fig. 3), in contrast to a recent report that all chloroquine-sensitive DRG cells in mouse also responded to histamine as well as capsaicin (Liu et al. 2009). In the present study, the majority of histamine-sensitive TG and DRG cells were also activated by capsaicin, in agreement with the report of Liu et al. (2009).
Vc recordings.
Our search strategy was intended to maximize the probability of finding 5-HT-responsive Vc neurons (Akiyama et al. 2009a, 2009b, 2010a; Jinks and Carstens 2002). Units identified in this manner exhibited more prolonged responses to the higher than the lower dose of 5-HT, as well as tachyphylaxis to repeated intradermal injections of 5-HT as previously reported for responses of lumbar superficial dorsal neurons to hindpaw intradermal injections of 5-HT in rat (Jinks and Carstens 2002) and mouse (Akiyama et al. 2009b). Also consistent with these latter reports, the majority of 5-HT-sensitive Vc units were presently categorized as WDR or NS, with a small percentage being mechanically insensitive. Importantly, the vast majority of 5-HT-responsive units additionally responded to the algogens AITC and capsaicin (Table 3), consistent with previous studies from our laboratory as well as studies of histamine- or cowhage-sensitive primate spinothalamic tract neurons that always also responded to capsaicin (Davidson et al. 2007; Simone et al. 2004).
Central mechanisms of itch and pain.
Recent studies have advanced our understanding of itch and pain mechanisms (Davidson and Giesler 2010; Ma 2010; Patel and Dong 2010). GRP was implicated as a spinal neuropeptide transmitter for itch, since mice lacking GRP receptors (Sun and Chen 2007) or with neurotoxic ablation of GRP receptor-expressing neurons in the superficial dorsal horn (Sun et al. 2009) exhibit attenuated scratching behavior. Substance P is also implicated, since rats with neurotoxic ablation of NK-1-expressing neurons in the superficial medullary and cervical dorsal horn exhibited a significant reduction in 5-HT-evoked scratching (Carstens et al. 2010). Relief of itch by scratching is presumably mediated by intraspinal inhibition of itch-signaling neurons (Davidson et al. 2009). Mice lacking the transcription factor Bhlhb5 exhibited enhanced scratching behavior associated with a specific loss of inhibitory spinal interneurons (Ross et al. 2010). Such inhibitory interneurons are thought to be activated by glutamatergic input from nociceptive primary afferents. Mice lacking the VGlut2 vesicular transporter in Nav1.8-expressing primary afferents exhibited a reduction in nociception and enhancement of scratching (Lagerström et al. 2010; Liu et al. 2010), presumably due to the loss of nociceptive drive that normally activates inhibitory interneurons to suppress itch transmission.
It is currently uncertain whether a specific population of pruritogen-selective neurons signals itch (Andrew and Craig 2001) or if itch is signaled by a population coding mechanism involving neurons that receive both pruritic and nociceptive input (Akiyama et al. 2009a, 2009b, 2010a). In the latter scenario, itch may be signaled when pruritogens excite neurons that respond to both pruritic and noxious stimuli. Noxious stimuli also excite this population of neurons but additionally recruit a population of nociceptive neurons that is unresponsive to pruritic stimuli. It is suggested that excitation of both of these neuronal populations by a noxious stimulus signals pain. At the same time, the noxious stimulus also activates inhibitory interneurons to selectively inhibit itch-signaling neurons. In Vc, the large majority of pruritogen-sensitive neurons additionally responded to algogens, with little evidence for pruritogen-selective neurons (Akiyama et al. 2010a and present data). By this population coding mechanism, cheek injection of pruritogens elicits hindimb scratching by activating Vc neurons responsive to both pruritic and algesic stimuli, which ultimately engage descending propriospinal pathways that activate “scratch reflex” circuitry in the lumbar spinal cord. Cheek injection of algogens activates nociceptive Vc neurons that are ultimately connected to cervical motor circuits that control the ipsilateral forelimb wiping movement. The anatomic circuits underlying these distinct behavioral responses remain to be worked out.
GRANTS
This work was supported by National Institutes of Health Grants AR-57194 and DE-021183.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance provided by M. Ivanov, S. Cheung, C. Joe, C. Cleary, M. Tran, C. Kwok, and T. Le.
REFERENCES
- Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol 37: 539–540, 1989 [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol 104: 2442–2450, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol 102: 2176–2183, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and μ-opioid modulation in mice. Acta Derm Venereol 90: 575–581, 2010b [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain 151: 378–383, 2010c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther 329: 945–951, 2009c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci 29: 6691–6699, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin KC, Simons CT. Psychophysical evaluation of a sanshool derivative (alkylamide) and the elucidation of mechanisms subserving tingle. PLoS One 5: e9520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4: 72–77, 2001 [DOI] [PubMed] [Google Scholar]
- Carstens E. Neurobiology of itch and pain: scratching for answers. In: Current Topics in Pain: 12th World Congress on Pain, edited by Castro-Lopes J. Seattle, WA: IASP, 2009, p. 73–93 [Google Scholar]
- Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. Neuroreport 21: 303–308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Kuraishi Y. Animal models of itch: scratching away at the problem. In: Itch: Basic Mechanisms and Therapy, edited by Yosipovitch G, Greaves MW, Fleischer AB, McGlone F. Monticello, NY: Dekker, 2004, p. 35–50 [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci 33: 550–558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci 12: 544–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain 53: 323–334, 1993 [DOI] [PubMed] [Google Scholar]
- Hachisuka J, Furue H, Furue M, Yoshimura M. Responsiveness of C neurons in rat dorsal root ganglion to 5-hydroxytryptamine-induced pruritic stimuli in vivo. J Neurophysiol 104: 271–279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 126: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA 106: 11330–11335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Nagao M, Igeta K, Kawasaki H, Kim JF, Nagai H. Scratching behavior in various strains of mice. Skin Pharmacol Appl Skin Physiol 14: 87–96, 2001 [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol 87: 1280–1289, 2002 [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci 27: 7490–7497, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Carstens MI, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, Carstens E. Self- and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem Senses 36: 199–208, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Sawyer CM, Zanotto KL, Ivanov MA, Cheung S, Carstens MI, Furrer S, Simons CT, Slack JP, Carstens E. A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats. J Neurophysiol 105: 1701–1710, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol 275: 229–233, 1995 [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, Olund C, Smith C, Mendez JA, Chen ZF, Wood JN, Wallén-Mackenzie A, Kullander K. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 68: 529–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 101: 1430–1443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Ji Q, Ji W. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neurosci Lett 492: 175–178, 2011 [DOI] [PubMed] [Google Scholar]
- Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci 31: 1410–1418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68: 543–556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest 120: 3773–3778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Nojima H, Kuraishi Y. Itch-associated responses of afferent nerve innervating the murine skin: different effects of histamine and serotonin in ICR and ddY mice. Jpn J Pharmacol 84: 462–466, 2000 [DOI] [PubMed] [Google Scholar]
- Namer B, Seifert F, Handwerker HO, Maihöfner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport 16: 955–959, 2005 [DOI] [PubMed] [Google Scholar]
- Nicolson TA, Bevan S, Richards CD. Characterisation of the calcium responses to histamine in capsaicin-sensitive and capsaicin-insensitive sensory neurones. Neuroscience 110: 329–338, 2002 [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. 5-hydroxytryptamine (5-HT)2 receptor involvement in acute 5-HT-evoked scratching but not in allergic pruritus induced by dinitrofluorobenzene in rats. J Pharmacol Exp Ther 306: 245–252, 2003a [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. J Neurosci Methods 126: 137–143, 2003b [DOI] [PubMed] [Google Scholar]
- Nojima H, Simons CT, Cuellar JM, Carstens MI, Moore JA, Carstens E. Opioid modulation of scratching and spinal c-fos expression evoked by intradermal serotonin. J Neurosci 23: 10784–1090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MC, Pelegrini-da-Silva A, Parada CA, Tambeli CH. 5-HT acts on nociceptive primary afferents through an indirect mechanism to induce hyperalgesia in the subcutaneous tissue. Neuroscience 145: 708–714, 2007 [DOI] [PubMed] [Google Scholar]
- Patel KN, Dong X. An itch to be scratched. Neuron 68: 334–339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). San Diego, CA: Academic, 1998 [Google Scholar]
- Purcell WM, Cohen DL, Hanahoe TH. Comparison of histamine and 5-hydroxytryptamine content and secretion in rat mast cells isolated from different anatomical locations. Int Arch Allergy Appl Immunol 90: 382–386, 1989 [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28: 4331–4335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65: 886–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer CM, Carstens MI, Simons CT, Slack J, McCluskey TS, Furrer S, Carstens E. Activation of lumbar spinal wide-dynamic range neurons by a sanshool derivative. J Neurophysiol 101: 1742–1748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci 17: 8003–8008, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Cavanaugh DJ, Lee H, Anderson DJ, Basbaum AI. Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors. Pain 151: 422–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 27: 2331–2337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 530: 281–283, 2006 [DOI] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144: 66–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004 [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448: 700–703, 2007 [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 325: 1531–1534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience 48: 485–490, 1992 [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol 81: 250–254, 2001 [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Simonsen L, Benfeldt E, Jensen SB, Serup J. The effect of topically applied salicylic compounds on serotonin-induced scratching behaviour in hairless rats. Exp Dermatol 11: 370–375, 2002 [DOI] [PubMed] [Google Scholar]
- Tsujii K, Andoh T, Ui H, Lee JB, Kuraishi Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J Pharmacol Sci 109: 388–395, 2009 [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Nieves AL, Spada CS, Williams LS, Tuckett RP. Characterization of a behavioral model for peripherally evoked itch suggests platelet-activating factor as a potent pruritogen. J Pharmacol Exp Ther 272: 758–765, 1995 [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res 35: 77–83, 1999 [DOI] [PubMed] [Google Scholar]
- Zanotto KL, Iodi Carstens M, Carstens E. Cross-desensitization of responses of rat trigeminal subnucleus caudalis neurons to cinnamaldehyde and menthol. Neurosci Lett 430: 29–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol 97: 966–978, 2007 [DOI] [PubMed] [Google Scholar]