Abstract
As the use of genetically engineered mice has become increasingly prevalent in neurobiological research, evidence has steadily accumulated that substantial differences exist between strains. Although a number of studies have reported effects of genetic background on behavior, few have focused on differences in neurophysiology. The postburst afterhyperpolarization (AHP) is an important determinant of intrinsic neuronal excitability and has been suggested to play a critical role in the cellular mechanisms underlying learning and memory. Using whole cell current-clamp recordings of CA1 pyramidal neurons, we examined the magnitude of different phases of the AHP (peak, medium, and slow) in two commonly used genetic backgrounds, C57BL/6 (B6) and 129SvEv (129), as well as in an F2 hybrid B6:129 background (F2). We found that neurons from B6 and F2 animals exhibited a significantly larger AHP compared with 129 animals and that this difference was consistent across all phases. Furthermore, our recordings revealed a marked dichotomy in the shape of the AHP waveform, which was independent of genetic background. Approximately 60% of cells exhibited an AHP with a sharp transition between the peak AHP and medium AHP, whereas the remaining 40% exhibited a more gradual transition. Our data add to the growing body of work suggesting that genetic background can affect neuronal function as well as behavior. In addition, these results highlight the innate heterogeneity of CA1 pyramidal neurons, even within a single genetic background. These differences should be taken into consideration during the analysis and comparison of experimental results.
Keywords: mouse strains, whole cell, hippocampus
transgenic mice represent a powerful tool with which to investigate the cellular and molecular mechanisms that underlie basic neurobiological processes (Desai and Clapham 2005; Picciotto et al. 1999) as well as the development of neurodegenerative diseases (Heng et al. 2008; Pallas et al. 2008). In the majority of experiments, transgenic mice are generated on a defined isogenic background, in which many generations of successive inbreeding have created mice that are homozygous at every gene loci and thus considered genetically identical. It has become increasingly clear that many aspects of mouse behavior can be affected by genetic background, including depressive and anxiety-like behaviors and cognitive function (Bucan and Abel 2002; Holmes et al. 2002; Toescu and Vreugdenhil 2010). In addition, there is mounting evidence that genetic background can influence certain forms of synaptic plasticity (reviewed by Nguyen 2006). However, relatively little is known about the effect of genetic background on intrinsic excitability in mice.
The postburst afterhyperpolarization (AHP) is an important determinant of neuronal excitability, contributing to the repolarization of the membrane potential following action potential firing and serving to limit depolarization in response to sustained input. Modulation of the AHP has been suggested to play a critical role in the cellular processes underlying learning and memory. For example, successful learning of a hippocampus-dependent task, such as trace eye blink conditioning, has been demonstrated to reduce the magnitude of the AHP in CA1 pyramidal neurons compared with neurons from both nonlearners as well as naive animals (Moyer et al. 1996, 2000). Age-related increases in the magnitude of the AHP have been demonstrated in rabbits, rats, and mice (Matthews et al. 2009; Moyer et al. 1992; Murphy et al. 2006), and this increase is associated with age-dependent impairments in hippocampus-dependent memory formation (Disterhoft and Oh 2007; Tombaugh et al. 2005). Furthermore, genetic or pharmacological manipulations that reduce the magnitude of the AHP have been correlated with enhanced synaptic plasticity (Murphy et al. 2004; Power et al. 2001) and improved performance in learning and memory paradigms (Disterhoft et al. 1999; Disterhoft and Oh 2003).
To successfully employ transgenic mouse models of aging and cognitive disorders, it is important to understand the extent to which genetic background influences learning-related electrophysiological properties. One recent study investigated passive properties and action potential characteristics in C57BL/6 Jax and 129SvEv Tac mice (and Sprague-Dawley rats) (Routh et al. 2009), but to date there have been no direct investigations of the impact of genetic background on the AHP.
Therefore, we made blind whole cell current-clamp recordings from CA1 pyramidal neurons in acute hippocampal slices to examine whether the AHP was differentially modulated in two commonly used genetic backgrounds in mice, C57BL/6 (B6) and 129SvEv (129), as well as in F2 hybrid mice made by intercrossing nonsibling pairs of B6 and 129 mice (F2). Interestingly, we found that genetic background had a significant effect on the magnitude of the AHP, which was maintained across all phases. Furthermore, the unbiased sampling provided by the blind patch-clamp method revealed striking differences in the shape of the AHP waveform even within a single genetic background. Together, these experiments demonstrate the effect of genetic background and reveal marked heterogeneity in a functional property that may contribute to learning and memory in the mammalian brain: the postburst AHP.
MATERIALS AND METHODS
Animals.
All procedures were approved by and performed in accordance with the University Committee on the Use and Care of Animals at the University of Michigan. Young (2–6 mo old) wild-type mice were group housed and maintained on a 14:10-h light-dark cycle with ad libitum access to food and water. C57BL/6 (B6) and 129SvEv (129) mice were ordered from Taconic (Cambridge City, IN); F2 hybrid mice (F2) were created in-house by nonsibling intercrosses between B6 and 129 mice. Unless otherwise noted, the sample sizes are as follows for all experiments: B6, 28 cells from 13 animals; F2, 35 cells from 15 animals; and 129, 29 cells from 13 animals. Approximately equal numbers of male and female mice were used for experiments, and no differences in recordings were found between sexes (including analysis of passive properties, active properties, and the magnitude of the AHP); thus the data were combined for subsequent analysis and presentation.
Solutions.
Sucrose solution consisted of (in mM) 206 sucrose, 26 NaHCO3, 10 d-glucose, 2.8 KCl, 2 MgSO4, 1.25 NaH2PO4, 1 MgCl2, 1 CaCl2, and 0.4 ascorbic acid. Artificial cerebrospinal fluid (ACSF) consisted of (in mM) 125 NaCl, 25 NaHCO3, 25 d-glucose, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 0.4 ascorbic acid. Sucrose solution and ACSF were oxygenated by constant bubbling with 95% O2-5% CO2. All chemicals for external solutions were obtained from Fisher Scientific (Pittsburgh, PA). Internal solution consisted of (in mM) 120 KMeSO4, 20 KCl, 10 HEPES, 7 Na-phosphocreatine, 4 Na2-ATP, 2 MgCl2, 0.3 Tris-GTP, 0.2 EGTA, and 0.1% biocytin (for subsequent morphological evaluation). Intracellular solution had a pH of 7.3 (obtained using 1 M KOH) and an osmolarity of 305–310 mosmol/l. All chemicals for internal solution were obtained from Sigma-Aldrich (St. Louis, MO), except KMeSO4 (Pfaltz & Bauer, Waterbury, CT).
Slices.
Experimental animals were deeply anesthetized with isoflurane and then rapidly decapitated. The brain was removed and bisected; each hemisphere was affixed to a stage submerged in ice-cold, oxygenated sucrose or ACSF solution (approximately equal numbers of slices were made in sucrose or ACSF solutions; no differences were found between recordings from slices made in sucrose or ACSF, so data were combined for subsequent analysis and presentation). Sagittal slices (300 μm) through the dorsal hippocampal region were collected (Leica VT1000; Wetzlar, Germany) and stored in a holding chamber containing room temperature oxygenated ACSF until used for recording. Slices were allowed to recover for at least an hour before use.
Electrophysiology.
For recording, individual slices were transferred to a nylon net and submerged in warmed (31–32°C) oxygenated ACSF, which was constantly perfused into the bath. Patch pipettes, fashioned from borosilicate glass (OD 1.2 mm, ID 0.8 mm; Warner Instruments, Hamden, CT) on a Flaming-Brown P-97 pipette puller (Sutter Instruments, Novato, CA), were filled with KMeSO4-based internal solution and had an open-tip resistance of 3–6 MΩ. Blind whole cell recordings were obtained using a MS314 micromanipulator (MW, Wetzlar, Germany) to slowly advance the patch pipette into stratum pyramidale of CA1 until a sharp increase in tip resistance was observed. Negative pressure was applied to achieve a gigaohm seal, after which brief mouth-suction pulses were used to break into the cell for recording.
After whole cell configuration was achieved, the amplifier was switched to current-clamp mode. Series resistance and capacitance compensation were monitored and adjusted throughout the duration of the experiment. Data were sampled at 50 kHz by using pClamp 10 software (Molecular Devices, Sunnyvale, CA) with a BVC 700A amplifier (Dagan, Minneapolis, MN), filtered at 5 kHz, and stored via a Digidata 1400 analog-to-digital board (Molecular Devices) on a Dell personal computer. Recordings were not adjusted for the calculated liquid junction potential of +10.6 mV.
Neurons were accepted for recording if the resting membrane potential was less than −60 mV at break-in and action potentials evoked with a 500-ms step current injection displayed spike frequency accommodation (indicative of recording from pyramidal neurons, not interneurons). A hyperpolarizing current step (−200 pA, 500 ms) from the resting membrane potential was used to calculate input resistance by measuring the steady-state voltage change. Sag ratio, indicative of the amount of Ih (a voltage-gated conductance activated by hyperpolarization and a primary contributor to subthreshold excitability), was determined by dividing the steady-state voltage change by the peak voltage change. The membrane time constant was estimated as the time required to reach 1/e of the peak voltage.
The AHP was evoked by a 50-ms step current injection, at an amplitude sufficient to elicit five action potentials, from a holding potential of −60 mV. Three successive sweeps were collected and averaged to determine the magnitude of the AHP. The voltage difference relative to the precurrent injection holding potential was measured at three points to represent different phases of the AHP (Sah 1996): 1) the peak AHP (pAHP), occurring at any time after current injection offset, was the most hyperpolarized membrane potential reached; 2) the medium AHP (mAHP) was represented by the voltage deflection at 200 ms after current injection offset; and 3) the slow AHP (sAHP) was represented by the voltage deflection at 1,000 ms after current injection offset.
Electrophysiological properties were measured for each of the five action potentials in the burst that elicited the AHP. Threshold was determined by a sharp increase in dV/dt; amplitude was the most positive voltage reached (measured relative to threshold); and half-width was measured at 50% amplitude. The depolarizing envelope of the burst was determined by integrating the voltage between the onset and offset of the 50-ms current step.
After recording, slices were fixed in 4% paraformaldehyde. One to two weeks after electrophysiological characterization, a diaminobenzidine reaction (DAB; kit from Sigma-Aldrich) was performed to stain biocytin-filled neurons for subsequent morphological characterization.
Analysis.
Off-line analysis was performed using custom-programmed macros in Igor Pro (Wavemetrics, Lake Oswego, OR). Statistical significance was determined for group data using Student's t-test, one-factor repeated-measures ANOVA, or χ2 test, as appropriate. Where a main effect was detected with ANOVA, a Newman-Keuls post hoc test was used to determine significance between pairwise comparisons (GraphPad Prism5; La Jolla, CA). Group data are means ± SE.
RESULTS
Genetic background in wild-type mice affects the magnitude of the AHP.
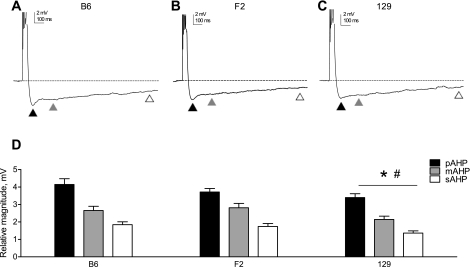
Blind whole cell current-clamp recordings were made from CA1 pyramidal neurons in acute hippocampal slices prepared from B6, 129, or F2 young adult mice to examine the effect of genetic background on the magnitude of the postburst AHP. A 50-ms step current injection at an amplitude that elicited five action potentials was delivered from a membrane holding potential of −60 mV, and the magnitude of the resulting hyperpolarization (relative to the precurrent injection membrane potential) was quantified at three points, representing different phases of the AHP (Sah 1996). The pAHP, due primarily to activation of voltage-gated K+ channels, occurs relatively quickly and was measured when the most hyperpolarized membrane potential was reached (Fig. 1, A–C, solid triangles); the mAHP, due to the activation of both voltage-activated and Ca2+-activated K+ conductances, has a slower time course and was measured 200 ms after current injection offset (Fig. 1, A–C, shaded triangles); the sAHP, due primarily to Ca2+-activated K+ conductances, has the longest latency and was measured 1,000 ms after current injection offset (Fig. 1, A–C, open triangles). Our recordings revealed that genetic background significantly affected the magnitude of the AHP: the pAHP, mAHP, and sAHP were all larger in neurons from B6 and F2 mice compared with 129 mice (Fig. 1D).
Fig. 1.
Genetic background affects the magnitude of the AHP. A–C: a representative trace of the afterhyperpolarization (AHP) evoked at −60 mV (by a 50-ms current injection sufficient to elicit 5 action potentials) is shown for each genetic background examined: B6 (A), F2 (B), and 129 (C). In all panels, the dashed line represents the baseline membrane potential before current injection and the triangles indicate different phases of the AHP as follows: peak AHP (pAHP; solid triangles), medium AHP (mAHP; shaded triangles), and slow AHP (sAHP; open triangles). Note that action potentials are truncated for clarity. D: group data (means ± SE) indicating the magnitudes of the pAHP, mAHP, and sAHP in each genetic background (B6, n = 28; F2, n = 35; 129, n = 29). The asterisk indicates a significant difference compared with B6, and the pound sign indicates a significant difference compared with F2 (P = 0.02, 1-factor repeated-measures ANOVA with Newman-Keuls post hoc test).
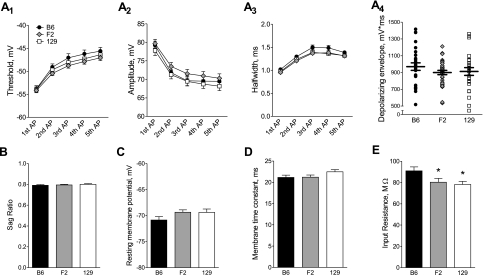
Background-specific alterations in active or passive electrophysiological properties may contribute to the observed differences in the magnitude of the AHP. Active properties refer to the activation (and inactivation/deactivation) of voltage-gated channels, which contribute to action potential firing and repolarization, such as INa and IKA, as well as to subthreshold excitability. Analysis of the stimulus used to evoke the AHP revealed no differences between genetic backgrounds in action potential threshold (Fig. 2A 1), amplitude (Fig. 2A2), or half-width (Fig. 2A3) or in the total depolarizing envelope of the burst (Fig. 2A4). In addition, the sag ratio, indicative of the amount of Ih, was identical between genetic backgrounds (Fig. 2B).
Fig. 2.
Investigation of active and passive neuronal properties that may underlie the effect of genetic background on the magnitude of the AHP. For A–E, B6 is represented by solid symbols or bars (n = 28), F2 is represented by shaded symbols or bars (n = 35), and 129 is represented by open symbols or bars (n = 29). A: the threshold (A1), amplitude (A2), and half-width (A3), shown as means ± SE, were measured for each of the 5 action potentials in the burst stimulus used to evoke the AHP. There were no differences in any of these measures between genetic backgrounds (1-factor repeated-measures ANOVA: P = 0.38 for threshold, P = 0.35 for amplitude, and P = 0.06 for half-width). The depolarizing envelope for the burst stimulus (A4), calculated as the integrated voltage from current onset to current offset, is shown for individual cells with the mean ± SE indicated by horizontal lines. A 1-factor ANOVA revealed no differences between genetic backgrounds (P = 0.37). B–E: a 1-factor ANOVA of the group data (means ± SE) revealed no differences between genetic backgrounds for the sag ratio (B; P = 0.64), resting membrane potential (C; P = 0.13), or membrane time constant (D; P = 0.14). Input resistance (E) was significantly smaller in F2 and 129 backgrounds compared with B6 (P = 0.03, 1-factor ANOVA with Newman-Keuls post hoc test; *P < 0.05 compared with B6).
Passive properties of neurons are mediated by the intrinsic activation of non-voltage-gated conductances, such as IKleak, and are primarily responsible for determining the resting membrane potential, the membrane time constant, and the input resistance. Neither the resting membrane potential, recorded at break-in, nor the membrane time constant, estimated by a hyperpolarizing current injection, was significantly different between any of the genetic backgrounds (Fig. 2, C and D). Conversely, there was a main effect of genetic background on input resistance; a post hoc examination revealed that the input resistance in B6 neurons was significantly higher than that observed in F2 and 129 neurons (Fig. 2E). Importantly, however, the input resistance was not different between F2 and 129 neurons (although the magnitude of AHP was), suggesting that differences in input resistance cannot fully account for the observed differences in the magnitude of the AHP.
Magnitude of the AHP is independent of location and morphology.
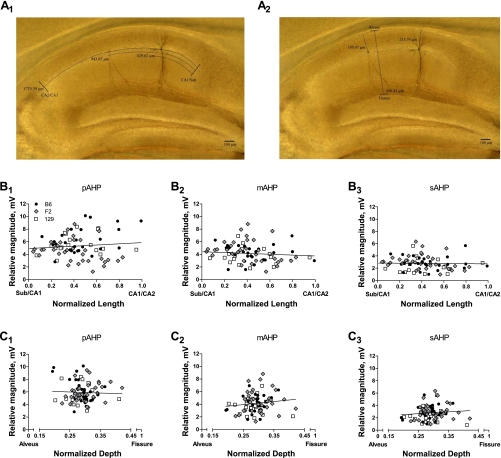
After recording, slices underwent histological processing during which biocytin-filled neurons (i.e., those from which recordings were obtained) were stained via a DAB reaction (see materials and methods). The position of the cell body for each recorded neuron was normalized to the total distance on both the proximal-distal axis (from the CA2/CA1 border to the CA1/subiculum border; Fig. 3A 1) and the superficial-deep axis (from the alveus to the hippocampal fissure; Fig. 3A2). A post hoc examination showed that our recordings encompassed a representative population of pyramidal neurons, with cell bodies located in stratum pyramidale (constituting a relatively narrow band on the superficial-deep axis) and spanning the entire length of CA1 (distributed across the proximal-distal axis). Furthermore, these data demonstrate that there was no differential sampling of neurons from a particular location in any genetic background.
Fig. 3.
AHP magnitude is not differentially affected by location within CA1. A: a representative image of a hippocampal slice after diaminobenzidine (DAB) processing. Note that in this slice, recordings were obtained from 2 individual pyramidal neurons. The position of each cell body is determined along the length axis (from the CA2/CA1 border to the CA1/subiculum border; A1) and the depth axis (from the alveus to the hippocampal fissure; A2). B and C: the location of each cell was normalized to the total length and depth of CA1 in the slice from which it was recorded and plotted against the magnitude of the pAHP (B1, C1), mAHP (B2, C2), and sAHP (B3, C3). On all graphs, each point represents 1 recorded cell, classified by symbol with respect to genetic background (key in B1 applies to all panels in B and C; B6, n = 28; F2, n = 35; 129, n = 29). On each graph, the solid line represents the best linear fit to the data from all genetic backgrounds; there were no correlations between the magnitude of any phase of the AHP and location on either the length or depth axis (the slope of each best-fit line is not different from 0).
Next, we examined whether the AHP was correlated with location by plotting the magnitude of each phase (pAHP, Fig. 3, B1 and C1; mAHP, Fig. 3, B2 and C2; sAHP, Fig. 3, B3 and C3) against the normalized position on both axes for every cell. No significant relationship between magnitude and location was observed for any phase of the AHP in any genetic background. Together, these data demonstrate that the magnitude of the AHP is not differentially modulated by position within the CA1 region and suggest that the effects of genetic background are manifested globally in CA1 pyramidal neurons.
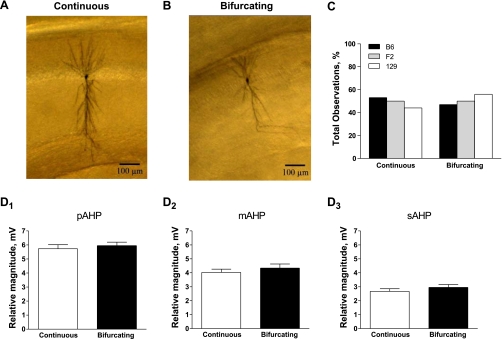
Another observation, readily apparent on examination of individual neurons after DAB processing, was that the morphology varied substantially from cell to cell. In particular, these neurons could be grouped into two classes based on whether the primary apical dendrite remained continuous through stratum radiatum until stratum lacunosum moleculare (Fig. 4A) or bifurcated within stratum radiatum (Fig. 4B). However, the number of observations of a continuous or bifurcated primary apical dendrite did not differ between genetic backgrounds (Fig. 4C). In addition, the morphology did not affect the magnitude of any phase of the AHP (Fig. 4, D1–D3), nor did it correlate with position along the proximal-distal or superficial-deep axis in CA1 (data not shown; Student's t-test, P = 0.16 for the length axis, P = 1.0 for the depth axis).
Fig. 4.
AHP magnitude is not differentially affected by the morphology of the primary apical dendrite. A and B: magnification of the cells shown in Fig. 3. After DAB processing, cells could be readily divided into 2 groups based on whether the primary apical dendrite remained continuous (A) or bifurcated (B) within stratum radiatum. C: the distribution of continuous (B6, n = 9; F2, n = 19; 129, n = 8) vs. bifurcating primary apical dendrites (B6, n = 8; F2, n = 16; 129, n = 10) did not differ between genetic backgrounds (P = 0.43, χ2). D: a Student's t-test comparing the magnitude of each phase of the AHP (mean ± SE) showed that the pAHP (D1; P = 0.57), mAHP (D2; P = 0.41), and sAHP (D3; P = 0.30) were not different between neurons with continuous (n = 36) or bifurcating primary apical dendrites (n = 34). Because there were no differences across genetic background (see C), data from B6, F2, and 129 animals are combined in these graphs.
Heterogeneity in the shape of the AHP is not dependent on genetic background.
One of the unique advantages of the blind patch-clamp technique is that it avoids biases inherent in visualized recordings, including the selection of cells based on a specific appearance and the exclusion of cells that cannot be resolved with the limited optics of the microscope. These subpopulations of neurons may represent distinct functional classes of cells, and thus electrophysiological properties may vary depending on which cells are chosen.
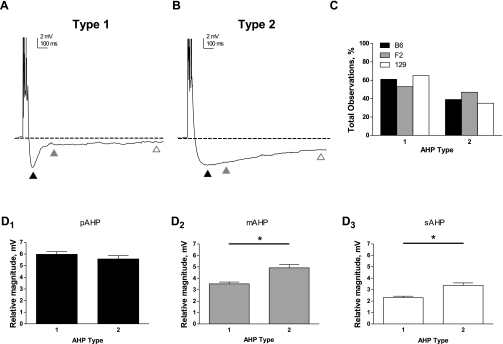
Our recordings, which provide an unbiased sampling of CA1 pyramidal neurons, revealed intriguing observations about the heterogeneity of the AHP. We noted that the AHP waveform could generally be classified into one of two discrete shapes: type 1 had a sharper inflection point between the pAHP and mAHP (Fig. 5A), whereas type 2 had a more gradual transition between these phases (Fig. 5B). Not surprisingly, this difference in shape resulted in a larger magnitude mAHP (Fig. 5D2) and sAHP (Fig. 5D3) in type 2 compared with type 1 recordings, whereas the pAHP was unaffected (Fig. 5D1). Interestingly, both types of AHPs were observed in each genetic background, but there were no differences in the number of occurrences of type 1 or type 2 AHPs between backgrounds (Fig. 5C).
Fig. 5.
Heterogeneity in the shape of the AHP waveform is independent of genetic background. A and B: representative traces illustrating 2 discrete shapes of the evoked AHP. For both A and B, the dashed line represents the baseline membrane potential and the triangles indicate different phases of the AHP as follows: pAHP, solid triangles; mAHP, shaded triangles; and sAHP, open triangles. Note that action potentials are truncated for clarity. A type 1 AHP (A) was characterized by a sharp transition in voltage between the pAHP and mAHP, whereas a type 2 AHP (B) was characterized by a more gradual change in the voltage between the pAHP and mAHP. C: the distribution of type 1 (B6, n = 18; F2, n = 19; 129, n = 20) vs. type 2 AHPs (B6, n = 10; F2, n = 9; 129, n = 16) did not differ between genetic backgrounds (P = 0.21, χ2). D: quantification of the magnitude (means ± SE) of the pAHP (D1), mAHP (D2), and sAHP (D3) in type 1 (n = 57) and type 2 AHPs (n = 35). *P < 0.0001, significant difference between type 1 and type 2 (Student's t-test). Because there were no differences in the distribution of type 1 vs. type 2 AHPs across genetic background (see C), data from B6, F2, and 129 animals are combined in these graphs.
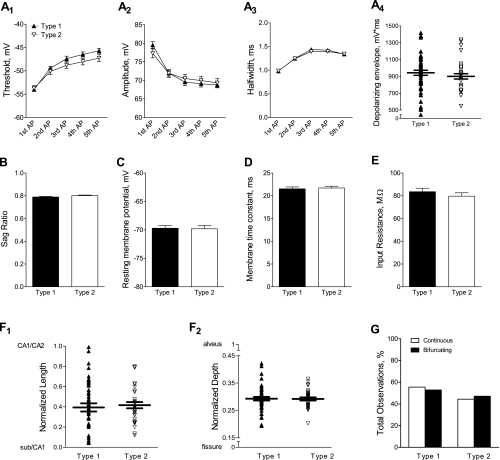
To investigate factors that may contribute to neuron-specific differences in the shape of the AHP, we analyzed electrophysiological and morphological properties of neurons exhibiting a type 1 or type 2 AHP. There were no differences in the action potential threshold (Fig. 6A 1), amplitude (Fig. 6A2), or half-width (Fig. 6A3) or in the depolarizing envelope of the AHP-evoking burst stimulus (Fig. 6A4) between type 1 and type 2 recordings. Likewise, the sag ratio, resting membrane potential, membrane time constant, and input resistance were unaltered between type 1 and type 2 AHPs (Fig. 6, B–E). In addition, the observation of a type 1 or type 2 AHP did not correlate with position on either the proximal-distal (Fig. 6F1) or superficial-deep axis in CA1 (Fig. 6F2). Finally, neither type 1 nor type 2 AHPs were preferentially associated with a continuous or bifurcating primary apical dendrite (Fig. 6G).
Fig. 6.
Investigation of neuronal properties and morphological characteristics that may contribute to differences in the shape of the AHP waveform. Because the shape of the AHP waveform was not differentially affected by genetic background (see Fig. 5), the data were combined for the following analysis. For A–F, type 1 AHPs are represented by solid symbols or bars (n = 57) and type 2 AHPs are represented by open symbols or bars (n = 35). A: the threshold (A1), amplitude (A2), and half-width (A3), shown as means ± SE, were measured for each of the 5 action potentials in the burst stimulus used to evoke the AHP. A 1-factor repeated-measures ANOVA revealed no differences between type 1 and type 2 AHPs (P = 0.21 for threshold, P = 0.99 for amplitude, and P = 0.67 for half-width). The depolarizing envelope for the burst stimulus (A4), calculated as the integrated voltage from current onset to current offset, is shown for individual cells with the mean ± SE indicated by horizontal lines. A Student's t-test revealed no difference between AHP types (P = 0.34). B–E: a Student's t-test of the group data (means ± SE) revealed no differences between type 1 and type 2 AHPs for the sag ratio (B; P = 0.12), resting membrane potential (C; P = 0.90), membrane time constant (D; P = 0.28), or input resistance (E; P = 0.37). F: the location of each cell body on the length axis (F1) and the depth axis (F2) was plotted for type 1 and type 2 AHPs. The mean position was not different for type 1 vs. type 2 AHPs (length axis, P = 0.58; depth axis, P = 1.0; Student's t-test). G: neither type 1 (n = 38) nor type 2 (n = 32) AHPs were preferentially associated with a continuous or bifurcating primary apical dendrite (P = 0.83, χ2).
Together, these results suggest that the function of specific conductances underlying discrete phases of the AHP, especially the mAHP and sAHP, may be differentially regulated within individual neurons. This modulation is not restricted to a particular subregion but appears to be distributed throughout CA1, highlighting the heterogeneity of pyramidal neurons even within a single genetic background.
DISCUSSION
Transgenic mice are a valuable tool that can be used to examine the roles of candidate genes in both physiological processes and pathological conditions. Genetic mutations are introduced into isogenic lines in which mice are considered genetically identical because of many generations of successive inbreeding. However, it has become increasingly clear that the effects of mutations can be altered by genetic background and that different strains of mice have unique characteristics that can profoundly affect the phenotype under investigation (Holmes et al. 2002).
One area in which transgenic mice have been extensively employed is in the study of the neurobiology underlying learning and memory. A growing number of experiments have demonstrated behavioral differences in wild-type mice that are due solely to genetic background (Holmes et al. 2002; Yang et al. 2007), and although at least one study has described some differences in electrophysiological properties (Routh et al. 2009), very few have examined the effect of genetic background on intrinsic neuronal excitability.
Genetic background in wild-type mice differentially affects AHP magnitude.
The AHP plays a critical role in regulating neuronal excitability, constraining the frequency and amount of action potential firing, by repolarizing the membrane potential and limiting voltage summation during sustained or repetitive input. In addition, modulation of the AHP has been suggested as a general cellular mechanism contributing to learning and memory in the mammalian brain. Animals that successfully learn a hippocampus-dependent task exhibit a transiently smaller AHP in CA1 neurons, and this modulation appears to play a permissive role in the memory formation (Moyer et al. 1996). Furthermore, aged animals that perform poorly in hippocampus-dependent tasks have a larger magnitude AHP compared with young animals (Disterhoft and Oh 2007). Interestingly, in both young and aged animals, compounds that reduce the magnitude of the AHP facilitate memory formation and enhance behavioral performance (Disterhoft et al. 1999; Moyer et al. 1992; Power et al. 2001).
Although it is clear that the AHP plays a critical role in regulating neuronal firing patterns and may contribute substantially to memory formation, there has yet to be a direct investigation of the extent to which genetic background influences the AHP. Therefore, we examined the magnitude of the AHP in wild-type mice from two commonly used genetic backgrounds in mice (B6 and 129), as well as an F2 hybrid cross of these two backgrounds (F2). Interestingly, we found that neurons from B6 and F2 animals had a larger magnitude AHP than neurons from 129 animals and that this effect persisted across all phases of the AHP (peak, medium, and slow). Although the overall effect was small (0.5–2 mV), differences of this magnitude have previously been shown to profoundly affect action potential firing in hippocampal neurons (Fernandez and White 2010; Royeck et al. 2008; Zhang et al. 2010) and thus may represent a physiologically relevant difference in these cells.
Because a reduction in the magnitude of the AHP has been shown to be correlated with enhanced performance in learning and memory tasks in mice, it is tempting to hypothesize that 129 mice, which exhibit a smaller AHP, may show superior behavioral performance compared with B6 and F2 mice. Although 129 mice have been shown to exhibit higher levels of freezing during fear conditioning compared with B6 mice (Camp et al. 2009), they do not learn to extinguish their freezing behavior as well as B6 mice (Hefner et al. 2008). Similarly, B6 and F2 animals may be expected to have comparable behavioral performance because the magnitude of the AHP is indistinguishable between these two groups. However, F2 animals have been shown to outperform B6 animals in the Morris water maze (de Bruin et al. 2006). These observations suggest that the magnitude of the AHP is not sufficient to determine the relative strength of learning and memory in different genetic backgrounds across all behavioral paradigms. It is possible that in these cases, the AHP contributes to learning and memory in a task-specific manner (for example, contributing to acquisition of conditioned fear but not its extinction or water maze learning) or, alternatively, that the AHP may be required to work in concert with other functional neuronal properties to regulate the levels of learning and memory.
Differential function of passive and/or active conductances may underlie background-specific differences in AHP magnitude.
Because the AHP was evoked by somatic current injection, independent of synaptic activation, the differences in the magnitude of the AHP between genetic backgrounds must be due to differences in intrinsic properties of the neurons. Neither the resting membrane potential nor the membrane time constant was differentially affected by genetic background, but neurons from B6 mice exhibited a significantly larger input resistance compared with F2 and 129 neurons. Because a higher input resistance would potentiate the magnitude of any voltage deflection, it is possible that this difference may contribute to the larger magnitude AHP in B6 compared with 129 neurons. Importantly, however, F2 neurons also exhibited a larger AHP compared with 129 neurons, but input resistance was not different between these two groups. These results demonstrate that although input resistance may contribute to a larger AHP, it is not sufficient for the differences in magnitude observed across genetic backgrounds. Alternatively, background-specific changes in input resistance may be mediated by a separate mechanism and may be completely independent of the differences in the magnitude of the AHP. In either case, alterations in voltage- or Ca2+-gated conductances, in combination with or separate from changes in input resistance, likely contribute substantially to the differential effect of genetic background on AHP magnitude.
To probe for differences in the function of active conductances, we measured parameters indicative of subthreshold and suprathreshold excitability. We found no effect of genetic background on the sag ratio, the action potential threshold, amplitude, or half-width, or the depolarizing envelope of the burst stimulus used to evoke the AHP. These data suggest that voltage-gated channels active at the resting membrane potential, such as Ih, or which contribute to action potential firing and repolarization, such as voltage-gated sodium and voltage-gated potassium conductances, do not underlie the observed differences in the magnitude of the AHP. Instead, it is likely that voltage- or Ca2+-gated channels active after the termination of action potential firing contribute substantially to the differential effect of genetic background on the AHP. Candidates for these affected conductances include the big- and small-conductance voltage-gated K+ channels (BK and SK, respectively), which are thought to underlie the pAHP and mAHP (Storm 1990; Villalobos et al. 2004), L-type Ca2+ channels, which are primarily responsible for providing the source of Ca2+ necessary to open the Ca2+-activated K+ channels underlying the sAHP (Gamelli et al. 2009; Norris et al. 1998; Power et al. 2002), and these as-yet unidentified Ca2+-activated K+ channels themselves (Bond et al. 2004; Lima and Marrion 2007).
Although our experiments focused on differences in the magnitude of the AHP evoked by somatic current injection, it is important to note that suprathreshold synaptic stimulation also induces an afterhyperpolarization (Gant and Thibault 2009; Wu et al. 2004), which is thought to be regulated by a unique cohort of conductances located throughout the dendritic arbor. Because it is likely that hyperpolarizations evoked by somatic depolarization or synaptic stimulation play different roles in governing neuronal excitability, it will be important to determine whether this synaptically induced AHP is also differentially modulated by genetic background.
Magnitude of the AHP is not correlated with location or morphology.
Pyramidal neurons in CA1 have a well-characterized topographical arrangement; efferent projections from different subregions innervate discrete parts of target regions. For example, neurons in proximal CA1 project to distal subiculum (farther away from CA1/subiculum border), whereas neurons in distal CA1 project to proximal subiculum (near the CA1/subiculum border) (Amaral et al. 1991). In addition, gradients in intrinsic neuronal properties and functional characteristics of pyramidal neurons have been described along the proximal-distal axis (Jarsky et al. 2008). Together, these previous observations raise the possibility that the magnitude of the AHP may also be correlated with location in CA1 and differential gradients could account for the effect of genetic background.
Therefore, after recording, we performed histological processing (via a DAB staining procedure) to map the position of each recorded neuron on both the length (from the CA2/CA1 border to the CA1/subiculum border) and depth axes (from the alveus to the hippocampal fissure). This post hoc analysis confirmed that our experiments encompassed a representative population of pyramidal neurons distributed across the length and depth of stratum pyramidale in CA1. Importantly, these data showed that there was no differential bias of recording position between genetic backgrounds. Furthermore, the magnitudes of the pAHP, mAHP, and sAHP were independent of location in all genetic backgrounds, demonstrating that a differential gradient does not account for differences in the magnitude of the AHP.
Pyramidal neurons in CA1 have previously been divided into one of two groups, characterized by the morphology of the primary apical dendrite (Bannister and Larkman 1995). After histological processing, we also observed this dichotomy: in some cases, the apical dendrite remained continuous through stratum radiatum to the stratum lacunosum moleculare border, whereas in others, the apical dendrite exhibited a bifurcation within stratum radiatum. In agreement with previous reports (Jarsky et al. 2008), we found no correlation between the location of the recorded neuron and the morphology of the primary apical dendrite. In addition, we found that the number of observations of a continuous or bifurcated primary apical dendrite did not differ between genetic backgrounds. Finally, there was no correlation between the morphology of the primary apical dendrite and the magnitude of any phase of the AHP, which is perhaps not surprising, because it has been suggested that the channels underlying the AHP are located relatively proximally to the cell soma rather than distally along the apical dendrite (Bekkers 2000; Lima and Marrion 2007; Sah and Bekkers 1996). It is important to note that this finding does not rule out the contribution of other morphological differences (for example, in the number or branching pattern of basal dendrites) to background- or neuron-specific alterations in the magnitude of the AHP.
Heterogeneity in the shape of the AHP is independent of genetic background.
Classically, CA1 pyramidal neurons have been viewed as a relatively homogenous population, but more recent evidence has suggested that smaller functional subpopulations exist. Blind whole cell patch-clamp recording has the distinct advantage of allowing an unbiased examination of cells within a particular region. This technique afforded the opportunity to observe marked heterogeneity in the AHP, even within a single genetic background.
Our recordings revealed two distinct profiles in the shape of the AHP waveform: Type 1 AHPs exhibited a sharp transition in voltage between the pAHP and mAHP, whereas type 2 AHPs exhibited a more gradual transition. These differences could be easily observed by eye but were also reflected in the quantification of the magnitudes of different phases of the AHP: both the mAHP and sAHP (but not pAHP) were significantly larger in type 2 than in type 1 AHPs, reflecting the slower time course of voltage decay. In general, type 1 AHPs were observed more often than type 2 AHPs (∼3:2), but, interestingly, this distribution was not differentially affected by genetic background. As was the case for the magnitude of the AHP, the type of AHP observed did not correlate with location on either the length or depth axis or with the morphology of the primary apical dendrite (continuous or bifurcated), suggesting that the heterogeneity in this property is distributed across the CA1 region.
The shape of the waveform likely reflects the function of the specific conductances that underlie distinct phases of the AHP, as opposed to alterations in conductances that contribute to more general neuronal excitability. This assertion is supported by the lack of any differences in passive properties (such as the input resistance) or parameters that reflect activation of conductances contributing to subthreshold excitability or action potential firing (such as sag ratio or action potential threshold) between type 1 and type 2 AHPs. Because we found no difference in the magnitude of the pAHP between types, it is also unlikely that channels mediating this phase, such as BK, are substantially altered in neurons displaying different types AHPs. On the other hand, the mAHP and sAHP both exhibited significantly smaller magnitudes in type 1 compared with type 2 AHPs, and thus channels that contribute to these phases [such as SK, L-type Ca2+, and the unidentified channel(s) mediating the sAHP] are likely to be differentially regulated. In addition, because Ca2+ is an important factor in determining the magnitude of the sAHP (and possibly the mAHP), differences in intracellular Ca2+ dynamics, for example, by differential expression and activity of Ca2+-binding proteins such as calbindin (Klausberger and Somogyi 2008), may also contribute to the neuron-specific appearance of a type 1 or type 2 AHP.
Together, our results demonstrate the heterogeneity of an important neurophysiological property: the postburst AHP. Genetic background significantly affects the magnitude of the AHP, even in the absence of genetic manipulations. In addition, within a single genetic background, the shape of the AHP varies substantially from cell to cell. As we gain a deeper understanding of hippocampal function, the importance and impact of these innate differences between and within genetic backgrounds is likely to become increasingly clear.
GRANTS
Support for this research was provided by National Institute on Aging Grants R01-AG28488 (to G. G. Murphy) and T32-AG000114 (to S. J. Moore).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to the members of the Murphy laboratory for helpful discussions regarding this project. In addition, we thank Austin Graves for critical reading of and comments on the manuscript.
REFERENCES
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus 1: 415–435, 1991 [DOI] [PubMed] [Google Scholar]
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurones from the rat hippocampus: I. Branching patterns. J Comp Neurol 360: 150–160, 1995 [DOI] [PubMed] [Google Scholar]
- Bekkers JM. Distribution of slow AHP channels on hippocampal CA1 pyramidal neurons. J Neurophysiol 83: 1756–1759, 2000 [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24: 5301–5306, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet 3: 114–123, 2002 [DOI] [PubMed] [Google Scholar]
- Camp M, Norcross M, Whittle N, Feyder M, D'Hanis W, Yilmazer-Hanke D, Singewald N, Holmes A. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes Brain Behav 8: 744–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin N, Mahieu M, Patel T, Willems R, Lesage A, Megens A. Performance of F2 B6 × 129 hybrid mice in the Morris water maze, latent inhibition and prepulse inhibition paradigms: comparison with C57Bl/6J and 129sv inbred mice. Behav Brain Res 172: 122–134, 2006 [DOI] [PubMed] [Google Scholar]
- Desai BN, Clapham DE. TRP channels and mice deficient in TRP channels. Pflügers Arch 451: 11–18, 2005 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Kronforst-Collins M, Oh MM, Power JM, Preston AR, Weiss C. Cholinergic facilitation of trace eyeblink conditioning in aging rabbits. Life Sci 64: 541–548, 1999 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 6: 327–336, 2007 [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Modulation of cholinergic transmission enhances excitability of hippocampal pyramidal neurons and ameliorates learning impairments in aging animals. Neurobiol Learn Mem 80: 223–233, 2003 [DOI] [PubMed] [Google Scholar]
- Fernandez FR, White JA. Gain control in CA1 pyramidal cells using changes in somatic conductance. J Neurosci 30: 230–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamelli AE, McKinney BC, White JA, Murphy GG. Deletion of the L-type calcium channel CaV1.3 but not CaV1.2 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus 21: 133–141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Thibault O. Action potential throughput in aged rat hippocampal neurons: regulation by selective forms of hyperpolarization. Neurobiol Aging 30: 2053–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci 28: 8074–8085, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Detloff PJ, Albin RL. Rodent genetic models of Huntington disease. Neurobiol Dis 32: 1–9, 2008 [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav 1: 55–69, 2002 [DOI] [PubMed] [Google Scholar]
- Jarsky T, Mady R, Kennedy B, Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J Comp Neurol 506: 535–547, 2008 [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321: 53–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima PA, Marrion NV. Mechanisms underlying activation of the slow AHP in rat hippocampal neurons. Brain Res 1150: 74–82, 2007 [DOI] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci 29: 4750–4755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci 20: 5476–5482, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol 68: 2100–2109, 1992 [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 16: 5536–5546, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol 14: 1907–1915, 2004 [DOI] [PubMed] [Google Scholar]
- Murphy GG, Shah V, Hell JW, Silva AJ. Investigation of age-related cognitive decline using mice as a model system: neurophysiological correlates. Am J Geriatr Psychiatry 14: 1012–1021, 2006 [DOI] [PubMed] [Google Scholar]
- Nguyen PV. Comparative plasticity of brain synapses in inbred mouse strains. J Exp Biol 209: 2293–2303, 2006 [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci 18: 3171–3179, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Camins A, Smith MA, Perry G, Lee HG, Casadesus G. From aging to Alzheimer's disease: unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). J Alzheimers Dis 15: 615–624, 2008 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1, Suppl 2: S121–S125; discussion S139–S140, 1999 [DOI] [PubMed] [Google Scholar]
- Power JM, Oh MM, Disterhoft JF. Metrifonate decreases sI(AHP) in CA1 pyramidal neurons in vitro. J Neurophysiol 85: 319–322, 2001 [DOI] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci 22: 7234–7243, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Harris K, Chitwood RA. Anatomical and electrophysiological comparison of CA1 pyramidal neurons of the rat and mouse. J Neurophysiol 102: 2288–2302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol 100: 2361–2380, 2008 [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci 16: 4537–4542, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990 [DOI] [PubMed] [Google Scholar]
- Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW. Calcium dysregulation in neuronal aging and Alzheimer's disease: history and new directions. Cell Calcium 24: 417–433, 1998 [DOI] [PubMed] [Google Scholar]
- Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell Calcium 47: 158–164, 2010 [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25: 2609–2616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci 24: 3537–3542, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Chan CS, Disterhoft JF. Slow afterhyperpolarization governs the development of NMDA receptor-dependent afterdepolarization in CA1 pyramidal neurons during synaptic stimulation. J Neurophysiol 92: 2346–2356, 2004 [DOI] [PubMed] [Google Scholar]
- Yang S, Farias M, Kapfhamer D, Tobias J, Grant G, Abel T, Bucan M. Biochemical, molecular and behavioral phenotypes of Rab3A mutations in the mouse. Genes Brain Behav 6: 77–96, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bertaso F, Yoo JW, Baumgartel K, Clancy SM, Lee V, Cienfuegos C, Wilmot C, Avis J, Hunyh T, Daguia C, Schmedt C, Noebels J, Jegla T. Deletion of the potassium channel Kv12.2 causes hippocampal hyperexcitability and epilepsy. Nat Neurosci 13: 1056–1058, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]