Abstract
Two of the biggest health problems facing us today are addiction to nicotine and the increased prevalence of obesity. Interestingly, nicotine attenuates obesity, but the underlying mechanism is not clear. Here we address the hypothesis that if weight-reducing actions of nicotine are mediated by anorexigenic proopiomelanocortin (POMC) neurons of the hypothalamic arcuate nucleus, nicotine should excite these cells. Nicotine at concentrations similar to those found in smokers, 100–1,000 nM, excited POMC cells by mechanisms based on increased spike frequency, depolarization of membrane potential, and opening of ion channels. This was mediated by activation of both α7 and α4β2 nicotinic receptors; by itself, this nicotine-mediated excitation could explain weight loss caused by nicotine. However, in control experiments nicotine also excited the orexigenic arcuate nucleus neuropeptide Y (NPY) cells. Nicotine exerted similar actions on POMC and NPY cells, with a slightly greater depolarizing action on POMC cells. Immunocytochemistry revealed cholinergic axons terminating on both cell types. Nicotine actions were direct in both cell types, with nicotine depolarizing the membrane potentials and reducing input resistance. We found no differences in the relative desensitization to nicotine between POMC and NPY neurons. Nicotine inhibited excitatory synaptic activity recorded in NPY, but not POMC, cells. Nicotine also excited hypocretin/orexin neurons that enhance cognitive arousal, but the responses were smaller than in NPY or POMC cells. Together, these results indicate that nicotine has a number of similar actions, but also a few different actions, on POMC and NPY neurons that could contribute to the weight loss associated with smoking.
Keywords: acetylcholine, hypothalamus, feeding, arcuate nucleus
smoking and obesity are two leading causes of morbidity and mortality worldwide (Haslam and James 2005; Mokdad et al. 2004). Obesity increases the risk for secondary health complications (Peeters et al. 2003). Smokers tend to have a lower body weight than nonsmokers; people who quit smoking are likely to gain weight (Ward et al. 2001; Williamson et al. 1991). Smoking cessation leads to increased food intake, decreased resting metabolic rate, and decreased physical activity (Carney and Goldberg 1984; Ferrara et al. 2001; Hofstetter et al. 1986). Nicotine, the major addictive component of tobacco, increases energy expenditure, reduces appetite, and alters feeding patterns (Grunberg 1986; Grunberg et al. 1986; Miyata et al. 2001), which typically results in reduced body weight. The mechanism by which nicotine reduces body weight is unclear. Neuronal nicotine acetylcholine (ACh) receptors (nAChRs) are transmitter-gated ion channels (Karlin and Akabas 1995). A number of different subtypes of nAChRs exist, each with an individual pharmacological and physiological profile and distinct anatomic distribution in the brain (McGehee and Role 1995; Paterson and Nordberg 2000).
Neurons of the hypothalamic arcuate nucleus that synthesize and release proopiomelanocortin (POMC) peptides play a key role in reducing food intake (Wisse and Schwartz 2001). In contrast, the nearby neurons that synthesize neuropeptide Y (NPY) and agouti-related protein are thought to enhance food intake and positive caloric balance by orexigenic actions within the hypothalamus (Elmquist et al. 1999; Saper et al. 2002; Schwartz et al. 2000; Seeley and Woods 2003; Spiegelman and Flier 2001). The hypothalamus receives a rich cholinergic innervation and has a diverse expression of nicotinic α and β nAChR subunits (Britto et al. 1992; Davila-Garcia et al. 1999; Harfstrand et al. 1988; Hatton and Yang 2002; O'Hara et al. 1998; Okuda et al. 1993; Pabreza et al. 1991; Shioda et al. 1997). The physiological actions of nicotine on arcuate POMC and NPY neuronal activity are unknown. Nicotine may have an effect on NPY expression, but the data are inconsistent, with nicotine reducing food intake and lowering arcuate nucleus NPY and NPY mRNA levels (Jang et al. 2003), raising expression (Li et al. 2000a, 2000b), or raising mRNA but decreasing the peptide (Frankish et al. 1995); duration of nicotine exposure may be one factor that may explain these seemingly contradictory results.
Hypocretin/orexin cells in the perifornical/lateral hypothalamic area (de Lecea et al. 1998) have also been reported to modulate food intake (Sakurai et al. 1998). Hypocretin neurons enhance the wake state and cognitive arousal (Hagan et al. 1999). As smoking enhances cognitive arousal, it is possible that one site of nicotine action is on the hypocretin cell. Nicotine has been suggested to modulate hypocretin neurons or neurons innervated by hypocretin axons (Hollander et al. 2008; Plaza-Zabala et al. 2010), but little electrophysiological analysis of this in hypocretin neurons has been done. Interestingly, hypocretin cells may play an important proaddiction role for a number of unrelated drugs including opiates, cocaine, alcohol, and nicotine, each of which acts at different receptors (Boutrel 2008; España et al. 2010; Georgescu et al. 2003; Harris et al. 2005; Hollander et al. 2008).
On the basis of an immunocytochemical study presented here that reveals an abundant cholinergic innervation of the hypothalamic POMC and NPY neurons, we tested the hypothesis that nicotine might inhibit food intake by activating the anorexigenic POMC cells. For control purposes, we also studied the effect on NPY neurons. In this study, we used whole cell voltage- and current-clamp recordings to study the cellular effects of nicotine on the activity of identified POMC and NPY neurons in hypothalamic arcuate nucleus slices from green fluorescent protein (GFP)-expressing transgenic mice.
MATERIALS AND METHODS
Preparation of hypothalamic slices.
Experiments were performed on hypothalamic slices (250–350 μm) obtained from NPY-GFP (van den Pol et al. 2009), hypocretin-GFP (Li et al. 2002), or POMC-GFP transgenic mice. POMC-GFP mice have been described elsewhere (Cowley et al. 2001), and were kindly provided by Dr. Malcolm Low. Young 14- to 21-day-old or adult 6- to 7-wk-old mice maintained in a 12:12-h light-dark cycle were given an overdose of pentobarbital sodium (100 mg/kg) during the light part of the cycle (11:00 AM to 4:00 PM). Brains were then removed rapidly and placed in an ice-cold, oxygenated (95% O2-5% CO2) high-sucrose solution that contained (in mM) 220 sucrose, 2.5 KCl, 6 MgCl2, 1 CaCl2, 1.23 NaH2PO4, 26 NaHCO3, and 10 d-glucose, pH 7.4 (with an osmolarity of 300–305 mosM). A block of tissue containing the hypothalamus was isolated, and coronal slices were cut on a vibratome. After a 1- to 2-h recovery period, slices were moved to a recording chamber mounted on a BX51WI upright microscope (Olympus, Tokyo, Japan) equipped with video-enhanced infrared-differential interference contrast (DIC) and fluorescence. Slices were perfused with a continuous flow of gassed artificial cerebrospinal fluid (ACSF; 95% O2 and 5% CO2) that contained (in mM) 124 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.23 NaH2PO4, 26 NaHCO3, and 10 glucose, pH 7.4. Bath temperature in the recording chamber was maintained at 33 ± 1°C with a dual-channel heat controller (Warner Instruments, Hamden, CT). Neurons were visualized with an Olympus Optical ×40 water-immersion lens. The Yale University Committee on Animal Care and Use approved all procedures used in this study.
Patch-clamp recording.
Whole cell current- and voltage-clamp recordings were performed with pipettes with 4- to 6-MΩ resistance after being filled with pipette solution. The pipettes were made of borosilicate glass (World Precision Instruments, Sarasota, FL) with a PP-83 vertical puller (Narishige, Tokyo, Japan). For most recordings, the composition of the pipette solution was as follows (in mM): 145 KMeSO4 [or KCl for inhibitory postsynaptic currents (IPSCs)], 1 MgCl2, 10 HEPES, 1.1 EGTA, 2 Mg-ATP, 0.5 Na2-GTP, 5 Na2-phosphocreatine, pH 7.3 with KOH (with an osmolarity of 290–295 mosM). Residual series resistance was 3–5 mΩ after electronic compensation. Potentials were corrected for liquid junction potential, which in our system is −9.5 mV when calculated with JPCalc software (Barry 1994). Slow and fast capacitance compensation was automatically performed with Pulse software (HEKA Elektronik, Lambrecht/Pfalz, Germany). Access resistance was continuously monitored during the experiments. Only those cells in which access resistance was stable (changes <10%) were included in the analysis. As unhealthy neurons can show a positive shift in resting membrane potential (RMP) (Ceranik et al. 1997; Manuel et al. 2009; Stucky and Lewin 1999), we did not experiment on any neurons with an RMP positive to −45 mV. An EPC10 amplifier and Pulse software were used for data acquisition (HEKA Elektronik). PulseFit (HEKA Elektronik), Axograph (Axon instruments, Foster City, CA), and Igor Pro (WaveMetrics, Lake Oswego, OR) software were used for analysis. Both excitatory and inhibitory spontaneous postsynaptic currents were detected and measured with an algorithm in Axograph, and only those events with amplitude >5 pA were used, as described in detail previously (Gao and van den Pol 2001). The frequency of action potentials was measured with Axograph as well. Data are expressed as means ± SE. Group statistical significance was assessed with Student's t-test for comparison of two groups and with one-way ANOVA followed by a Bonferroni post hoc test for three or more groups. P < 0.05 was considered statistically significant.
Drugs and drug application.
(−)-Nicotine hydrogen tartrate, d-tubocurarine (d-TC), dihydro-β-erythroidine hydrobromide (DHBE), methyllycaconitine citrate (MLA), and mecamylamine hydrochloride (MEC) were purchased from Tocris Bioscience (Ellisville, MO); 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), dl-2-amino-5-phosphonopentanoic acid (APV), bicuculline (BIC), and acetylcholine chloride (ACh) were purchased from Sigma (St. Louis, MO). Tetrodotoxin (TTX) was obtained from Alomone Labs (Jerusalem, Israel), and ipratropium bromide (atropine) was obtained from Tocris Bioscience. All drugs were given by large-diameter (500 μm) flow pipette, directed at the recorded cell, unless otherwise noted. When a drug was not being administered, normal ACSF continuously flowed from the flow pipe. Drug solutions were prepared by diluting the appropriate stock solution with ACSF.
Immunocytochemistry.
To identify cholinergic axons, we used an antibody against the cholinergic vesicular transporter VAChT (ACh vesicular transporter). Transgenic mice expressing GFP selectively in POMC and NPY neurons were heavily anesthetized with pentobarbital and then perfused transcardially with saline followed by 4% paraformaldehyde. Fifteen- to twenty-five-micrometer-thick coronal sections were cut on a cryostat, immersed in normal PBS, and then placed in guinea pig anti-VAChT (Chemicon) at a dilution of 1:2,500 overnight (Gras et al. 2002); this antibody labeled a single band on a Western blot of the expected size for VACh, and this band was absent with VAChT antigen incubation. After washing five times in normal buffer, sections were placed in secondary antiserum of donkey anti-guinea pig conjugated to CY5 at a dilution of 1:200 for 1–2 h, washed, and mounted on glass slides. Sections were studied on an Olympus IX70 inverted fluorescence microscope. Micrographs were recorded on a Spot digital camera (Diagnostic Imaging), and contrast and brightness were corrected with Photoshop CS2.
RESULTS
Cholinergic axons innervate arcuate nucleus.
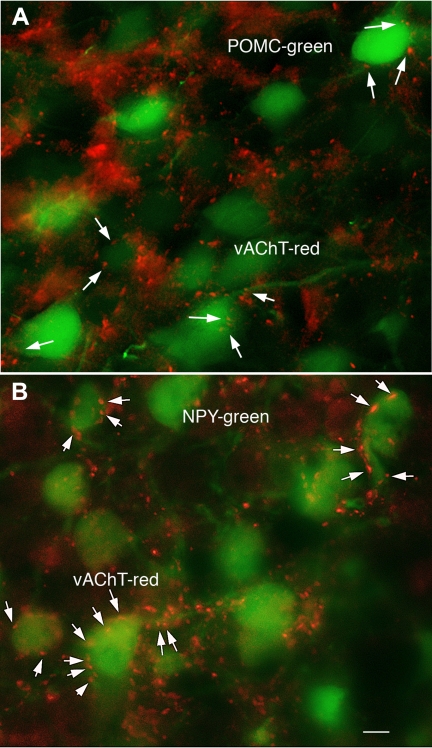
The present study focuses on the physiological response to nicotine, a substance that selectively activates the nicotinic type of ACh receptor. We first examined the possibility that ACh was found in axons in the arcuate nucleus with antisera against the ACh vesicular transporter (VAChT), a marker for cholinergic neurons and axons. A high density of VAChT-immunoreactive axons was found throughout the arcuate nucleus (Fig. 1). POMC (Fig. 1A) and NPY (Fig. 1B) neurons, visualized by GFP expression, were surrounded by red immunoreactive axons that appeared to be in contact with green fluorescent cell bodies or proximal dendrites, suggesting that arcuate POMC and NPY neurons may receive cholinergic innervation.
Fig. 1.
Cholinergic axons innervate arcuate nucleus. Cholinergic axons were identified with immunostaining against the vesicular acetylcholine (ACh) transporter VAChT. Red VAChT immunoreactive axons and boutons (arrows) surround a neuron expressing green fluorescent protein (GFP) under regulation of the proopiomelanocortin (POMC; A) and neuropeptide Y (NPY; B) promoter. Scale bar, 6 μm.
Nicotine excites POMC neurons.
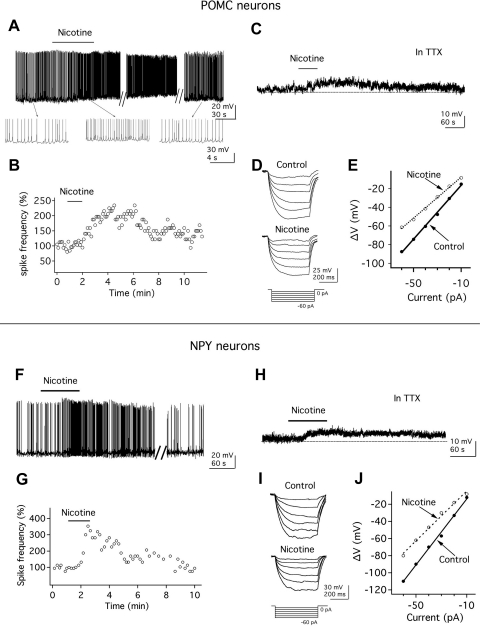
Whole cell recording was then used to study the effect of nicotine on anorexigenic POMC neuronal activity in hypothalamic slices from 2- to 3-wk-old transgenic mice. The effect of nicotine on POMC neurons was tested in transgenic mice that expressed GFP selectively in POMC neurons. Application of nicotine at 1 μM, roughly equivalent to the arterial blood nicotine concentration shortly after smoking a few cigarettes (Henningfield et al. 1993), excited POMC neurons. As shown in Fig. 2A, nicotine (1 μM) depolarized the membrane potential (change by 6.5 ± 0.9 mV; n = 7, P < 0.05) and increased the spike frequency by 93.2 ± 30.0% (n = 7, P < 0.05). With these low concentrations of nicotine, responses peaked between 30 and 90 s after initiation of application. After nicotine application, six of seven cells studied showed an increase >20% in spike frequency; one cell showed no effect. All cells tested are included in the statistical analysis. After nicotine washout, the membrane potential and spike frequency returned toward prenicotine control levels (Fig. 2, A and B). These results indicate that activation of nicotine receptors has a strong excitatory action on POMC neurons.
Fig. 2.
Nicotine excites POMC and NPY neurons. A: nicotine (1 μM) increases the spike frequency of a POMC neuron. B: time course of the nicotine effect on spike frequency in the above POMC neuron; 100% was defined as the mean spike frequency in the minute preceding nicotine application. C: in the presence of tetrodotoxin (TTX), nicotine depolarizes the membrane potential of a POMC neuron. Resting membrane potential (RMP), −61.5 mV. D: nicotine decreases the voltage response of POMC neurons to hyperpolarizing current steps (shown below response). E: current-voltage (I-V) relationship in the absence (●) and presence (○) of nicotine in POMC neurons. F: nicotine (1 μM) increases the spike frequency of a NPY neuron. G: time-course of effect of nicotine on spike frequency in the above NPY neuron. H: in the presence of TTX, nicotine depolarizes the membrane potential of a NPY neuron. RMP, −60.5 mV. I: nicotine decreases the voltage response of NPY neurons after hyperpolarizing current steps (shown below response). J: current-voltage relationship in the absence (●) and presence (○) of nicotine in NPY neurons.
Nicotine excites NPY neurons.
Actions of nicotine on nearby orexigenic NPY neurons were also studied. Nicotine (1 μM) depolarized GFP-expressing NPY neurons (membrane depolarization: 4.7 ± 0.4 mV; n = 11) and increased the frequency of action potentials by 63.2 ± 18.5% (from 1.1 ± 0.2 to 1.7 ± 0.3 Hz; n = 11, P < 0.05; Fig. 2F). In these 11 cells studied, 9 cells showed an increase >20% in spike frequency following nicotine application; the remaining 2 cells showed little or no effect. After nicotine washout, the NPY neurons returned toward control values (Fig. 2, F and G).
Postsynaptic actions of nicotine on POMC and NPY neurons.
To investigate whether nicotine had a direct postsynaptic effect on POMC neurons, we examined the actions of nicotine on the membrane potential of POMC neurons in the presence of the sodium channel blocker TTX. In the presence of TTX (0.5 μM), nicotine depolarized the membrane potential by 5.2 ± 0.7 mV (n = 7, P < 0.05; Fig. 2C), suggesting that nicotine has a direct effect on POMC neurons.
To determine whether the excitatory actions of nicotine on POMC neurons were accompanied by changes in the whole cell input resistance, we delivered negative current steps (from −10 to −60 pA during 500 ms; increments of 10 pA) through the recording pipette and evaluated changes in the membrane potential before and after nicotine application in the presence of TTX (0.5 μM). In the presence of nicotine (1 μM), the hyperpolarizing shifts in the membrane potential in response to the injection of negative current steps were reduced (Fig. 2D), and the current-voltage relationship showed a consistent alteration compared with control prenicotine conditions (Fig. 2E). A linear function was fitted to the current-voltage relationship, and a significant decrease in the slope was observed after nicotine application, consistent with a reduction in the whole cell input resistance from 1.4 ± 0.2 to 1.1 ± 0.1 GΩ (n = 6, P < 0.05; Fig. 2E), suggesting that the mechanism of nicotine excitation is due to opening of ion channels.
Similar nicotine actions were also observed on NPY neurons. In the presence of TTX, nicotine (1 μM) depolarized the membrane potential by 4.6 ± 0.5 mV (n = 7, P < 0.05; Fig. 2H). As in POMC neurons, the whole cell input resistance was also significantly reduced in NPY neurons from 1.2 ± 0.08 to 0.9 ± 0.09 GΩ (n = 9; P < 0.05; Fig. 2, I and J) after nicotine (1 μM) application.
Nicotine-induced depolarization in POMC and NPY neurons is mediated via both α4β2 and α7 nicotinic receptors.
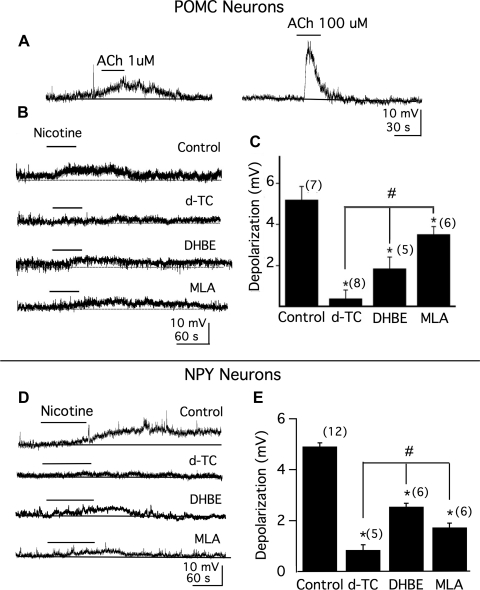
Nicotine acts on cholinergic receptors. We next tested whether ACh would directly excite POMC neurons. In the presence of 5 μM atropine, an irreversible muscarinic receptor antagonist, 1 μM ACh evoked a membrane depolarization of 3.2 ± 0.6 mV (n = 6; Fig. 3A, left) and 100 μM ACh evoked a depolarization of 8.8 ± 0.6 mV (n = 6; Fig. 3A, right).
Fig. 3.
Both α4β2 and α7 nicotinic receptors mediate nicotine-induced membrane depolarization in POMC and NPY neurons. A: action of ACh (1 μM, left and 100 μM, right) on membrane potentials of POMC neurons in the presence of atropine (5 μM), TTX (0.5 μM), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), AP-5 (50 μM), and bicuculline (BIC, 30 μM). B: actions of nicotine (1 μM) on POMC neuron membrane potential in the absence (control; RMP, −62.5 mV) or presence of broad-spectrum nicotine receptor antagonist d-tubocurarine (d-TC, 10 μM; RMP, −59.0 mV), α4β2 nicotine receptor antagonist dihydro-β-erythroidine hydrobromide (DHBE, 1 μM; RMP, −60.5 mV), or α7 nicotine receptor antagonist methyllycaconitine (MLA, 20 nM; RMP, −61.5 mV). C: mean effect of nicotine on membrane potential obtained from B. Number of cells is shown in parentheses. Error bars indicate SE. D: action of nicotine (1 μM) on NPY neuron membrane potential in the absence (control; RMP, −65 mV) or presence of broad-spectrum nicotine receptor antagonist d-TC (10 μM; RMP, −66 mV), α4β2 nicotine receptor antagonist DHBE (1 μM; RMP, −70 mV), or α7 nicotine receptor antagonist MLA (20 nM; RMP, −60 mV). E: mean effect of nicotine on membrane potential obtained from D. Number of cells is shown in parentheses. Error bars indicate SE. *Each of the 3 test groups was significantly different from control. #Response to nicotine in the presence of either DHBE or MLA alone was significantly different from that in the presence of d-TC.
We then studied the pharmacology of the nicotine receptors in POMC neurons. The experiments were conducted in the presence of TTX (0.5 μM). In control conditions, the depolarization by nicotine was 5.2 ± 0.7 mV (n = 7, P < 0.05; Fig. 3B, 1st trace). In all the neurons tested (n = 8), the excitatory response to nicotine was significantly blocked by d-TC, a broad-spectrum nicotinic receptor antagonist (Fig. 3B, 2nd trace). When the slice was pretreated for 10–15 min with d-TC, the depolarization evoked by nicotine was blocked (change by 0.4 ± 0.4 mV; P < 0.05 compared with control, n = 8). To determine the specific nicotinic receptor subtype(s) involved, we tested the effect of subtype-selective receptor antagonists. As shown in Fig. 3B (3rd trace), when the slice was pretreated for 10–15 min with DHBE, an α4β2 nicotine receptor antagonist, the depolarization by nicotine was significantly reduced. In the presence of DHBE (1 μM), the depolarization by nicotine was 1.9 ± 0.6 mV (n = 5, P < 0.05 compared with control, ANOVA; Fig. 3C). Similarly, when the slice was pretreated with the α7-preferring antagonist MLA, the depolarization by nicotine was also significantly reduced (Fig. 3B, 4th trace). With MLA (20 nM) in the bath for 10–15 min, the depolarization by nicotine was 3.5 ± 0.4 mV (n = 6, P < 0.05 compared with control, ANOVA; Fig. 3C). When comparing the effect on the depolarization of d-TC with either DHBE or MLA, a statistically significant difference was detected (Fig. 3C).
We also investigated the pharmacology of the nicotine receptors in NPY neurons. Ten micromolar d-TC almost completely blocked the depolarization evoked by nicotine in NPY neurons (Fig. 3D, 2nd trace). In control conditions, the depolarization by nicotine was 4.9 ± 0.1 mV (n = 12; Fig. 3D, 1st trace) in NPY neurons. In the presence of d-TC, the depolarization evoked by nicotine was 0.8 ± 0.2 mV (n = 5, P < 0.05 compared with control; Fig. 3E). When the slice was pretreated with the α4β2 nicotine receptor antagonist DHBE for 10–15 min, the depolarization by nicotine was significantly reduced (Fig. 3D, 3rd trace). In 1 μM DHBE, the depolarization by nicotine was 2.6 ± 0.1 mV (n = 6; P < 0.05 compared with control; ANOVA, Fig. 3E). MLA (20 nM) also significantly reduced the response induced by nicotine (Fig. 3D, 4th trace). The depolarization by nicotine in MLA was 1.7 ± 0.2 mV (n = 6; P < 0.05 compared with control, ANOVA; Fig. 3E).
These data provide pharmacological evidence that nicotine receptors containing α4β2 and α7 subunits are functionally expressed in both POMC and NPY neurons and both α4β2 and α7 subunits of nicotine receptors are involved in nicotine-induced membrane depolarization.
Nicotine excites immature and adult POMC and NPY neurons.
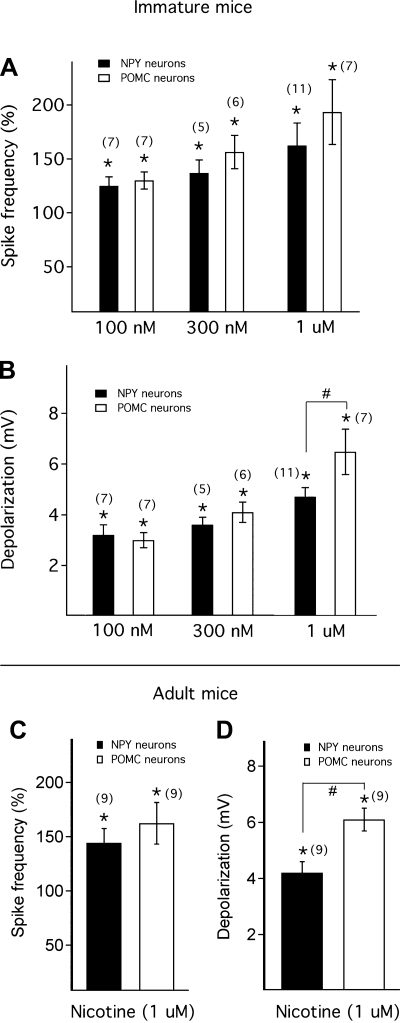
NPY and POMC neurons have opposite actions on food intake and energy metabolism (Elmquist et al. 1999; Wisse and Schwartz 2001). To investigate the net effect of nicotine on NPY and POMC neurons, we compared the effect of nicotine on these two types of neurons in 2- to 3-wk-old mice. As shown in Fig. 4, A and B, 1 μM nicotine evoked a greater effect on spike frequency and membrane potential (P < 0.05) in POMC than in NPY neurons. In addition, we studied the effect of lower concentrations of nicotine (100 nM and 300 nM) on both POMC and NPY neurons. Nicotine at 100 nM and 300 nM depolarized the membrane potential of POMC neurons by 3.0 ± 0.3 mV (n = 7) and 4.1 ± 0.4 mV (n = 6) and increased the spike frequency by 29.6 ± 8.0% and 56.2 ± 15.5%, respectively (Fig. 4, A and B). Similarly, nicotine at 100 nM and 300 nM depolarized the membrane potential of NPY neurons by 3.2 ± 0.4 mV (n = 7) and 3.6 ± 0.3 mV (n = 5) and increased the spike frequency by 24.5 ± 8.4% and 37.0 ± 12.1%, respectively (Fig. 4, A and B). When data from all concentrations (100 nM to 1 μM) were pooled (n = 43), nicotine's depolarizing action on POMC cells was significantly greater than on NPY cells (P < 0.05).
Fig. 4.
Nicotine exerts slightly greater magnitude depolarizing actions in POMC neurons than in NPY neurons. A: mean spike frequency after nicotine application at different concentrations, including 100 nM, 300 nM, and 1 μM, in NPY and POMC neurons from young mice. B: mean depolarization of membrane potential evoked by nicotine at concentrations of 100 nM, 300 nM, and 1 μM in NPY and POMC neurons from young mice. C: action of nicotine (1 μM) on spike frequency in NPY and POMC neurons from adult mice. D: action of nicotine (1 μM) on depolarization of membrane potential in NPY and POMC neurons from adult mice. Numbers of cells are shown in parentheses. Error bars indicate SE. *Each of the test groups was significantly different from the control (A–D). #Membrane depolarization induced by nicotine in POMC neurons was significantly higher than in NPY neurons (B and D).
We also studied the effect of nicotine in 6- to 7-wk-old adult NPY and POMC transgenic mice. Nicotine at 1 μM significantly increased the spike frequency of POMC neurons by 62.4 ± 19.2% and depolarized the membrane potential by 6.1 ± 0.4 mV (from an initial membrane potential of −59.0 ± 2.2 mV) (n = 9, P < 0.05; Fig. 4, C and D). In NPY neurons, nicotine (1 μM) significantly increased the spike frequency by 44.2 ± 13.2% and depolarized the membrane potential by 4.2 ± 0.4 mV (from an initial membrane potential of −59.6 ± 3.1 mV) (n = 9, P < 0.05; Fig. 4, C and D). Nicotine evoked a significantly greater effect on membrane depolarization in POMC neurons than in NPY neurons (P < 0.05; Fig. 4D). These results indicate that nicotine has a slightly greater excitatory action on POMC neurons than on NPY neurons at a 1 μM concentration. Although the nicotine responses appeared slightly greater in the younger mice, this difference was not statistically significant (P > 0.05, unpaired t-test).
POMC and NPY neurons show similar desensitization to nicotine.
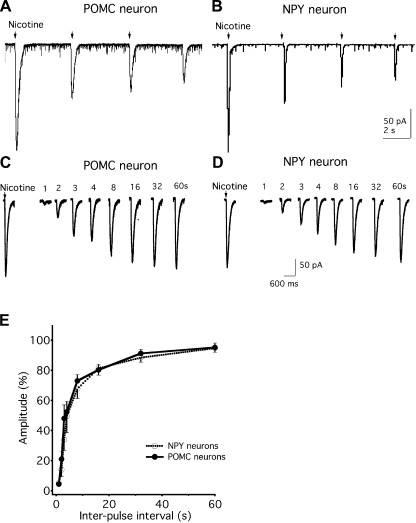
The response to nicotine can desensitize with repeated exposure (Quick and Lester 2002). One possibility that could underlie different effects of nicotine on the two cell types is different levels of desensitization. In the first experiments testing both POMC and NPY neurons, after repetitive nicotine microapplications (10 mM for 1 s) from a micropipette, a similar progressive decrease in the amplitude of the nicotine-induced current was recorded (Fig. 5, A and B), suggesting similar receptor desensitization in both cell types after repetitive activation.
Fig. 5.
Similar rates of desensitization to nicotine application exist between POMC and NPY neurons. A and B: nicotine-induced currents desensitize during repeated application of nicotine (10 mM, 1-s duration) in POMC (A) and NPY (B) neurons. C: traces show the currents induced by equimolar applications of nicotine at different intervals, including 1 s, 2 s, 3 s, 4 s, 8 s, 16 s, 32 s, and 60s, in POMC neurons. D: traces show the currents induced by equimolar nicotine applications at different intervals, including 1 s, 2 s, 3 s, 4 s, 8 s, 16 s, 32 s, and 60 s, in NPY neurons. E: ratio of the amplitude of the 2nd nicotine-induced current divided by that of the 1st one with increasing intervals as shown in C. There was no significant difference in the rate of desensitization between POMC and NPY neurons.
The properties of desensitization were then studied by a somewhat different second approach in which a two-pulse protocol of identical nicotine pulses (10 mM, 1-s duration) was delivered at increasing time intervals (from 1 s to 60 s) from a micropipette. The desensitization was assessed by calculating the ratio of the amplitude of the second nicotine-induced current divided by that of the first one. As shown in Fig. 5, C and D, both POMC and NPY neurons developed quick desensitization and mostly recovered by 60 s after the first nicotine application. When the desensitization ratios at 1 s, 2 s, 3 s, 4 s, 8 s, 16 s, 32 s, and 60 s after the first nicotine application were compared, there was no significant difference between POMC and NPY neurons (P > 0.05; Fig. 5E). These results suggest that nicotine has similar desensitizing actions in both POMC and NPY neurons.
Nicotine depresses excitatory postsynaptic currents in NPY neurons but not in POMC neurons.
We next investigated the effect of nicotine on synaptic transmission. First we tested nicotine actions on excitatory postsynaptic currents (EPSCs) in POMC neurons. In these experiments, BIC (30 μM) was added to the bath to block GABAA receptors. Nicotine showed no detectable effect on frequency (change by 0.2 ± 4.8% of control) or amplitude (change by 3.1 ± 2.9% of control) of spontaneous EPSCs (sEPSCs) (n = 7, P > 0.05; Fig. 6, A and C). Figure 6B shows the time course of the nicotine effect on the frequency of sEPSCs. Further application of the glutamate receptor antagonists CNQX (10 μM) and APV (50 μM) completely suppressed the synaptic currents, confirming the glutamatergic nature of these currents (n = 3; data not shown). In addition, the effect of nicotine on spontaneous IPSCs (sIPSCs) in POMC neurons was studied. Nicotine at 1 μM exerted no significant effect on the frequency (change by −2.9 ± 10.5%; n = 9, P > 0.5) or amplitude (change by −4.9 ± 5.2%; n = 9, P > 0.5) of sIPSCs in POMC neurons (data not shown). The GABAA receptor antagonist BIC completely suppressed the inhibitory synaptic currents, confirming that they are attributable to the activation of GABAA receptors (n = 3; data not shown).
Fig. 6.
Nicotine attenuates excitatory synaptic input to NPY cells but not POMC cells. A: traces show that nicotine (1 μM) evokes little effect on spontaneous excitatory postsynaptic currents (sEPSCs) in a POMC neuron. B: time-course effect of nicotine on the frequency of sEPSCs as shown in A. C: mean effect of nicotine on the frequency and amplitude of sEPSCs in POMC neurons. D: traces show that nicotine (1 μM) decreases sEPSCs in a NPY neuron. E: time-course effect of nicotine on the frequency of sEPSCs as shown in D. F: mean effect of nicotine on the frequency and amplitude of sEPSCs in NPY neurons. Nicotine significantly decreases the frequency and amplitude of sEPSCs in NPY neurons. G: traces show sEPSC before (control) and during nicotine (1 μM) in the presence of 3 nAChR antagonists: MEC (1 μM), the broad-spectrum nicotinic receptor antagonist, MLA (20 nM), an α7 nicotinic receptor antagonist, and DHBE (1 μM), an α4β2 nicotinic receptor antagonist. H: effectiveness of nAChR antagonists in blocking the nicotine-induced inhibition in sEPSC frequency and amplitude in NPY neurons. sEPSCs were significantly decreased by nicotine both in frequency and in amplitude, and this nicotine-mediated reduction was partially blocked by MEC and almost blocked by a combination of MEC, MLA, and DHBE. Number of cells is shown in parentheses. Error bars indicate SE. *Each of the test groups was significantly different from the control (F and H). #Statistical significance between nicotine and nicotine+MEC or between nicotine and nicotine+MEC+MLA+DHBE (H). Ctrl, control; Nic, nicotine; W, washout.
The effect of nicotine on sEPSCs in NPY neurons was then studied. As shown in Fig. 6, D and F, nicotine (1 μM) significantly decreased the frequency of sEPSCs by 37.9 ± 6.1% (from 3.6 ± 0.6 Hz to 2.4 ± 0.5 Hz; n = 14, P < 0.05). Furthermore, nicotine also decreased the amplitude of sEPSCs by 11.6 ± 2.7% (n = 14, P < 0.05; Fig. 6, D and F). The time course of the nicotine effect is shown in Fig. 6E. In 12 of 14 cells tested, the decrease was >20%; 2 cells showed no effect. In a follow-up experiment with TTX (0.5 μM) in the bath, nicotine (1.0 μM) showed no significant effect on miniature EPSCs (to 117.9 ± 7.1% and 103.9 ± 2.3% of control frequency and amplitude, respectively; n = 9; P > 0.05) (not shown). These results suggest that nicotine reduces glutamate release through actions on presynaptic cell bodies or dendrites, and probably not directly on the presynaptic axons.
We next asked whether the reduction in EPSCs in NPY neurons might be due to an initial increase and then a desensitization-mediated longer term decrease. However, we found no initial increase in EPSC frequency, but rather a continued reduction. During a 10-min application of 1 μM nicotine, by the 3rd minute EPSC frequency was decreased by 26.2 ± 5.0%, by the 6th minute 32.6 ± 5.6%, and by the 10th minute, EPSC frequency was reduced by 31.3 ± 5.4% (n = 4; data not shown). No significant difference in EPSC frequency reduction was found between the three intervals (P > 0.05).
To corroborate that the nicotine-induced inhibition of sEPSCs in NPY neurons was mediated by the activation of nicotinic receptors, we investigated the effect of nicotine on sEPSCs in the presence of the noncompetitive nicotinic receptor antagonist MEC (Rabenstein et al. 2006). Nicotine (1 μM) induced a significant decrease in sEPSC frequency and amplitude (Fig. 6H) with a mean reduction of 31.3 ± 0.6% and 27.2 ± 1.6%, respectively (n = 5, P < 0.05 compared with control, ANOVA). When the slice was pretreated with MEC (1 μM) for 10 min, this inhibition by nicotine was significantly reduced to 14.9 ± 0.4% in frequency and 16.2 ± 0.4% in amplitude (n = 7, P < 0.05 nicotine vs. nicotine + MEC, ANOVA; Fig. 6H). MEC may not block all nicotine receptors, for instance, α7 nAChR (Gao et al. 2010; Ishibashi et al. 2009), potentially explaining the absence of complete block by MEC. We therefore employed a cocktail of three nAChR antagonists (MEC, MLA, and DHBE). Treatment for 10 min with MEC (1 μM), MLA (20 nM), and DHBE (1 μM) blocked the nicotine (1 μM)-mediated reduction in sEPSC frequency and amplitude (n = 6, P > 0.5 control vs. nicotine + all 3 nAChR antagonists and P < 0.05 nicotine vs. nicotine + all 3 nAChR antagonists, ANOVA; Fig. 6, G and H). These data are consistent with the view that the reduction of glutamate release by nicotine application is modulated via nAChRs.
As we earlier found immunocytochemical evidence for cholinergic innervation of NPY and POMC neurons, to determine whether the nicotine effect on sEPSCs described above in NPY neurons might be modulated by ongoing ACh release, d-TC (10 μM), a broad-spectrum nicotine receptor antagonist, was applied to brain slices. d-TC had little effect on sEPSCs (n = 5, not shown) and no effect on baseline current. Similarly, we found no effect of d-TC (10 μM) on baseline current in voltage clamp in POMC neurons (n = 5). These data suggest that under our conditions, there was little ongoing intrinsic action of ACh on nicotinic receptors expressed by NPY or POMC neurons.
We also investigated the effect of nicotine on IPSCs in NPY neurons in the presence of the ionotropic glutamate receptor blockers APV (50 μM) and CNQX (10 μM). Nicotine (1 μM) showed no clear effect on the frequency or amplitude of sIPSCs (change by 6.0 ± 6.8% and 3.9 ± 5.6% of control frequency and amplitude, respectively; n = 6, P > 0.05) (data not shown).
Hypocretin cells.
POMC and NPY cells showed a somewhat similar sensitivity to nicotine; we also compared a third cell type, the hypocretin neuron from the lateral hypothalamus. The hypocretin cells play a role in enhancing cognitive arousal, and have also been suggested to modulate arousal related to energy homeostasis. Hypocretin cells project to, and excite, both POMC and NPY neurons (Acuna-Goycolea and van den Pol 2009; Horvath et al. 1999; van den Top et al. 2004). Nicotine (1 μM) significantly increased the spike frequency by 19.1 ± 3.3% (n = 10, P < 0.05). In the 10 hypocretin cells tested here, 6 cells showed an increase >20% of control spike frequency following nicotine application; the remaining 4 cells showed no effect. Nicotine at 300 nM evoked only a modest increase in spike frequency (increase by 11.3 ± 6.8%; n = 4). Because of the continuous spiking of hypocretin cells and the consequent variable membrane potential (Li et al. 2002), we did not examine depolarization in the absence of TTX. In the presence of TTX (0.5 μM), nicotine (1 μM) evoked a 1.8 ± 0.8 mV (n = 5) depolarization. The increase in spike frequency evoked by 1 μM nicotine and the depolarization in TTX were significantly less in hypocretin neurons than in NPY or POMC neurons (P < 0.05).
DISCUSSION
In the present study, we used voltage- and current-clamp whole cell recording to study the actions of nicotine on GFP-expressing POMC and NPY neurons in hypothalamic slices in vitro. Nicotine evoked a slightly greater depolarization in POMC cells than in NPY cells, but with TTX no difference was found between these two cell types. POMC and NPY neurons showed similar desensitization rates upon repeated exposure to nicotine. Nicotine exerted an indirect inhibitory effect on the synaptic release of glutamate onto NPY, but not POMC, neurons. The modestly bigger direct excitatory action on anorexigenic POMC neurons and indirect inhibitory actions on orexigenic NPY neurons suggest that nicotine actions on these cells may contribute to multiple mechanisms by which body weight and food intake are reduced by nicotine.
Actions of nicotine on POMC and NPY neurons.
POMC and NPY neurons both appear quite sensitive to even low (nanomolar) concentrations of nicotine. Low concentrations (100 and 300 nM) of nicotine evoked a similar membrane depolarization and spike frequency increase in the two cell types. Furthermore, the desensitization profiles of these two cell types to prolonged or repeated nicotine application were similar. The depolarizing action on membrane potential continues in the presence of the sodium channel blocker TTX, suggesting that this is a direct postsynaptic effect. In addition, the nicotine-mediated decrease in input resistance in both cell types suggests that the excitatory effect of nicotine on these cells probably results from the opening of ion channels. The depolarizing actions on membrane potential in POMC cells are significantly decreased by MLA, an α7 nAChR antagonist, and by DHBE, a selective α4β2 nAChR antagonist, suggesting that both α7 and α4β2 nAChRs coexist in these neurons.
Two primary differences in nicotine responses were found in POMC and NPY neurons. The first was that, with higher concentrations (1 μM), nicotine evoked a slightly greater depolarizing action on POMC than on NPY cells. The second difference was that nicotine evoked an inhibitory effect on synaptic release of glutamate onto NPY neurons, whereas it had no detectable effect on glutamate release onto POMC neurons. Whereas nicotine generally enhances synaptic release (Jo et al. 2005; Kawa 2002; Neff et al. 1998; Wu et al. 2003), in this case we find an inhibitory effect. Although this inhibitory effect is unusual, it is not without precedent (Fisher and Dani 2000; Levy et al. 2006; Maggi et al. 2004; Zhu and Chiappinelli 1999). In most cases, such a decrease has been attributed to excitation of GABA neurons that then inhibit glutamate release; this is consistent with the lack of effect of nicotine on miniature EPSCs in NPY cells, suggesting that nicotine did not act directly on glutamatergic axon terminals to inhibit release. In other brain regions, there is some evidence for a nicotine-mediated reduction in NMDA responses (Fisher and Dani 2000), and a possible presynaptic inhibition has been suggested, particularly for high probability-release synapses (Levy et al. 2006; Maggi et al. 2004).
The inhibitory effect on glutamate release onto NPY neurons might result from the depolarization-induced release of cannabinoids from the postsynaptic neurons, which in turn could inhibit the release of glutamate from the presynaptic cells. However, this mechanism is not likely, since in our previous study the cannabinoid type 1 receptor agonist WIN55,212-2 produced no effect on NPY neuronal activity, either on membrane potential or frequency of sIPSCs or sEPSCs (van den Pol et al. 2009). Previous studies have examined nicotine excitatory actions on GABA interneurons (de Rover et al. 2002; Frazier et al. 1998; Maggi et al. 2001; Porter et al. 1999; Takeda et al. 2007), and these actions increase inhibitory synaptic transmitter release (Buhler and Dunwiddie 2002). As both NPY and POMC cells may also contain GABA, and are excited by nicotine and maintain local axon collaterals, nicotine would increase GABA release within the arcuate nucleus. However, activation of GABA interneurons by nicotine is unlikely to underlie the phenomenon here because it was found in the presence of GABA receptor antagonists.
If nicotine reduces excitatory synaptic transmission to NPY cells, it would tend to reduce the activity of the NPY cells, similar to the reduced spike frequency in arcuate neurons evoked by glutamate receptor antagonists (Acuna-Goycolea and van den Pol 2005). Compared with other regions of the brain, the weak blood-brain barrier in the arcuate nucleus may allow a faster or greater accumulation of nicotine in the extracellular space near the POMC and NPY cells than in cells outside the arcuate nucleus, making these neurons a potential critical target in nicotine-mediated weight regulation. This, however, does not imply that cells in other sites within the hypothalamus or other regions of the brain that respond to nicotine might not also play a role in modulating energy homeostasis (Jo et al. 2002, 2005).
Nicotine excites hypocretin neurons.
We also studied perifornical/lateral hypothalamic neurons that synthesize hypocretin. Nicotine depolarized the membrane potential and increased spike frequency in these cells. The response in hypocretin neurons was less substantial than that found in either POMC or NPY neurons. As hypocretin cells are responsible for maintaining a wake state, and are associated with enhanced cognitive arousal, the nicotine-mediated excitation could underlie some of the behavioral actions of nicotine on arousal (Mineur and Picciotto 2010; Picciotto et al. 2000). Growing evidence also suggests a key role for hypocretin cells in supporting addiction to a number of drugs, including nicotine (Boutrel 2008; España et al. 2010; Georgescu et al. 2003; Harris et al. 2005; Hollander et al. 2008). The modest response to nicotine suggests that in addition to a direct effect on the hypocretin cells, indirect effects, including possible presynaptic effects on hypocretin axon terminals, or nicotine actions on cells postsynaptic to hypocretin axons are involved in the interaction of hypocretin and nicotine.
Functional relevance.
Nicotine is the primary addictive agent in tobacco, the most widespread substance of abuse; tobacco is responsible for a wide variety of health problems. A second major health problem today is the growing incidence of obesity and its own secondary health problems. In the context of the worldwide obesity epidemic and a high prevalence of smoking, the relation between smoking and obesity thus has major public health relevance. These two major health risks are not independent. Nicotine is an appetite suppressant, and reduced smoking often leads to an increase in eating and body weight (Ward et al. 2001; Williamson et al. 1991). In this study, we found that nicotine not only excited POMC neurons but also had an excitatory effect on NPY neurons. The excitatory effect on adult POMC neurons was modestly greater than on NPY neurons, and this greater excitatory effect on these anorexigenic cells may contribute to nicotine-mediated weight loss. The absence of nicotine stimulation as a result of smoking cessation would tend to reduce the firing rate of anorexigenic POMC cells and reduce the inhibition of excitatory synaptic inputs to NPY neurons; the combination of these two effects may constitute one mechanism for the increase in body weight occurring when nicotine intake is stopped. A caveat here is that the difference in POMC and NPY cell responses was blocked by TTX and was not very large; desensitization profiles for nicotine were remarkably similar for both cell types. Additionally, our experiments were done on brain slices, sometimes in the presence of various transmitter receptor antagonists. Study of nicotine responses by NPY and POMC cells in vivo would help clarify the complexity of differential actions in the two cell types. Thus it seems probable that nicotine actions on other neuron types also contribute to the appetite-suppressant actions of nicotine.
We show here that nicotine excites hypocretin cells, but this is unlikely to explain the nicotine-mediated reduced food intake, since hypocretin cells have been suggested to be orexigenic. On the other hand, the nicotine activation of the hypocretin cell could serve as a partial explanation for the increased cognitive arousal associated with nicotine. Another cell type that may play a role in food intake is the orexigenic hypothalamic melanin concentrating hormone (MCH) neuron. Nicotine increases the inhibitory synaptic activity to MCH cells (Jo et al. 2005), which would be consistent with reduced food intake.
A number of papers have reported opposing actions of several signaling molecules relevant to energy homeostasis in POMC and NPY cells. For instance, POMC cells are excited by leptin and serotonin, whereas NPY cells are inhibited by both (Coll et al. 2007; Cowley et al. 2001; Heisler et al. 2002, 2006). In addition, POMC cells are inhibited by ghrelin, whereas NPY neurons are excited (Cowley et al. 2003). In contrast, both cell types show somewhat similar responses to many other signaling molecules, including glutamate, GABA, bombesin, neuromedin B, gastrin-releasing peptide, NPY, PYY3–36, and hypocretin (Acuna-Goycolea and van den Pol 2009; Roseberry et al. 2004; van den Pol et al. 2009; van den Top et al. 2004). One possibility that could still explain a differential action of a neuromodulator is that the pattern or strength of innervation may differ between the two cell types. Alternately, the membrane properties of the two cells, including RMP, input resistance, and plasma membrane nicotine receptor density, will also influence the amplitude of nicotine actions in vivo. But the question remains as to whether there may be some physiological conditions in which similar excitatory or inhibitory actions of a single compound on both cell types may provide a functional benefit.
Although most likely only part of the critical circuit by which nicotine affects feeding, the roles of POMC and NPY in food intake and energy metabolism suggest that nicotine effects on these neurons may be one potential site of action for selective pharmacological intervention to prevent the increase of body weight after smoking cessation. Future studies examining the physiological response of POMC, NPY, and hypocretin cells to chronic nicotine exposure will be of interest.
The question remains as to the role of nicotine receptors in the normal functioning of the cells studied here. As we find axons expressing the vesicular ACh transporter, a marker for cholinergic axons, abutting both POMC and NPY cells, ACh may be released directly onto these two cells or nearby axons. When we added a broad-spectrum nicotinic antagonist to the POMC or NPY cells, we found no obvious change in baseline membrane current. This could suggest that synaptically released ACh does not exert a substantive effect on these cells; however, there are alternate interpretations: Most cholinergic neuron cell bodies were probably eliminated from the slices we used, and continuity of cell body to axon would be required for normal functioning of cholinergic input. Additionally, nicotine receptors on axons presynaptic to POMC or NPY cells may be key to the role of endogenous ACh, consistent with the view that ACh functions as a presynaptic neuromodulator more often than as a primary fast transmitter in other regions of the brain (Dani and Bertrand 2007; Lambe et al. 2005). That all three neuron types examined, POMC, NPY, and hypocretin, show responses to nicotine at the cell body suggests that the axon terminals of these same cells may also respond to nicotine, and the presynaptic responses of those axons may be critical for the endogenous action of axonally released ACh.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-41454 and NS-48476.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Y. Yang, V. Rogulin, and J. N. Davis for technical assistance.
REFERENCES
- Acuna-Goycolea C, van den Pol AN. Peptide YY(3–36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis. J Neurosci 25: 10510–10519, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna-Goycolea C, van den Pol AN. Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin. J Neurosci 29: 1503–1515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51: 107–116, 1994 [DOI] [PubMed] [Google Scholar]
- Boutrel B. A neuropeptide-centric view of psychostimulant addiction. Br J Pharmacol 154: 343–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto LR, Keyser KT, Lindstrom JM, Karten HJ. Immunohistochemical localization of nicotinic acetylcholine receptor subunits in the mesencephalon and diencephalon of the chick (Gallus gallus). J Comp Neurol 317: 325–340, 1992 [DOI] [PubMed] [Google Scholar]
- Buhler AV, Dunwiddie TV. Alpha7 nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J Neurophysiol 87: 548–557, 2002 [DOI] [PubMed] [Google Scholar]
- Carney RM, Goldberg AP. Weight gain after cessation of cigarette smoking. A possible role for adipose-tissue lipoprotein lipase. N Engl J Med 310: 614–616, 1984 [DOI] [PubMed] [Google Scholar]
- Ceranik K, Bender R, Geiger JR, Monyer H, Jonas P, Frotscher M, Lübke J. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J Neurosci 17: 5380–5394, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell 129: 251–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37: 649–661, 2003 [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47: 699–729, 2007 [DOI] [PubMed] [Google Scholar]
- Davila-Garcia MI, Houghtling RA, Qasba SS, Kellar KJ. Nicotinic receptor binding sites in rat primary neuronal cells in culture: characterization and their regulation by chronic nicotine. Brain Res Mol Brain Res 66: 14–23, 1999 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: two hypothalamic peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci 16: 2279–2290, 2002 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221–232, 1999 [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 31: 336–48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara CM, Kumar M, Nicklas B, McCrone S, Goldberg AP. Weight gain and adipose tissue metabolism after smoking cessation in women. Int J Obes Relat Metab Disord 25: 1322–1326, 2001 [DOI] [PubMed] [Google Scholar]
- Fisher JL, Dani JA. Nicotinic receptors on hippocampal cultures can increase synaptic glutamate currents while decreasing the NMDA-receptor component. Neuropharmacology 39: 2756–2769, 2000 [DOI] [PubMed] [Google Scholar]
- Frankish HM, Dryden S, Wang Q, Bing C, MacFarlane IA, Williams G. Nicotine administration reduces neuropeptide Y and neuropeptide Y mRNA concentrations in the at hypothalamus: NPY may mediate nicotine's effects on energy balance. Brain Res 694: 139–146, 1995 [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci 18: 1187–1195, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci 30: 13814–13825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol 533: 237–252, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23: 3106–3111, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci 22: 5442–5451, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE. Nicotine as a psychoactive drug: appetite regulation. Psychopharmacol Bull 22: 875–881, 1986 [PubMed] [Google Scholar]
- Grunberg NE, Bowen DJ, Winders SE. Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology (Berl) 90: 101–105, 1986 [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911–10916, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfstrand A, Adem A, Fuxe K, Agnati L, Andersson K, Nordberg A. Distribution of nicotinic cholinergic receptors in the rat tel- and diencephalon: a quantitative receptor autoradiographical study using [3H]-acetylcholine, [alpha-125I]bungarotoxin and [3H]nicotine. Acta Physiol Scand 132: 1–14, 1988 [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559, 2005 [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet 366: 1197–1209, 2005 [DOI] [PubMed] [Google Scholar]
- Hatton GI, Yang QZ. Synaptic potentials mediated by alpha7 nicotinic acetylcholine receptors in supraoptic nucleus. J Neurosci 22: 29–37, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science 297: 609–611, 2002 [DOI] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51: 239–249, 2006 [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend 33: 23–29, 1993 [DOI] [PubMed] [Google Scholar]
- Hofstetter A, Schutz Y, Jequier E, Wahren J. Increased 24-hour energy expenditure in cigarette smokers. N Engl J Med 314: 79–82, 1986 [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA 105: 19480–19485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) containing neurons and arcuate nucleus NPY-producing cells in rodent and primate—a new hypothalamic circuit implicated in energy homeostasis. J Neurosci 19: 1072–1087, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Leonard CS, Kohlmeier KA. Nicotinic activation of laterodorsal tegmental neurons: implications for addiction to nicotine. Neuropsychopharmacology 34: 2529–2547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Kim KH, Cho SY, Bahn GH, Kim EH, Kim CJ. Nicotine administration decreases neuropeptide Y expression and increases leptin receptor expression in the hypothalamus of food-deprived rats. Brain Res 964: 311–315, 2003 [DOI] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol 53: 618–632, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Wiedl D, Role LW. Cholinergic modulation of appetite-related synapses in mouse lateral hypothalamic slice. J Neurosci 25: 11133–11144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron 15: 1231–1244, 1995 [DOI] [PubMed] [Google Scholar]
- Kawa K. Acute synaptic modulation by nicotinic agonists in developing cerebellar Purkinje cells of the rat. J Physiol 538: 87–102, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci 25: 5225–5229, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RB, Reyes AD, Aoki C. Nicotinic and muscarinic reduction of unitary excitatory postsynaptic potentials in sensory cortex: dual intracellular recording in vitro. J Neurophysiol 95: 2155–2166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res 867: 157–164, 2000a [DOI] [PubMed] [Google Scholar]
- Li MD, Parker SL, Kane JK. Regulation of feeding-associated peptides and receptors by nicotine. Mol Neurobiol 22: 143–165, 2000b [DOI] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron—a potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36: 1169–1181, 2002 [DOI] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol 536: 89–100, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J Physiol 559: 863–874, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Iglesias C, Donnet M, Leroy F, Heckman CJ, Zytnicki D. Fast kinetics, high-frequency oscillations, and subprimary firing range in adult mouse spinal motoneurons. J Neurosci 29: 11246–11256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57: 521–546, 1995 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31: 580–586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav 74: 169–176, 2001 [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 291: 1238–1245, 2004 [DOI] [PubMed] [Google Scholar]
- Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circ Res 83: 1241–1247, 1998 [DOI] [PubMed] [Google Scholar]
- O'Hara BF, Edgar DM, Cao VH, Wiler SW, Heller HC, Kilduff TS, Miller JD. Nicotine and nicotinic receptors in the circadian system. Psychoneuroendocrinology 23: 161–173, 1998 [DOI] [PubMed] [Google Scholar]
- Okuda H, Shioda S, Nakai Y, Nakayama H, Okamoto M, Nakashima T. Immunocytochemical localization of nicotinic acetylcholine receptor in rat hypothalamus. Brain Res 625: 145–151, 1993 [DOI] [PubMed] [Google Scholar]
- Pabreza LA, Dhawan S, Kellar KJ. [3H]cytisine binding to nicotinic cholinergic receptors in brain. Mol Pharmacol 39: 9–12, 1991 [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol 61: 75–111, 2000 [DOI] [PubMed] [Google Scholar]
- Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 138: 24–32, 2003 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology 22: 451–465, 2000 [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martín-García E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine, and induce reinstatement of nicotine-seeking behavior. J Neurosci 30: 2300–2310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J Neurosci 9: 5228–5235, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457–478, 2002 [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 189: 395–401, 2006 [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 41: 711–722, 2004 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998 [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199–211, 2002 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci 4: 901–909, 2003 [DOI] [PubMed] [Google Scholar]
- Shioda S, Nakajo S, Hirabayashi T, Nakayama H, Nakaya K, Matsuda K, Nakai Y. Neuronal nicotinic acetylcholine receptor in the hypothalamus: morphological diversity and neuroendocrine regulations. Brain Res Mol Brain Res 49: 45–54, 1997 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 104: 531–543, 2001 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci 19: 6497–6505, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda D, Nakatsuka T, Gu JG, Yoshida M. The activation of nicotinic acetylcholine receptors enhances the inhibitory synaptic transmission in the deep dorsal horn neurons of the adult rat spinal cord. Mol Pain 3: 26, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci 29: 4622–4639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7: 493–494, 2004 [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC, Vander Weg MW. Cessation of smoking and body weight. In: International Textbook of Obesity, edited by Bj̈orntop P. Chichester, UK: Wiley, 2001, p. 323–336 [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med 324: 739–745, 1991 [DOI] [PubMed] [Google Scholar]
- Wisse BE, Schwartz MW. Role of melanocortins in control of obesity. Lancet 358: 857–859, 2001 [DOI] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci 18: 1155–1168, 2003 [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Chiappinelli VA. Nicotine modulates evoked GABAergic transmission in the brain. J Neurophysiol 82: 3041–3045, 1999 [DOI] [PubMed] [Google Scholar]