Abstract
The goal of this study was to evaluate the influence of wrist tendon vibration on a multijoint elbow/shoulder tracking task. We hypothesized that tendon vibration applied at the wrist musculature would improve upper arm tracking performance in chronic stroke survivors through increased, Ia-afferent feedback to the central nervous system (CNS). To test this hypothesis, 10 chronic stroke and 5 neurologically intact subjects grasped the handle of a planar robot as they tracked a target through a horizontal figure-8 pattern. A total of 36 trials were completed by each subject. During the middle trials, 70-Hz tendon vibration was applied at the wrist flexor tendons. Position, velocity, and electromyography data were evaluated to compare the quality of arm movements before, during, and after trials with concurrent vibration. Despite tracking a target that moved at a constant velocity, hand trajectories appeared to be segmented, displaying alternating intervals of acceleration and deceleration. Segments were identifiable in tangential velocity data as single-peaked, bell-shaped speed pulses. When tendon vibration was applied at the wrist musculature, stroke subjects experienced improved tracking performance in that hand path lengths and peak speed variability decreased, whereas movement smoothness increased. These performance improvements were accompanied by decreases in the muscle activity during movement. Possible mechanisms behind improved movement control in response to tendon vibration may include improved sensorimotor integration or improved cortical modulation of spinal reflex activity.
Keywords: movement smoothness, shoulder, electromyography, sensory feedback
the purpose of this study was to determine whether vibratory stimuli applied to the wrist/finger flexors (FF) improves the control of hemiparetic arm movement during a manual tracking task. Results from a previous study demonstrate that vibration applied to the forearm improves hand stability at the end of rapid point-to-point arm movements (Conrad et al. 2011). This previous experimental paradigm allowed us to evaluate effects of wrist vibratory sensory stimuli during rapid arm movement as well as during arm stabilization at the end of a targeted movement. During these previous experiments, we found that wrist tendon vibration (TV) had a greater effect during the terminal (stabilization) phase of rapid-reaching movements than the initial (transition) phase, consistent with the idea that TV might enhance feedback control of arm position. In the present study, we evaluated a task requiring manual tracking of a target through space. The manual tracking task was selected for study because it requires continual feedback of position and velocity information and integration with motor planning. We postulated that if TV enhanced stabilization at the end of a reach, it would also be effective in enhancing corrections in a dynamic tracking movement. This has important implications to the rehabilitation of the control of movement in people poststroke.
People with stroke demonstrate a number of impairments in hand movement that are related to both feedforward and feedback control mechanisms. In healthy individuals, motor planning of coordinated movement appears to optimize smoothness of movement, thereby affecting motor features such as muscle force and jerk (Soechting and Flanders 1998). This coordination is disrupted poststroke, resulting in movement that is slower, less smooth, and has longer path lengths and a reduced overall range of motion (Cirstea and Levin 2000; Kamper et al. 2002). Furthermore, when hemiparetic stroke survivors make circular drawing motions, they require a longer amount of time for motor planning, produce arm movements that are not smooth, and exhibit a tendency to segment hand trajectory (Fang et al. 2007; Krebs et al. 1999). These problems in motor control could involve challenges to both the generation of motor commands as well as the processing of sensory feedback.
The addition of sensory signals from afferents not directly involved in the motor task may be an effective method for improving (i.e., reducing) the segmentation of hemiparetic arm movements. In particular, additional sensory stimuli may aid in overcoming impaired proprioception (Connell et al. 2008) and is one factor contributing to the poor control of arm movements in poststroke hemiparesis. TV is a powerful means of providing proprioceptive sensory input to the central nervous system (CNS) and has traditionally been thought to target Ia-afferent firing (Cordo et al. 1995; Roll et al. 1989). However, high-amplitude TV has the capacity to provide additional proprioceptive input through Golgi tendon organs (Fallon and Macefield 2007) and muscle spindle secondaries (Burke et al. 1976) during induced muscle contractions. TV, by increasing proprioceptive input, also has the ability to increase activity in the sensorimotor cortex (Golaszewski et al. 2006; Radovanovic et al. 2002; Romaiguère et al. 2003) and can enhance cortical influence on motor systems (Kossev et al. 1999; Smith and Brouwer 2005). Additionally, recent training paradigms incorporating vibratory stimuli during therapy suggest the enhanced sensory input can lead to improved motor function (Cordo et al. 2009). Thus activating sensorimotor pathways, even sensory pathways not directly involved in the motion, might have the capacity to alter the control of arm motion.
Vibration at one location on a limb can have effects that extend beyond the vibrated muscle and its antagonists. For example, multijoint reflexes exist between the forearm and shoulder muscles at both the spinal (Cavallari and Katz 1989) and supraspinal level (Alexander and Harrison 2003; Gracies et al. 1991), and coupling between these joints may be exaggerated poststroke (Sangani et al. 2007; Trumbower et al. 2008). Vibration can also have extended effects on more complex motor patterns as evidenced by the initiation of locomotor-like patterns of movement throughout the leg in response to vibration of a single, remote muscle (Gurfinkel et al. 1998). In this study, TV was applied at the wrist to determine whether exaggerated multijoint coupling could be exploited in such a way that stimulation of one joint (in this case, the wrist) could actually affect performance of tasks at other joints.
We hypothesized that a vibratory stimulus applied to the wrist would improve performance of arm (combined elbow and shoulder) tracking tasks in subjects with chronic hemiparesis through increased afferent feedback to the CNS. To test this hypothesis, we evaluated the kinematics of arm movement while chronic stroke (S) and neurologically intact control (C) subjects tracked a target through a planar figure-8 pattern. TV was applied to the wrist musculature, providing a proprioceptive input to the CNS during some trials. Based on the improvements in arm stabilization at the end of planar point-to-point movements using a similar sensory stimulus (Conrad et al. 2011), we expected smoother kinematics during tracking with TV. This study offers valuable information regarding a potential sensory therapy that might improve coordination of arm movement during everyday tasks.
MATERIALS AND METHODS
Subject population.
Ten chronic S and five age-matched neurologically intact C subjects participated in this study. S subjects (4 male, 6 female; aged 44–62 yr) were required to be at least 1 yr poststroke and to experience upper extremity hemiparesis. Exclusion criteria included the diagnoses of any other neurological disorder or recent treatment that interfered with neuromuscular function, such as botulinum toxin injection. S subjects completed the experiment using their hemiparetic arm, whereas the C participants used their dominant arm. The impairment level of S subjects was measured by the upper extremity Fugl-Meyer Assessment (FMA; maximum score, 66; Fugl-Meyer et al. 1975). FMA scores ranged from 21 to 63, and patients reported being between 1 and 22 yr poststroke. C subjects (2 male, 3 female; aged 59–61 yr) reported no history of stroke or any other upper extremity pathology. Informed consent was obtained from all subjects before participation in the study. The experimental protocol was approved by the Marquette University Institutional Review Board, and procedures complied with the ethical standards outlined by the Declaration of Helsinki. Table 1 provides detailed demographic data for all subjects.
Table 1.
Demographic and clinical data for the subjects participating in the present study
| Subject Identifier | Sex | Age, yr | Time After Stroke, yr | Arm Tested | Fugl-Meyer |
|---|---|---|---|---|---|
| S1 | F | 51 | 15 | R | 63 |
| S2 | F | 57 | 4 | L | 50 |
| S3 | F | 62 | 4 | L | 21 |
| S4 | F | 57 | 17 | R | 57 |
| S5 | F | 60 | 6 | L | 62 |
| S6 | M | 44 | 1 | R | 56 |
| S7 | M | 51 | 6 | L | 40 |
| S8 | M | 56 | 9 | L | 42 |
| S9 | M | 59 | 4 | L | 23 |
| S10 | F | 58 | 22 | L | 30 |
| C1 | F | 59 | L | ||
| C2 | M | 61 | L | ||
| C3 | M | 56 | R | ||
| C4 | F | 55 | R | ||
| C5 | F | 50 | R |
Subject identifiers are categorized as either stroke (S) or control (C) subjects. The amount of time between the occurrence of the subject's stroke and the time at which the experiment was conducted (“Time After Stroke”) is expressed in years. The “Arm Tested” in this experiment was the hemiparetic side for S subjects and the dominant hand for C subjects. “Fugl-Meyer” indicates the subject's Fugl-Meyer upper extremity motor score out of a maximum of 66. F, female; M, male; R, right; L, left.
Test apparatus.
The planar manual tracking task was conducted within the workspace of a two-joint, five-bar linkage robot arm (Scheidt et al. 2010). The planar robot is a real-time system powered by two brushless direct-current motors (Kollmorgan, Northampton, MA) and is capable of applying forces and measuring movement at the manipulandum. When seated at the robot, seatbelts strapped the subject into the high-backed chair, minimizing trunk movement. A horizontal screen obstructed the subject's view of their hand and arm. Below the horizontal screen, the base of the subject's wrist was affixed to the robot handle via a wrist brace.
During the trials, as the hand moved the robot arm, two 17-bit optical encoders (Gurley Precision Instruments, Troy, NY) provided angular position data used for calculating movement characteristics. Hand position was projected onto the horizontal screen for visual display. A 12-degree-of-freedom load cell (JR3, Woodland, CA) measured forces, moments, as well as linear and angular accelerations at the robot handle. The system used the xPC real-time kernel for control of the motors (The MathWorks, Natick, MA) and for data collection at 1,000 Hz. Analog inputs passed through a passive hardware filter with low-pass cutoff frequency of 500 Hz before sampling. Program commands and data logging were completed on a host computer running MATLAB, Simulink, and Stateflow software (The MathWorks).
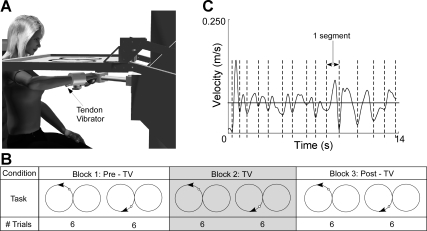
A custom-made tendon vibrator was affixed to the skin adjacent to the forearm flexor tendons (Fig. 1A). The forearm flexor region was selected because the vibrator could be easily held against the forearm at this location due to the mechanical configuration of the wrist splint. The vibrator consisted of an offset mass that rotated about the shaft of a motor (Faulhaber Group, Clearwater, FL) and was enclosed in a Teflon casing. Vibration was applied at 70 Hz, initiated 1 s before each tracking trial and remaining on throughout each trial. Vibration (70 Hz) was targeted as the stimulus frequency because it lies within the range of frequencies that will activate the most muscle spindles at the highest response rate (Roll et al. 1989). Vibration remained off during the ∼1-min break between trials and between blocks.
Fig. 1.
Experimental setup. A: subjects were seated at a planar robot. Tendon vibrators were affixed adjacent to the wrist flexor tendons and secured with a wrist brace that eliminated wrist movement. The tracking task and cursor representing hand position were projected onto a horizontal screen. B: a single repetition consisted of 1 trace of the figure-8 pattern. A sample set of trials consisted of 6 counterclockwise (CCW) repetitions followed by 6 clockwise (CW) repetitions. The set of trials was repeated with the application of tendon vibration (TV). The final set of trials was completed without TV. The order of CCW and CW trials was randomized between subjects to eliminate an order effect. C: segmentation in movement was identified as multiple peaks in velocity data. A single segment was defined as the movement occurring between 2 adjacent local minima in velocity data.
Muscle activity was measured from arm, forearm, and shoulder musculature using a commercially available surface electromyography (EMG) system (AMT-16; Bortec Biomedical, Calgary, Alberta, Canada). The skin adjacent to the muscle belly of the wrist/FF (flexor carpi radialis) of the forearm, wrist/finger extensor (FE; extensor carpi ulnaris) of the forearm, brachioradialis (BRD), biceps brachii long head (BI), triceps brachii lateral head (TRI), anterior deltoid (AD), posterior deltoid (PD), and pectoralis major (PEC) was cleaned and lightly abraded before attaching disposable Ag/AgCl electrodes (Vermed Medical, Bellows Falls, VT). EMG signals were amplified (500–1,000) and filtered (10–500 Hz) before sampling at 1,000 Hz.
Experimental protocol.
Before the session, subjects were briefed on the experiment, equipment, and objectives. After the subject was strapped into the robot, EMG data were recorded during maximum voluntary contractions (MVCs) targeting movements made in the planar workspace. MVCs consisted of two trials each of isometric wrist flexion, wrist extension, elbow flexion, elbow extension, shoulder flexion, and shoulder extension efforts. Individual trial EMG data were later normalized to the mean peak MVC of the appropriate muscle group for each individual subject (see Data analysis).
Experiment instructions were projected onto the horizontal screen in front of the subject. First, each subject conducted a minimum of 8 practice trials. All subjects were allowed additional practice trials until they were comfortable with the task. This minimized learning effects during the blocks of experimental trials. At the beginning of each trial, the robot moved the subject's hand to a target in the center of the workspace. An auditory tone signaled the beginning of a trial. When the tone sounded, the central target (diameter = 1 cm) began moving in a horizontal figure-8 pattern. The 440-Hz tone was adjusted to a comfortable listening volume for each subject. The tone sounded throughout the duration of each trial. The figure-8 was constructed of 2 side-by-side circles (radius = 14 cm; Fig. 1) with the intersection of the circles centered ∼24 cm in front of the sternum. As the target moved (0.91 rad/s), the subject was instructed to follow the target, attempting to keep a red cursor (diameter = 0.5 cm), representing their hand position, in the center of the target circle. The target moved through the figure-8 pattern 3 times in a row so that 3 repetitions were conducted consecutively without pause. At the end of the set of 3 trials, the robot returned the subject to the central target location to wait for the next set of trials to be initiated at an auditory cue. A total of 36 repetitions (3 repetitions per trial × 4 trials per block × 3 blocks) were completed by each subject. The goal of this study was to assess the coordination of arm movements made during and after application of the sensory stimulus. Therefore, vibration was applied during the 2nd block of trials allowing for kinematic comparisons before (Pre-TV), during (TV), and after (Post-TV) vibration. Within each block, the tracking movement began in the clockwise (CW) direction for half of the trials and in the counterclockwise (CCW) direction for the other half of the trials. Thus, for half of the trials, subjects were tracking the circle in the left side of their workspace in a CCW direction, tracking the circle in the right side of their workspace in a CW direction, and vice versa for the other half of the trials. The order of trials beginning with CCW and CW directions were randomized among subjects to eliminate an order effect.
Five S subjects returned for a second experimental session providing additional control data for the study. In this session, the planar tracking experiment was repeated using the previously described experimental protocol. However, during this session, a “sham” vibration was applied in place of wrist vibration. The 70-Hz sham vibration was administered to the left plantar flexor tendons just proximal to the ankle joint. The purpose of the control experiment was to assess whether changes in tracking performance were in fact due to wrist TV or whether other contributing factors existed (such as motor learning).
Data analysis.
Data analysis focused on hand path kinematics and muscle activity during target tracking. Mean absolute error ( ε̄ ), or the mean distance between the target location and the hand position, quantified movement accuracy. Mean and standard deviation of tangential velocity, derived from the x- and y-position data, evaluated fluctuations in movement speed. MATLAB trapz function (The MathWorks) was used to calculate the area under the tangential velocity curve, or the total path length of the hand per trial. To identify the beginning and end of each segment, we identified each local minimum of tangential velocity falling below the mean velocity. A single submovement was identified as the movement occurring during the period between consecutive local minimum (Fig. 1B). The number of segments (N) were counted for each trial and averaged across trials.
The index of movement smoothness (IMS; equivalent to the magnitude of change in velocity about the target velocity) and the error frequency (Fe; median frequency of endpoint velocity while tracking the target) were also computed from the tangential velocity. The area under the power spectral density (PSD) of the tangential velocity between 1 and 5 Hz was calculated to determine the IMS of hand position. Movements with a larger IMS corresponded to a greater movement about the target. The Fe is the median frequency of the same PSD between 1 and 5 Hz. The Fe was later used to evaluate the magnitude of muscle activity at the frequency of hand movement. Correlations between movement quality and functional ability were evaluated by plotting IMS, total path length, ε̄, and the mean number of segments against the upper extremity FMA scores recorded for each S subject.
Muscle activity was quantified using the EMG signals to identify the strategy used to produce changes in movement smoothness during target tracking. All EMG signals were forward and backward pass-filtered using second order Butterworth filters. First, the data were band-pass filtered (1–500 Hz) and then notch filtered for line noise (59–61 Hz) and artifact resulting from the vibratory stimulus (68–72 and 136–144 Hz). The root-mean square (RMS) signals of both trial and MVC data were calculated using a 100-ms sliding window. A peak RMS value was taken as the largest peak value calculated from 1-s windows taken in the middle of each MVC trial. The RMS data from each muscle group in each tracking trial were normalized to the respective peak RMS value from the MVC for that muscle group. Finally, a PSD of the normalized RMS EMG data was plotted between 1 and 5 Hz. The power of the EMG at Fe, the median frequency of hand movement, was identified and compared between Pre-TV, TV, and Post-TV trials.
Statistical analysis.
Statistical analysis was completed using SPSS software (SPSS, Chicago, IL). Multifactor ANOVAs were conducted to determine the effect of TV on the mean number of segments, total path length, absolute error (Ae), movement smoothness, average velocity, standard deviation of velocity, and EMG power. Tukey post hoc test was used to identify the differences among the three experimental conditions (Pre-TV, TV, and Post-TV). A linear regression analysis was performed to identify correlations between FMA scores and performance data. For all analyses, significance was accepted at P < 0.05. Unless otherwise indicated, all data values are reported as mean ± 1 SD.
RESULTS
Tracking motion: baseline performance.
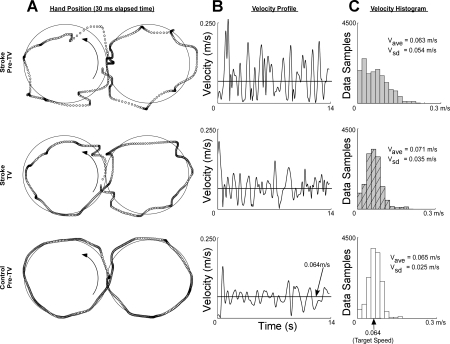
All subjects were able to track the target as it moved through a horizontal figure-8 pattern. Compared with C subjects, the S subjects experienced larger tracking errors and more fragmented hand trajectories (Fig. 2A). Increased error was evidenced by longer hand paths (lS,pre = 1.065 ± 0.060 m, lC,pre = 0.943 ± 0.058 m; P = 0.032), larger Ae (AeS,pre = 0.020 ± 0.003 m, AeC,pre = 0.007 ± 0.001 m; P = 0.051), and smoother movement trajectories (IMSS,pre = 3.21 ± 0.55, IMSC,pre = 1.19 ± 0.12; P = 0.024).
Fig. 2.
Tracking response to TV. A: plots of single trial trajectories made by a chronic stroke (S) subject before TV, the same S subject during TV, and a neurologically intact control (C) subject. Both S and C subjects segmented movement, slowing or stopping at several points throughout the trial. B: segmentation was more pronounced for the S subjects who spent more time at the beginning/end of each segment. C: histograms depicting the distribution of velocity (V) during each trial indicate that TV caused S movement to become normally distributed around the target velocity similar to the tracking behavior of C subjects. ave, Average; sd, standard deviation.
Although the target moved at a constant rate of 0.064 m/s, all subject data displayed fragmented hand movements, characterized by pulses in hand speed (Fig. 2B). Whereas the mean number of segments per trial did not vary between the subject groups (NS,pre = 18 ± 1, NC,pre = 17 ± 1; P = 0.109), segmentation was more pronounced for S subjects as evidenced by larger variances in hand speed. We acknowledge S velocity data were not normally distributed, but review of the mean velocity (V) and standard deviation of velocity (Vsd) provides some understanding of the differences in segmentation between S and C subjects. At baseline, S subjects had higher mean V (VS,pre = 0.076 m/s, VC,pre = 0.068; P = 0.035) and Vsd (VsdS,pre = 0.015, VsdC,pre = 0.010; P = 0.006) than the C group. To describe further the pronounced segmentation in S subjects, histograms of representative trials by S and C subjects were constructed. The histograms verified that at baseline, S subjects' hands spent more time at very low velocities and had a tendency to reach higher velocities (e.g., Fig. 2C). This suggested that the S hand spent more time at the beginning/end of a segment and tended to reach higher midsegment movement velocities.
Tracking performance during and after TV.
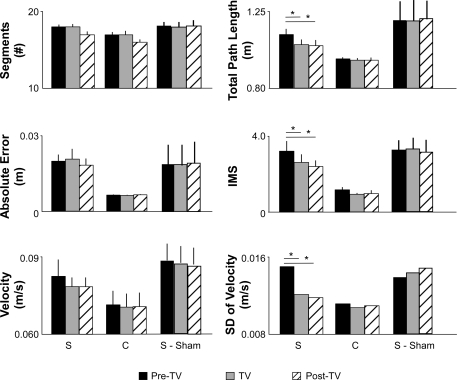
The addition of TV caused a marked improvement in tracking performance for the S group (Fig. 2A). Figure 3 plots differences in tracking performance between the Pre-TV, TV, and Post-TV conditions for S and C subjects. The mean segmentation (NS,pre = 18 ± 1, NC,pre = 17 ± 1) did not significantly change in response to TV for either S or C individuals (NS,TV = 18 ± 1, NS,post = 17 ± 1; NC,TV = 17 ± 1, NC,post = 16 ± 1; ANOVA P = 0.594). Likewise, there were no improvements in Ae (means ± SD) for either group (AeS,pre = 0.020 ± 0.003 m, AeS,TV = 0.021 ± 0.004 m, AeS,post = 0.018 ± 0.002 m; AeC,pre = 0.007 ± 0.001 m, AeC,TV = 0.006 ± 0.001 m, AeC,post = 0.007 ± 0.001 m; ANOVA; P = 0.324). However, compared with Pre-TV values (reported above), in the S group, TV elicited significant decreases in total path length (means ± SD; lS,TV = 1.014 ± 0.058 m, lS,post = 1.008 ± 0.058 m; Tukey, P = 0.011 and 0.006, respectively) and movement smoothness (IMSS,TV = 2.60 ± 0.43, IMSS,post = 2.38 ± 0.34; Tukey, P = 0.020 and 0.003, respectively) during both TV and Post-TV trials. No change in mean velocity occurred in the S group (VS,TV = 0.073 m/s, VS,post = 0.073 m/s; P = 0.345 and 0.391, respectively); however, decreases in Vsd were significant (VsdS,TV = 0.012 m/s, VsdS,post = 0.011 m/s; Tukey, P = 0.041 and 0.006, respectively). Histograms of hand velocity from representative data trials confirm that although mean velocity remained unchanged in response to TV, the velocity at each point in time became more tightly clustered about the target speed (Fig. 2C). The change to a narrower histogram more closely emulated the behavior of velocity data observed during C subjects' trials.
Fig. 3.
Tracking performance. Performance (means ± SD) measures for S (n = 10), C (n = 5) and S-sham trials Pre-TV, during TV, and Post-TV. S subject's tracking performance significantly improved (ANOVA; *P < 0.05) in response to TV as evidenced by decreased total path length, improved movement smoothness, and decreased standard deviation of velocity. The mean number of segments, absolute error, and velocity did not significantly change in response to TV for S or C subjects. No effect on tracking performance occurred with the sham vibration. IMS, index of movement smoothness.
In contrast, the S subjects who participated in the sham vibration experiment exhibited no improvements in any of the performance measures we analyzed. We found no significant improvements in the number of segments (NSham,pre = 17 ± 1, NSham,TV = 17 ± 1; NSham,post = 17 ± 2), Ae (AeSham,pre = 0.023 ± 0.009 m, AeSham,TV = 0.022 ± 0.010 m, AeSham,post = 0.023 ± 0.010 m), mean velocity (VSham,pre = 0.089 m/s, VSham,TV = 0.087 m/s, VSham,post = 0.087 m/s), standard deviation of velocity (VsdSham,pre = 0.010 m/s, VsdSham,TV = 0.011 m/s, VsdSham,post = 0.012 m/s), IMS (IMSSham,pre = 3.24 ± 0.51, IMSSham,TV = 3.27 ± 0.57, IMSSham,post = 3.12 ± 0.63), or total path length (lSham,pre = 1.224 ± 0.144 m, lSham,TV = 1.206 ± 0.156 m, lSham,post = 1.198 ± 0.166 m) associated with the sham vibration (P > 0.05 for all tests). Thus the performance improvements reported for TV were specific to that experimental manipulation and were not a simple effect of prolonged training.
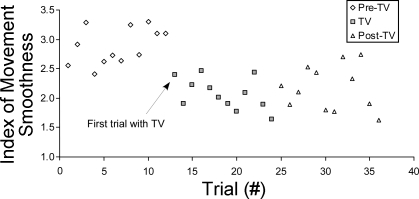
To develop a further understanding of the movement response to TV, we plotted the median IMS from all S data on a trial-by-trial basis (Fig. 4). The plot indicates the response to TV occurs immediately at the onset of TV. The lack of a trend in the initial Pre-TV trials further supports the conclusion that learning or fatigue is not likely a contributing factor to the response observed in TV and Post-TV trials.
Fig. 4.
Plotting the median IMS value across stroke subject data for each trial indicates improvements in IMS occur immediately with the application of TV to the forearm muscles.
For C subjects, compared with Pre-TV values (reported above), modest decreases in total path length (lC,TV = 0.935 ± 0.056 m, lC,post = 0.937 ± 0.059 m), movement smoothness (IMSC,TV = 0.95 ± 0.05, IMSC,post = 0.96 ± 0.20), mean velocity (VS,TV = 0.068 m/s, VS,post = 0.068 m/s), and standard deviation of velocity (VsdC,TV = 0.068 ± 0.009 m/s, VsdC,post = 0.068 ± 0.009 m/s) were not statistically significant (P > 0.05 for all tests).
Muscle activations.
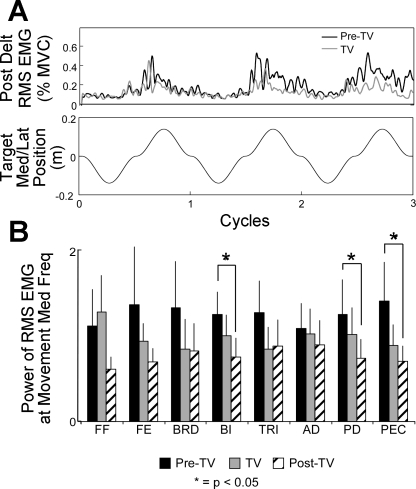
In the shoulder, muscle activity cyclically increased corresponding with the x (medial/lateral)-position data. For example, in Fig. 5A, increased PD activity occurred for a right-handed subject as they moved laterally (+x-direction) through the workspace. In the S group, the power of RMS EMG at the frequency of hand movement decreased at the elbow and shoulder (FE, BRD, BI, TRI, AD, PD, and PEC) in response to TV and remained lower Post-TV (Fig. 5B). However, the difference between Pre-TV and Post-TV muscle activity was only significant for the BI, PD, and PEC muscles (P = 0.023, 0.011, and 0.009, respectively). As a result, the change in strategy of movement that improved the movement smoothness with TV did not appear to involve increased muscle activation (e.g., as might occur with a coactivation strategy). The power of the RMS EMG for the FF muscles increased during vibration trials, suggesting vibration of these muscles elicited a tonic vibration reflex (Hagbarth and Eklund 2004).
Fig. 5.
Muscle activity while tracking. A: modulation of electromyography (EMG) activity in the posterior deltoid (Post Delt; PD) muscles during vibration for a single chronic S subject. The magnitude of the root-mean square (RMS) signal fluctuated at a frequency corresponding to the frequency of hand movement. The magnitude of fluctuation decreased during TV. Med/Lat, medial/lateral; MVC, maximum voluntary contraction. B: changes in the power of the RMS EMG activity (means ± SD) recorded at the median frequency (Freq) of hand movement for all muscles. Significant decreases in biceps brachii long head (BI), PD, and pectoralis major (PEC) activity was recorded between Pre-TV and Post-TV trials.
Correlations between performance and functional ability.
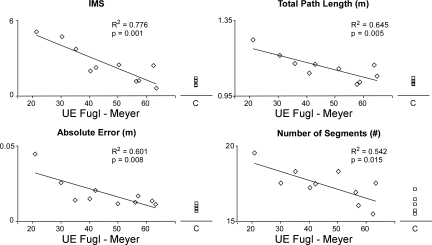
For S subjects, correlations were observed between functional ability and performance measures. Figure 6 displays that the mean movement smoothness, total path length, Ae, and the number of movement segments (averaged across Pre-TV, TV, and Post-TV trials) all negatively correlated with upper extremity FMA scores (r2 = 0.776, 0.645, 0.601, and 0.542, respectively; P < 0.01 in all cases).
Fig. 6.
Correlations between tracking performance measures and Fugl-Meyer scores. A negative correlation existed between tracking performance and functional ability level for stroke subjects. UE, upper extremity.
DISCUSSION
This study evaluated movement deficits in a tracking task and demonstrated that wrist vibration improved performance on manual tracking tasks for chronic stroke survivors. S subjects did not perform as well as C subjects during the figure-8 tracking task, which required control of the shoulder and elbow musculature. When TV was applied at the wrist, S subjects significantly improved tracking performance. Data supporting the improved quality of hand movement included decreased hand path length, a decrease in the standard deviation of hand velocity, and improvement in movement smoothness. Improvement in tracking performance in these subjects was accompanied by a decrease in the amount of shoulder muscle activity required to complete the task. These improvements were specific to the vibration applied to the wrist because no improvements were observed when a sham was applied to the left plantar flexor tendons proximal to the ankle.
Segmentation of coordinated hand movement.
Both S and C subjects failed to produce smooth and continuous movements despite the fact that the target they were tracking moved at a constant speed. Rather, hand movement was segmented into submovements that can be identified in tangential velocity data as many single-peaked, bell-shaped speed profiles or speed pulses (Milner 1992). Segmentation of complex movements into submovements has previously been observed in experiments requiring manual tracking by humans (Pasalar et al. 2005) and nonhuman primates (Roitman et al. 2004). Submovements are characterized by a linear relationship between peak speed and the duration of the movement (Pasalar et al. 2005; Roitman et al. 2004). The slope of this (positive) proportional relationship increases when experimental conditions introduce larger tracking errors in the experiment, such as in the presence of force fields or when movement speed is amplified (Pasalar et al. 2005). This suggests that larger movement errors are characterized by greater fluctuations in movement velocity. This indeed was what we found in this study: movements made by S subjects had higher Ae, whereas hand speed was more variable and attained higher peak speeds than in C subjects. Although C subjects also produced segmented movements, they were better able to maintain hand speeds normally distributed within a tight band about the desired target speed, thus allowing these subjects to keep the hand close to the target throughout each trial. As a result, C subjects performed the task with shorter average path lengths and lower Ae compared with stroke survivors.
Differences in submovement quality were observed both at the ends of segments as well as during the submovements. The first observation was that S subjects spent longer periods of time moving at slow velocities at the beginning and end of each submovement. The second observation was that movement occurring midsegment had a tendency to reach higher velocities for S subjects. Previously, Krebs and colleagues (1999) observed distinct submovements made by stroke patients during what should have been continuous arm movements and attributed the lack of smoothness to an inability to coordinate and overlap movement segments as typically done by healthy individuals. Results from the present study indicate that each S submovement was more pronounced compared with C submovements and exhibited an increased range in velocity.
Possible mechanisms for improved movement with TV.
When TV was applied at the wrist flexor tendons, submovement generation in trajectories made by S subjects became more consistent with that observed in the control group. The variability of velocity decreased and movement smoothness increased, indicating that S subjects made consistently smoother trajectories with vibration than without. Corresponding with improved quality of movement, the power of the EMG signal decreased and remained significantly lower in upper arm musculature during the 12 Post-TV trials.
Submovement generation is not abolished by the removal of visual feedback in goal-directed movement tasks (Novak et al. 2002), and thus proprioceptive feedback suffices to drive the feedback control actions needed to ensure movement accuracy. Insight into the origins of poor tracking performance in stroke survivors may foster understanding of the role proprioception plays in limb movement control. At least three possibilities related to the cortical control of movement exist. First, abnormal segmentation of movements may arise after stroke due to deficits in proprioceptive sensation itself (Carey et al. 1993; Niessen et al. 2008; Smith et al. 1983) since cortical feedback control actions that are dependent on any given sensory signal will be compromised if that signal is absent or corrupted by noise. This does not appear to be the primary issue with our subjects because application of vibration at the wrist (i.e., adding noise to the proprioceptive signals encoding limb state; cf. Cordo et al. 1995; Roll et al. 1989) acts to improve kinematic performance. Second, many S subjects have deficits integrating proprioceptive feedback into the motor commands driving limb movement (Trombly 1992). This might not be surprising because many strokes involve the middle cerebral artery, which compromises sensorimotor cortical areas (including primary sensorimotor cortex) and associated white matter tracts. Recent experimental evidence from functional imaging experiments implicates primary sensorimotor cortex in the moment-by-moment feedback control of limb position (Suminski et al. 2007), areas that also demonstrate responsivity to vibration (Naito et al. 1999; Romaiguère et al. 2003; see also Siggelkow et al. 1999) and passive manipulation of the limbs (Kocak et al. 2009; Reddy et al. 2001; Suminski et al. 2007) as well as functional reorganization associated with recovery of motor function poststroke (Nudo and Milliken 1996).
It is possible that TV improves feedback control of tracking movements by elevating excitability of sensorimotor cortical regions participating in the reorganized, but still suboptimal, control of arm motion. Although cortical excitability is expected to be greatest in somatomotor regions of brain that code the vibrated muscle (e.g., Smith and Brouwer 2005), there is also evidence of cortical excitation throughout the brain. Vibratory activation of motor areas was initially documented using PET, in which motor cortical areas were active during biceps TV at 70 Hz (Naito et al. 1999). Vibration of the palm at 50 Hz at a level that generates a TV reflex also activates a number of motor areas of the cortex based on functional MRI (fMRI) recordings (Golaszewski et al. 2002). fMRI recordings during vibration of the biceps (150 Hz) yields similar activation of motor areas (Gizewski et al. 2005), and results demonstrating activation of motor areas have been observed using MEG recordings during vibration (Casini et al. 2006). The cortical areas activated by vibration are capable of integrating sensory inputs throughout the limb for planning movements. Such excitation could facilitate recruitment of cortical neurons with beneficial but quiescent control potential at neighboring joints in the limb. Finally, impaired kinematic performance during target tracking might instead reflect deficits in the selective and coordinated activation of limb muscles throughout the limb. In this case, TV might instead improve feedback control of tracking movements through intracortical inhibition of “spillover” motor efferent activation in neighboring brain regions associated with the elbow and shoulder control. The current experimental findings cannot differentiate between these last two possibilities.
It is also possible that TV could have had an effect on spinal reflex contributions to the task, although the reduction in muscle activity associated with the vibration makes this unlikely. Indeed, abnormal reflex modulation is very common poststroke. Typically, spinal reflex activity in healthy individuals is modulated by the motor cortex through regulation of brain-stem pathways as well as by direct corticospinal input to Ia inhibitory interneurons (Lundberg 1979). However, this descending inhibition of spinal reflexes is often compromised in S subjects, leading to deficits in the coordination of activation between muscles groups in the limb (Dewald et al. 1995), abnormal reflex coupling between the elbow and shoulder joints (Sangani et al. 2007), and an inability to regulate muscle stiffness through the entire range of motion of a joint (Levin et al. 2000; Schmit et al. 2000). Wrist TV may also modify control of movement through changes in the excitability of spinal reflex pathways. However, we observed a reduction in the activity of proximal limb muscles during TV applied to the wrist, an effect that persisted through the 12 washout trials performed after vibration ceased. Intuitively, this observed reduction in muscle activity does not favor the notion of an increase in spinal reflexes as an explanation for improved movement performance.
Perhaps the most important result of this study is the sustained improvements in arm tracking that were maintained Post-TV. Studies evaluating posteffects of vibration indicate the stimulus has the capacity to sustain altered proprioception and muscle contractions on removal of the stimulus (Duclos et al. 2007; Rogers et al. 1985; Wierzbicka et al. 1998). The postvibratory effects to a 30-s vibration period can last from 3 min to 3 h (Wierzbicka et al. 1998) and can produce muscle contractions in the vibrated muscle for several minutes (Gilhodes et al. 1992). In the current study, vibration was applied for briefer periods of time and repeated, but the postvibratory effects were long lasting, consistent with the time course of changes in proprioception (Rogers et al. 1985) or posture (Duclos et al. 2007; Wierzbicka et al. 1998) observed previously. The aftereffects of TV are generally opposite the illusion produced by vibration (Kito et al. 2006; Seizova-Cajic et al. 2007); however, in the current study, improvements in movement smoothness were similar in both the TV and Post-TV periods. Furthermore, there was no evidence of degradation of smoothness over the Post-TV period in the current study, as might be expected as an aftereffect wears off. At present, it is unknown how long the beneficial effects of vibration might last.
Similar to the effects during vibration, postvibration effects have been associated with supraspinal interpretation of proprioceptive cues. Although there may be spinal postvibratory effects associated with decreases in spindle firing of the vibrated muscle (Ribot-Ciscar et al. 1998), the spinal effects on elbow and shoulder muscles following vibration of wrist flexors are largely unknown. Muscle spindle afferent signals from vibrated wrist muscles alter elbow movement (Kasai et al. 1992). Furthermore, multijoint reflexes are known to exist between the forearm and shoulder muscles at both the spinal (Cavallari and Katz 1989) and supraspinal level (Alexander and Harrison 2003; Gracies et al. 1991). Despite these observations, the importance of the supraspinal processing of the postvibratory effect is emphasized by observations that illusions produced by vibration or postvibration are altered by visual and auditory information (Feldman and Latash 1982). TMS applied during the postvibratory period suggests a change in the excitability of motor cortical areas associated with the vibrated muscle (Kito et al. 2006). Thus the Post-TV effects on tracking in subjects with stroke suggest an adaptation that could be linked to postvibration effects described previously. Future studies are needed to identify the duration of the effects and the illusory impact of the wrist vibration both at the wrist as well as throughout the arm.
Clinical implications.
The ability to improve motor control of slow, controlled movements has the capacity to improve the quality of life of people with stroke. Gains in manual tracking abilities are directly applicable to many activities of daily living such as the use of a steering wheel to drive a car, a joystick to operate a wheelchair, or a mouse to navigate a computer screen. Because performance measures on the figure-8 tracking task were significantly correlated with a measure of functional motor impairment (upper extremity FMA scores), the development of therapeutic interventions aimed at improving functional ability should continue to be explored. Electrical stimulation has previously been used as a nonspecific sensory stimulus to improve motor performance on functional tasks (Conforto et al. 2007; Wu et al. 2006). In a similar context, the use of TV should continue to be investigated as a rehabilitative tool used to enhance motor function during complex arm movements.
GRANTS
This work was funded through the National Institutes of Health (NIH) Research Grant R01-NS-052509. Support for the robot used to conduct the study was provided by NIH National Institute of Child Health and Human Development Grant R01-HD-053727.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Allison Hyngstrom, PT, PhD, for completing clinical assessments of the study participants.
REFERENCES
- Alexander CM, Harrison PJ. Reflex connections from forearm and hand afferents to shoulder girdle muscles in humans. Exp Brain Res 148: 277–282, 2003 [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol 261: 695–711, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil 74: 602–611, 1993 [DOI] [PubMed] [Google Scholar]
- Casini L, Romaiguère P, Ducorps A, Schwartz D, Anton JL, Roll JP. Cortical correlates of illusory hand movement perception in humans: a MEG study. Brain Res 1121: 200–206, 2006 [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Exp Brain Res 78: 465–478, 1989 [DOI] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain 123: 940–953, 2000 [DOI] [PubMed] [Google Scholar]
- Conforto AB, Cohen LG, dos Santos RL, Scaff M, Marie SK. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol 254: 333–339, 2007 [DOI] [PubMed] [Google Scholar]
- Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil 22: 758–767, 2008 [DOI] [PubMed] [Google Scholar]
- Conrad MO, Scheidt RA, Schmit BD. Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis. Neurorehabil Neural Repair 25: 61–70, 2011 [DOI] [PubMed] [Google Scholar]
- Cordo P, Gurfinkel VS, Bevan L, Kerr GK. Proprioceptive consequences of tendon vibration during movement. J Neurophysiol 74: 1675–1688, 1995 [DOI] [PubMed] [Google Scholar]
- Cordo P, Lutsep H, Cordo L, Wright WG, Cacciatore T, Skoss R. Assisted movement with enhanced sensation (AMES): coupling motor and sensory to remediate motor deficits in chronic stroke patients. Neurorehabil Neural Repair 23: 67–77, 2009 [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118: 495–510, 1995 [DOI] [PubMed] [Google Scholar]
- Duclos C, Roll R, Kavounoudias A, Roll JP, Forget R. Vibration-induced post-effects: a means to improve postural asymmetry in lower leg amputees? Gait Posture 26: 595–602, 2007 [DOI] [PubMed] [Google Scholar]
- Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve 36: 21–29, 2007 [DOI] [PubMed] [Google Scholar]
- Fang Y, Yue GH, Hrovat K, Sahgal V, Daly JJ. Abnormal cognitive planning and movement smoothness control for a complex shoulder/elbow motor task in stroke survivors. J Neurol Sci 256: 21–29, 2007 [DOI] [PubMed] [Google Scholar]
- Feldman AG, Latash MI. Inversions of vibration-induced senso-motor events caused by supraspinal influences in man. Neurosci Lett 31: 147–151, 1982 [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- Gilhodes JC, Gurfinkel VS, Roll JP. Roll of Ia afferents in post-contraction and post-vibration motor effect genesis. Neuroscience Lett 135: 247–251, 1992 [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Koeze O, Uffmann K, de Greiff A, Ladd ME, Forsting M. Cerebral activation using a MR-compatible piezoelectric actuator with adjustable vibration frequencies and in vivo wave propagation control. Neuroimage 24: 723–730, 2005 [DOI] [PubMed] [Google Scholar]
- Golaszewski SM, Siedentopf CM, Baldauf E, Koppelstaetter F, Eisner W, Unterrainer J, Guendisch GM, Mottaghy FM, Felber SR. Functional magnetic resonance imaging of the human sensorimotor cortex using a novel vibrotactile stimulator. Neuroimage 17: 421–430, 2002 [DOI] [PubMed] [Google Scholar]
- Golaszewski SM, Siedentopf CM, Koppelstaetter F, Fend M, Ischebeck A, Gonzalex-Felipe V, Haala I, Struhal W, Mottaghy FM, Gallasch E, Felber SR, Gerstenbrand F. Human brain structures related to plantar vibrotactile stimulation: a functional magnetic resonance imaging study. Neuroimage 29: 923–929, 2006 [DOI] [PubMed] [Google Scholar]
- Gracies JM, Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of propriospinal-like excitation to different species of human upper limb motoneurones. J Physiol 434: 151–167, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA. Locomotor-like movements evoked by leg muscle vibration in humans. Eur J Neurosci 10: 1608–1612, 1998 [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Eklund G. Tonic vibration reflexes (TVR) in spasticity. Brain Res 2: 201–203, 1966 [DOI] [PubMed] [Google Scholar]
- Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil 83: 702–707, 2002 [DOI] [PubMed] [Google Scholar]
- Kasai T, Kawanishi M, Yahagi S. The effects of wrist muscle vibration on human voluntary elbow flexion-extension movements. Exp Brain Res 90: 217–220, 1992 [DOI] [PubMed] [Google Scholar]
- Kito T, Hashimoto T, Yoneda T, Katamoto S, Naito E. Sensory processing during kinesthetic aftereffect following illusory hand movement elicited by tendon vibration. Brain Res 1114: 75–84, 2006 [DOI] [PubMed] [Google Scholar]
- Kocak M, Ulmer JL, Sahin Ugurel M, Gaggl W, Prost RW. Motor homunculus: passive mapping in healthy volunteers by using functional MR imaging–initial results. Radiology 251: 485–492, 2009 [DOI] [PubMed] [Google Scholar]
- Kossev A, Siggelkow S, Schubert M, Wohlfarth K, Dengler R. Muscle vibration: different effects on transcranial magnetic and electrical stimulation. Muscle Nerve 22: 946–948, 1999 [DOI] [PubMed] [Google Scholar]
- Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proc Natl Acad Sci USA 96: 4645–4649, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML. Neurophysiological Basis of Movement. Champaign, IL: Human Kinetics, 2008 [Google Scholar]
- Levin MF, Selles RW, Verheul MH, Meijer OG. Deficits in the coordination of agonist and antagonist muscles in stroke patients: implications for normal motor control. Brain Res 853: 352–369, 2000 [DOI] [PubMed] [Google Scholar]
- Lundberg A. Multisensorial control of spinal reflex pathways. Prog Brain Res 50: 11–28, 1979 [DOI] [PubMed] [Google Scholar]
- Milner TE. A model for the generation of movements requiring endpoint precision. Neuroscience 49: 487–496, 1992 [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE. Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19: 6134–6144, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen MH, Veeger DH, Koppe PA, Konijnenbelt MH, van Dieen J, Janssen TW. Proprioception of the shoulder after stroke. Arch Phys Med Rehabil 89: 333–338, 2008 [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LL, Houk JC. The use of overlapping submovements in the control of rapid hand movements. Exp Brain Res 144: 351–364, 2002 [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996 [DOI] [PubMed] [Google Scholar]
- Paillard J. Fast and slow feedback loops for the visual correction of spatial errors in a pointing task: a reappraisal. Can J Physiol Pharmacol 74: 401–417, 1996 [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Ebner TJ. Effects of speeds and force fields on submovements during circular manual tracking in humans. Exp Brain Res 163: 214–225, 2005 [DOI] [PubMed] [Google Scholar]
- Radovanovic S, Korotkov A, Ljubisavljevic M, Lyskov E, Thunbers J, Kataeva G, Danko S, Roudas M, Pakhomov S, Medvedev S, Johansson H. Comparison of brain activity during different types of proprioceptive inputs: a positron emission tomography study. Exp Brain Res 143: 276–285, 2002 [DOI] [PubMed] [Google Scholar]
- Reddy H, Floyer A, Donaghy M, Matthews PM. Altered cortical activation with finger movement after peripheral denervation: comparison of active and passive tasks. Exp Brain Res 138: 484–491, 2001 [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Rossi-Durand C, Roll JP. Muscle spindle activity following muscle tendon vibration in man. Neurosci Lett 258: 147–150, 1998 [DOI] [PubMed] [Google Scholar]
- Rogers DK, Bendrups AP, Lewis MM. Disturbed proprioception following a period of muscle vibration in humans. Neurosci Lett 57: 147–152, 1985 [DOI] [PubMed] [Google Scholar]
- Roitman AV, Massaquoi G, Takahashi K, Ebner TJ. Kinematic analysis of manual tracking in monkeys: characterization of movement intermittencies during a circular tracking task. J Neurophysiol 91: 901–911, 2004 [DOI] [PubMed] [Google Scholar]
- Roitman AV, Pasalar S, Ebner TJ. Single trial coupling of Purkinje cell activity to speed and error signals during circular manual tracking. Exp Brain Res 192: 241–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76: 213–222, 1989 [DOI] [PubMed] [Google Scholar]
- Romaiguère P, Anton JL, Roth M, Casini L, Roll JP. Motor and parietal cortical areas both underlie kinaesthesia. Brain Res Cogn Brain Res 16: 74–82, 2003 [DOI] [PubMed] [Google Scholar]
- Sangani S, Starsky A, McGuire J, Schmit B. Multijoint reflexes of the stroke arm: neural coupling of the elbow and shoulder. Muscle Nerve 36: 694–703, 2007 [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Lillis KP, Emerson SJ. Visual, motor and attentional influences on proprioceptive discrimination between straight and curved hand paths in reaching. Exp Brain Res 204: 239–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit BD, Dewald JP, Rymer WZ. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil 81: 269–278, 2000 [DOI] [PubMed] [Google Scholar]
- Seizova-Cajic T, Smith JL, Taylor JL, Gandevia SC. Proprioceptive movement illusions due to prolonged stimulation: reversals and aftereffects. PLoS One 2: e1033, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggelkow S, Kossev A, Schubert M, Kappels H, Wolf W, Dengler R. Modulation of motor evoked potentials by muscle vibration: the role of vibration frequency. Muscle Nerve 22: 1544–1548, 1999 [DOI] [PubMed] [Google Scholar]
- Smith DL, Akhtar AJ, Garraway WM. Proprioception and spatial neglect after stroke. Age Ageing 12: 63–69, 1983 [DOI] [PubMed] [Google Scholar]
- Smith L, Brouwer B. Effectiveness of muscle vibration in modulating corticospinal excitability. J Rehabil Res Dev 42: 787–794, 2005 [DOI] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Flexible strategies for sensory integration during motor planning. Nat Neurosci 8: 490–497, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting FJ, Flanders M. Movement planning: kinematics, dynamics, both or neither? In: Vision and Action, edited by Harris L, Jenkin M. New York: Cambridge Univ. Press, 1998, p. 352–371 [Google Scholar]
- Suminski AJ, Rao SM, Mosier KM, Scheidt RA. Neural and electromyographic correlates of wrist posture control. J Neurophysiol 97: 1527–1545, 2007 [DOI] [PubMed] [Google Scholar]
- Trombly CA. Deficits of reaching in subjects with left hemiparesis: a pilot study. Am J Occup Ther 46: 887–897, 1992 [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Ravichandran VJ, Krutky MA, Perreault EJ. Altered multijoint reflex coordination is indicative of motor impairment level following stroke. Conf Proc IEEE Eng Med Biol Soc 3558–3561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka MM, Gilhodes JC, Roll JP. Vibration-induced postural posteffects. J Neurophysiol 79: 143–150, 1998 [DOI] [PubMed] [Google Scholar]
- Wu CW, Seo H, Cohen LG. Influence of electric somatosensory stimulation on paretic-hand function in chronic stroke. Arch Phys Med Rehabil 87: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain 127: 1035–1046, 2004 [DOI] [PubMed] [Google Scholar]