Abstract
Study Objectives:
Sleeping 7 to 8 hours per night appears to be optimal, since both shorter and longer sleep times are related to increased morbidity and mortality. Depressive disorder is almost invariably accompanied by disturbed sleep, leading to decreased sleep duration, and disturbed sleep may be a precipitating factor in the initiation of depressive illness. Here, we examined whether, in healthy individuals, sleep duration is associated with genes that we earlier found to be associated with depressive disorder.
Design:
Population-based molecular genetic study.
Setting:
Regression analysis of 23 risk variants for depressive disorder from 12 genes to sleep duration in healthy individuals.
Participants:
Three thousand, one hundred, forty-seven individuals (25–75 y) from population-based Health 2000 and FINRISK 2007 samples.
Measurements and Results:
We found a significant association of rs687577 from GRIA3 on the X-chromosome with sleep duration in women (permutation-based corrected empirical P = 0.00001, β = 0.27; Bonferroni corrected P = 0.0052; f = 0.11). The frequency of C/C genotype previously found to increase risk for depression in women was highest among those who slept for 8 hours or less in all age groups younger than 70 years. Its frequency decreased with the lengthening of sleep duration, and those who slept for 9 to 10 hours showed a higher frequency of C/A or A/A genotypes, when compared with the midrange sleepers (7-8 hours) (permutation-based corrected empirical P = 0.0003, OR = 1.81).
Conclusions:
The GRIA3 polymorphism that was previously found to be associated with depressive disorder in women showed an association with sleep duration in healthy women. Mood disorders and short sleep may share a common genetic background and biologic mechanisms that involve glutamatergic neurotransmission.
Citation:
Utge S; Kronholm E; Partonen T; Soronen P; Ollila HM; Loukola A; Perola M; Salomaa V; Porkka-Heiskanen T; Paunio T. Shared genetic background for regulation of mood and sleep: association of GRIA3 with sleep duration in healthy Finnish women. SLEEP 2011;34(10):1309-1316.
Keywords: Sleep duration, short sleep, long sleep, depressive disorder, GRIA3, glutamatergic
INTRODUCTION
Sleep is an important contributor to our general health and well-being. Sleeping from 7 to 8 hours per night is considered optimal for health, since, in cross-sectional studies, both shorter and longer sleep have been related to poor health outcomes, including obesity, heart disease, neuroticism, anxiety, and death,1–7 as well as, in prospective studies, to increased morbidity and mortality.8–10
Both short and long sleep are common; according to a cross-sectional population-based study conducted in the United States, 28.3% of the respondents reported having a sleep duration of 6 hours or less, whereas 8.5% reported sleep durations of 9 hours or more.11 The prevalence of short sleepers (≤ 6 hours) in the Finnish adult population has been estimated to be 14.5% (16.7% of men and 12.5% of women) and that of long sleepers (≥ 9 hours) to be 13.5% (10.5% of men and 16.1% of women).12 The proportion of both long and short sleepers appears to be relatively stable, even when the average sleep duration at the population level decreases.13 However, these epidemiologic studies have not revealed what proportion of the short sleepers are natural short sleepers and which suffer have sleep restriction.
Animal studies have shown that sleep duration is at least partially under genetic control.14 The Shaker mutation in Drosophila melanogaster reduces the daily 9- to 15-hour sleep duration to 4 to 5 hours,15 and a mutation in a transcriptional factor, basic helix-loop-helix family, a member of e41, BHLHE41 (DEC2) gene, has been associated with short sleep in both humans and mice.16 Sleep can be characterized by a range of attributes, such as sleep length, sleep intensity (slow wave sleep activity or electroencephalographic [EEG] spectral power), latency to sleep onset, early morning awakenings, and sleep quality. Many of these features have a strong genetic component; for example, the heritability of EEG power spectrum in non-rapid eye movement (NREM) sleep has been found to be as high as 96%.17 According to twin studies in Finland and Australia, even self-reported sleep duration has a relatively high heritability estimate: 44% and 33%, respectively.18,19
Depressive disorder is almost invariably accompanied by disturbed sleep, typically with early morning awakenings, leading to decrease in sleep duration,20 but, in some forms of depression (e.g., seasonal affective disorder), sleep duration can be increased.21 In bipolar depression, a decrease in sleep duration can lead to a switch into mania.22 Depressive disorder has also been linked with several genes and their variants, e.g., serotonin transporter.23 However, these findings have been inconsistent and have not been replicated in other populations.24 It is possible that, even with a genetic vulnerability for depressive disorder, one needs a triggering factor for the onset of the disease itself.
Our recent findings suggest that insufficient or disturbed sleep may be one of such triggering factors. To clarify whether disturbed sleep precedes depressed mood, we studied a nationwide cohort of same-sex Finnish twins at 5-year intervals. Those with sleep complaints at an earlier study point had an increased risk for developing life dissatisfaction, which is a surrogate for depressed mood, at a later time point, whereas life dissatisfaction at an earlier study point did not increase the risk for developing sleep complaints at the later study point. Furthermore, the risk of life dissatisfaction was significantly increased among those who slept less than 7 hours per night.25 We observed that variants of a number of genes regulating serotonergic, glutamatergic, neural plasticity, the hypothalamic-pituitary-adrenal (HPA) axis, and circadian systems were associated with mood disorders with sleep disturbances, whereas those who slept normally did not show associations to the same extent.26,27 The associated genes included tryptophan hydroxylase 2 (TPH2); glutamate decarboxylase 1 (GAD1); glutamate receptor, ionotrophic, AMPA 3 (GRIA3); brain-derived neurotrophic factor (BDNF); corticotropin releasing hormone receptor 1 (CRHR1); and timeless homolog (Drosophila) (TIMELESS). This finding could be explained by the hypothesis that disturbed sleep, by leading to sleep loss, is a precipitating factor in the initiation of depression in those individuals who also have a genetic vulnerability to depression. Another possibility is that the same genetic factors that regulate mood also regulate sleep duration and quality; in other words, the genetic variants that associate with depression also associate with features of sleep.
In the present study, we asked whether sleep duration in healthy individuals associates with genes that we have previously found to be associated with depressive disorder. We tested this hypothesis in a population-based sample from Finland from which we selected all healthy individuals with information on their sleep duration. Our data demonstrated that one of the candidate genes, GRIA3, is strongly associated with sleep duration in healthy women, raising the possibility that some of the genetic mechanisms underlying the regulation of sleep duration contribute to depressive disorder.
MATERIALS AND METHODS
Study Samples
The participants were recruited from the population-based national health interview and examination survey Health 2000 (http://www.terveys2000.fi/indexe.html) and FINRISK study 2007 survey (http://www.ktl.fi/portal/4168) carried out in Finland. The health status of individuals was assessed with a health examination monitored by physicians and trained nurses at a local health care center.
Health 2000
The health status of individuals was evaluated by the research version of the Composite International Diagnostic Interview using the DSM-IV criteria for psychiatric disorders.28 All the included individuals had answered the sleep-duration questionnaire. Individuals with no depression and no complaint of disturbed sleep, comprising the sample of 1135 healthy sleepers (610 women and 525 men) were selected for the study (Healthy-sleeper sample). In addition, 1357 individuals (690 women and 667 men) from the complete Health 2000 samples, originally selected for a case-control study on the metabolic syndrome, were included after exclusion of 141 cases with depressive disorder (Genmets (D-) sample). Within that sample, 285 women and 271 men had metabolic syndrome. The criteria for metabolic syndrome were defined as follows: the waist circumference had to be at least 94 cm in men and 80 cm in women. In addition, the subjects had to fulfill two of the following four criteria: (1) blood triglyceride levels of at least 1.7 mmol/l, (2) blood high-density lipoprotein cholesterol level in men less than 1.03 mmol/L or in women less than 1.29 mmol/L, (3) systolic blood pressure at least 130 mm Hg or diastolic blood pressure at least 85 mm Hg or medication for treating blood pressure, and (4) glucose concentration at least 5.6 mmol/L. Therefore, metabolic syndrome (n = 556) was controlled as a covariate in the analyses.
FINRISK 2007
The sample was collected from the Helsinki-Vantaa region as part of the Dietary, Lifestyle, and Genetic determinants of Obesity and Metabolic syndrome (DILGOM) study, an extension of the FINRISK 2007 study. A total of 655 healthy individuals (326 women and 329 men) with no cardiovascular disease were included in the study (FINRISK 2007 sample). All of them had answered the sleep-duration questionnaire.
These participants from the two population-based cohorts were also characterized for symptoms of depressed mood as defined by a quantitative sum score of Beck's Depression Inventory. The Beck Depression Inventory comprises 21 questions, of which we had 13 available in our data set, allowing us to assess the main depression component of the Beck Depression Inventory. The questions available were: feeling sad, hopelessness about the future, feelings about failing in life, dissatisfaction with life, disappointment in oneself, self criticism and uselessness, suicidal thoughts, lack of interest in other people, problems in decision making, disappointment in one's own appearance, professional capability, tiredness, and appetite. The study individuals were also characterized for symptoms of insomnia, which was evaluated in both cohorts with the question “Do you suffer from insomnia?”; answers ranging from often, sometimes, and never were used as variables in both cohorts. In the secondary analyses, symptoms of a depressed mood (n = 2691) as well as symptoms of insomnia (n = 1689) were used as covariates.
Finally, the complete study sample comprised 3147 healthy subjects: 1626 women (mean age 50 y) and 1521 men (mean age 49 y) (Table 1).
Table 1.
Distribution of sleep duration groups across study samples by sex (n = 3147).
| Study Samples | Women |
Men |
||||||
|---|---|---|---|---|---|---|---|---|
| All | Short sleepers | Midrange sleepers | Long sleepers | All | Short sleepers | Midrange sleepers | Long sleepers | |
| Healthy Sleepers | 610 (100%) | 35 (6%) | 502 (83%) | 73 (11%) | 525 (100%) | 58 (11%) | 431 (82%) | 36 (7%) |
| Genmets (D-) | 690 (100%) | 114 (17%) | 484 (70%) | 92 (13%) | 667 (100%) | 130 (20%) | 486 (73%) | 51 (8%) |
| FINRISK 2007 | 326 (100%) | 46 (14%) | 234 (72%) | 46 (14%) | 329 (100%) | 46 (14%) | 239 (73%) | 44 (13%) |
| All | 1626 | 195 | 1220 | 211 | 1521 | 234 | 1156 | 131 |
| Age, Mean ± SD | 51 ± 13 | 54 ± 15 | 50 ± 13 | 47 ± 16 | 49 ± 13 | 49 ± 11 | 48 ± 13 | 55 ± 14 |
Short sleepers, ≤ 6 hours sleep; midrange sleepers, 7-8 hours sleep; long sleepers, ≥ 9 hours sleep.
Sleep Duration
Sleep duration was assessed with the question “How many hours do you sleep on average during 24 hours?” It was applied in statistical analyses as quantitative sleep duration. We also classified the subjects into those who sleep 6 hours or less (short sleepers), those who sleep 7 to 8 hours (midrange sleepers), and those who sleep 9 hours or more (long sleepers). Altogether, 195 women and 234 men were short sleepers, 1220 women and 1156 men were midrange sleepers, and 211 women and 131 men were long sleepers (Table 1). The particulars of the self-reported sleep-duration measurement in the Health 2000 and FINRISK study surveys have been previously described.12,13
The study was approved by the Institutional Review Board of Helsinki and Uusimaa Hospital District. All the participants provided written informed consent for the collection of samples and subsequent analyses.
Selection of SNPs and Genotyping
The genes that in our earlier studies had associated significantly (P < 0.05) with depressive disorder and disturbed sleep were selected.26,27 We examined 23 single nucleotide polymorphisms (SNPs) (Supplementary Table S1) from 12 genes; TPH2 (rs12229394), solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 (SLC6A4) (rs4251417), GRIA3 (rs687577, rs526716), disrupted in schizophrenia 1 (DISC1) (rs3738401), BDNF (rs6265, rs1491850), CRHR1 (rs173365), neuronal PAS domain protein 2 (NPAS2) (rs12712083), nuclear factor, interleukin 3 regulated (NFIL3) (rs1619450), aryl hydrocarbon receptor nuclear translocator-like (ARNTL) (rs6486121, rs3816358, rs969485), aryl hydrocarbon receptor nuclear translocator-like 2 (ARNTL2) (rs4964060, rs7304939, rs1037921, rs2289709), TIMELESS (rs2291738, rs1082214), and RAR-related orphan receptor A (RORA) (rs4774370, rs8027829, rs1568717, rs4774388).
After the single SNP analyses had shown a strong association between sleep duration and GRIA3, we selected 54 variants of this gene (Supplementary Table S2), genotyped within Genmets (D-) and FINRISK 2007 samples, for a post-hoc linkage disequilibrium block and haplotype analysis.
Genotyping of the sample of the healthy sleepers was performed using MassARRAY technology (Sequenom, Inc., San Diego, CA), as has been previously described.26,27 The Genmets (D-) and FINRISK 2007 samples were genotyped with the Illumina 610 K platform (Illumina, Inc., San Diego, CA), with a greater than 95% call rate cutoff for both individuals and markers. The markers with Hardy-Weinberg equilibrium P > 1×10-6 had been included in the analyses.
Statistical Analysis
We tested the association of the selected SNPs with the self-reported habitual sleep duration in men and women separately by using three different models. First, a linear-regression model was used to analyze sleep duration. Second, the groups with short sleep duration (≤ 6 hours) versus midrange sleep duration (7-8 hours) and, third, long sleep duration (≥ 9 hours) versus midrange sleep duration (7-8 hours) were analyzed using a logistic-regression model. We included age and metabolic syndrome status as covariates in all of these primary analyses. Association P values were corrected by simulating the data set 10,000 times, and P values were adjusted to the number of models (three models in both sexes, altogether six models) by the Bonferroni correction. Association was considered significant when both the permutation-based corrected empirical P values and Bonferroni corrected P values were P < 0.05. We implemented all these analyses in the PLINK software package, web-based version 1.06.29
Subsequently, we performed descriptive analyses of variant rs687577 from GRIA3 that gave evidence for a significant association in the primary analyses. Using a linear regression model we analyzed separately those females who slept for less than 10 hours, and using a logistic regression model we compared those who slept for 9-10 hours to midrange sleepers (7-8 hours). We also performed secondary analyses in the complete study sample including symptoms for depressed mood and insomnia as covariates and analyzed sleep duration using a linear regression model.
We performed post-hoc haplotype based association tests using the sliding window approach as implemented in the PLINK (V.1.06),29 and determined the LD structure of GRIA3 by using the Haploview program (V.4.1).30 For analysis of the transcription factor binding sites we used the tool ConSite, a platform-independent web resource.31 We first retrieved the corresponding transcript region of humans and mice by using a genome browser EnsEMBL (www.ensembl.org) and then examined the transcription factor binding sites shared by this gene as described in Utge et al.27
RESULTS
Single SNP Analysis
Out of the 23 SNPs, seven showed suggestive associations (pointwise P < 0.05) either with sleep duration or with short or long sleep (Table 2, whole data presented in Supplementary Table S1). Association with only one variant, rs687577 from GRIA3, survived correction for multiple testing in females. We found the strongest evidence for association with sleep duration (complete study sample: pointwise P = 0.00001, permutation-based corrected empirical P = 0.00001, β = 0.27; Bonferroni corrected P = 0.0052, f = 0.11), and with long sleep in females (complete study sample: pointwise P = 0.00001, permutation-based corrected empirical P = 0.0003, OR = 1.89; Bonferroni corrected P = 0.039, f = 0.18). The distribution of the rs687577 minor allele and genotype frequencies in the whole sample, in short sleepers, midrange sleepers, and long sleepers is provided in Supplementary Table S3.
Table 2.
Single marker association analyses between sleep duration, short and long sleepers and allelic replication with depressive disorder
| Chr | Gene | SNP | A1/A2 | Gender | Sleep duration (linear model) |
Short sleep (logistic model midrange sleepers as reference) |
Long sleep (logistic model midrange sleepers as reference) |
†Association with depression – disturbed sleep |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF | β (95% CI) | P | MAF | OR (95% CI) | P | MAF | OR (95% CI) | P | Group (OR) | Gender | |||||
| 15 | RORA | rs4774388 | A/G | Male | 0.21 | -0.08 (-0.18-0.02) | 0.123 | 0.19 | 1.42 (1.08-1.86) | 0.010 | 0.24 | 0.94 (0.63-1.38) | 0.89 | D+FAT+ (0.61) | Female |
| 12 | TPH2 | rs12229394 | G/A | Male | 0.07 | 0.01 (-0.06-0.09) | 0.697 | 0.08 | 1.28 (1.03-1.59) | 0.024 | 0.10 | 1.33 (1.00-1.76) | 0.041 | D+ (1.28) D+EMA+ (1.40) | Female |
| 12 | TIMELESS | rs2291738 | G/A | Male | 0.11 | -0.03 (-0.10-0.03) | 0.339 | 0.11 | 1.22 (1.00-1.50) | 0.048 | 0.08 | 1.05 (0.80-1.36) | 0.706 | D+FAT+ (1.52) | Female |
| 12 | TIMELESS | rs1082214 | C/T | Male | 0.48 | 0.08 (-0.05-0.21) | 0.238 | 0.47 | 1.23 (0.85-1.78) | 0.266 | 0.48 | 1.61 (1.04-2.50) | 0.026 | D+EMA+ (2.70) D+FAT+ (1.72) | Male |
| X | GRIA3 | rs687577 | C/A | Female | 0.11 | 0.27 (0.16-0.38) | 0.00001* | 0.08 | 0.84 (0.57-1.23) | 0.382 | 0.18 | 1.89 (1.42-2.52) | 0.00001** | D+ (0.70) | Female |
| 17 | SLC6A4 | rs4251417 | G/A | Female | 0.07 | 0.15 (0.01-0.28) | 0.027 | 0.05 | 0.74 (0.46-1.19) | 0.214 | 0.09 | 1.33 (0.92-1.92) | 0.114 | D+ (1.49) D+FAT+ (1.46) | Female |
| 15 | RORA | rs4774370 | T/C | Female | 0.30 | 0.05 (-0.03-0.14) | 0.211 | 0.31 | 0.72 (0.53-0.97) | 0.037 | 0.29 | 1.06 (082-1.38) | 0.616 | D+ (0.75) | Female |
Chr, Chromosome; A1/A2, Major allele/Minor allele; MAF, Minor allele frequency; β, Regression coefficient; OR, Odds ratio. 95% CI, Lower and upper bound confidence interval for β or odds ratio. SNPs (single nucleotide polymorphisms) which showed suggestive association from quantitative and dichotomous analysis (P < 0.05) are marked with bold type.
Permutation-based corrected empirical P = 0.00001, Bonferroni P = 0.000038, P-values adjusted to the number of models (three models in both genders, altogether six models) (Bonferroni corrected P = 0.0052) for association with sleep duration in females.
Permutation-based corrected empirical P = 0.0003, Bonferroni P = 0.00028, P-values adjusted to the number of models (three models in both genders, altogether six models) (Bonferroni corrected P = 0.039) for association with long sleep in females.
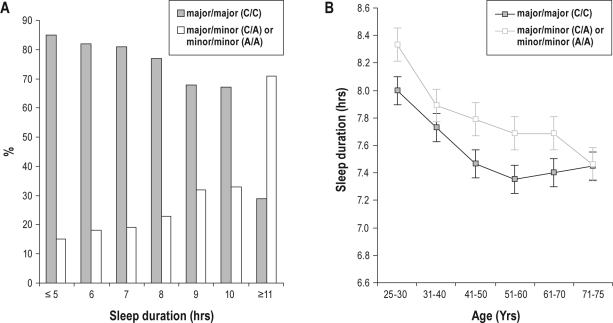
The frequency of rs687577 genotype major/major (C/C) was highest among those women who slept ≤ 8 hours (Figure 1A) in all age groups except those over 70 years of age (Figure 1B). The complete data with the number of subjects in each group of Figure 1A and Figure 1B are given in Supplementary Table S4. Figure 1A also shows that the proportion of C/C genotypes decreases by lengthening sleep duration. Although the relative decrease was found to be most striking among those sleeping ≥ 11 hours, the association with sleep duration remained significant also after excluding the extreme long sleepers (permutation-based corrected empirical P = 0.0002, β = 0.21). Females who slept for 9-10 hours, as compared to midrange sleepers (7-8 hours), showed a higher proportion of C/A or A/A genotypes (permutation-based corrected empirical P = 0.0003, OR = 1.81).
Figure 1.
(A) Self-reported sleep duration in women with respect to their genotype at rs687577 from GRIA3. (B) Self-reported sleep duration with respect to genotype at rs687577 from GRIA3 in different age groups of women. The complete data for the number of subjects in each group are given in Table S4.
The association of rs687577 with sleep duration was robust, and it emerged in females in all three subsamples (“Healthy sleepers” sample: permutation-based corrected empirical P = 0.002 and β = 0.24, “Genmets (D-)” sample: permutation-based corrected empirical P = 0.004 and β = 0.30, and “FINRISK 2007” sample: permutation-based corrected empirical P = 0.04 and β = 0.31). The results also remained significant when models were adjusted for symptoms of depressed mood (complete study sample: permutation-based corrected empirical P = 0.0001, β = 0.28), and symptoms of insomnia (complete study sample: permutation-based corrected empirical P = 0.0001, β = 0.28).
Haplotype Analysis of GRIA3
We then proceeded to demarcation of the region in GRIA3 behind the association signal in single SNP analysis by defining the LD structure of the gene and by haplotype analysis. With the aid of all available (N = 54) genotyped variants of GRIA3 in the “Genmets (D-)” and “FINRISK 2007” samples, we identified 11 haploblocks by using the algorithm solid spine.30 RS687577 was located on haploblock 9 that ranged from intron 11 to 12 (Supplementary Figure S1, whole haploblocks presented in Supplementary Figure S2) Haplotype analysis utilizing rs3848874 and rs687577 (pairwise LD: D' = 0.84, r2 = 0.018) in block 9 revealed an equivalent association signal to sleep duration as compared with that obtained with rs687577 alone in females from the “Healthy sleepers” subsample of the Health 2000 cohort (G-A of rs3848874 and rs687577, P = 0.0011, β = 0.24). Addition of rs526716 from block 8 (Supplementary Figure S1) dismantled the signal between two allelic haplotypes that differed at rs526716 (A-G-A or G-G-A of rs526716-rs3848874-rs687577, P = 0.030, β = 0.31 and P = 0.007, β = 0.25, respectively). Similarly, haplotype analysis of the “Genmets (D-)” and “FINRISK 2007” samples demarcated the association signal to haploblock 9 (for example, A-A of rs10521721-rs687577, P = 0.00004, β = 0.31) while adding variants from adjacent blocks (A-A-A or G-A-A of rs526716-rs10521721-rs687577, P = 0.042 and P = 0.0001; β = 0.28 and β = 0.39, respectively) diluted or dismantled the signal between different allelic haplotypes. Thus, the association signal initially observed by rs687577 in single SNP analysis to sleep duration in females apparently reflects allelic diversity on haploblock 9 ranging from intron 11 to intron 12 of GRIA3 (Supplementary Figure S1).
DISCUSSION
In the present study we assessed the shared genetic background for regulation of mood and sleep. Out of variants from the 12 candidate genes that had been identified in our previous studies,26,27 GRIA3, encoding for inotorphic glutamatergic receptor, associated strongly with sleep duration in healthy females. This finding was statistically robust and consistent in all three subpopulations included in the study. The major allele C of GRIA3 variant rs687577, previously associated with depression in females 26, associated here for short sleep and minor allele A robustly associated in the present study with long sleep in healthy females (Table 2). The distribution of rs687577 genotypes also differed significantly in relation to sleep duration in women. We observed a systematic decrease in the proportion of C/C genotype carriers according to lengthening in sleep duration per each hour. Thus, females with C/A or A/A genotypes were more likely to have longer sleep duration. The difference in average sleep duration between women with C/C genotype as compared to those with C/A or A/A was present in all age groups below 70 years. The absence of correlation in the oldest age group is likely to reflect the age-related changes in sleep32 and the effect of medical comorbidities on sleep duration and quality.33
According to epidemiological studies, poor quality of sleep and insomnia are predictive for depression.34 To further elucidate the mechanism of GRIA3 in the interplay of regulation of mood, insomnia, and sleep duration, we adjusted the analyses on sleep duration with reported symptoms of depressive mood and insomnia. The effect of rs687577 on sleep duration was maintained even when symptoms of depressive mood and insomnia were taken into account. These findings suggest that the association of an allelic variant of GRIA3 is specific to sleep duration.
Glutamate is the main excitatory neurotransmitter in the central nervous system. Approximately 70% of the synapses in the mammalian brain contain N-methyl-d-aspartate (NMDA) or α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors,35 which are also potential therapeutic targets for medication of major depressive disorder.36 GRIA3 (GluR3) is one of the four AMPA receptor subunits, and it is expressed e.g., in the reticular nucleus of the thalamus and the cerebral cortex,37,38 which are important areas in the regulation of sleep and wakefulness.39 In a genome-wide scan aiming to identify the natural gene variants that contribute to quantitative traits, Gria3 was found to affect aggressive behavior in inbred mouse strains.40 Recently, its functional role has been addressed by using GluR3-knockout mice, which demonstrate reduced motor coordination and reduced exploratory behavior but increased mobility in the forced swimming test, as well as increased seizure activity.41 The EEG recordings evidenced decreased power in the lower frequency ranges (0.5-4 Hz) particularly during NREM sleep, suggesting that the GluR3 subunit may play a role in the generation of cortical slow oscillations.41 On the other hand, the mRNA level of GluR3 in the cortex was elevated during sleep deprivation and declined during sleep.42 Activation of NMDA and AMPA receptors using their respective agonists induce robust waking,43,44 whereas the infusion of the antagonists has the opposite effect.44 Thus the role of the glutamatergic system in the brain is to induce or maintain wakefulness.This is the first study showing that human genetic variation of GRIA3 gene is associated with the regulation of sleep. GRIA3 has been previously associated with schizophrenia,45 bipolar disorder,46 mental retardation,46,47 migraine,48 sexual dysfunction during major depressive disorder,49 and to suicidal ideation emerging during citalopram treatment of major depression.50,51 However, all these associations locate towards the 5'end of the gene, whereas haplotype analysis in the present study demarcated the associating region of sleep duration to a haploblock ranging from intron 11 to intron 12, towards the 3' end of the gene (Supplementary Figure S1). In a previous study we identified suggestive association of variants from the same haploblock of GRIA3, the minor allele A from rs3848874 and the major allele C of rs687577 as putative susceptibility factors for depression with sleep disturbance while, haplotype G-A of rs3848874 and rs687577 appeared as protective for depression.26 Here, in coherence with findings from the single SNP analysis, we found an association between the haplotype G-A to longer sleep duration. Interestingly, we did not observe any association between variants from GRIA3 gene, located on X-chromosome, with sleep duration in males. Similarly, in our previous work on depression27 and in accordance with findings from twin studies,52 a consistent difference between the genetic liability factors for depression in the two genders has been observed.
These sex differences can be explained by several molecular and neurobiological mechanisms. Gender-related differences in norepinephrine neurons at locus coeruleus may render women more vulnerable to developing stress-related pathologies.53 Various neurotransmitter systems interact with estrogen and can induce sex-specific changes in mood and sleep.54 Estrogen also regulates expression of corticotrophin-releasing factor, a primary mediator of the stress response.55
Interestingly, at the promoter region of GRIA3, there is a binding site for GATA-3 (GATA binding protein 3) (Supplementary Figure S1), a transcription factor that plays an important role in normal cellular development56 and contains one estrogen receptor (ERα) binding site near the 3'end.57 In addition, gender influences methylation of genes on X-chromosome and autosomes, and sex-specific differences in GRIA3 methylation have been detected.58 Previous studies have also identified a methylation sensitive site (HpaII-site) at the promoter region of GRIA3 that has been found to be involved in X-chromosome inactivation in a female patient with bipolar disorder and mental retardation,46 and in a male patient with mental retardation.47 It remains to be clarified whether these sites are involved in the mechanisms of gender-specific regulation of sleep via the GRIA3 haplotype revealed in the present study.
According to both epidemiologic and experimental studies, short sleep is associated with several unfavorable health outcomes, including obesity, compromised cardiovascular health, type 2 diabetes, anxiety, substance use, and a depressed mood.59–62 Short sleepers reported higher rates of difficulty for falling to sleep, awakenings across the nights, awakening in the very early morning, awakening unrefreshed, and feeling sleepy during the day.2 We have previously identified a time-bound link between short sleep and depressed mood so that among individuals with normal mood at baseline, the risk for depressed mood was significantly, albeit modestly (1.31-fold), increased for those who slept less than 7 hours per night at the baseline.25 A disadvantage of epidemiological studies is that the only available data is the self-reported sleep duration. One limitation of the current study is that we do not have information on possible naps during the day. Whether the self-reported sleep duration represents genuine sleep need (true short sleepers) or results from voluntary restriction of sleep remains open. The present results raise the possibility that mood disorders and short sleep may share at least partially common mechanisms and a genetic background which involve glutamatergic neurotransmission. Our findings also make GRIA3 an appealing candidate gene to study a number of nonpsychiatric (somatic) features and traits related to short sleep, such as obesity, cardiovascular disease, and type 2 diabetes.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Partonen has participated in speaking engagements for Janssen-Cilag, Leiras Finland, Nordea, and Servier Finland. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We wish to thank all the participants of the Health 2000 and FINRISK study 2007 survey. This study was supported by grants from the European Union (LSHM-CT-2005-518189 and MCRTN-CT-2004-512362), and Helsinki University Central Hospital (EVO, TYH6254), and this support is gratefully acknowledged. Dr. Anu Loukola and Dr. Timo Partonen were supported by an Academy of Finland post-doctoral research fellowship. We thank Prof. Markus Perola, the late Prof. Leena Peltonen, and Prof. Veikko Salomaa for providing us with the genotype data of the independent set of individuals from Health 2000 and the 2007 FINRISK survey.
ABBREVIATIONS
- GRIA3 (GluR3)
glutamate receptor, ionotrophic, AMPA 3
- BHLHE41 (DEC2)
basic helix-loop-helix family, member e41
- EEG
electroencephalographic
- NREM
non-rapid eye movement
- HPA
hypothalamic pituitary adrenal
- TPH2
tryptophan hydroxylase 2
- GAD1
glutamate decarboxylase 1
- BDNF
brain-derived neurotrophic factor
- CRHR1
corticotropin releasing hormone receptor 1
- TIMELESS
timeless homolog (Drosophila)
- CIDI
Composite International Diagnostic Interview
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders IV
- DILGOM
Dietary, Lifestyle, and Genetic determinants of Obesity and Metabolic syndrome
- SLC6A4
solute carrier family 6 (neurotransmitter transporter, serotonin), member 4
- DISC1
disrupted in schizophrenia 1
- NPAS2
neuronal PAS domain protein 2
- NFIL3
nuclear factor, interleukin 3 regulated
- ARNTL
aryl hydrocarbon receptor nuclear translocator-like
- ARNTL2
aryl hydrocarbon receptor nuclear translocator-like 2
- RORA
RAR-related orphan receptor A
- LD
linkage disequilibrium
- SNP
single nucleotide polymorphism
- NMDA
N-methyl-d-aspartate
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- GATA-3
GATA binding protein 3
Schematic presentation of GRIA3 gene. The 5'promoter region (P), 15 introns, and 16 exons (gray blocks) are represented with approximate locations. The bold SNPs indicate those variants that were included in the core haplotype that gave statistically the strongest evidence for association to sleep duration. A bent arrow indicates the transcription factor binding site for GATA-3, 10kb upstream (at 122135839bp) from promoter starting (GRIA3 starts at 122145839bp). The figure also summarizes previous evidence for association of GRIA3 with mental retardation and bipolar disorder in women (Gecz J. et al., 1999); mental retardation in men (Chiyonobu T. et al., 2007); schizophrenia, mental retardation, and autism (Guilmatre A. et al., 2009); migraine in women (Formicola D. et al., 2010), schizophrenia in women (Magri C. et al., 2008), sexual dysfunction during major depressive disorder (Perlis, R.H. et al., 2009), and suicidal ideation emerging during citalopram treatment of major depression (Laje G. et al., 2007 and 2009).
Linkage disequilibrium and haploblocks structure of GRIA3 gene. The linkage disequilibrium and haploblock structure of 54 SNPs of GRIA3 in the Genmets (D-) and FINRISK 2007 samples created using the algorithm solid spine.30 Blocks 1-3 are positioned within intron 2. Block 4 includes variants from intron 2 and 3. Block 5 is the largest block spanning a 68kb region located within introns 3 and 4. Block 6 ranges from intron 4 to 5. Block 7 is located within intron 5. Block 8 includes variants from introns 5,7,10, and 11. Block 9 extends over a 17kb region and includes variants from introns 11 and 12, and the most significantly associated variant, rs687577, is located within this block. Blocks 10 and 11 are positioned in the region of intron 12.
Table S1.
Complete results for single marker association analyses of sleep duration as well as short and long sleep in females (N = 1626) and males (N = 1521) from the population-based Health 2000 and FINRISK study 2007 samples.
| Chr | Gene | SNPs | BP | A1/A2 | Females |
Males |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep durationa |
Short sleep vs.midrange sleepb |
Long sleep vs.midrange sleepb |
Sleep durationa |
Short sleep vs.midrange sleepb |
Long sleep vs.midrange sleepb |
|||||||||||||||||
| MAF | β (95% CI) | P | MAF | OR (95% CI) | P | MAF | OR (95% CI) | P | MAF | β (95% CI) | P | MAF | OR (95% CI) | P | MAF | OR (95% CI) | P | |||||
| 1 | DISC1 | rs3738401 | 228137030 | G/A | 0.28 | -0.051 (-0.12-0.025) | 0.190 | 0.31 | 1.11 (0.88-1.40) | 0.353 | 0.26 | 0.90 (0.71-1.13) | 0.374 | 0.29 | 0.005 (-0.07-0.08) | 0.891 | 0.29 | 1.02 (0.82-1.27) | 0.819 | 0.30 | 1.05 (0.79-1.39) | 0.705 |
| 2 | NPAS2 | rs12712083 | 100875331 | A/G | 0.43 | 0.005 (-0.06-0.076) | 0.873 | 0.43 | 0.96 (0.77-1.2) | 0.740 | 0.42 | 0.92 (0.75-1.14) | 0.479 | 0.43 | 0.052 (-0.02-0.12) | 0.154 | 0.42 | 0.94 (0.77-1.15) | 0.595 | 0.43 | 1.05 (0.81-1.37) | 0.689 |
| 9 | NFIL3 | rs1619450 | 93208976 | T/C | 0.14 | -0.008 (-0.122-0.105) | 0.887 | 0.15 | 1.18 (0.83-1.67) | 0.353 | 0.16 | 1.3 (0.94-1.78) | 0.108 | 0.13 | -0.021 (-0.13-0.09) | 0.721 | 0.17 | 1.01 (0.73-1.40) | 0.909 | 0.12 | 1.11 (0.74-1.65) | 0.627 |
| 11 | ARNTL | rs6486121 | 13312346 | C/T | 0.10 | -0.016 (-0.087-0.054) | 0.648 | 0.11 | 1.05 (0.84-1.31) | 0.630 | 0.12 | 1.12 (0.91-1.38) | 0.288 | 0.10 | -0.035 (-0.10-0.03) | 0.330 | 0.10 | 1.01 (0.83-1.24) | 0.855 | 0.11 | 0.95 (0.73-1.23) | 0.708 |
| 11 | ARNTL | rs3816358 | 13348048 | C/A | 0.49 | 0.013 (-0.088-0.116) | 0.789 | 0.48 | 0.84 (0.60-1.17) | 0.325 | 0.47 | 0.99 (0.73-1.35) | 0.996 | 0.49 | 0.042 (-0.05-0.14) | 0.418 | 0.49 | 0.95 (0.71-1.27) | 0.748 | 0.49 | 1.05 (0.73-1.52) | 0.768 |
| 11 | ARNTL | rs969485 | 13359619 | A/G | 0.14 | -0.053 (-0.137-0.030) | 0.204 | 0.13 | 1.13 (0.88-1.46) | 0.325 | 0.13 | 0.96 (0.75-1.24) | 0.804 | 0.14 | 0.0005 (-0.08-0.08) | 0.991 | 0.13 | 0.89 (0.70-1.14) | 0.374 | 0.14 | 1.02 (0.75-1.39) | 0.895 |
| 11 | BDNF | rs6265 | 27636492 | C/T | 0.22 | 0.036 (-0.061-0.134) | 0.461 | 0.24 | 1.05 (0.77-1.42) | 0.742 | 0.21 | 1.05 (0.79-1.40) | 0.696 | 0.22 | -0.043 (-0.14-0.05) | 0.382 | 0.20 | 1.2 (0.92-1.56) | 0.176 | 0.22 | 1.04 (0.72-1.49) | 0.822 |
| 11 | BDNF | rs1491850 | 27706301 | T/C | 0.15 | 0.067 (-0.003-0.139) | 0.060 | 0.15 | 0.87 (0.69-1.09) | 0.232 | 0.15 | 1.03 (0.84-1.28) | 0.715 | 0.15 | -0.038 (-0.10-0.03) | 0.286 | 0.17 | 1.18 (0.97-1.45) | 0.090 | 0.14 | 1.08 (0.83-1.40) | 0.549 |
| 12 | ARNTL2 | rs4964060 | 27424634 | G/A | 0.41 | -0.006 (-0.078-0.065) | 0.862 | 0.38 | 1.03 (0.82-1.29) | 0.758 | 0.42 | 0.90 (0.73-1.12) | 0.381 | 0.43 | 0.019 (-0.05-0.09) | 0.599 | 0.47 | 1.03 (0.84-1.26) | 0.732 | 0.44 | 0.88 (0.67-1.14) | 0.348 |
| 12 | ARNTL2 | rs7304939 | 27435612 | C/T | 0.41 | 0.033 (-0.088-0.155) | 0.586 | 0.41 | 0.83 (0.55-1.24) | 0.395 | 0.39 | 0.87 (0.60-1.25) | 0.461 | 0.43 | -0.066 (-0.18-0.05) | 0.276 | 0.44 | 1.16 (0.84-1.60) | 0.337 | 0.39 | 0.73 (0.44-1.20) | 0.228 |
| 12 | ARNTL2 | rs1037921 | 27444833 | A/G | 0.09 | 0.006 (-0.127-0.140) | 0.921 | 0.07 | 0.82 (0.52-1.28) | 0.394 | 0.08 | 0.88 (0.59-1.33) | 0.563 | 0.09 | -0.097 (-0.23-0.03) | 0.155 | 0.11 | 1.21 (0.85-1.71) | 0.289 | 0.07 | 0.67 (0.38-1.18) | 0.163 |
| 12 | ARNTL2 | rs2289709 | 27464900 | C/T | 0.07 | 0.006 (-0.101-0.115) | 0.898 | 0.06 | 1.03 (0.73-1.44) | 0.851 | 0.06 | 0.94 (0.68-1.31) | 0.739 | 0.07 | -0.029 (-0.14-0.08) | 0.604 | 0.09 | 1.01 (0.74-1.37) | 0.941 | 0.05 | 0.73 (0.46-1.15) | 0.179 |
| 12 | TIMELESS/ | rs2291738 | 55101548 | G/A | 0.11 | 0.025 (-0.046-0.097) | 0.491 | 0.11 | 0.81 (0.65-1.02) | 0.081 | 0.10 | 0.97 (0.78-1.21) | 0.844 | 0.11 | -0.035 (-0.10-0.03) | 0.339 | 0.11 | 1.22 (1.00-1.50) | 0.048 | 0.08 | 1.05 (0.80-1.36) | 0.706 |
| 12 | TIMELESS | rs1082214 | 55132757 | C/T | 0.44 | -0.037 (-0.164-0.089) | 0.560 | 0.40 | 1.24 (0.86-1.78) | 0.242 | 0.44 | 1.05 (0.72-1.53) | 0.775 | 0.48 | 0.082 (-0.05-0.21) | 0.238 | 0.47 | 1.23 (0.85-1.78) | 0.266 | 0.48 | 1.61 (1.04-2.50) | 0.026 |
| 12 | TPH2 | rs12229394 | 70679181 | G/A | 0.07 | -0.002 (-0.077-0.073) | 0.956 | 0.09 | 1.04 (0.83-1.32) | 0.681 | 0.07 | 0.92 (0.73-1.16) | 0.521 | 0.07 | 0.015 (-0.06-0.09) | 0.697 | 0.08 | 1.28 (1.03-1.59) | 0.024 | 0.10 | 1.33 (1.00-1.76) | 0.041 |
| 15 | RORA | rs4774370 | 58680723 | T/C | 0.30 | 0.056 (-0.032-0.145) | 0.211 | 0.31 | 0.72 (0.53-0.97) | 0.037 | 0.29 | 1.06 (0.82-1.38) | 0.616 | 0.30 | 0.060 (-0.02-0.15) | 0.184 | 0.34 | 0.86 (0.66-1.11) | 0.257 | 0.35 | 1.07 (0.78-1.47) | 0.653 |
| 15 | RORA | rs8027829 | 58961163 | C/T | 0.20 | -0.015 (-0.090-0.059) | 0.687 | 0.15 | 0.96 (0.76-1.21) | 0.774 | 0.21 | 0.99 (0.79-1.24) | 0.975 | 0.19 | 0.005 (-0.06-0.07) | 0.877 | 0.17 | 0.90 (0.73-1.11) | 0.332 | 0.21 | 0.99 (0.75-1.30) | 0.952 |
| 15 | RORA | rs1568717 | 59149739 | G/T | 0.35 | -0.051 (-0.135-0.031) | 0.233 | 0.33 | 1.02 (0.78-1.32) | 0.883 | 0.35 | 0.82 (0.63-1.07) | 0.147 | 0.35 | 0.069 (-0.01-0.15) | 0.115 | 0.32 | 0.89 (0.69-1.15) | 0.397 | 0.34 | 1.18 (0.87-1.60) | 0.281 |
| 15 | RORA | rs4774388 | 59254290 | A/G | 0.23 | 0.005 (-0.09-0.104) | 0.905 | 0.24 | 1.12 (0.83-1.50) | 0.438 | 0.20 | 1.13 (0.84-1.50) | 0.403 | 0.21 | -0.081 (-0.18-0.02) | 0.125 | 0.19 | 1.42 (1.08-1.86) | 0.010 | 0.24 | 0.94 (0.63-1.38) | 0.760 |
| 17 | SLC6A4 | rs4251417 | 25575984 | G/A | 0.07 | 0.153 (0.017-0.288) | 0.027 | 0.05 | 0.74 (0.46-1.19) | 0.214 | 0.09 | 1.33 (0.92-1.92) | 0.114 | 0.08 | 0.053 (-0.07-0.18) | 0.422 | 0.07 | 0.96 (0.66-1.39) | 0.824 | 0.09 | 1.27 (0.81-1.97) | 0.281 |
| 17 | CRHR1 | rs173365 | 41256855 | G/A | 0.32 | 0.008 (-0.068-0.085) | 0.831 | 0.32 | 0.98 (0.77-1.25) | 0.903 | 0.32 | 0.97 (0.77-1.23) | 0.861 | 0.29 | -0.014 (-0.09-0.06) | 0.722 | 0.31 | 1.10 (0.88-1.37) | 0.388 | 0.30 | 1.10 (0.83-1.47) | 0.492 |
| X | GRIA3 | rs687577 | 122304639 | C/A | 0.11 | 0.270 (0.160-0.381) | 0.00001* | 0.08 | 0.84 (0.57-1.23) | 0.382 | 0.18 | 1.89 (1.42-2.52) | 0.00001** | 0.11 | 0.0003 (-0.16-0.16) | 0.995 | 0.08 | 0.68 (0.41-1.12) | 0.129 | 0.07 | 0.62 (0.31-1.22) | 0.186 |
| X | GRIA3 | rs526716 | 122374650 | G/A | 0.16 | -0.008 (-0.103-0.086) | 0.860 | 0.18 | 1.20 (0.91-1.60) | 0.181 | 0.18 | 1.19 (0.91-1.57) | 0.187 | 0.17 | -0.017 (-0.15-0.11) | 0.794 | 0.17 | 1.06 (0.73-1.54) | 0.750 | 0.17 | 0.97 (0.59-1.59) | 0.985 |
Chr, chromosome; BP, base pair, reported by NCBI dbSNP database build 125 to 130; A1/A2, Major allele / Minor allele; MAF, Minor allele frequency; β, Regression coefficient; OR, Odds ratio; 95% CI, Lower and upper bound confidence interval for β or odds ratio.
Linear regression analysis with age and presence of metabolic disorder as covariates;
Logestic regression analysis with age and presence of metabolic disorder as covariates; Gene and SNPs with P-values < 0.05 as well as the corresponding MAF, β/OR -values are bolded.
Permutation-based corrected empirical P = 0.00001, Bonferoni P = 0.000038, P-values adjusted to the number of models (3 models in both genders, altogether 6 models) (Bonferoni corrected P = 0.0052) for association with sleep duration in females.
Permutation-based corrected empirical P = 0.0003, Bonferoni P = 0.00028, P-values adjusted to the number of models (3 models in both genders, altogether 6 models) (Bonferoni corrected P = 0.039) for association with long sleep in females.
Table S2.
List of 54 GRIA3 SNPs genotyped in the Health 2000 “Genmets (D-)” and “FINRISK 2007” samples.
| Chr | SNPs | BP | Region | A1/A2 | MAF |
|---|---|---|---|---|---|
| X | rs3761557 | 122143150 | Promoter | G/A | 0.311 |
| X | rs3761555 | 122144118 | Promoter | A/G | 0.269 |
| X | rs4825836 | 122153999 | INTRON2 | G/A | 0.307 |
| X | rs2040404 | 122160177 | INTRON2 | A/C | 0.257 |
| X | rs4825838 | 122160374 | INTRON2 | A/G | 0.382 |
| X | rs5911547 | 122162359 | INTRON2 | G/A | 0.247 |
| X | rs5909975 | 122171306 | INTRON2 | A/C | 0.467 |
| X | rs4825840 | 122175690 | INTRON2 | G/A | 0.227 |
| X | rs2157292 | 122182364 | INTRON2 | A/G | 0.149 |
| X | rs5958198 | 122192643 | INTRON2 | C/A | 0.395 |
| X | rs5911557 | 122194317 | INTRON2 | A/G | 0.099 |
| X | rs6608062 | 122195582 | INTRON2 | A/G | 0.395 |
| X | rs989638 | 122200788 | INTRON2 | A/G | 0.329 |
| X | rs983007 | 122203281 | INTRON2 | G/A | 0.417 |
| X | rs12557782 | 122205419 | INTRON2 | A/G | 0.418 |
| X | rs12353611 | 122209224 | INTRON2 | A/C | 0.373 |
| X | rs4825847 | 122215515 | INTRON3 | A/C | 0.379 |
| X | rs5909978 | 122218494 | INTRON3 | A/C | 0.128 |
| X | rs2157272 | 122220788 | INTRON3 | A/G | 0.128 |
| X | rs2157271 | 122220831 | INTRON3 | A/G | 0.347 |
| X | rs695214 | 122224942 | INTRON3 | G/A | 0.138 |
| X | rs17330407 | 122232036 | INTRON3 | G/A | 0.053 |
| X | rs5911569 | 122232568 | INTRON3 | G/A | 0.252 |
| X | rs5909981 | 122234703 | INTRON3 | A/G | 0.307 |
| X | rs5958217 | 122257803 | INTRON3 | A/G | 0.257 |
| X | rs4825476 | 122269160 | INTRON3 | A/G | 0.309 |
| X | rs5911592 | 122290243 | INTRON4 | G/A | 0.231 |
| X | rs10521718 | 122294219 | INTRON4 | G/A | 0.229 |
| X | rs7891744 | 122301013 | INTRON4 | A/G | 0.12 |
| X | rs5909995 | 122303507 | INTRON4 | A/C | 0.452 |
| X | rs3827431 | 122316845 | INTRON5 | A/G | 0.181 |
| X | rs4825857 | 122324045 | INTRON5 | C/A | 0.431 |
| X | rs12857357 | 122330260 | INTRON5 | A/G | 0.132 |
| X | rs7052053 | 122331410 | INTRON5 | G/A | 0.151 |
| X | rs5910001 | 122336974 | INTRON5 | G/A | 0.215 |
| X | rs5911609 | 122338964 | INTRON5 | A/G | 0.482 |
| X | rs11260429 | 122340630 | INTRON5 | A/G | 0.156 |
| X | rs550640 | 122356484 | INTRON5 | G/A | 0.292 |
| X | rs612595 | 122363807 | INTRON7 | A/C | 0.304 |
| X | rs549895 | 122371307 | INTRON10 | G/A | 0.301 |
| X | rs526716 | 122374650 | INTRON10 | G/A | 0.166 |
| X | rs12836469 | 122386662 | INTRON11 | A/G | 0.271 |
| X | rs651595 | 122389038 | INTRON11 | G/A | 0.356 |
| X | rs6649011 | 122398392 | INTRON12 | C/A | 0.064 |
| X | rs524654 | 122398611 | INTRON12 | C/A | 0.231 |
| X | rs625074 | 122403594 | INTRON12 | G/A | 0.125 |
| X | rs545958 | 122405251 | INTRON12 | G/A | 0.281 |
| X | rs549580 | 122405634 | INTRON12 | G/A | 0.282 |
| X | rs10521721 | 122405854 | INTRON12 | A/G | 0.135 |
| X | rs687577 | 122406785 | INTRON12 | C/A | 0.116 |
| X | rs5911622 | 122410604 | INTRON12 | G/A | 0.433 |
| X | rs5911623 | 122414093 | INTRON12 | G/A | 0.369 |
| X | rs6608087 | 122415252 | INTRON12 | G/A | 0.492 |
| X | rs616364 | 122417727 | INTRON12 | A/G | 0.18 |
Chr, chromosome; BP, base pair, reported by NCBI dbSNP database build 125 to 130; A1/A2, Major allele / Minor allele; MAF, Minor allele frequency.
Table S3.
Distribution of rs687577 minor allele frequency and genotype counts across the samples of sleep duration, short sleepers, long sleepers and midrange sleepers
| Females |
Males |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | MAF | Genotype count CC/CA/AA | β/OR | P-value | Samples | MAF | Genotype count CC/CA/AA | β/OR | P-value |
| All females | 0.11 | 1258 / 342 / 20 (N = 1620) 77.7% / 21.1% / 1.2% | 0.27 | 0.00001 | All males | 0.11 | 1346 / 0 / 167 (N = 1513) 89.0% / 0% / 11.0% | 0.0003 | 0.995 |
| Female short sleepers | 0.08 | 161 / 33 / 1 (N = 195) 82.6% / 16.9% / 0.5% | 0.84 | 0.382 | Male short sleepers | 0.08 | 213 / 0 / 20 (N = 233) 91.4% / 0% / 8.6% | 0.68 | 0.129 |
| Female long sleepers | 0.18 | 140 / 62 / 9 (N = 211) 66.4% / 29.4% / 4.3% | 1.89 | 0.00001 | Male long sleepers | 0.07 | 120 / 0 / 10 (N = 130) 92.3% / 0% / 7.7% | 0.62 | 0.186 |
| Female midrange sleepers | 0.11 | 957 / 247 / 10 (N = 1214) 78.8% / 20.3% / 0.8% | na | na | Male midrange sleepers | 0.11 | 1013 / 0 / 137 (N = 1150) 88.1% / 0% / 11.9% | na | na |
MAF, minor allele frequency (major allele ‘C’, minor allele ‘A’); β, Regression coefficient; OR, Odds ratio; na, not applicable. The β/OR and P-values were generated by using linear regression model (for sleep duration) and logistic regression model (for short sleepers vs. midrange sleepers as well as long sleepers vs. midrange sleepers).
Table S4.
| Self-reported sleep duration in females in respect to their genotype at rs687577 from GRIA3 (Figure 1A) | ||||||
|---|---|---|---|---|---|---|
| Hours of sleep | MAJ/MAJ (C/C) |
MAJ/MIN (C/A) or MIN/MIN (A/A) |
||||
| Number of subjects | Age (Average ± SD) | Frequency of C/C | Number of subjects | Age (Average ± SD) | Frequency of C/A or A/A | |
| ≤ 5 | 29 | 58.65 ± 11.38 | 85% | 5 | 60.2 ± 14.72 | 15% |
| 6 | 132 | 53 ± 14.66 | 82% | 29 | 52.34 ± 16.02 | 18% |
| 7 | 450 | 50.37 ± 12.70 | 81% | 107 | 47.48 ± 14 | 19% |
| 8 | 507 | 49.66 ± 12.81 | 77% | 150 | 47.38 ± 13.48 | 23% |
| 9 | 114 | 46.51 ± 16.14 | 68% | 54 | 44.68 ± 16.40 | 32% |
| 10 | 24 | 52.41 ± 18.68 | 67% | 12 | 48.75 ± 13.53 | 33% |
| ≥ 11 | 2 | 45 ± 12.72 | 29% | 5 | 46.6 ± 15.59 | 71% |
| Self-reported sleep duration in respect to genotype at rs687577 from GRIA3 in different age group of females (Figure 1B) | ||||||
| Age groups | MAJ/MAJ (C/C) |
MAJ/MIN(C/A) or MIN/MIN (A/A) |
||||
| Number of subjects | Sleep duration (Average ± SD) | Number of subjects | Sleep duration (Average ± SD) | |||
| 25-30 | 23 | 8 ± 1.04 | 15 | 8.33 ± 1.17 | ||
| 31-40 | 268 | 7.74 ± 0.87 | 94 | 7.89 ± 1.22 | ||
| 41-50 | 338 | 7.46 ± 0.88 | 92 | 7.79 ± 0.84 | ||
| 51-60 | 302 | 7.35 ± 0.96 | 80 | 7.68 ± 1.38 | ||
| 61-70 | 235 | 7.4 ± 1.04 | 61 | 7.68 ± 1.28 | ||
| 71-75 | 80 | 7.45 ± 1.13 | 13 | 7.46 ± 1.33 | ||
REFERENCES
- 1.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Grandner MA, Kripke DF. Self-reported sleep complaints with long and short sleep: a nationally representative sample. Psychosom Med. 2004;66:239–41. doi: 10.1097/01.psy.0000107881.53228.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann E, Baekeland F, Zwilling GR. Psychological differences between long and short sleepers. Arch Gen Psychiatry. 1972;26:463–8. doi: 10.1001/archpsyc.1972.01750230073014. [DOI] [PubMed] [Google Scholar]
- 4.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Vaidya AK. Neuroticism in short and long sleepers. Percept Mot Skills. 1982;54:962. doi: 10.2466/pms.1982.54.3.962. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Vaidya AK. Anxiety as a personality dimension of short and long sleepers. J Clin Psychol. 1984;40:197–8. doi: 10.1002/1097-4679(198401)40:1<197::aid-jclp2270400137>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN, Bixler EO, Chrousos GP, Pejovic S. Obesity and sleep disturbances: meaningful sub-typing of obesity. Arch Physiol Biochem. 2008;114:224–36. doi: 10.1080/13813450802521507. [DOI] [PubMed] [Google Scholar]
- 8.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011 doi: 10.1016/j.sleep.2010.07.021. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–53. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallander MA, Johansson S, Ruigomez A, Garcia Rodriguez LA, Jones R. Morbidity Associated With Sleep Disorders in Primary Care: A Longitudinal Cohort Study. Prim Care Companion J Clin Psychiatry. 2007;9:338–45. doi: 10.4088/pcc.v09n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronholm E, Harma M, Hublin C, Aro AR, Partonen T. Self-reported sleep duration in Finnish general population. J Sleep Res. 2006;15:276–90. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 13.Kronholm E, Partonen T, Laatikainen T, et al. Trends in self-reported sleep duration and insomnia-related symptoms in Finland from 1972 to 2005: a comparative review and re-analysis of Finnish population samples. J Sleep Res. 2008;17:54–62. doi: 10.1111/j.1365-2869.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 14.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–60. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirelli C, Bushey D, Hill S, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 16.He Y, Jones CR, Fujiki N, et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009;325:866–70. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Gennaro L, Marzano C, Fratello F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–60. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 18.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 19.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 20.Leventhal AM, Rehm LP. The empirical status of melancholia: implications for psychology. Clin Psychol Rev. 2005;25:25–44. doi: 10.1016/j.cpr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Partonen T, Lonnqvist J. Seasonal affective disorder. Lancet. 1998;352:1369–74. doi: 10.1016/S0140-6736(98)01015-0. [DOI] [PubMed] [Google Scholar]
- 22.Doghramji K. Treatment strategies for sleep disturbance in patients with depression. J Clin Psychiatry. 2003;64(Suppl 14):24–9. [PubMed] [Google Scholar]
- 23.Willeit M, Praschak-Rieder N, Neumeister A, et al. A polymorphism (5-HTTLPR) in the serotonin transporter promoter gene is associated with DSM-IV depression subtypes in seasonal affective disorder. Mol Psychiatry. 2003;8:942–6. doi: 10.1038/sj.mp.4001392. [DOI] [PubMed] [Google Scholar]
- 24.Johansson C, Willeit M, Levitan R, et al. The serotonin transporter promoter repeat length polymorphism, seasonal affective disorder and seasonality. Psychol Med. 2003;33:785–92. doi: 10.1017/s0033291703007372. [DOI] [PubMed] [Google Scholar]
- 25.Paunio T, Korhonen T, Hublin C, et al. Longitudinal study on poor sleep and life dissatisfaction in a nationwide cohort of twins. Am J Epidemiol. 2009;169:206–13. doi: 10.1093/aje/kwn305. [DOI] [PubMed] [Google Scholar]
- 26.Utge S, Soronen P, Partonen T, et al. A population-based association study of candidate genes for depression and sleep disturbance. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:468–76. doi: 10.1002/ajmg.b.31002. [DOI] [PubMed] [Google Scholar]
- 27.Utge SJ, Soronen P, Loukola A, et al. Systematic analysis of circadian genes in a population-based sample reveals association of TIMELESS with depression and sleep disturbance. PLoS One. 2010;5:e9259. doi: 10.1371/journal.pone.0009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirkola SP, Isometsa E, Suvisaari J, et al. DSM-IV mood-, anxiety- and alcohol use disorders and their comorbidity in the Finnish general population--results from the Health 2000 Study. Soc Psychiatry Psychiatr Epidemiol. 2005;40:1–10. doi: 10.1007/s00127-005-0848-7. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 31.Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res. 2004;32(Web Server issue):W249–52. doi: 10.1093/nar/gkh372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 33.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell ND, Baker GB. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr Scand. 2009 doi: 10.1111/j.1600-0447.2009.01529.x. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–23. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Beneyto M, Meador-Woodruff JH. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol. 2004;468:530–54. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- 38.Sato K, Kiyama H, Tohyama M. The differential expression patterns of messenger RNAs encoding non-N-methyl-D-aspartate glutamate receptor subunits (GluR1-4) in the rat brain. Neuroscience. 1993;52:515–39. doi: 10.1016/0306-4522(93)90403-3. [DOI] [PubMed] [Google Scholar]
- 39.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Brodkin ES, Goforth SA, Keene AH, Fossella JA, Silver LM. Identification of quantitative trait Loci that affect aggressive behavior in mice. J Neurosci. 2002;22:1165–70. doi: 10.1523/JNEUROSCI.22-03-01165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steenland HW, Kim SS, Zhuo M. GluR3 subunit regulates sleep, breathing and seizure generation. Eur J Neurosci. 2008;27:1166–73. doi: 10.1111/j.1460-9568.2008.06078.x. [DOI] [PubMed] [Google Scholar]
- 42.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 43.Manfridi A, Brambilla D, Mancia M. Stimulation of NMDA and AMPA receptors in the rat nucleus basalis of Meynert affects sleep. Am J Physiol. 1999;277:R1488–92. doi: 10.1152/ajpregu.1999.277.5.R1488. 5 Pt 2. [DOI] [PubMed] [Google Scholar]
- 44.Wigren HK, Schepens M, Matto V, Stenberg D, Porkka-Heiskanen T. Glutamatergic stimulation of the basal forebrain elevates extracellular adenosine and increases the subsequent sleep. Neuroscience. 2007;147:811–23. doi: 10.1016/j.neuroscience.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 45.Magri C, Gardella R, Valsecchi P, et al. Study on GRIA2, GRIA3 and GRIA4 genes highlights a positive association between schizophrenia and GRIA3 in female patients. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:745–53. doi: 10.1002/ajmg.b.30674. [DOI] [PubMed] [Google Scholar]
- 46.Gecz J, Barnett S, Liu J, et al. Characterization of the human glutamate receptor subunit 3 gene (GRIA3), a candidate for bipolar disorder and nonspecific X-linked mental retardation. Genomics. 1999;62:356–68. doi: 10.1006/geno.1999.6032. [DOI] [PubMed] [Google Scholar]
- 47.Chiyonobu T, Hayashi S, Kobayashi K, et al. Partial tandem duplication of GRIA3 in a male with mental retardation. Am J Med Genet A. 2007;143A:1448–55. doi: 10.1002/ajmg.a.31798. [DOI] [PubMed] [Google Scholar]
- 48.Formicola D, Aloia A, Sampaolo S, et al. Common variants in the regulative regions of GRIA1 and GRIA3 receptor genes are associated with migraine susceptibility. BMC Med Genet. 2010;11:103. doi: 10.1186/1471-2350-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perlis RH, Laje G, Smoller JW, Fava M, Rush AJ, McMahon FJ. Genetic and clinical predictors of sexual dysfunction in citalopram-treated depressed patients. Neuropsychopharmacology. 2009;34:1819–28. doi: 10.1038/npp.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–8. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 51.Laje G, Allen AS, Akula N, Manji H, Rush A John, McMahon FJ. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. 2009;19:666–74. doi: 10.1097/FPC.0b013e32832e4bcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–14. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 53.Bangasser DA, Curtis A, Reyes BA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dzaja A, Arber S, Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usui T, Preiss JC, Kanno Y, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–66. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–97. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PLoS One. 2010;5:e10028. doi: 10.1371/journal.pone.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14:239–47. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 61.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic presentation of GRIA3 gene. The 5'promoter region (P), 15 introns, and 16 exons (gray blocks) are represented with approximate locations. The bold SNPs indicate those variants that were included in the core haplotype that gave statistically the strongest evidence for association to sleep duration. A bent arrow indicates the transcription factor binding site for GATA-3, 10kb upstream (at 122135839bp) from promoter starting (GRIA3 starts at 122145839bp). The figure also summarizes previous evidence for association of GRIA3 with mental retardation and bipolar disorder in women (Gecz J. et al., 1999); mental retardation in men (Chiyonobu T. et al., 2007); schizophrenia, mental retardation, and autism (Guilmatre A. et al., 2009); migraine in women (Formicola D. et al., 2010), schizophrenia in women (Magri C. et al., 2008), sexual dysfunction during major depressive disorder (Perlis, R.H. et al., 2009), and suicidal ideation emerging during citalopram treatment of major depression (Laje G. et al., 2007 and 2009).
Linkage disequilibrium and haploblocks structure of GRIA3 gene. The linkage disequilibrium and haploblock structure of 54 SNPs of GRIA3 in the Genmets (D-) and FINRISK 2007 samples created using the algorithm solid spine.30 Blocks 1-3 are positioned within intron 2. Block 4 includes variants from intron 2 and 3. Block 5 is the largest block spanning a 68kb region located within introns 3 and 4. Block 6 ranges from intron 4 to 5. Block 7 is located within intron 5. Block 8 includes variants from introns 5,7,10, and 11. Block 9 extends over a 17kb region and includes variants from introns 11 and 12, and the most significantly associated variant, rs687577, is located within this block. Blocks 10 and 11 are positioned in the region of intron 12.
Table S1.
Complete results for single marker association analyses of sleep duration as well as short and long sleep in females (N = 1626) and males (N = 1521) from the population-based Health 2000 and FINRISK study 2007 samples.
| Chr | Gene | SNPs | BP | A1/A2 | Females |
Males |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep durationa |
Short sleep vs.midrange sleepb |
Long sleep vs.midrange sleepb |
Sleep durationa |
Short sleep vs.midrange sleepb |
Long sleep vs.midrange sleepb |
|||||||||||||||||
| MAF | β (95% CI) | P | MAF | OR (95% CI) | P | MAF | OR (95% CI) | P | MAF | β (95% CI) | P | MAF | OR (95% CI) | P | MAF | OR (95% CI) | P | |||||
| 1 | DISC1 | rs3738401 | 228137030 | G/A | 0.28 | -0.051 (-0.12-0.025) | 0.190 | 0.31 | 1.11 (0.88-1.40) | 0.353 | 0.26 | 0.90 (0.71-1.13) | 0.374 | 0.29 | 0.005 (-0.07-0.08) | 0.891 | 0.29 | 1.02 (0.82-1.27) | 0.819 | 0.30 | 1.05 (0.79-1.39) | 0.705 |
| 2 | NPAS2 | rs12712083 | 100875331 | A/G | 0.43 | 0.005 (-0.06-0.076) | 0.873 | 0.43 | 0.96 (0.77-1.2) | 0.740 | 0.42 | 0.92 (0.75-1.14) | 0.479 | 0.43 | 0.052 (-0.02-0.12) | 0.154 | 0.42 | 0.94 (0.77-1.15) | 0.595 | 0.43 | 1.05 (0.81-1.37) | 0.689 |
| 9 | NFIL3 | rs1619450 | 93208976 | T/C | 0.14 | -0.008 (-0.122-0.105) | 0.887 | 0.15 | 1.18 (0.83-1.67) | 0.353 | 0.16 | 1.3 (0.94-1.78) | 0.108 | 0.13 | -0.021 (-0.13-0.09) | 0.721 | 0.17 | 1.01 (0.73-1.40) | 0.909 | 0.12 | 1.11 (0.74-1.65) | 0.627 |
| 11 | ARNTL | rs6486121 | 13312346 | C/T | 0.10 | -0.016 (-0.087-0.054) | 0.648 | 0.11 | 1.05 (0.84-1.31) | 0.630 | 0.12 | 1.12 (0.91-1.38) | 0.288 | 0.10 | -0.035 (-0.10-0.03) | 0.330 | 0.10 | 1.01 (0.83-1.24) | 0.855 | 0.11 | 0.95 (0.73-1.23) | 0.708 |
| 11 | ARNTL | rs3816358 | 13348048 | C/A | 0.49 | 0.013 (-0.088-0.116) | 0.789 | 0.48 | 0.84 (0.60-1.17) | 0.325 | 0.47 | 0.99 (0.73-1.35) | 0.996 | 0.49 | 0.042 (-0.05-0.14) | 0.418 | 0.49 | 0.95 (0.71-1.27) | 0.748 | 0.49 | 1.05 (0.73-1.52) | 0.768 |
| 11 | ARNTL | rs969485 | 13359619 | A/G | 0.14 | -0.053 (-0.137-0.030) | 0.204 | 0.13 | 1.13 (0.88-1.46) | 0.325 | 0.13 | 0.96 (0.75-1.24) | 0.804 | 0.14 | 0.0005 (-0.08-0.08) | 0.991 | 0.13 | 0.89 (0.70-1.14) | 0.374 | 0.14 | 1.02 (0.75-1.39) | 0.895 |
| 11 | BDNF | rs6265 | 27636492 | C/T | 0.22 | 0.036 (-0.061-0.134) | 0.461 | 0.24 | 1.05 (0.77-1.42) | 0.742 | 0.21 | 1.05 (0.79-1.40) | 0.696 | 0.22 | -0.043 (-0.14-0.05) | 0.382 | 0.20 | 1.2 (0.92-1.56) | 0.176 | 0.22 | 1.04 (0.72-1.49) | 0.822 |
| 11 | BDNF | rs1491850 | 27706301 | T/C | 0.15 | 0.067 (-0.003-0.139) | 0.060 | 0.15 | 0.87 (0.69-1.09) | 0.232 | 0.15 | 1.03 (0.84-1.28) | 0.715 | 0.15 | -0.038 (-0.10-0.03) | 0.286 | 0.17 | 1.18 (0.97-1.45) | 0.090 | 0.14 | 1.08 (0.83-1.40) | 0.549 |
| 12 | ARNTL2 | rs4964060 | 27424634 | G/A | 0.41 | -0.006 (-0.078-0.065) | 0.862 | 0.38 | 1.03 (0.82-1.29) | 0.758 | 0.42 | 0.90 (0.73-1.12) | 0.381 | 0.43 | 0.019 (-0.05-0.09) | 0.599 | 0.47 | 1.03 (0.84-1.26) | 0.732 | 0.44 | 0.88 (0.67-1.14) | 0.348 |
| 12 | ARNTL2 | rs7304939 | 27435612 | C/T | 0.41 | 0.033 (-0.088-0.155) | 0.586 | 0.41 | 0.83 (0.55-1.24) | 0.395 | 0.39 | 0.87 (0.60-1.25) | 0.461 | 0.43 | -0.066 (-0.18-0.05) | 0.276 | 0.44 | 1.16 (0.84-1.60) | 0.337 | 0.39 | 0.73 (0.44-1.20) | 0.228 |
| 12 | ARNTL2 | rs1037921 | 27444833 | A/G | 0.09 | 0.006 (-0.127-0.140) | 0.921 | 0.07 | 0.82 (0.52-1.28) | 0.394 | 0.08 | 0.88 (0.59-1.33) | 0.563 | 0.09 | -0.097 (-0.23-0.03) | 0.155 | 0.11 | 1.21 (0.85-1.71) | 0.289 | 0.07 | 0.67 (0.38-1.18) | 0.163 |
| 12 | ARNTL2 | rs2289709 | 27464900 | C/T | 0.07 | 0.006 (-0.101-0.115) | 0.898 | 0.06 | 1.03 (0.73-1.44) | 0.851 | 0.06 | 0.94 (0.68-1.31) | 0.739 | 0.07 | -0.029 (-0.14-0.08) | 0.604 | 0.09 | 1.01 (0.74-1.37) | 0.941 | 0.05 | 0.73 (0.46-1.15) | 0.179 |
| 12 | TIMELESS/ | rs2291738 | 55101548 | G/A | 0.11 | 0.025 (-0.046-0.097) | 0.491 | 0.11 | 0.81 (0.65-1.02) | 0.081 | 0.10 | 0.97 (0.78-1.21) | 0.844 | 0.11 | -0.035 (-0.10-0.03) | 0.339 | 0.11 | 1.22 (1.00-1.50) | 0.048 | 0.08 | 1.05 (0.80-1.36) | 0.706 |
| 12 | TIMELESS | rs1082214 | 55132757 | C/T | 0.44 | -0.037 (-0.164-0.089) | 0.560 | 0.40 | 1.24 (0.86-1.78) | 0.242 | 0.44 | 1.05 (0.72-1.53) | 0.775 | 0.48 | 0.082 (-0.05-0.21) | 0.238 | 0.47 | 1.23 (0.85-1.78) | 0.266 | 0.48 | 1.61 (1.04-2.50) | 0.026 |
| 12 | TPH2 | rs12229394 | 70679181 | G/A | 0.07 | -0.002 (-0.077-0.073) | 0.956 | 0.09 | 1.04 (0.83-1.32) | 0.681 | 0.07 | 0.92 (0.73-1.16) | 0.521 | 0.07 | 0.015 (-0.06-0.09) | 0.697 | 0.08 | 1.28 (1.03-1.59) | 0.024 | 0.10 | 1.33 (1.00-1.76) | 0.041 |
| 15 | RORA | rs4774370 | 58680723 | T/C | 0.30 | 0.056 (-0.032-0.145) | 0.211 | 0.31 | 0.72 (0.53-0.97) | 0.037 | 0.29 | 1.06 (0.82-1.38) | 0.616 | 0.30 | 0.060 (-0.02-0.15) | 0.184 | 0.34 | 0.86 (0.66-1.11) | 0.257 | 0.35 | 1.07 (0.78-1.47) | 0.653 |
| 15 | RORA | rs8027829 | 58961163 | C/T | 0.20 | -0.015 (-0.090-0.059) | 0.687 | 0.15 | 0.96 (0.76-1.21) | 0.774 | 0.21 | 0.99 (0.79-1.24) | 0.975 | 0.19 | 0.005 (-0.06-0.07) | 0.877 | 0.17 | 0.90 (0.73-1.11) | 0.332 | 0.21 | 0.99 (0.75-1.30) | 0.952 |
| 15 | RORA | rs1568717 | 59149739 | G/T | 0.35 | -0.051 (-0.135-0.031) | 0.233 | 0.33 | 1.02 (0.78-1.32) | 0.883 | 0.35 | 0.82 (0.63-1.07) | 0.147 | 0.35 | 0.069 (-0.01-0.15) | 0.115 | 0.32 | 0.89 (0.69-1.15) | 0.397 | 0.34 | 1.18 (0.87-1.60) | 0.281 |
| 15 | RORA | rs4774388 | 59254290 | A/G | 0.23 | 0.005 (-0.09-0.104) | 0.905 | 0.24 | 1.12 (0.83-1.50) | 0.438 | 0.20 | 1.13 (0.84-1.50) | 0.403 | 0.21 | -0.081 (-0.18-0.02) | 0.125 | 0.19 | 1.42 (1.08-1.86) | 0.010 | 0.24 | 0.94 (0.63-1.38) | 0.760 |
| 17 | SLC6A4 | rs4251417 | 25575984 | G/A | 0.07 | 0.153 (0.017-0.288) | 0.027 | 0.05 | 0.74 (0.46-1.19) | 0.214 | 0.09 | 1.33 (0.92-1.92) | 0.114 | 0.08 | 0.053 (-0.07-0.18) | 0.422 | 0.07 | 0.96 (0.66-1.39) | 0.824 | 0.09 | 1.27 (0.81-1.97) | 0.281 |
| 17 | CRHR1 | rs173365 | 41256855 | G/A | 0.32 | 0.008 (-0.068-0.085) | 0.831 | 0.32 | 0.98 (0.77-1.25) | 0.903 | 0.32 | 0.97 (0.77-1.23) | 0.861 | 0.29 | -0.014 (-0.09-0.06) | 0.722 | 0.31 | 1.10 (0.88-1.37) | 0.388 | 0.30 | 1.10 (0.83-1.47) | 0.492 |
| X | GRIA3 | rs687577 | 122304639 | C/A | 0.11 | 0.270 (0.160-0.381) | 0.00001* | 0.08 | 0.84 (0.57-1.23) | 0.382 | 0.18 | 1.89 (1.42-2.52) | 0.00001** | 0.11 | 0.0003 (-0.16-0.16) | 0.995 | 0.08 | 0.68 (0.41-1.12) | 0.129 | 0.07 | 0.62 (0.31-1.22) | 0.186 |
| X | GRIA3 | rs526716 | 122374650 | G/A | 0.16 | -0.008 (-0.103-0.086) | 0.860 | 0.18 | 1.20 (0.91-1.60) | 0.181 | 0.18 | 1.19 (0.91-1.57) | 0.187 | 0.17 | -0.017 (-0.15-0.11) | 0.794 | 0.17 | 1.06 (0.73-1.54) | 0.750 | 0.17 | 0.97 (0.59-1.59) | 0.985 |
Chr, chromosome; BP, base pair, reported by NCBI dbSNP database build 125 to 130; A1/A2, Major allele / Minor allele; MAF, Minor allele frequency; β, Regression coefficient; OR, Odds ratio; 95% CI, Lower and upper bound confidence interval for β or odds ratio.
Linear regression analysis with age and presence of metabolic disorder as covariates;
Logestic regression analysis with age and presence of metabolic disorder as covariates; Gene and SNPs with P-values < 0.05 as well as the corresponding MAF, β/OR -values are bolded.
Permutation-based corrected empirical P = 0.00001, Bonferoni P = 0.000038, P-values adjusted to the number of models (3 models in both genders, altogether 6 models) (Bonferoni corrected P = 0.0052) for association with sleep duration in females.
Permutation-based corrected empirical P = 0.0003, Bonferoni P = 0.00028, P-values adjusted to the number of models (3 models in both genders, altogether 6 models) (Bonferoni corrected P = 0.039) for association with long sleep in females.
Table S2.
List of 54 GRIA3 SNPs genotyped in the Health 2000 “Genmets (D-)” and “FINRISK 2007” samples.
| Chr | SNPs | BP | Region | A1/A2 | MAF |
|---|---|---|---|---|---|
| X | rs3761557 | 122143150 | Promoter | G/A | 0.311 |
| X | rs3761555 | 122144118 | Promoter | A/G | 0.269 |
| X | rs4825836 | 122153999 | INTRON2 | G/A | 0.307 |
| X | rs2040404 | 122160177 | INTRON2 | A/C | 0.257 |
| X | rs4825838 | 122160374 | INTRON2 | A/G | 0.382 |
| X | rs5911547 | 122162359 | INTRON2 | G/A | 0.247 |
| X | rs5909975 | 122171306 | INTRON2 | A/C | 0.467 |
| X | rs4825840 | 122175690 | INTRON2 | G/A | 0.227 |
| X | rs2157292 | 122182364 | INTRON2 | A/G | 0.149 |
| X | rs5958198 | 122192643 | INTRON2 | C/A | 0.395 |
| X | rs5911557 | 122194317 | INTRON2 | A/G | 0.099 |
| X | rs6608062 | 122195582 | INTRON2 | A/G | 0.395 |
| X | rs989638 | 122200788 | INTRON2 | A/G | 0.329 |
| X | rs983007 | 122203281 | INTRON2 | G/A | 0.417 |
| X | rs12557782 | 122205419 | INTRON2 | A/G | 0.418 |
| X | rs12353611 | 122209224 | INTRON2 | A/C | 0.373 |
| X | rs4825847 | 122215515 | INTRON3 | A/C | 0.379 |
| X | rs5909978 | 122218494 | INTRON3 | A/C | 0.128 |
| X | rs2157272 | 122220788 | INTRON3 | A/G | 0.128 |
| X | rs2157271 | 122220831 | INTRON3 | A/G | 0.347 |
| X | rs695214 | 122224942 | INTRON3 | G/A | 0.138 |
| X | rs17330407 | 122232036 | INTRON3 | G/A | 0.053 |
| X | rs5911569 | 122232568 | INTRON3 | G/A | 0.252 |
| X | rs5909981 | 122234703 | INTRON3 | A/G | 0.307 |
| X | rs5958217 | 122257803 | INTRON3 | A/G | 0.257 |
| X | rs4825476 | 122269160 | INTRON3 | A/G | 0.309 |
| X | rs5911592 | 122290243 | INTRON4 | G/A | 0.231 |
| X | rs10521718 | 122294219 | INTRON4 | G/A | 0.229 |
| X | rs7891744 | 122301013 | INTRON4 | A/G | 0.12 |
| X | rs5909995 | 122303507 | INTRON4 | A/C | 0.452 |
| X | rs3827431 | 122316845 | INTRON5 | A/G | 0.181 |
| X | rs4825857 | 122324045 | INTRON5 | C/A | 0.431 |
| X | rs12857357 | 122330260 | INTRON5 | A/G | 0.132 |
| X | rs7052053 | 122331410 | INTRON5 | G/A | 0.151 |
| X | rs5910001 | 122336974 | INTRON5 | G/A | 0.215 |
| X | rs5911609 | 122338964 | INTRON5 | A/G | 0.482 |
| X | rs11260429 | 122340630 | INTRON5 | A/G | 0.156 |
| X | rs550640 | 122356484 | INTRON5 | G/A | 0.292 |
| X | rs612595 | 122363807 | INTRON7 | A/C | 0.304 |
| X | rs549895 | 122371307 | INTRON10 | G/A | 0.301 |
| X | rs526716 | 122374650 | INTRON10 | G/A | 0.166 |
| X | rs12836469 | 122386662 | INTRON11 | A/G | 0.271 |
| X | rs651595 | 122389038 | INTRON11 | G/A | 0.356 |
| X | rs6649011 | 122398392 | INTRON12 | C/A | 0.064 |
| X | rs524654 | 122398611 | INTRON12 | C/A | 0.231 |
| X | rs625074 | 122403594 | INTRON12 | G/A | 0.125 |
| X | rs545958 | 122405251 | INTRON12 | G/A | 0.281 |
| X | rs549580 | 122405634 | INTRON12 | G/A | 0.282 |
| X | rs10521721 | 122405854 | INTRON12 | A/G | 0.135 |
| X | rs687577 | 122406785 | INTRON12 | C/A | 0.116 |
| X | rs5911622 | 122410604 | INTRON12 | G/A | 0.433 |
| X | rs5911623 | 122414093 | INTRON12 | G/A | 0.369 |
| X | rs6608087 | 122415252 | INTRON12 | G/A | 0.492 |
| X | rs616364 | 122417727 | INTRON12 | A/G | 0.18 |
Chr, chromosome; BP, base pair, reported by NCBI dbSNP database build 125 to 130; A1/A2, Major allele / Minor allele; MAF, Minor allele frequency.
Table S3.
Distribution of rs687577 minor allele frequency and genotype counts across the samples of sleep duration, short sleepers, long sleepers and midrange sleepers
| Females |
Males |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | MAF | Genotype count CC/CA/AA | β/OR | P-value | Samples | MAF | Genotype count CC/CA/AA | β/OR | P-value |
| All females | 0.11 | 1258 / 342 / 20 (N = 1620) 77.7% / 21.1% / 1.2% | 0.27 | 0.00001 | All males | 0.11 | 1346 / 0 / 167 (N = 1513) 89.0% / 0% / 11.0% | 0.0003 | 0.995 |
| Female short sleepers | 0.08 | 161 / 33 / 1 (N = 195) 82.6% / 16.9% / 0.5% | 0.84 | 0.382 | Male short sleepers | 0.08 | 213 / 0 / 20 (N = 233) 91.4% / 0% / 8.6% | 0.68 | 0.129 |
| Female long sleepers | 0.18 | 140 / 62 / 9 (N = 211) 66.4% / 29.4% / 4.3% | 1.89 | 0.00001 | Male long sleepers | 0.07 | 120 / 0 / 10 (N = 130) 92.3% / 0% / 7.7% | 0.62 | 0.186 |
| Female midrange sleepers | 0.11 | 957 / 247 / 10 (N = 1214) 78.8% / 20.3% / 0.8% | na | na | Male midrange sleepers | 0.11 | 1013 / 0 / 137 (N = 1150) 88.1% / 0% / 11.9% | na | na |
MAF, minor allele frequency (major allele ‘C’, minor allele ‘A’); β, Regression coefficient; OR, Odds ratio; na, not applicable. The β/OR and P-values were generated by using linear regression model (for sleep duration) and logistic regression model (for short sleepers vs. midrange sleepers as well as long sleepers vs. midrange sleepers).
Table S4.
| Self-reported sleep duration in females in respect to their genotype at rs687577 from GRIA3 (Figure 1A) | ||||||
|---|---|---|---|---|---|---|
| Hours of sleep | MAJ/MAJ (C/C) |
MAJ/MIN (C/A) or MIN/MIN (A/A) |
||||
| Number of subjects | Age (Average ± SD) | Frequency of C/C | Number of subjects | Age (Average ± SD) | Frequency of C/A or A/A | |
| ≤ 5 | 29 | 58.65 ± 11.38 | 85% | 5 | 60.2 ± 14.72 | 15% |
| 6 | 132 | 53 ± 14.66 | 82% | 29 | 52.34 ± 16.02 | 18% |
| 7 | 450 | 50.37 ± 12.70 | 81% | 107 | 47.48 ± 14 | 19% |
| 8 | 507 | 49.66 ± 12.81 | 77% | 150 | 47.38 ± 13.48 | 23% |
| 9 | 114 | 46.51 ± 16.14 | 68% | 54 | 44.68 ± 16.40 | 32% |
| 10 | 24 | 52.41 ± 18.68 | 67% | 12 | 48.75 ± 13.53 | 33% |
| ≥ 11 | 2 | 45 ± 12.72 | 29% | 5 | 46.6 ± 15.59 | 71% |
| Self-reported sleep duration in respect to genotype at rs687577 from GRIA3 in different age group of females (Figure 1B) | ||||||
| Age groups | MAJ/MAJ (C/C) |
MAJ/MIN(C/A) or MIN/MIN (A/A) |
||||
| Number of subjects | Sleep duration (Average ± SD) | Number of subjects | Sleep duration (Average ± SD) | |||
| 25-30 | 23 | 8 ± 1.04 | 15 | 8.33 ± 1.17 | ||
| 31-40 | 268 | 7.74 ± 0.87 | 94 | 7.89 ± 1.22 | ||
| 41-50 | 338 | 7.46 ± 0.88 | 92 | 7.79 ± 0.84 | ||
| 51-60 | 302 | 7.35 ± 0.96 | 80 | 7.68 ± 1.38 | ||
| 61-70 | 235 | 7.4 ± 1.04 | 61 | 7.68 ± 1.28 | ||
| 71-75 | 80 | 7.45 ± 1.13 | 13 | 7.46 ± 1.33 | ||