Abstract
Endogenously bursting neurons play central roles in many aspects of nervous system function, ranging from motor control to perception. The properties and bursting patterns generated by these neurons are subject to neuromodulation, which can alter cycle frequency and amplitude by modifying the properties of the neuron's ionic currents. In the stomatogastric ganglion (STG) of the spiny lobster, Panulirus interruptus, the anterior burster (AB) neuron is a conditional oscillator in the presence of dopamine (DA) and other neuromodulators and serves as the pacemaker to drive rhythmic output from the pyloric network. We analyzed the mechanisms by which DA evokes bursting in the AB neuron. Previous work showed that DA-evoked bursting is critically dependent on external calcium (Harris-Warrick RM, Flamm RE. J Neurosci 7: 2113–2128, 1987). Using two-photon microscopy and calcium imaging, we show that DA evokes the release of calcium from intracellular stores well before the emergence of voltage oscillations. When this release from intracellular stores is blocked by antagonists of ryanodine or inositol trisphosphate (IP3) receptor channels, DA fails to evoke AB bursting. We further demonstrate that DA enhances the calcium-activated inward current, ICAN, despite the fact that it significantly reduces voltage-activated calcium currents. This suggests that DA-induced release of calcium from intracellular stores activates ICAN, which provides a depolarizing ramp current that underlies endogenous bursting in the AB neuron.
Keywords: calcium-activated inward current, ryanodine, voltage clamp, central pattern generator
rhythmic activity plays central roles in many aspects of nervous system function, ranging from motor control to perception. The neural mechanisms underlying rhythmogenesis are not well understood but include both network-driven oscillators and neuronal oscillators (Harris-Warrick 2010). Neuronal oscillators are single neurons that generate an endogenous oscillatory voltage pattern based on the ionic currents they express. The bursting properties of these neurons are subject to neuromodulation, which can alter cycle frequency and amplitude by modifying the properties of the neuron's ionic currents (Harris-Warrick and Johnson 2010; Lotshaw et al. 1986; Marder and Bucher 2001; Marder et al. 2005; Partridge et al. 1990).
The pyloric network in the crustacean stomatogastric ganglion (STG) is an important system for studying the mechanisms of rhythmogenesis (Harris-Warrick 1993; Marder and Bucher 2001; Selverston and Ayers 2006). The pyloric circuit consists of 1 interneuron (the anterior burster, or AB) and 13 motoneurons. The AB neuron is a conditional oscillator: under the appropriate modulatory conditions, it generates intrinsic rhythmic oscillations and serves as the primary pacemaker to drive the network with a cycle frequency of 1–2 Hz. When isolated from all synaptic and modulatory input, the AB neuron loses its oscillatory capabilities. Addition of neuromodulators such as the monoamines dopamine (DA) and serotonin (5-HT) can restore rhythmic bursting in the isolated AB neuron. However, DA and 5-HT appear to use different sets of currents to drive the oscillations (Harris-Warrick and Flamm 1987). DA-induced bursting of the AB neuron is critically dependent on external calcium, as low external calcium or broad-spectrum calcium channel blockers halt it, while these blockers only slow the frequency of 5-HT-evoked bursting. In contrast, low external sodium concentration or application of tetrodotoxin (TTX) does not affect DA-induced oscillations but abolishes 5-HT-induced bursting (Harris-Warrick and Flamm 1987).
DA shapes the electrical properties of the AB neuron by affecting multiple currents in a cell-specific manner. DA reduces the tonic leak current, the transient potassium current (Peck et al. 2001), and the voltage-gated calcium current [ICa(V)] (Johnson et al. 2003). Additionally, DA enhances the hyperpolarization-activated inward current, Ih (Peck et al. 2006), which contributes to (but alone is not sufficient to trigger) the depolarizing ramp driving regenerative voltage oscillations. On the other hand, DA also enhances a slowly activating and deactivating inward-rectifying voltage-gated potassium current [IK(V)] (Gruhn et al. 2005), which may act to restrict the maximal spike frequency of the oscillating AB neuron (Harris-Warrick and Johnson 2010).
Here we have further studied the cellular mechanisms of DA-induced pacemaking in the AB neuron. The calcium dependence of DA-induced oscillations suggests an involvement of calcium currents and/or calcium-activated inward currents. However, DA reduces ICa(V) by up to 75% (Johnson et al. 2003), significantly reducing the influx of calcium ions into the cell. Since this does not appear to be the major source of calcium for DA-evoked bursting, we hypothesized that enhanced release of calcium from intracellular stores might be central to DA's actions.
In this paper, we show that DA does indeed evoke an accumulation of intracellular calcium by inositol trisphosphate (IP3) receptor (IP3R)- and ryanodine (Ry) receptor (RyR)-mediated release from intracellular stores. This increase in calcium concentration begins well before the emergence of voltage oscillations, and in turn appears to activate the calciumactivated nonselective current ICAN, which provides a depolarizing ramp current that contributes to the pacemaker oscillations in the AB neuron.
MATERIALS AND METHODS
Animals and preparation.
Adult California spiny lobsters (Panulirus interruptus) were supplied by Don Tomlinson Commercial Fishing (San Diego, CA) and kept in tanks with artificial seawater. Animal use followed a protocol approved by the Institutional Animal Care and Use Committee at Cornell University. Lobsters were anesthetized in ice; the stomatogastric nervous system (STNS) was dissected out, pinned on a Sylgard-coated petri dish, and superfused with oxygenated lobster saline at 16–17°C unless otherwise specified. The STG was desheathed, and cells were identified as previously described (Selverston et al. 1976). Experiments were primarily performed on the AB neuron, but some used the pyloric dilator (PD) and lateral pyloric (LP) neurons.
Solutions.
Panulirus physiological saline had the following composition (in mM): 479 NaCl, 12.8 KCl, 13.7 CaCl2, 3.9 Na2SO4, 10.0 MgSO4, 2 glucose, and 11.1 Tris base, pH 7.4 (Mulloney and Selverston 1972). To pharmacologically isolate the ionic currents of interest, we used 50 mM tetraethylammonium chloride (TEA) and 4 mM 4-aminopyridine (4-AP) to block potassium currents, 5 mM CsCl to block the hyperpolarization-activated inward current Ih, 0.1 μM TTX to block the sodium current, and 5 μM picrotoxin (PTX) to block glutamatergic synapses within the STG; the NaCl concentration was adjusted to compensate for high TEA. In addition to the bath-applied blockers, we loaded the cell body with intracellular K+ channel blockers by iontophoresis, injecting small negative current steps through electrodes loaded with 2 M TEA and 2 M CsCl for 1–1.5 h; the cell was allowed to recover for 1–1.5 h before the experiment started. Although these treatments reduced K+ currents by >99%, a detectable outward current still contaminated our voltage-clamp studies of the very small calcium-dependent currents. We used flufenamic acid (FFA, 0.1–30 μM) to block ICAN, 30 μM BAPTA-AM to chelate intracellular calcium ions, 10–100 nM Ry to block intracellular RyR channels, 1–10 μM xestospongin C or D to block intracellular IP3R channels, and 10 μM thapsigargin (Tg) to block the sarco/endoplasmic reticulum calcium-ATPase (SERCA) pump. FFA, Ry, xestospongin, and Tg were first dissolved in DMSO to make 100× stock solutions. Immediately before the experiment, the stock solution was diluted in saline to bring the concentration of the blockers to the desired level; the concentration of DMSO did not exceed 1% in the final solution. DMSO alone at this concentration did not have any significant effect on the physiology of the neuron. Chemicals were purchased from Sigma (BAPTA-AM, FFA, Ry), Tocris (Ry), or Cayman Chemical (xestospongin).
Two-photon calcium imaging.
Calcium indicator dyes were injected into the AB neuron by iontophoresis (Kloppenburg et al. 2000). Only AB neurons that maintained rhythmic activity and showed labeling in fine neurites 30 min after iontophoresis were retained for analysis. We labeled the AB neuron with potassium salts of Calcium Green-1 or Indo-1 (both from Invitrogen). Our two-photon microscope is based on an upright Olympus BX50WI unit (Díaz-Ríos et al. 2007; Kwan et al. 2009). The excitation, generated by a Ti:sapphire laser (Tsunami, Spectra-Physics), was scanned by a modified Bio-Rad Radiance scan head and focused onto the sample by a water immersion ×40/NA 0.80 objective (Olympus). The Tsunami was driven by a 10-W diode laser (Millenia Pro, SpectraPhysics), whose power was ∼1.7 W directly out of the laser cavity. The laser intensity was controlled by a Pockels cell (350-80LA, Conoptics). The typical after-objective laser power used in our experiments was 5–10 mW as measured with a standard thermopile sensor. For Calcium Green-1, the specimen was excited at 800 nm and the fluorescence was collected by a GaAsP photomultiplier tube (H7422, Hamamatsu) after a band-pass filter between 500 and 650 nm (Chroma). For Indo-1, the specimen was excited at 720 nm and fluorescence was separated by a DCLP440 dichroic mirror into two detection channels: 360–430 nm with a bialkali photomultiplier tube (HC125-02, Hamamatsu) and 485–505 nm with a GaAsP photomultiplier tube. The line scans were synchronized to the electrophysiological recordings by connecting the voltage output of the amplifier to the image acquisition system and recording membrane potential (Vm) on a third detection channel (Kloppenburg et al. 2000).
For the ratiometric calcium indicator Indo-1, we estimated the absolute free calcium concentration, [Ca2+]in, by calculating the ratio between fluorescence detected at the two emission channels, R = F395/F495, and using the equation [Ca2+] = Keff × (R − R0)/(R1 − R), where Keff is the in situ dissociation constant of Indo-1 and R0 and R1 are the fluorescence ratios at zero and saturating calcium concentrations. Background fluorescence was estimated from a region without neurites and subtracted from the actual fluorescence signals. Because the calcium dissociation constant within fine neurites of STG neurons is not known, we assumed that it is similar to the value measured in salt solution, so Keff ∼ Kd = 250 nM (Grynkiewiecz et al. 1985). To measure R1, we topically applied a calcium ionophore, 4-bromo-A-23187 (Invitrogen) to equilibrate the intra- and extracellular calcium concentrations. We tried to measure R0 by washing in saline with zero Ca2+ and 100 μM BAPTA-AM, but the fluorescence signals at both detection channels decreased rapidly and precluded meaningful measurement of R0. Instead of measuring R0, we assumed that [Ca2+]in is 97 nM as previously measured in STG neurons (Kloppenburg et al., 2000) and used Keff, R1, and Rsaline to calculate R0. We then used these calibration parameters to calculate [Ca2+]in under other conditions.
Electrophysiology.
Current clamp (CC) and two-electrode voltage clamp (TEVC) and were performed with an Axoclamp-2B amplifier, a Digidata 1440 data acquisition board, and pCLAMP10 software (all from Molecular Devices, Sunnyvale, CA). For the CC experiments, the AB neuron was synaptically isolated by 5,6-carboxyfluorescein photoablation of the three pyloric motoneurons that are electrically coupled to it—the ventricular dilator (VD) and two PD cells—as described by Miller and Selverston (1979). In some experiments, we blocked synaptic and neuromodulatory input to AB neuron by adding TTX (0.1 μM) and PTX (5 μM) to the saline instead of photoablation. This did not make any difference in DA-induced bursting besides removing the very small action potentials (see also Harris-Warrick and Flamm 1987); therefore, these results were pooled for data analysis.
ICAN measurements.
To measure ICAN activated by influx of extracellular calcium in voltage clamp, we delivered depolarizing presteps from Vh = −50 mV to 0 mV to activate ICa(V). This current in turn activates ICAN, which is detected as a slowly decaying inward tail current upon repolarization. All measured currents were leak subtracted before data analysis. For the determination of the ICAN reversal potential, an activating prestep was followed by a series of hyperpolarizing steps from −80 mV to −40 mV in 5-mV increments to evoke a tail current. The peak slow tail current amplitude was measured and plotted against the value of the voltage step and extrapolated to determine the reversal potential (Erev). The deactivating portion of the current trace was fitted with an exponential function using Clampex; the time constants were then plotted against voltage steps.
Data analysis.
Electrophysiological data analysis was performed with Clampfit10 (Molecular Devices). The calcium imaging data were analyzed with ImageJ (National Institutes of Health). Synchronization of imaging and physiology was done with custom-written MATLAB software. Statistical analyses were performed with JMP 7 (SAS Systems). Graphs and figures were made in Excel, Origin 6 (OriginLab, Northampton, MA), and Adobe Illustrator CS3 (Adobe Systems). All values are presented as means ± SE; the effect was considered statistically significant at P < 0.05.
RESULTS
Release of calcium from intracellular stores is essential for DA-induced bursting in the AB neuron.
In light of our previous results that DA induces calcium-dependent pacemaker oscillations (Harris-Warrick and Flamm 1987) while greatly inhibiting calcium influx via ICa(V) (Johnson et al. 2003), we hypothesized that these oscillations rely on intracellular sources of calcium ions. DA could enhance Ca2+ release from intracellular stores, inhibit Ca2+ sequestration and buffering in intracellular compartments, or both.
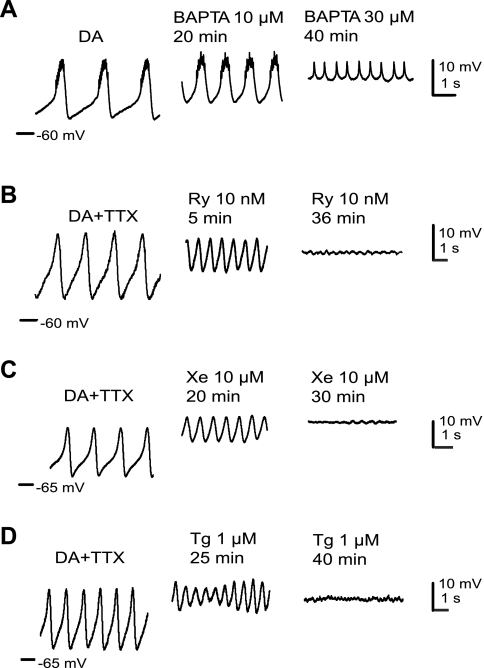
We initially assessed the involvement of intracellular calcium stores in induction of pacemaker oscillations by chelating the intracellular calcium with lipid-permeable BAPTA-AM (Fig. 1A). This compound diffuses into the neuron, where it is deesterified to yield BAPTA. With time of diffusion the AB oscillations became smaller, and eventually the neuron ceased to oscillate and remained at a depolarized voltage. Similar results were seen by blocking the intracellular calcium-release channels located on the endoplasmic reticulum (ER) membrane such as Ry- and IP3-sensitive intracellular calcium receptor channels, as well as SERCA, a calcium reuptake pump. Ry, a specific blocker of the intracellular calcium channels responsible for calcium-activated calcium release, blocked DA-induced AB neuron oscillations at concentrations of 10 nM or higher within 40 min (n = 7; Fig. 1B). Xestospongin C (Xe) is a specific blocker of the IP3R channel (Gafni et al. 1997); 10 μM Xe effectively halted the DA-evoked AB rhythm (n = 3; Fig. 1C). Finally, application of 1 μM Tg, a specific blocker of the SERCA pump, stopped an ongoing DA oscillation within 40 min (n = 3; Fig. 1D). These data suggest that release of stored intracellular calcium via RyRs and IP3Rs is essential for DA-induced oscillations in the AB neuron. Notably, as the DA-evoked bursting ceased with all of these blockers, the trough potential depolarized until the cell fell silent at a highly depolarized voltage (typically −40 to −42 mV). This was also observed when DA-evoked AB bursting was abolished by low extracellular calcium or calcium channel blockers (Harris-Warrick and Flamm 1987). This depolarization may be caused by an indirect block of calcium-sensitive potassium current [IK(Ca)] and subsequent depolarization of the Vm, as well as reflecting the effects of DA on other ionic currents such as inhibition of the leak conductance or activation of calcium-dependent inward currents (see below).
Fig. 1.
Release of calcium from intracellular stores is essential for dopamine (DA)-induced anterior burster (AB) oscillations. A: 30 μM BAPTA-AM (BAPTA) blocked 10−4 M DA-induced bursting in a synaptically isolated AB. In this experiment, the AB neuron was isolated from all synaptic and descending neuromodulatory inputs by photoablation of the pyloric dilator (PD) and ventricular dilator (VD) neurons and local application of tetrodotoxin (TTX) to a Vaseline pool around the stomatogastric nerve. B–D: 10 nM ryanodine (Ry), 10 μM xestospongin C (Xe), and 1 μM thapsigargin (Tg) blocked DA-induced oscillations in the AB. In these experiments, the AB neuron was isolated by application of TTX and picrotoxin (PTX) directly to the stomatogastric ganglion (STG).
Calcium imaging reveals DA-evoked increase of intracellular [Ca2+] in AB neurites.
To directly test the possibility that DA induces an increase in intracellular Ca2+ levels, we carried out two-photon laser microscopic imaging of intracellular calcium activity in the AB neuron on two different timescales, using two different calcium-sensitive indicator dyes (Calcium Green-1 and Indo-1). Briefly, we loaded the AB neuron soma with the calcium indicator dye via the intracellular electrode, let the dye diffuse throughout the dendritic arbor for up to 1 h, and performed synchronized two-photon calcium imaging and electrophysiological recordings.
Distribution and strength of calcium signal in AB neuron.
The dye-filled AB soma and neurites showed clear calcium fluorescence in both intact and DA-stimulated oscillating AB neurons. The signal from the cell body and the primary neurite was relatively constant, and we were unable to detect any oscillation-related fluorescence changes there. This is consistent with previous reports suggesting that calcium oscillations originate outside the soma in the fine neuropil (Kloppenburg et al. 2000, 2007; Ross and Graubard 1989). As a consequence, we made our measurements in small neurites with diameter <5 μm, at distances from the soma of >500 μm.
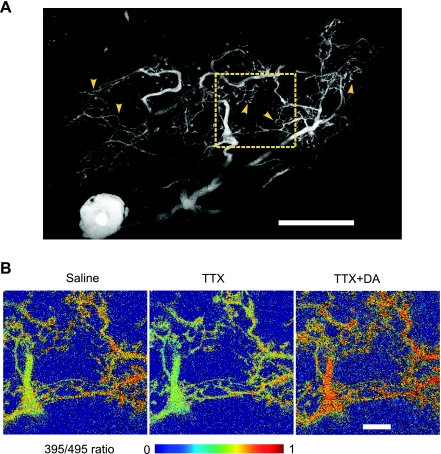
We performed timed (6–10 images/s) ratiometric measurements of Indo-1 fluorescence levels in fine neurites combined with simultaneous voltage recordings from the AB soma first in the absence and then in the presence of DA. Figure 2 shows an example of the AB soma and its extensive neuritic tree filled with Indo-1 (Fig. 2A, fluorescence from the 395-nm channel), where the yellow arrowheads indicate typical regions of neuropil used for calcium measurements and the dashed square demarcates the region enlarged in Fig. 2B. Within this region, the ratios of the fluorescence signals from the 395- and 495-nm channels were calculated and shown in Fig. 2B in pseudocolor. Additionally, the time course of the calcium signal from 19 regions of interest (ROIs) in neuropil was measured over the period of the whole experiment.
Fig. 2.
Calcium imaging in the AB neuron filled with the calcium indicator Indo-1. A: Z stack of the AB neuron labeled with Indo-1, showing fluorescence from the 395-nm channel. Cell body with a bright nucleus is at bottom left. The primary and secondary neurites, along with an extensive dendritic tree, are clearly labeled. Yellow arrowheads mark the approximate regions of fine neuropil where calcium measurements were typically taken. Size bar, 100 μm. Region in yellow square is shown enlarged in B. B: the same region of fine neuropil, showing the ratios of the calcium emission signal detected at 395 nm and 495 nm. Size bar, 25 μm. Measurements are repeated after blockade of synaptic input with TTX, and again after addition of 10−4 M DA.
Basal level of intracellular calcium rises during DA application.
Upon DA application, all visualized fine neurites displayed significant increases in calcium fluorescence of varying amplitude relative to their pre-DA levels (Fig. 2B and Fig. 3A). The calcium responses of 27 individual neurites from 3 different AB neurons were monitored as DA was applied to the bath. For individual ROIs in the fine neuropil, when averaged calcium measurements were taken at slow speed (6–10 images/s) the DA-induced increase in [Ca2+]in ranged from 136% to 555% of the pre-DA level with an average of 302 ± 153% increase. Thus different neurites of a single neuron showed marked variability in calcium handling both under control conditions and in the presence of DA.
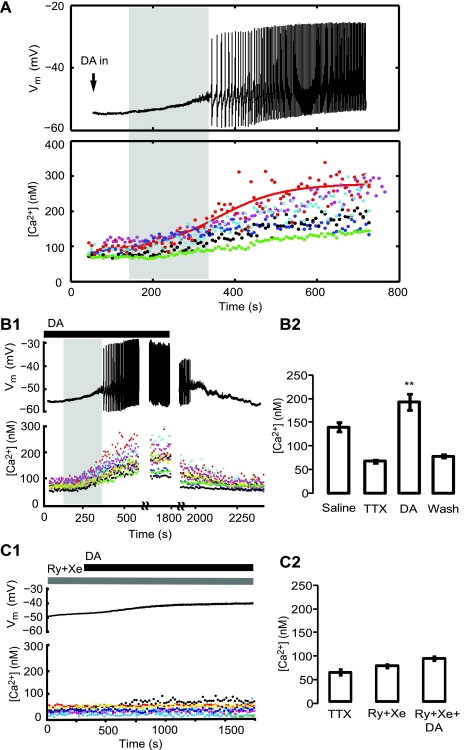
Fig. 3.
Onset of the DA-induced calcium rise precedes the onset of membrane potential (Vm) oscillations. A: time course of the voltage (top) and free Ca2+ concentration ([Ca2+]in; bottom) change during DA application; 10−4 M DA has reached the bath solution at the 50 s time point. Each dendrite [region of interest (ROI)] is indicated by different color; the data points from one of the neurites are fit with a sigmoid function (plotted as a red solid line). Note that in the time period highlighted with the vertical light gray bar, [Ca2+] started rising before emergence of bursting. Voltage oscillations initially are slower and accelerate as the DA concentration reaches steady state. B1: complete time course of AB oscillations and intracellular calcium measurements as DA is added and washed out. As DA was washed out of the bath solution, the voltage oscillations and calcium signal returned back to their pre-DA levels within 10–15 min. B2: summary of the changes in the average calcium levels under different conditions: physiological saline (140 ± 8 nM), TTX (67 ± 3 nM), 10−4 M DA (193 ± 17 nM), and washout of DA. The neuron was oscillating in saline and in the presence of DA and quiescent in TTX and after DA (wash). C: preincubation with intracellular calcium channel blockers (Ry+Xe) abolishes the DA-evoked rise in [Ca2+]in and oscillations in the AB neuron. C1: the AB neuron was preincubated with Ry+Xe for 30–60 min before DA application. Intracellular [Ca2+] did not change upon DA application; the neuron depolarized, because of DA's effects on other currents, but did not oscillate. C2: there was no significant difference in mean [Ca2+] under different conditions: 82 ± 11 nM before DA (in Ry+Xe) and 87 ± 17 nM in the presence of DA and Ry+Xe (P > 0.05, n = 10 ROIs).
Onset of basal [Ca2+]in rise precedes onset of voltage oscillations.
The changes in [Ca2+]in were monitored in parallel with voltage recordings; for the large majority of neurites (25 of 27), the rise in [Ca2+]in started well before the emergence of sustained voltage oscillations (Fig. 3A). The intracellular calcium levels began rising ∼3 min after DA reached the ganglion. The interval between the start of the calcium increase and the onset of voltage oscillations is highlighted with a vertical light gray bar in Fig. 3, A and B1. On average, voltage oscillations started 108 ± 12 s after the onset of calcium rise in fine neurites (n = 27 neurites, 3 AB neurons). This earlier onset of calcium rise in fine neurites, >1.5 min before the emergence of bursting, suggests that the rise in intracellular calcium might bring about the subsequent depolarization and rhythmic Vm oscillations. DA's effect was completely reversible: washing DA out of the bath solution abolished the Vm oscillations and brought the levels of [Ca2+]in back to pre-DA levels (Fig. 3B). Figure 3B2 presents a summary of the mean intracellular [Ca2+] under different conditions: in physiological saline, [Ca2+] in a spontaneously oscillating AB neuron was 140 ± 8 nM. When AB neuron was synaptically isolated (either by photoablation of the electrically coupled PD and VD neurons or pharmacologically by bath application of TTX and PTX), the mean [Ca2+] decreased to 67 ± 3 nM. Upon subsequent DA application the levels of calcium started rising within minutes; the voltage oscillatory activity usually started when the mean [Ca2+]in reached 135 ± 14 nM (not shown), while during stable DA oscillations time-averaged [Ca2+] reached 193 ± 17 nM (for all conditions, n = 27). Calcium levels during DA application were significantly different from control measurements before DA in TTX or after washout (ANOVA, P < 0.01, n = 27).
Effect of preincubation with ryanodine and xestospongin on DA-evoked calcium rise.
Our previous data on AB bursting predicted that application of the intracellular calcium channel blockers Ry and Xe would prevent or block the DA-induced increase in calcium levels and voltage oscillations. In three cells that were pretreated for 30–60 min with a cocktail of 10 μM Ry and 10 μM Xe, DA either elicited only a small calcium increase and a short-lived train of slow voltage oscillations or did not induce any significant increase in intracellular calcium and did not evoke voltage oscillations (Fig. 3C1). The mean [Ca2+] was 82 ± 11 nM in Ry + Xe and 87 ± 17 nM in the presence of DA together with Ry + Xe, which were not statistically different (Fig. 3C2) (P > 0.05, n = 10 ROIs). Although DA failed to evoke sustained voltage oscillations in these neurons, it did slightly depolarize the Vm, probably because of its effect on other ionic currents in the AB neuron (Gruhn et al. 2005; Johnson et al. 2003; Peck et al. 2001, 2006).
High-temporal-resolution calcium imaging with simultaneous recording of membrane potential.
We used Calcium Green-1 to track calcium changes on a faster timescale during spontaneous and DA-evoked AB oscillations. As noted above, we did not detect any calcium oscillations in the cell soma or primary neurites, but the fluorescence signals in fine neurites revealed clear oscillatory behavior at the same frequency as the Vm oscillations.
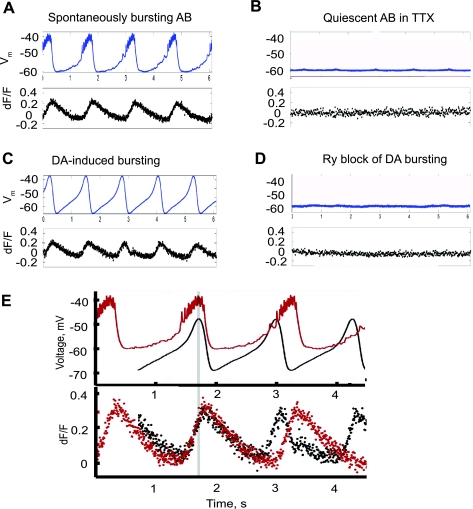
To monitor calcium changes on a cycle-by-cycle basis, we used fast line scan (frequency 164 Hz) to monitor calcium activity in single fine neurites along with synchronized electrophysiological recordings of Vm in the soma. In the spontaneously bursting AB (with intact modulatory input to the STG from the higher-order ganglia), [Ca2+], monitored as the change in fluorescence level (dF/F), oscillated at the same frequency as the voltage (Fig. 4A). Application of TTX and PTX to block the descending modulatory inputs effectively blocked both voltage and calcium oscillations within several minutes (Fig. 4B). Addition of DA to the bath saline restored rhythmic bursting of voltage along with calcium oscillations (Fig. 4C). Finally, Ry treatment of the bursting pacemaker in the continued presence of DA halted both calcium and voltage oscillations (Fig. 4D). The amplitude of the calcium oscillations was ∼20% of the basal level of calcium in the presence of DA. Notably, the calcium oscillations were slightly delayed relative to the voltage oscillations in both the spontaneously bursting AB neuron before isolation and the synaptically isolated AB treated with DA (Fig. 4E; n = 3). The maxima of the calcium oscillations were delayed by ∼150 ms relative to the peaks of the Vm oscillations. This delay seems to be too long to be explained by the slow kinetics of calcium activation of Calcium Green-1 (Parker and Yao 1996).
Fig. 4.
The AB neuron calcium signal oscillates in phase with voltage oscillations but is delayed in onset and peak levels. Data were collected at 164 Hz by a fast line scan over a single dendrite. Simultaneous recordings of calcium signal and membrane: Vm (top) and relative change in fluorescence (dF/F; bottom). Time markers, in seconds, are below the voltage trace. A: spontaneously bursting AB neuron in physiological saline. B: 10−7 M TTX+ 5 × 10−5 M PTX blocked both voltage and calcium oscillations in <10 min. C: 10−4 M DA induced Vm and calcium signal oscillations. D: application of 10 μM Ry stopped both voltage and calcium oscillations. E: overlay of voltage and calcium traces. Calcium oscillations had similar delayed time course and amplitude both in a spontaneously bursting AB neuron with no blockers (red traces) and in a synaptically isolated AB (treated with TTX and PTX) in the presence of DA (black traces). Top: voltage trace. Bottom: calcium signal change (dF/F).
Dopamine-induced oscillations are blocked by FFA, a blocker of the ICAN.
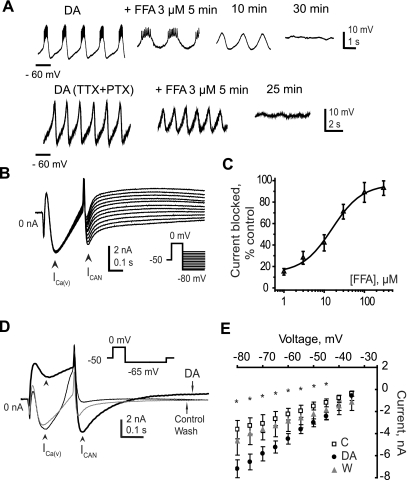
Our imaging studies show that DA evokes increases in intracellular calcium that could lead to depolarizations preceding the onset of voltage oscillations. One possible target of the rise in [Ca2+]in is the calcium-activated nonspecific current ICAN. We tested the importance of ICAN in DA-evoked bursting by application of a blocker of this current, FFA. Three micromolar FFA reliably blocked the ongoing DA oscillations in a synaptically isolated AB neuron (n = 5), regardless of the method used to synaptically isolate the AB (Fig. 5A). Notably, as the rhythm ceased, the trough potential depolarized until the cell fell silent at −40 to −42 mV. This was also observed when we blocked the rise in intracellular calcium (Fig. 1) and when DA-evoked AB bursting was abolished by low extracellular calcium or calcium channel blockers (Harris-Warrick and Flamm 1987). To rule out the possibility that this depolarization was responsible for the cessation of bursting, we injected a negative bias current (usually 1–5 nA) to bring the Vm down to its preblocker trough values of −55 to −60 mV. Although early during FFA application AB bursting could be rescued by hyperpolarizing the Vm, perhaps after enough time to allow equilibration of the FFA concentration, the cell became completely unable to burst or oscillate, even when hyperpolarized. This lack of burst rescue by hyperpolarization suggests that it is the block of the FFA-sensitive inward current and not the possible secondary effect of depolarization that causes disruption of the DA rhythm. Therefore, we next focused on the characterization of the properties and DA modulation of ICAN in the pacemaker neuron.
Fig. 5.
Calcium-activated inward current ICAN is a likely target of DA modulation. A: flufenamic acid (FFA) abolishes DA oscillations in a synaptically isolated AB neuron. Top: the AB neuron was isolated by photoablation, and bursting was evoked with 10−4 M DA. Bottom: the AB neuron was isolated from modulatory and synaptic input by application of 10−7 M TTX + 5 × 10−5 M PTX, and oscillations were evoked with 10−4 M DA. In both cases, 3 μM FFA stopped oscillations within 30 min. B: properties of ICAN in the AB neuron: the current is measured during voltage-clamp recordings as a slowly deactivating tail current evoked by an activating prestep to 0 mV and a series of hyperpolarizing steps (inset at right, voltage protocol). Arrowheads on the current traces mark the peak voltage-gated calcium current [ICa(V)] activated by the depolarizing prestep and the peak ICAN tail currents measured after repolarization of the cell. C: dose-response curve for FFA block of ICAN (IC50 = 24.7 ± 5 μM; n = 4). The amplitude of the blocked current was normalized by the corresponding control current amplitude. D: DA enhances the amplitude of ICAN while inhibiting ICa(V). Voltage-clamp current traces under control conditions (thin black trace), during application of 10−4 M DA (thick black trace), and after 30 min of washout (thin gray trace). DA inhibited ICa(V) while enhancing ICAN amplitude (arrowheads). Current traces are not leak subtracted. The voltage protocol used to measure ICAN is shown in the small inset. E: ICAN current-voltage relationship under control conditions (C, open squares), in the presence of DA (filled circles), and after washout (W, gray triangles). All data points are means ± SE. Asterisks mark the voltage steps where the difference between control and DA was statistically significant (n = 8, P < 0.05, Student's t-test).
Properties of ICAN in the anterior burster neuron.
We have carried out a physiological and pharmacological analysis of ICAN by measuring its Erev, deactivation time constant, and sensitivity to FFA. Because this current lacks intrinsic voltage sensitivity, and because of problems of space clamp in these highly branched neurons (Kloppenburg et al. 2000), we were unable to perform a full biophysical analysis. In the presence of blockers of ionic currents and synaptic input, we measured ICAN as a slowly deactivating tail current following a depolarizing prestep to 0 mV to activate ICa(V). The tail current was recorded over −80 mV to −40 mV in 5-mV increments. Although we blocked >99% of the total K+ currents, there was still some leak contamination of the very small calcium currents measured in the AB neuron (Fig. 5B). Figure 5B shows sample current traces without leak current subtraction. The peak amplitude of this tail current was measured at the beginning of the hyperpolarizing step, taking advantage of the much more rapid deactivation kinetics of the calcium current relative to that of ICAN in stomatogastric neurons (Johnson et al. 2003). At −65 mV, the mean amplitude of the peak leak-subtracted ICAN in the AB was −2.7 ± 0.7 nA (Fig. 5E, open squares; n = 8). The leak-subtracted tail current was plotted as a function of the hyperpolarizing voltage step, fitted with a linear function, and Erev was extrapolated for each cell. The average Erev was −29 ± 0.3 mV. The current decayed during the hyperpolarizing step, reflecting the time constant of channel deactivation and/or removal of intracellular calcium. The decay of the ICAN tail current was fitted with an exponential function, and resulting time constants τ were plotted against tail current voltage. We tested whether there was any voltage dependence of the deactivation rate. The current tended to decay somewhat more rapidly at voltages more depolarized than −60 mV, but there was no statistically significant difference between τ values at different voltages, with an average τ of 74 ± 14 ms.
As expected for ICAN, the tail current was sensitive to application of FFA or BAPTA or block of calcium entry by CdCl2. In the high-osmolarity lobster saline, FFA visibly precipitated out of solution at 16°C; thus we raised the experimental temperature to 21°C to maintain the drug in solution for full dose-response experiments. FFA blocked ICAN with an IC50 of 24.7 ± 5 μM, (n = 4; Fig. 5C). Preventing a rise in intracellular calcium with 50–70 μM BAPTA completely blocked ICAN. Finally, blockade of voltage-dependent calcium currents with 0.6 mM CdCl2 eliminated the step-activated ICa(V) and thus the resulting ICAN (not shown).
We next measured DA's effect on ICAN in the AB neuron. The mean peak amplitude of ICAN was increased in the presence of DA (Fig. 5D, thick black trace, arrowhead). For all eight neurons tested, this effect of DA was statistically significant at voltages more hyperpolarized than −45 mV (P < 0.05; Fig. 5E). This ICAN enhancement occurred despite DA's marked reduction of the amplitude of ICa(V) (by 68 ± 7%; Fig. 5D, thick black trace, arrowhead; see also Johnson et al. 2003). The maximal CAN conductance in the AB neuron significantly increased from 58 ± 9 nS under control conditions to 145 ± 12 nS in the presence of DA (248 ± 15% of control; n = 8, P < 0.05). We also tested DA's effects on ICAN in two other pyloric network neurons, the PD, which is electrically coupled to the AB neuron, and the LP neuron. DA did not modify ICAN amplitude in the PD (105.8 ± 25% of control; n = 3) or the LP (103.1 ± 29% of control; n = 3) neurons.
The time course of decay of ICAN most likely reflects the reduction in availability of free calcium ions in the intracellular space near channels mediating ICAN. Consistent with this, within each treatment (control, DA, and washout) there was no voltage dependence of the time constants of decay (ANOVA, P > 0.05, n = 7 for each treatment; not shown). However, when we compared the mean time constants between treatments (control and DA), the time constant in DA (150 ± 21 ms) was significantly slower compared with control (74 ± 14 ms) at −65 mV and more depolarized potentials (P < 0.05, n = 6). Thus, despite the fact that DA inhibits most of the ICa(V), the ICAN in DA is larger and deactivates more slowly than before DA application.
DA effects on other ionic currents.
As reported previously (Peck et al. 2001), DA also reduced the leak current, measured with 5-mV hyperpolarizing steps from −60 mV or −55 mV. Under our experimental conditions, the mean conductance of the leak current at −6 5 mV was 45 ± 17 pS in control, 11 ± 8 pS during DA application, and 41 ± 23 pS after DA washout. This ∼70% reduction in leak current in the presence of DA is larger than the 10% reduction we previously reported in the AB (Peck et al. 2001, 2006).
DISCUSSION
Our results suggest that DA-induced AB bursting depends on the release of calcium from intracellular stores, which in turn enhances ICAN to provide the additional ramp depolarization leading to the onset of each oscillation. Blockade of either of these steps eliminates or prevents pacemaker oscillations.
Dopamine-induced rise in intracellular calcium is central for pacemaker oscillations.
Many bursting neurons rely on intracellular calcium stores as a source of calcium in both invertebrates (Levy 1992; Partridge and Swandulla 1987; Partridge and Valenzuela 1999; Sawada et al. 1990; Swandulla and Lux 1985; Yazenian and Byerly 1989) and vertebrates (Del Negro et al. 2008; Pace et al. 2007a, 2007b; Peña et al. 2004; Rubin et al. 2009). We show that in the pyloric pacemaker AB neuron chelation of calcium ions or block of calcium release from the intracellular stores prevents or eliminates ongoing DA-evoked pacemaker oscillations. Calcium imaging of the AB neuron with a ratiometric dye shows that DA elevates basal calcium levels in the neurites by more than twofold over the quiescent state (67 ± 3 nM pre-DA, 193 ± 17 nM in DA). We are able to detect this DA-induced elevation of [Ca2+]in beginning more than a minute and a half before the emergence of sustained voltage oscillations (Fig. 3A) and, in some cells, even before the neuron starts to depolarize.
During AB oscillations, fast line scanning of neurites filled with Calcium Green-1 shows that the intracellular calcium level oscillates with amplitude of ∼20% of the basal level of calcium in the presence of DA and with the same frequency as the voltage oscillations. However, the calcium oscillations are delayed from the voltage oscillations by ∼150 ms (0.08 of the period). One interpretation of this result is that a tonic modulator-evoked release of calcium from intracellular stores activates ICAN, which acts as a major contributor to the tonic ramp current, depolarizing the membrane voltage; above a voltage threshold, this depolarization activates the small DA-insensitive portion of ICa(V), which then participates (possibly along with ICAN) in the rapid rise of the oscillation to its peak. The essential role of calcium release from intracellular stores is maintained even during stable oscillations, since Ry blockade of calcium-induced calcium release abolishes these ongoing calcium oscillations along with voltage oscillations (Fig. 4D).
Cross talk between IP3 and Ry receptors and their respective pathways.
The ER calcium stores appear to play a critical role in DA-induced AB bursting, since blockade of Ry-sensitive and IP3 receptors or depletion of stores by blockade of the SERCA inhibits this bursting. The mechanisms by which DA enhances calcium release from these stores are not yet known. Clark and colleagues cloned three types of DA receptors from the lobster STG: D1αPan coupled with Gs and Gq, D1βPan coupled with Gs, and D2αPan coupled with Gi proteins (Clark and Baro 2006, 2007; Clark et al. 2008). All three receptor types were reported to localize to the synaptic neuropil (Clark et al. 2008), where we made our calcium measurements. In vertebrates, DA can trigger mobilization of intracellular calcium through concurrent activation of D1 and D2 receptors or a D1–D2 heteromer, via activation of Gq, phospholipase C (PLC), and the IP3 cascade (Hasbi et al. 2010; Undie et al. 1994), which in turn provides calcium to activate further calcium release via the RyRs. In Drosophila photoreceptors, this signaling cascade leads to calcium release from IP3Rs and calcium influx across the cell membrane via two types of TRP channels (Hardie and Minke 1993). Thus, in the AB neuron, DA most likely acts on the D1αPan receptor or its heteromer to activate the Gq-PLC-IP3 pathway.
In addition, DA could also activate D1αPan or D1βPan, which are coupled to the Gs-cAMP-PKA pathway (Clark et al. 2008), leading to increased phosphorylation of RyR protein and calcium release from the ER, as demonstrated in heart sinoatrial node pacemaker cells (Vinogradova et al. 2006). Since both types of ER receptor channels are sensitive to calcium, they are subject to positive autofeedback, leading to regenerative opening of RyRs and/or IP3Rs beyond a certain threshold of [Ca2+]in and amplification of the initial calcium signal (Verkhratski 2005). Replenishment of the intracellular calcium stores is critically important to prolonged DA bursting; this may occur via ICAN, ICa(V), and/or store-operated calcium entry (SOCE). Although we did not test this directly in the AB neuron, in rat hepatocytes SOCE appears to be a more effective way of refilling the stores than ICAN (Gregory et al. 2003).
The FFA-sensitive ICAN is enhanced by DA and contributes to sustained pacemaker oscillations.
We showed that ICAN plays an important role in DA-induced AB bursting. First, FFA terminated ongoing dopamine-evoked oscillations and blocked ICAN with an IC50 of 24 μM (Fig. 5A). FFA is routinely used at 100–500 μM to reduce ICAN in both invertebrates and vertebrates (Derjean et al. 2005; Ghamari-Langroudi and Bourque 2002; Green and Cottrell 1997; Lee and Tepper 2007; Morisset and Nagy 1999; Pace et al. 2007a; Partridge and Valenzuela 2000). At these high concentrations, above 100 μM, FFA has multiple side effects, including partial inhibition of calcium channels and NMDA receptors (Wang et al. 2006) and induction of calcium release from ER (Gardam et al. 2008; Lee et al. 1996; Shaw et al. 1995) and mitochondria (Tu et al. 2009). We avoided these side effects by using much lower concentrations (below 10 μM). In our system, FFA block causes the membrane potential to stabilize around −40 to −42 mV (Fig. 5A), near the upper voltage limit of the slow oscillatory wave. This end-point depolarization was also seen in the experiments in which we blocked AB oscillations by disruption of intracellular calcium dynamics (Fig. 1). While we do not understand the basis for this depolarization, it may be the result of calcium compartmentalization into separate domains and limiting the calcium supply to calcium-activated potassium [K(Ca)] channels that normally stabilize the potential near its resting state. This was not the main cause of the cessation of bursting, as the AB neuron also failed to burst upon artificial hyperpolarization to its initial resting potential.
A second argument for the role of ICAN in DA-evoked AB bursting is that DA significantly enhances this current's maximal conductance and slows its deactivation rate. The ICAN peak amplitude almost doubled during DA application (Fig. 5, D and E), despite DA inhibition of ICa(V) (Johnson et al. 2003), which would normally act to reduce ICAN. Monoamine-induced modulation of ICAN was previously described in the Aplysia R15 neuron (Lotshaw et al. 1986) and Helix bursting neurons (Partridge et al. 1990), as well as in the DG neuron of the Cancer STNS (Zhang et al. 1995). In the AB neuron, DA could modulate ICAN either directly, via second messenger-mediated modulation of these channels, or indirectly by elevating intracellular calcium levels, which would interact cooperatively with calcium entering from the extracellular space; our data suggest that at least this second mechanism occurs. Alternatively, DA could slow the rate of reuptake of calcium into intracellular stores after entering via voltage-activated channels, thus increasing the maximal ICAN and slowing its deactivation rate.
Model of DA-induced bursting of the lobster pyloric pacemaker neuron.
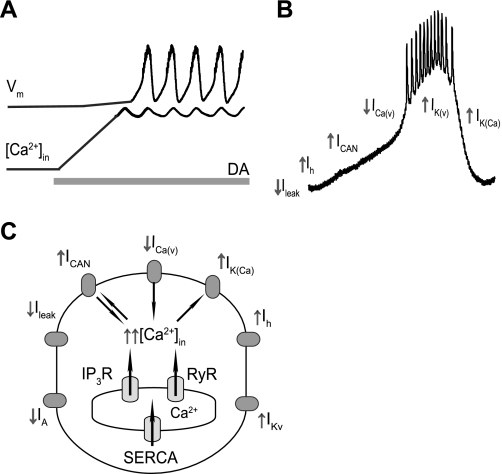
Based on previous work and our data, we suggest the following model of DA-induced bursting in AB neurons (Fig. 6).
Fig. 6.
Multiple effects of DA on the ionic currents in AB neuron. A: schematics of the changes in the voltage and [Ca2+] during DA application, based on our results with indo-1 and Calcium Green-1 imaging. B: summary of DA effects on AB neuron. DA inhibits transient potassium current IA, leak current (Ileak), and ICa(V), enhances IK(V) and hyperpolarization-activated current Ih, and increases intracellular [Ca2+]. C: mechanism of the DA-induced oscillations in the AB neuron (see discussion for the description of the mechanism of action of DA). IP3R, inositol trisphosphate receptor; RyR, Ry receptor.
1) DA binds to D1αPan and/or D1βPan receptors and increases the intracellular [Ca2+] in the fine neuropil. The ER calcium channels and the SERCA play a major part in this process. IP3Rs are likely activated via the Gq-PLC-phosphatidylinositol 4,5-bisphosphate (PIP2) pathway with a subsequent activation of additional IP3Rs and RyRs as calcium is released from the ER.
2) This rise in intracellular calcium activates ICAN. DA increases the peak current amplitude and slows the rate of deactivation of the current, probably due to elevated levels of cytoplasmic calcium, although a direct modulatory action on the channels is also possible.
3) ICAN activation acts as a ramp current in addition to Ih to depolarize the cell after the termination of the previous burst. This ramp brings the cell to voltage threshold to activate other inward currents possibly including the DA-insensitive portion of ICa(V) to initiate additional calcium entry and the full burst. This additional influx of Ca2+ could also recruit more ICAN during the burst phase to contribute to the burst itself.
4) Depolarization and high intracellular calcium activate calcium-dependent outward currents, such as IK(Ca), which must have either slower activation kinetics or a higher calcium concentration dependence compared with ICAN. Other currents, such as sodium-sensitive potassium currents [IK(Na)] or pump currents, may also contribute to this accumulation of outward currents. The outward currents eventually prevail over the inward currents driving the depolarization; this repolarizes the cell, ending the cycle.
5) Extracellular calcium is essential for replenishment of the intracellular calcium stores and is carried inside via ICAN, ICa(V), and/or SOCE.
6) In addition to these effects, previous work in our laboratory has shown that DA also reduces the tonic leak current, which will make the neuron electrically tighter and more compact, enhancing the effects of the rather small ICAN and other subthreshold currents (Fig. 6B). DA reduces the transient potassium current (Peck et al. 2001), which would normally counteract the ramp ICAN current. DA also enhances the hyperpolarization-activated inward current Ih (Peck et al. 2006), which would also contribute to the depolarizing ramp driving regenerative oscillations in AB. Paradoxically, DA also enhances a slowly activating and deactivating inward-rectifying IK(V) (Gruhn et al. 2005), which may act to restrict the maximal spike frequency of the oscillating AB neuron (Harris-Warrick and Johnson 2010; Fig. 6).
Multiple pacemaking mechanisms.
There are multiple possible ionic mechanisms to generate bursting in neurons, and the relative contribution of each may vary under different conditions (Harris-Warrick and Flamm 1987; Harris-Warrick and Johnson 2010; Peña et al. 2004). In the vertebrate respiratory central pattern generator (CPG), there may exist different groups of pacemaker neurons, each with a distinct rhythmogenic mechanism—either sodium dependent or calcium dependent (Del Negro et al. 2002; Pace et al. 2007a, 2007b; Peña et al. 2004). Our work shows how DA evokes AB bursting by a calcium-dependent mechanism (Harris-Warrick and Flamm 1987). However, the same AB neuron uses a completely different mechanism to induce bursting in the presence of 5-HT, which is driven by a sodium-dependent mechanism (Harris-Warrick and Flamm 1987; Kadiri and Harris-Warrick, unpublished observations). Thus if in the respiratory CPG there may be different groups of pacemaker neurons in the same CPG, in the pyloric CPG a single pacemaker neuron can employ two quite distinct ionic mechanisms to drive rhythmic behaviors.
GRANTS
This work was supported by National Institutes of Health (NIH) predoctoral grant T32 GM-007469 to L. R. Kadiri, NIH Grant 9-P41-EB-001976 to W. W. Webb, and NIH Grant NS-17323 to R. Harris-Warrick.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank B. R. Johnson and W. R. Zipfel for their feedback on the experiments.
Present addresses: L. R. Kadiri, Cold Spring Harbor Laboratory, One Bungtown Rd., Cold Spring Harbor, NY 11724; A. C. Kwan, Division of Neurobiology, Department of Molecular and Cell Biology, Helen Wills Neuroscience Institute, University of California, Berkeley, CA 94120.
REFERENCES
- Büschges A, Wikström MA, Grillner S, El Manira A. Roles of high-voltage-activated calcium channel subtypes in a vertebrate spinal locomotor network. J Neurophysiol 84: 2758–2766, 2000 [DOI] [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Molecular cloning and characterization of crustacean type-one dopamine receptors: D1alphaPan and D1betaPan. Comp Biochem Physiol B Biochem Mol Biol 143: 294–301, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Arthropod D2 receptors positively couple with cAMP through the Gi/o protein family. Comp Biochem Physiol B Biochem Mol Biol 146: 9–19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Khan R, Baro DJ. Crustacean dopamine receptors: localization and G protein coupling in the stomatogastric ganglion. J Neurochem 104: 1006–1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Hayes JA. A “group pacemaker” mechanism for respiratory rhythm generation. J Physiol 586: 2245–2246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties, and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol 88: 2242–2250, 2002 [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Pace RW, Hayes JA. What role do pacemakers play in the generation of respiratory rhythm? Adv Exp Med Biol 605: 88–93, 2008 [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Nagy F, Shefchyk SJ. Plateau potentials and membrane oscillations in parasympathetic preganglionic neurones and intermediolateral neurones in the rat lumbosacral spinal cord. J Physiol 563: 583–596, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Ríos M, Dombeck DA, Webb WW, Harris-Warrick RM. Serotonin modulates dendritic calcium influx in commissural interneurons in the mouse spinal locomotor network. J Neurophysiol 98: 2157–2167, 2007 [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733, 1997 [DOI] [PubMed] [Google Scholar]
- Gardam KE, Geiger JE, Hickey CM, Hung AY, Magoski NS. Flufenamic acid affects multiple currents and causes intracellular Ca2+ release in Aplysia bag cell neurons. J Neurophysiol 100: 38–49, 2008 [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol 545: 537–542, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KA, Cottrell GA. Modulation of ligand-gated dopamine channels in Helix neurones. Pflügers Arch 434: 313–22, 1997 [DOI] [PubMed] [Google Scholar]
- Gregory RB, Sykiotis D, Barritt GJ. Evidence that store-operated Ca2+ channels are more effective than intracellular messenger-activated non-selective cation channels in refilling rat hepatocyte intracellular Ca2+ stores. Cell Calcium 34: 241–251, 2003 [DOI] [PubMed] [Google Scholar]
- Gruhn M, Guckenheimer J, Land B, Harris-Warrick RM. Dopamine modulation of two delayed rectifier potassium currents in a small neural network. J Neurophysiol 94: 2888–2900, 2005 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- Hardie RC, Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci 16: 371–376, 1993 [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM. Pattern generation. Curr Opin Neurobiol 3: 982–988, 1993 [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM. General principles of rhythmogenesis in central pattern generator networks. Prog Brain Res 187: 213–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Flamm RE. Multiple mechanisms of bursting in a conditional bursting neuron. J Neurosci 7: 2113–2128, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR. Checks and balances in neuromodulation. Front Behav Neurosci 4: 47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol 10: 93–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Kloppenburg P, Harris-Warrick RM. Dopamine modulation of calcium currents in pyloric neurons of the lobster stomatogastric ganglion. J Neurophysiol 90: 631–643, 2003 [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Zipfel WR, Webb WW, Harris-Warrick RM. Heterogeneous effects of dopamine on highly localized, voltage-induced Ca2+ accumulation in identified motoneurons. J Neurophysiol 98: 2910–2917, 2007 [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Zipfel WR, Webb WW, Harris-Warrick RM. Highly localized Ca2+ accumulation revealed by multiphoton microscopy in an identified motoneuron and its modulation by dopamine. J Neurosci 20: 2523–2533, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan AC, Dietz SB, Webb WW, Harris-Warrick RM. Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. J Neurosci 29: 11601–11613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci 27: 6531–6541, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Shaw T, Sandquist M, Partridge LD. Mechanism of action of the non-steroidal anti-inflammatory drug flufenamate on [Ca2+]i and Ca2+-activated currents in neurons. Cell Calcium 19: 431–438, 1996 [DOI] [PubMed] [Google Scholar]
- Levi R, Samoilova M, Selverston AI. Calcium signaling components of oscillating invertebrate neurons in vitro. Neuroscience 118: 283–296, 2003 [DOI] [PubMed] [Google Scholar]
- Levy S. Effect of intracellular injection of inositol trisphosphate on cytosolic calcium and membrane currents in Aplysia neurons. J Neurosci 12: 2120–2129, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotshaw DP, Levitan ES, Levitan IB. Fine tuning of neuronal electrical activity: modulation of several ion channels by intracellular messengers in a single identified nerve cell. J Exp Biol 124: 307–322, 1986 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001 [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol 15: R685–R699, 2005 [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston A. Rapid killing of single neurons by irradiation of intracellularly injected dye. Science 206: 702–704, 1979 [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci 19: 7309–7316, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloney B, Selverston A. Antidromic action potentials fail to demonstrate known interactions between neurons. Science 177: 69–72, 1972 [DOI] [PubMed] [Google Scholar]
- Oginsky MF, Rodgers EW, Clark MC, Simmons R, Krenz WD, Baro DJ. D2 receptors receive paracrine neurotransmission and are consistently targeted to a subset of synaptic structures in an identified neuron of the crustacean stomatogastric nervous system. J Comp Neurol 518: 255–276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol 582: 113–125, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBötzinger complex neurons and respiratory rhythm generation. J Physiol 580: 485–496, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J Physiol 491: 663–668, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Swandulla D. Single Ca-activated cation channels in bursting neurons of Helix. Pflügers Arch 410: 627–631, 1987 [DOI] [PubMed] [Google Scholar]
- Partridge LD. Cytoplasmic Ca2+ activity regulation as measured by a calciumactivated current. Brain Res 647: 76–82, 1994 [DOI] [PubMed] [Google Scholar]
- Partridge LD, Swandulla D, Müller TH. Modulation of calcium-activated non-specific cation currents by cyclic AMP-dependent phosphorylation in neurones of Helix. J Physiol 429: 131–145, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Ca2+ store-dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. J Physiol 521: 617–627, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Block of hippocampal CAN channels by flufenamate. Brain Res 867: 143–148, 2000 [DOI] [PubMed] [Google Scholar]
- Peck JH, Gaier E, Stevens E, Repicky S, Harris-Warrick RM. Amine modulation of Ih in a small neural network. J Neurophysiol 96: 2931–2940, 2006 [DOI] [PubMed] [Google Scholar]
- Peck JH, Nakanishi ST, Yaple R, Harris-Warrick RM. Amine modulation of the transient potassium current in identified cells of the lobster stomatogastric ganglion. J Neurophysiol 86: 2957–2965, 2001 [DOI] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004 [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci 24: 7549–7556, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528: 107–113, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross WN, Graubard K. Spatially and temporally resolved calcium concentration changes in oscillating neurons of crab stomatogastric ganglion. Proc Natl Acad Sci USA 86: 1679–1683, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci USA 106: 2939–2944, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Ichinose M, Maeno T. Activation of a non-specific cation conductance by intracellular injection of inositol 1,3,4,5-tetrakisphosphate into identified neurons of Aplysia. Brain Res 512: 333–338, 1990 [DOI] [PubMed] [Google Scholar]
- Selverston AI, Ayers J. Oscillations and oscillatory behavior in small neural circuits. Biol Cybern 95: 537–554, 2006 [DOI] [PubMed] [Google Scholar]
- Selverston AI, Russell DF, Miller JP. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7: 215–290, 1976 [DOI] [PubMed] [Google Scholar]
- Shaw T, Lee RJ, Partridge LD. Action of diphenylamine carboxylate derivatives, a family of non-steroidal anti-inflammatory drugs, on [Ca2+]i and Ca2+-activated channels in neurons. Neurosci Lett 190: 121–124, 1995 [DOI] [PubMed] [Google Scholar]
- Swandulla D, Lux HD. Activation of a nonspecific cation conductance by intracellular Ca2+ elevation in bursting pacemaker neurons of Helix pomatia. J Neurophysiol 54: 1430–1443, 1985 [DOI] [PubMed] [Google Scholar]
- Tu P, Brandolin G, Bouron A. The anti-inflammatory agent flufenamic acid depresses store-operated channels by altering mitochondrial calcium homeostasis. Neuropharmacology 56: 1010–1016, 2009 [DOI] [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem 62: 2045–2048, 1994 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279, 2005 [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006 [DOI] [PubMed] [Google Scholar]
- Wang D, Grillner S, Wallén P. Effects of flufenamic acid on fictive locomotion, plateau potentials, calcium channels and NMDA receptors in the lamprey spinal cord. Neuropharmacology 51: 1038–1046, 2006 [DOI] [PubMed] [Google Scholar]
- Yazejian B, Byerly L. Voltage-independent barium-permeable channel activated in Lymnaea neurons by internal perfusion or patch excision. J Membr Biol 107: 63–75, 1989 [DOI] [PubMed] [Google Scholar]
- Zhang H, Rodgers EW, Krenz WD, Clark MC, Baro DJ. Cell specific dopamine modulation of the transient potassium current in the pyloric network by the canonical D1 receptor signal transduction cascade. J Neurophysiol 104: 873–884, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wootton JF, Harris-Warrick RM. Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. II. Calcium-activated slow inward current. J Neurophysiol 74: 1938–1946, 1995 [DOI] [PubMed] [Google Scholar]