Abstract
Study Objectives:
To examine the association of objectively and subjectively measured sleep characteristics with cognition in older men.
Design:
A population-based cross-sectional study.
Setting:
6 centers in the United States.
Participants:
3,132 community-dwelling older men (mean age 76.4 ± 5.6 years).
Interventions:
None.
Measurements and Results:
Objectively measured sleep predictors from wrist actigraphy were total sleep time (TST), sleep efficiency (SE), and wake after sleep onset (WASO). Subjective sleep predictors were self-reported poor sleep (Pittsburgh Sleep Quality Index [PSQI] > 5), excessive daytime sleepiness (EDS, Epworth Sleepiness Scale Score > 10), and TST. Cognitive outcomes were measured with the Modified Mini-Mental State examination (3MS), the Trails B test, and the Digit Vigilance Test (DVT). After adjustment for multiple potential confounders, WASO was modestly related to poorer cognition. Compared to those with WASO < 90 min, men with WASO ≥ 90 min took 6.1 sec longer to complete the Trails B test and had a 0.9-point worse 3MS score, on average (P < 0.05). Actigraphically measured long sleepers had a slightly worse 3MS score compared to those with 7-8 h of sleep, but had similar Trails B and DVT completion times. Compared to those who self-reported sleeping 7-8 h, long sleepers (> 8 h) on average took 8.6 sec more to complete the Trails B test, had a 0.6-point worse 3MS score, and took 46 sec longer to complete the DVT (P < 0.05). PSQI and EDS were not independently related to cognitive outcomes.
Conclusions:
There were modest cross-sectional associations of WASO and self-reported long sleep with cognition among older community-dwelling men. EDS and PSQI were not related to cognition.
Citation:
Blackwell T; Yaffe K; Ancoli-Israel S; Redline S; Ensrud KE; Stefanick ML; Laffan A; Stone KL. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS Sleep Study. SLEEP 2011;34(10):1347-1356.
Keywords: Sleep fragmentation, total sleep time, cognitive function, aging
INTRODUCTION
At least 10% of people 65 years old or older will develop cognitive impairment, with the rate rising exponentially with advancing age.1,2 With life expectancies rising this number will grow in future years, increasing the importance of identifying factors related to cognitive impairment among older adults which may lead to preventative strategies. As many as 50% of older adults report habitual sleep problems, including chronic insomnia.3–5 The association between both subjectively and objectively measured sleep characteristics and cognitive function has received little attention.
Past studies of the relation of sleep characteristics to cognition have predominantly focused on self-reported total sleep time (TST) and sleep quality,6–12 with most finding an association between both self-reported long sleep duration and self-reported sleep complaints and lower levels of cognitive function. A few studies examined the association of objectively measured TST and sleep fragmentation with cognitive function and found little association between TST and cognition and conflicting results regarding sleep fragmentation.13–18 Of note, most previous studies of the association of sleep characteristics and cognitive function have been done in specific populations with clinical disorders, such as patients with insomnia or sleep disordered breathing.15,17,19,20 Few large studies of community-dwelling older adults have focused on objective measures of sleep.13,16,18
While these past studies have added to the understanding of the relationship between sleep and cognition, none have addressed the association of both subjectively and objectively measured sleep parameters and cognition in a large cohort of community-dwelling men. In particular, no study has examined both self-report and objectively measured TST in relation to cognition. This analysis examined the cross-sectional relationship of subjective and objective sleep parameters and cognitive function with data gathered in the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study.
METHODS
Participants
During the Osteoporotic Fractures in Men Study (MrOS) baseline examination from 2000 to 2002, 5,994 community-dwelling men 65 years or older were enrolled at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.21,22 In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement.
The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, was conducted between December 2003 and March 2005 and recruited 3,135 of these participants (exceeding the goal of 3,000 participants) for a comprehensive sleep assessment. Men were screened for nightly use of mechanical devices during sleep, including pressure mask for sleep apnea (CPAP or BiPAP), mouthpiece for snoring or sleep apnea, or oxygen therapy and were generally excluded; however, the study sample includes 49 men who reported use of one of these devices. Of the 2,859 MrOS men who did not participate in this ancillary study, 344 died before the sleep visit, 36 had already terminated the study, 332 were not asked because recruitment goals had already been met, 150 were not eligible, and 1,997 refused. Cognitive function data was available for 3,132 men. All of these men had data on subjective sleep measures, and 3,053 underwent wrist actigraphy.
All men provided written informed consent, and the study was approved by the institutional review board at each site.
Sleep Parameters
Objective actigraphic parameters of sleep-wake patterns
Objective characteristics of sleep-wake patterns were estimated using an actigraph (SleepWatch-O, Ambulatory Monitoring, Inc., Ardsley, NY) worn continuously for a minimum of 5 nights (mean ± SD, 5.2 ± 0.9 nights). Participants were instructed to wear the actigraph securely fastened around their non-dominant wrist, to be removed only when bathing or during water sports. The actigraph is similar in size and weight to a standard wristwatch, and movement is detected via a piezoelectric bimorph-ceramic cantilever beam that generates a voltage each time the actigraph is moved. These voltages are gathered continuously and summarized over 1-min intervals. Actigraphy has been shown to provide a reliable estimate of sleep-wake patterns.23 Data were collected in 3 modes but are reported here based on digital integration mode (also known as proportional integration mode).24 ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to analyze the actigraphy data.25 Details of the actigraphy scoring algorithms utilized in the study have been published elsewhere.26,27
Participants completed sleep diaries for the time period they wore the actigraph. The diaries included time into and time out of bed and times the actigraph was removed. This information was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep (after “lights off”), and to delete time when the actigraph was removed. Inter-scorer reliability for editing the actigraphy data files has been previously found to be high in our group (intra-class coefficient = 0.95), and actigraphy has been shown to have good concordance with TST from polysomnography.26,28
Variables estimated from actigraphy used in this analysis included: (1) total sleep time (TST): h per night spent sleeping while in bed after “lights off”; (2) sleep efficiency (SE): percentage of time in bed after “lights off” spent sleeping; (3) wake after sleep onset (WASO): minutes of wake after sleep onset during the in bed interval, with sleep onset defined as the point when the participant achieved a 20-min continuous block of sleep after “lights off”; (4) number of long wake episodes (LWEP): number of awakenings ≥ 5 min in duration while in bed. All exposure variables from actigraphy reflect data averaged over all nights they wore the device in order to obtain a more representative characterization of usual sleep patterns.
Self-reported sleep parameters
Participants completed the Pittsburgh Sleep Quality Index (PSQI), a validated measure of subjective sleep quality and sleep disturbances over a one-month time period. Global PSQI scores range from 0-21, and a score > 5 is indicative of poor sleep.29 The Epworth Sleepiness Scale (ESS), a self-administered questionnaire, was used to classify subjective daytime sleepiness. Scores on the ESS range from 0-24, with a score > 10 indicating excessive daytime sleepiness (EDS).30,31 In addition, participants were asked about TST with the question “On most nights, how many hours do you sleep each night?”, with data collected rounded to the hour.
Cognitive Function
Three cognitive function tests were administered by trained clinic staff: the Modified Mini-Mental State examination (3MS), the Trail Making Test-Part B (Trails B), and the Digit Vigilance Test (DVT).
The 3MS is a global measurement of cognitive function, with components for orientation, concentration, language, praxis, and immediate and delayed memory. Scores on the 3MS range from 0 to 100, with higher scores representing better cognitive functioning.32
The Trails B is a timed test that measures attention, sequencing, visual scanning, and executive function. The participant continuously scans a page to identify numbers and letters in a specified sequence while shifting from number to letter sets.33,34 A faster time for completion (in seconds) represents better cognitive functioning.
The DVT is a paper-and-pencil test designed to measure vigilance during rapid visual tracking and accurate selection of target stimuli.35 The standard test requires participants cross out 6s which appear randomly within 59 rows of 35 single digits. This test was modified to increase difficulty by requiring participants to cross out only those 6s that are followed by a number higher than 6 (7, 8, or 9). A faster time for completion (in seconds) represents better cognitive functioning.
Other Measurements
All participants completed questionnaires, which included items about demographics, medical history, self-reported health status, physical activity, smoking, caffeine intake, and alcohol use. The number of prior medical conditions was calculated as the summed total of prior diagnoses of common chronic illnesses (stroke or transient ischemic attack, diabetes, Parkinson disease, chronic obstructive pulmonary disease (COPD), hypertension, coronary heart disease). Participants were asked to bring in all current medications used within the preceding 30 days. All prescription and nonprescription medications were entered into an electronic database and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).36 The use of antidepressants, benzodiazepines, and sleep medications (non-benzodiazepine, non-barbiturate sedative hypnotics) were categorized. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, with higher scores corresponding to higher levels of depression.37,38 Level of physical activity was assessed using the physical activity scale for the elderly (PASE).39 Functional status was assessed by collecting information on 5 instrumental activities of daily living (IADL), which included walking 2 to 3 blocks on level ground, climbing up to 10 steps, preparing meals, doing heavy housework, and shopping for groceries or clothing.40,41 Self-reported caffeine intake was calculated based on answers to questions regarding intake of caffeinated coffee, tea, and soda.42
A comprehensive examination included measurements of body weight and height. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Statistical Analysis
For the primary analyses, the predictor variables were expressed as categorical variables which were defined similarly to previous publications for comparability (TST ≤ 5 h, > 5 to 7 h, > 7 to 8 h, > 8 h; SE < 70% vs. ≥ 70%; WASO ≥ 90 min vs. < 90 min; number of long wake episodes ≥ 8 vs. < 8) or used standard cutpoints (PSQI > 5 vs. ’ 5; ESS > 10 vs. ≥ 10). Analyses were also performed to evaluate the linear relationship of the sleep parameters and cognition, with the sleep parameters expressed as continuous variables.
Characteristics of participants were compared across categories of objectively measured TST using χ2 tests for categorical variables, analysis of variance (ANOVA) for normally distributed continuous variables, and Kruskal-Wallis tests for continuous variables with skewed distributions. Similar comparisons were performed across categories of the other sleep predictors (data not shown).
The association between a given sleep characteristic and cognitive function outcome was examined with linear regression models. The cognitive scores were transformed to meet model requirements (cube transformation for 3MS, log transformation for both Trails B and DVT) and back-transformed for display of results. All models were adjusted for multiple confounders. Covariates were included in the multivariable models if they were related to one or more of the cognitive outcomes and one or more of the sleep parameters at P < 0.10. Results are presented as adjusted means and 95% confidence intervals for the categorical representation of the predictors, β coefficients and p-values for the continuous representation of the predictors. The β coefficients are presented as a 1 SD decrease for SE, a 30-min decrease for WASO, a 1-h decrease for TST, and a 1 SD increase for the PSQI and ESS. The R2 for the models are presented to show the amount of variation of the outcome that was explained by all variables in the model combined, along with the partial r2 for the specific sleep predictor to show the amount of variation that was explained by the sleep predictor in addition to all other covariates in the model.
In secondary analyses, models with the predictor of TST were further adjusted by WASO to determine if associations were driven by underlying sleep fragmentation. Models with the predictor of sleep fragmentation were further adjusted by ESS to determine if associations were driven by daytime sleepiness. To enable comparability to other studies with much older populations, the interactions of the sleep parameters with age (< 80 vs ≥ 80 years old) were explored. Stratifications by age category was performed where appropriate (when interaction P < 0.10). Analyses were performed examining the association of the sleep predictors significantly related to the 3MS score with the subscores of the 3MS to determine if a specific component was driving the association. Lastly, although all analyses were prespecified, a Bonferroni correction was applied to all significant P-values in the primary analysis to examine if the associations observed held after correction for multiple comparisons.
All significance levels reported were 2-sided and all analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the Study Population
The analysis cohort comprised 3,132 men with data for cognitive outcomes and data for one or more sleep predictors. Of the 3,053 men with actigraphy data, 251 (8.2%) had < 5 nights of data collected: 181 had 4 nights of data, 49 had 3 nights of data, 10 had 2 nights of data, and 11 had 1 night of data. The men had an average age of 76 ± 6 years, and 90% were Caucasian. Almost half of the men (44%) had self-reported poor sleep quality defined as PSQI > 5, and 13% had self-reported excessive daytime sleepiness (ESS > 10, Table 1). The overall average of both self-reported and objectively measured TST was similar (self-reported 6.9 ± 1.2 h; objectively measured 6.4 ± 1.2 h), with approximately 12% of men with ≤ 5 h of sleep per night (self-reported 11.7%, objective 12.3%) and about 6% had a long sleep duration (> 8 h per night; self-reported 5.5%, objective 7.2%). Though rates of long and short sleep duration were similar for the self-reported and objectively measured TST, there was only 50% agreement between the 2 measures across the categories, and the correlation of the linear representations was modest (r = 0.31). Among categories of TST, those with self-reported long and short sleep duration had the highest levels of WASO on average (TST ≤ 5: 85.4 min; TST > 5 to 7: 75.6; TST > 7 to 8: 78.4 min; TST > 8: 93.0 min; P < 0.001) while the amount of WASO decreased linearly across categories of objectively measured TST (TST ≤ 5: 128.6 min; TST > 5 to 7: 78.4; TST > 7 to 8: 58.8 min; TST > 8: 58.7 min; P < 0.001). Men had an average SE of 78%, an average WASO of 78 min, and an average of 7 long wake episodes. The mean of the cognitive function outcomes for the cohort were as follows: 92.6 ± 6.4 points for the 3MS, 122.2 ± 55.3 sec for Trails B, and 558.4 ± 191.7 sec for the DVT.
Table 1.
Summary of sleep predictors and cognitive outcomes
| Characteristic | All Participants (N = 3132) |
|---|---|
| Objective sleep | |
| Sleep efficiency, % | 78.1 ± 12.0 |
| Sleep efficiency < 70% | 582 (19.1) |
| WASO, min | 78.4 ± 44.3 |
| WASO ’ 90 min | 977 (32.0) |
| # of long wake episodes | 6.9 ± 3.3 |
| ≥ 8 long wake episodes | 1006 (33.0) |
| Actigraphic TST, h | 6.4 ± 1.2 |
| ≤ 5 h | 376 (12.3) |
| >5 to 7 h | 1712 (56.1) |
| >7 to 8 h | 745 (24.4) |
| >8 h | 220 (7.2) |
| Subjective sleep | |
| Self-reported TST, h | 6.9 ± 1.2 |
| ≤ 5 h | 365 (11.7) |
| >5 to 7 h | 1780 (56.9) |
| >7 to 8 h | 813 (26.0) |
| >8 hrs | 173 (5.5) |
| PSQI (range 0-21) | 5.6 ± 3.3 |
| PSQI > 5 | 1383 (44.2) |
| ESS (range 0-24) | 6.2 ± 3.7 |
| ESS > 10 | 405 (12.9) |
| Cognitive outcomes | |
| 3MS score (range 0-100) | 92.6 ± 6.4 |
| Trails B completion time, sec | 122.2 ± 55.3 |
| DVT completion time, sec | 558.4 ± 191.7 |
Data shown as mean ± SD or n (%).
WASO, wake after sleep onset; TST, total sleep time; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; 3MS, Modified Mini-Mental State examination; DVT, Digit Vigilance Test.
Many covariates differed significantly across categories of objectively measured TST (Table 2). Those with long sleep duration (> 8 h) on average were older, had higher rates of antidepressant use, were less physically active, and had better self-reported health (P < 0.03). Those with short sleep duration (≤ 5 h) were on average heavier, more likely to be a minority, had a higher number of medical conditions including diabetes and COPD, were less educated, and were more likely to smoke (P < 0.05). There was a slight U-shaped pattern for some covariates: There were higher rates of IADL impairment, hypertension, stroke, and depression in both the long and short sleepers (P < 0.05).
Table 2.
Baseline characteristics by objectively measured TST
| Characteristic | ≤ 5 h (N = 376) | 5 to 7 h (N = 1712) | 7 to 8 h (N = 745) | 8+ h (N = 220) | P-value |
|---|---|---|---|---|---|
| Age, y | 76.5 ± 5.7 | 76.1 ± 5.4 | 76.5 ± 5.7 | 77.8 ± 5.9 | < 0.01 |
| BMI, kg/m2 | 29.3 ± 4.8 | 27.1 ± 3.7 | 26.5 ± 3.5 | 26.8 ± 3.5 | < 0.01 |
| Race/Ethnicity | |||||
| Caucasian | 326 (86.7) | 1537 (89.8) | 682 (91.5) | 202 (91.8) | < 0.01 |
| African American | 28 (7.5) | 62 (3.6) | 19 (2.6) | 6 (2.7) | |
| Other | 22 (5.9) | 113 (6.6) | 44 (5.9) | 12 (5.5) | |
| Any IADLs | 112 (29.8) | 347 (20.3) | 141 (18.9) | 50 (22.7) | < 0.01 |
| Medical conditions | |||||
| Hypertension | 214 (56.9) | 837 (48.9) | 359 (48.2) | 113 (51.4) | 0.03 |
| Stroke or TIA | 55 (14.6) | 157 (9.2) | 90 (12.1) | 31 (14.1) | < 0.01 |
| Diabetes | 68 (18.1) | 219 (12.8) | 93 (12.5) | 29 (13.2) | 0.04 |
| Parkinson disease | 6 (1.6) | 16 (0.9) | 11 (1.5) | 5 (2.3) | 0.27 |
| COPD | 29 (7.7) | 94 (5.5) | 27 (3.6) | 10 (4.6) | 0.03 |
| CHD* | 145 (38.7) | 541 (31.7) | 235 (31.5) | 70 (31.8) | 0.06 |
| # of medical conditions** | |||||
| None | 182 (48.4) | 1029 (60.1) | 444 (59.6) | 134 (60.9) | < 0.01 |
| 1-2 | 106 (28.2) | 439 (25.7) | 189 (25.4) | 55 (25.0) | |
| 3+ | 88 (23.4) | 243 (14.2) | 112 (15.0) | 31 (14.1) | |
| Antidepressant use | 35 (9.3) | 122 (7.1) | 52 (7.0) | 31 (14.2) | < 0.01 |
| Benzodiazepine use | 19 (5.1) | 67 (3.9) | 37 (5.0) | 15 (6.9) | 0.19 |
| Sleep medication use | 9 (2.4) | 36 (2.1) | 15 (2.0) | 2 (0.9) | 0.64 |
| GDS score | 2.2 ± 2.2 | 1.7 ± 2.1 | 1.8 ± 2.3 | 1.9 ± 2.2 | < 0.01 |
| Education | |||||
| < High school | 28 (7.5) | 93 (5.4) | 26 (3.5) | 15 (6.8) | < 0.01 |
| High school | 76 (20.2) | 273 (16.0) | 104 (14.0) | 35 (15.9) | |
| >High school | 272 (72.3) | 1346 (78.6) | 615 (82.6) | 170 (77.3) | |
| Alcohol use, drinks/week | |||||
| 0-2 | 226 (60.6) | 1030 (60.4) | 415 (55.9) | 129 (59.5) | 0.06 |
| 3-13 | 124 (33.2) | 592 (34.7) | 283 (38.1) | 68 (31.3) | |
| 14+ | 23 (6.2) | 83 (4.9) | 45 (6.1) | 20 (9.2) | |
| Smoking | |||||
| Never | 124 (33.0) | 688 (40.2) | 299 (40.1) | 93 (42.3) | < 0.01 |
| Past | 234 (62.2) | 994 (58.1) | 433 (58.1) | 125 (56.8) | |
| Current | 18 (4.8) | 30 (1.8) | 13 (1.7) | 2 (0.9) | |
| Caffeine use, mg/day | 261 ± 266 | 237 ± 249 | 229 ± 241 | 200 ± 196 | 0.13 |
| PASE physical activity score | 140.0 ± 75.0 | 150.2 ± 72.3 | 140.6 ± 69.3 | 138.3 ± 69.6 | < 0.01 |
| Self-rated health good/excellent | 311 (82.7) | 1499 (87.6) | 636 (85.4) | 197 (89.6) | 0.03 |
Data shown as mean ± SD or n (%). P-values for continuous data are from an ANOVA for normally distributed data, Kruskal-Wallis test for skewed data. P-values for categorical data are from a χ2 test for homogeneity.
Coronary heart disease includes myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty, or heart valve replacement.
Medical conditions include stoke/TIA, coronary heart disease, diabetes mellitus, COPD, Parkinson disease, and hypertension.
TST, total sleep time; BMI, body mass index; IADL, instrumental activities of daily living; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; GDS, geriatric depression scale; PASE, physical activity scale for the elderly.
Associations between Objectively Measured Sleep Parameters and Cognition
Objectively measured long sleep duration was associated with the 3MS score, with long sleepers having about a 1-point decrease in 3MS score, on average, compared to those sleeping > 7 to 8 h per night after multivariate adjustment for age, race, clinic, education, depression, BMI, number of IADLs, comorbidities, antidepressant use, benzodiazepine use, alcohol use, smoking, physical activity, and self-reported health status (Table 3). This association was not seen for Trails B and DVT (Tables 4 and 5). The multivariate model including TST explained about 21% of the overall variance in 3MS score, with long sleep explaining 0.4%. The association of long sleep duration and the 3MS persisted after further adjustment for WASO. Long sleep duration was associated to the 3MS subscore for delayed recall, but no other subscores. Short sleep duration showed no associations to cognitive function in this cohort (Table 3–Table 5).
Table 3.
Sleep characteristics and 3MS score
| Sleep Characteristic | Adjusted Means (95% CI) or β Coefficient (P-value) | R2 for Model | Partial r2 for the predictor | % of R2 Explained by this predictor |
|---|---|---|---|---|
| Objective actigraphic parameters | ||||
| Sleep efficiency | ||||
| < 70% | 93.1 (92.7, 93.5) | 0.2105 | 0.0021 | 1.00 |
| ≥ 70% (ref) | 93.1 (92.9, 93.3) | – | – | – |
| Continuous, per 1 SD decrease | −0.19 (0.06) | 0.2115 | 0.0071 | 3.35 |
| Wake after sleep onset | ||||
| < 90 min (ref) | 93.3 (93.1, 93.5) | – | – | – |
| ≥ 90 min | 92.7 (92.4, 93.0) | 0.2130 | 0.0085 | 3.98 |
| Continuous, per 30-min increase | -0.29 (< 0.01) | 0.2153 | 0.0184 | 8.56 |
| Number of long wake episodes | ||||
| < 8 (ref) | 93.3 (93.1, 93.5) | – | – | – |
| ≥ 8 | 92.7 (92.4, 93.0) | 0.2126 | 0.0070 | 3.29 |
| Continuous, per 1 episode increase | -0.11 (< 0.01) | 0.2139 | 0.0136 | 6.35 |
| Actigraphic total sleep time | ||||
| ≤ 5 h | 93.2 (92.7, 93.7) | 0.2137 | 0.0004 | 0.18 |
| > 5 to 7 h | 93.3 (93.0, 93.5) | 0.2137 | 0.0028 | 1.32 |
| > 7 to 8 h (ref) | 93.0 (92.6, 93.3) | – | – | – |
| > 8 h | 92.1 (91.4, 92.7) | 0.2137 | 0.0042 | 1.96 |
| Continuous, per 1-h decrease | 0.20 (0.01) | 0.2122 | 0.0009 | 0.42 |
| Subjective sleep | ||||
| Self-reported total sleep time | ||||
| ≤ 5 h | 92.4 (91.9, 92.9) | 0.2200 | 0.0094 | 4.27 |
| > 5 to 7 h | 93.2 (93.0, 93.4) | 0.2200 | 0.0009 | 0.43 |
| > 7 to 8 h (ref) | 93.2 (92.8, 93.5) | – | – | – |
| > 8 h | 92.3 (91.5, 93.0) | 0.2200 | 0.0058 | 2.66 |
| Continuous, per 1-h decrease | -0.06 (0.41) | 0.2169 | 0.0020 | 0.93 |
| Pittsburgh Sleep Quality Index | ||||
| ≤ 5 (ref) | 93.1 (92.9, 93.3) | – | – | – |
| > 5 | 93.0 (92.7, 93.3) | 0.2169 | 0.0061 | 2.81 |
| Continuous, per 1 SD increase | -0.06 (0.55) | 0.2169 | 0.0110 | 5.09 |
| Epworth Sleepiness Scale | ||||
| ≤ 10 (ref) | 93.1 (92.9, 93.2) | – | – | – |
| > 10 | 93.0 (92.6, 93.5) | 0.2168 | 0.0018 | 0.85 |
| Continuous, per 1 SD increase | 0.22 (0.01) | 0.2183 | 0.0001 | 0.05 |
Bold = P > 0.05. All results adjusted for age, race, clinic, BMI, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
Table 4.
Sleep characteristics and Trail Making B test completion time (sec)
| Sleep Characteristic | Adjusted Means (95% CI) or β Coefficient (P-value) | R2 for Model | Partial r2 for the predictor | % of R2 Explained by this predictor |
|---|---|---|---|---|
| Objective actigraphic parameters | ||||
| Sleep efficiency | ||||
| < 70% | 114.4 (110.8, 118.1) | – | – | – |
| ≥ 70% (ref) | 110.8 (109.2, 112.5) | 0.1972 | 0.0065 | 3.28 |
| Continuous, per 1 SD decrease | 2.24 (0.03) | 0.1978 | 0.0097 | 4.92 |
| Wake after sleep onset | ||||
| < 90 min (ref) | 109.6 (107.8, 111.4) | – | – | – |
| ≥ 90 min | 115.7 (112.9, 118.5) | 0.2000 | 0.0122 | 6.12 |
| Continuous, per 30-min increase | 3.26 (< 0.01) | 0.2026 | 0.0235 | 11.62 |
| Number of long wake episodes | ||||
| < 8 (ref) | 110.5 (108.7, 112.3) | – | – | – |
| ≥ 8 | 113.6 (110.9, 116.3) | 0.1973 | 0.0063 | 3.20 |
| Continuous, per 1 episode increase | 0.81 (< 0.01) | 0.1983 | 0.0122 | 6.14 |
| Actigraphic total sleep time | ||||
| ≤ 5 h | 111.8 (107.5, 116.3) | 0.1994 | 0.0011 | 0.57 |
| > 5 to 7 h | 109.7 (107.8, 111.7) | 0.1994 | 0.0039 | 1.96 |
| > 7 to 8 h (ref) | 113.4 (110.4, 116.5) | – | – | – |
| > 8 h | 118.9 (113.0, 125.0) | 0.1994 | 0.0026 | 1.30 |
| Continuous, per 1-h decrease | −1.53 (0.06) | 0.1974 | 0.0003 | 0.16 |
| Subjective sleep | ||||
| Self-reported total sleep time | ||||
| ≤ 5 h | 115.9 (111.4, 120.6) | 0.1993 | 0.0062 | 3.12 |
| > 5 to 7 h | 110.7 (108.8, 112.6) | 0.1993 | 0.0008 | 0.42 |
| > 7 to 8 h (ref) | 111.0 (108.2, 114.0) | – | – | – |
| > 8 h | 119.4 (112.7, 126.5) | 0.1993 | 0.0060 | 2.99 |
| Continuous, per 1-h decrease | 0.59 (0.49) | 0.1969 | 0.0014 | 0.71 |
| Pittsburgh Sleep Quality Index | ||||
| ≤ 5 (ref) | 111.8 (109.8, 113.9) | – | – | – |
| > 5 | 111.8 (109.5, 114.1) | 0.1968 | 0.0053 | 2.68 |
| Continuous, per 1 SD increase | 0.84 (0.34) | 0.1970 | 0.0134 | 6.80 |
| Epworth Sleepiness Scale | ||||
| ≤ 10 (ref) | 112.1 (110.5, 113.7) | – | – | – |
| > 10 | 109.9 (105.9, 114.2) | 0.1970 | 0.0001 | 0.06 |
| Continuous, per 1 SD increase | -1.94 (0.02) | 0.1983 | < 0.0001 | 0.01 |
Bold = P > 0.05. All results adjusted for age, race, clinic, BMI, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
Table 5.
Sleep characteristics and DVT test completion time (sec)
| Sleep Characteristic | Adjusted Means (95% CI) or β Coefficient (P-value) | R2 for Model | Partial r2 for the predictor | % of R2 Explained by this predictor |
|---|---|---|---|---|
| Objective actigraphic parameters | ||||
| Sleep efficiency | ||||
| < 70% | 531.5 (519.5, 543.8) | 0.1505 | 0.0038 | 2.53 |
| ≥ 70% (ref) | 530.6 (525.0, 536.4) | – | – | – |
| Continuous, per 1 SD decrease | 1.18 (0.65) | 0.1505 | 0.0056 | 3.72 |
| Wake after sleep onset | ||||
| < 90 min (ref) | 527.8 (521.7, 534.1) | – | – | – |
| ≥ 90 min | 537.4 (528.1, 546.9) | 0.1513 | 0.0068 | 4.46 |
| Continuous, per 30-min increase | 4.8 (< 0.01) | 0.1526 | 0.0152 | 9.98 |
| Number of long wake episodes | ||||
| < 8 (ref) | 530.2 (524.0, 536.5) | – | – | – |
| ≥ 8 | 532.1 (523.1, 541.4) | 0.1505 | 0.0034 | 2.26 |
| Continuous, per 1 episode increase | 0.59 (0.45) | 0.1506 | 0.0069 | 4.56 |
| Actigraphic total sleep time | ||||
| ≤ 5 h | 530.9 (515.9, 546.2) | 0.1513 | 0.0012 | 0.81 |
| > 5 to 7 h | 527.6 (520.9, 534.4) | 0.1513 | 0.0012 | 0.78 |
| >7 to 8 h (ref) | 534.6 (524.3, 545.2) | – | – | – |
| > 8 h | 543.2 (523.7, 563.3) | 0.1513 | 0.0011 | 0.72 |
| Continuous, per 1-h decrease | −1.98 (0.34) | 0.1507 | < 0.0001 | 0.01 |
| Subjective sleep | ||||
| Self-reported total sleep time | ||||
| ≤ 5 h | 532.6 (517.5, 548.1) | 0.1523 | 0.0015 | 0.98 |
| > 5 to 7 h | 529.3 (522.6, 536.0) | 0.1523 | 0.0020 | 1.35 |
| > 7 to 8 h (ref) | 530.3 (520.5, 540.4) | – | – | – |
| > 8 h | 576.5 (552.7, 601.2) | 0.1523 | 0.0083 | 5.43 |
| Continuous, per 1-h decrease | −4.61 (0.12) | 0.1488 | 0.0002 | 0.11 |
| Pittsburgh Sleep Quality Index | ||||
| ≤ 5 (ref) | 534.1 (527.1, 541.2) | – | – | – |
| > 5 | 530.2 (522.3, 538.1) | 0.1483 | 0.0038 | 2.54 |
| Continuous, per 1 SD increase | −2.54 (0.39) | 0.1483 | 0.0073 | 4.93 |
| Epworth Sleepiness Scale | ||||
| ≤ 10 (ref) | 531.7 (526.3, 537.2) | – | – | – |
| > 10 | 537.1 (522.5, 552.0) | 0.1483 | 0.0017 | 1.14 |
| Continuous, per 1 SD increase | 0.49 (0.86) | 0.1481 | 0.0015 | 0.98 |
Bold = P > 0.05. All results adjusted for age, race, clinic, BMI, instrumental activities of daily living, comorbidities, antidepressant use, benzodiazepine use, Geriatric Depression Scale score, education, alcohol use, smoking, physical activity, and self-reported health status.
After adjustment for multiple confounders, a number of measures of increased sleep fragmentation were associated with worse levels of cognitive function. WASO was associated to the Trails B test (men with WASO ≥ 90 min took 6.1 sec longer to complete on average) and the 3MS score (men with WASO ≥ 90 min had an 0.6 worse average score). The number of long wake episodes was associated to the 3MS score (men with ≥ 8 episodes had 0.6 worse average score). Multivariate models explained about 21% of the overall variance in the 3MS score, with WASO ≥ 90 min explaining about 0.8%, and 20% of the overall variance for Trails B with WASO ≥ 90 min explaining 1.2%. For comparison, the partial r2 for age in these models was about 7% for the 3MS outcome and 10% for the Trials B outcome (Tables 3 and 4). These associations of sleep fragmentation with cognition persisted after further adjustment for sleepiness (ESS). Sleep fragmentation measures were associated with two 3MS subscores related to memory (delayed recall and temporal orientation) and naming.
When objectively measured sleep parameters were modeled continuously their associations with cognition were largely similar, but there was greater evidence supporting independent associations and larger percentages of variation explained by the continuous predictors. When examined as a continuous predictor, the associations of SE and the number of long wake episodes with the Trails B was also significant. There was a 2.24 longer Trails B test time, on average, for each decrease of 1 SD (12%) in SE and a 0.81-sec average longer test completion time per 1 episode increase in the number of long wake episodes. The association of WASO and DVT was also significant, with a 4.8-sec average longer test completion time per 30-min increase in WASO (Table 3–Table 5).
Associations between Self-Reported Sleep Parameters and Cognition
After adjustment, there were no significant associations between PSQI or excessive daytime sleepiness (ESS > 10) and cognition (Table 3–Table 5). There was an apparent U-shaped association of self-reported sleep time with cognition as assessed by the 3MS (Tables 3). Those men with short (≤ 5 h) and long (> 8 h) sleep duration had lower levels of cognition, on average, compared to those with > 7 to 8 h of self-reported sleep per night. Self-reported sleep was related to the 3MS subscores for spatial orientation, writing, and immediate recall. Long but not short self-reported sleep duration was related to longer Trails B and DVT test completion times after multivariate adjustment. Long sleepers took an average of 8.4 sec longer to complete the Trails B test and 46.2 sec longer for the DVT than those who reported sleeping > 7 to 8 h per night. Further adjustment by WASO attenuated these results. There was no longer an association with long sleep duration and Trails B, and the association of long sleep duration with the 3MS was no longer statistically significant (P = 0.056).
Linear associations with the subjective sleep parameters and cognition were largely similar. After multivariate adjustment there was no linear association with self-reported sleep and cognition.
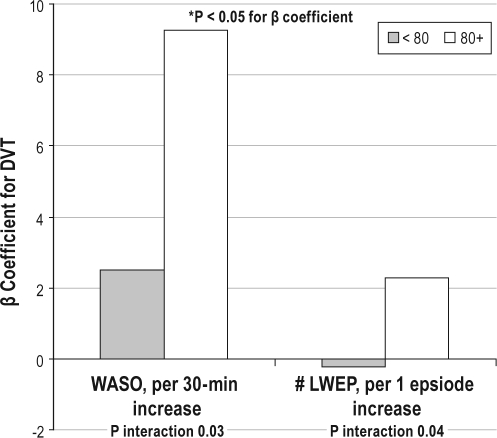
Interactions with Age
There was a significant interaction of age with WASO and the number of long wake episodes for the continuous outcome of the DVT completion time (P ≤ 0.04, Figure 1). Those aged ≥ 80 years showed a larger association with the sleep fragmentation measures and DVT than those < 80. Among those ≥ 80, there was an average 9.26-sec longer time to complete the DVT per 1 SD decrease in SE (P = 0.01).
Figure 1.
Interaction of Age with Sleep Fragmentation on DVT
Bonferroni Correction
In Table 3–Table 5 we performed 14 models per outcome, a total of 42 models. Adjusting the significance level of 0.05 with a Bonferroni correction would lead to a significance cutpoint of P < 0.0011 (= 0.05/42). After applying this more stringent cutpoint for significance to the these results a number of significant associations persisted: WASO used as a continuous predictor with both the 3MS and Trails B, the categorical representation of WASO and Trails B, the continuous representation of the number of long wake episodes and the 3MS, and self-reported long sleep duration and the DVT.
DISCUSSION
This cross-sectional analysis of 3,132 older community-dwelling men suggests a modest association of cognition with objectively measured sleep fragmentation (WASO, SE) and self-reported long sleep duration. These men had high levels of cognitive functioning. While they had a high rate of self-reported poor sleep quality, the average TST was between 6 and 7 h per night and the average SE was 78%.
Impaired sleep continuity may cause sleepiness, which although not detectable by subjective reports, may result in reduced vigilance. This reduced vigilance may negatively influence executive functioning.20,43,44 Such effects may be anticipated to be magnified in settings of increased vulnerability.45 The results of our secondary analyses are consistent with this, demonstrating larger sleep related cognitive deficits in the very elderly members of our cohort. While sleep fragmentation may be related to sleepiness, sleepiness did not completely mediate the association of sleep fragmentation with cognition. In this cohort, the associations persisted after further adjustment for daytime sleepiness; however, we did not formally test for mediation in these models.
Of the associations between sleep fragmentation and cognition, the most consistent relationship was seen between cognition and WASO, which explained more of the overall variability in the cognitive outcome measures. The somewhat less consistent findings observed for SE may relate to greater measurement error due to inaccuracies in identifying “lights off” time. Our findings are consistent with those of other research that have similarly found modest associations with objectively measured sleep fragmentation and cognition.15,16 Similar analyses were performed in the Study of Osteoporotic Fractures (SOF) on a much older population of community-dwelling women (average age 83.5 years).13 The associations with sleep fragmentation and cognition were larger in magnitude in the SOF study than those found in this younger MrOS cohort. Consistent with that finding, in the current analysis there was a significant interaction between age and sleep fragmentation for the DVT test, with stronger associations seen in those men 80 years old or older.
The association of cognition with TST measured both subjectively and objectively was examined. A U-shaped relationship with self-reported TST and the 3MS score was observed, with those with short and long sleep duration scoring worse on average. The association of objectively measured TST and the 3MS score was not U-shaped, but showed lower scores on the 3MS for the long sleepers. This association with long sleep duration and lower cognitive function was also seen with self-reported TST and the DVT and Trails B, but not for objectively measured long sleep duration. These results indicate a consistent association between self-reported, but not objectively measured, sleep duration and multiple aspects of cognition. No study to our knowledge has compared both relationships. This analysis helps to clarify the discrepant findings among studies examining TST and cognition. As with the current study, most previous results show an association with self-reported8,9,12 but not objectively measured TST and cognition.13,14,16 This may be due to self-reported long sleepers perceiving TST to be much longer than it actually is due to awakenings throughout the night, or because they do spend more time in bed, but much of that time is spent awake. The average values for WASO showed a U-shaped pattern among categories of self-reported TST, which supports this idea. Also in support of this idea was the loss of association of self-reported long sleep duration with the Trails B and 3MS after further adjustment for WASO.
We found no association with self-reported poor sleep or excessive daytime sleepiness. This conflicts with other studies, which may be due to the differing populations studied.10,11,20
This study has several strengths. The study had a large population of community-dwelling older men who were not selected for inclusion based on sleep problems or cognitive impairment. There were a number of validated measures of sleep characteristics, including both subjective and objective measures. Adjustments for multiple potential confounding factors were made, suggesting these associations were not explained by other covariates including depression, comorbidities, medication use, education, or lifestyle.
This study also had several limitations. The findings may not be generalizable to populations groups other than community-dwelling older men. Causality cannot be established due to the cross-sectional study design. The association of cognition and sleep parameters may be bi-directional, so further research investigating direction of association on incident cognitive decline are needed. Adjustment for numerous covariates was performed, but there may be unmeasured confounders that may affect the results. An additional limitation is that our cognitive battery was somewhat limited and only included measures of global cognition, vigilance, and executive function. The effect sizes are relatively small, and clinical meaningfulness is uncertain.
These findings suggest there was a modest association with objectively measured sleep fragmentation and self-reported long sleep duration and cognition among older community-dwelling men. Although self-reported long sleep duration was associated with cognitive deficits, this relationship may have been mediated by poorer sleep among the self-reported long sleepers. Further study needs to be done to examine if these associations hold for longitudinal cognitive decline.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ancoli-Israel has consulted for or is on the advisory boards of Ferring Pharmaceuticals, GlaxoSmithKline, Merck, NeuroVigil, Pfizer, Philips Respironics, Sanofi-Aventis, Sepracor, Scherling-Plough, and Perdue and has received research support from Sepracor and Litebook. Dr. Redline is the incumbent of an endowed chair professorship donated to Harvard Medical School by Dr. Peter Farrell, the founder and board chairman of ResMed, Inc, has received research support from Dymedix, Inc, and has received equipment for use in research from Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Investigators in the Outcomes of Sleep Disorders in Older Men study (MrOS Sleep):
Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): K.L. Stone (Principal Investigator), D.C. Bauer (co-Investigator), S.R. Cummings (co-Investigator), N. Goldschlager (co-Investigator), P. Varosy (co-Investigator), K. Yaffe (co-Investigator), P.M. Cawthon (co-Investigator), R. Fullman (Project Director), R. Benard, T. Blackwell, L. Concepcion, J. Diehl, S. Ewing, C. Fox, M. Jaime-Chavez, E. Kwan, S. Litwack, W. Liu, L.Y. Lui, J. Schneider, R. Scott, D. Tanaka, J. Ziarno; Administrative Center (Oregon Health – Sciences University): E. Orwoll (Principal Investigator), K. Phipps (co-Investigator), L. Marshall (co-Investigator), J. Babich Blank (Project Director), L. Lambert, B. Chan, D. Neevel; University of Alabama, Birmingham: C.E. Lewis (Principal Investigator), J. Shikany (co-Investigator), P. Johnson (Project Director), C. Oden, S. House, N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, J. Smith, C. Sassaman, C. Collier, C. Atkins; University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (co-Investigator), D. King (Program Manager), N. Michaels (Asst. Program Manager), N. Nelson (Clinic Coordinator), C. Bird, D. Blanks, F. Imker-Witte, K. Moen, M. Paudel, M. Slindee; Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (co-Investigator), K. Kent, B. Malig, S. Wong; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, C. Newman; University of California, San Diego: E. Barrett-Connor (Principal Investigator), S. Ancoli-Israel (co-Investigator), T. Dam (co-Investigator), ML Carrion-Petersen (Project Director), P. Miller, N. Kamantigue; Case Western Reserve University: S. Redline (Principal Investigator), S. Surovec (Project Administrator), N. Scott (Chief Polysomnologist), N. Johnson (Programmer Analyst), J. Arnold (Polysomnologist), R. Nawabit (Polysomnologist), J. Romaniuk (Polysomnologist), S. Seacian (Polysomnologist).
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Cancer Institute (NCI), the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, UL1 RR024140, and AG08415. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Dr Yaffe received funding from K 24 grant AG031155. The analysis was performed at California Pacific Medical Center Research Institute, San Francisco, CA.
ABBREVIATIONS
- 3MS
Modified Mini-Mental State examination
- ANOVA
Analysis of variance
- BMI
Body mass index
- CHD
Coronary heart disease
- COPD
Chronic obstructive pulmonary disease
- DVT
Digit Vigilance Test
- EDS
Excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- GDS
Geriatric Depression Scale
- IDIS
Iowa Drug Information Service
- IADL
Instrumental activities of daily living
- LWEP
Long wake episodes
- MrOS
Osteoporotic Fractures in Men Study
- PASE
Physical activity scale for the elderly
- PSQI
Pittsburgh Sleep Quality Index
- SE
Sleep efficiency
- SOF
Study of Osteoporotic Fractures
- TST
Total sleep time
- WASO
Wake after sleep onset
REFERENCES
- 1.Ott A, Breteler MM, van Harskamp F, et al. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol. 1998;147:574–80. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16:349–59. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- 6.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 7.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 8.Faubel R, López-García E, Guallar-Castillón P, Graciani A, Banegas JR, Rodríguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–35. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 10.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 12.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 13.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49:1622–7. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 15.Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 16.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26:596–9. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 17.Moore P, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Association between polysomnographic sleep measures and health-related quality of life in obstructive sleep apnea. J Sleep Res. 2001;10:303–8. doi: 10.1046/j.1365-2869.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL Study of Osteoporotic Fractures Group. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69:237–42. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- 19.Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66:485–92. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 20.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163:1626–31. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 21.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 24.Motionlogger User's Guide: Act Millenium. Ardsley NY: Ambulatory Monitoring, Inc; [Google Scholar]
- 25.Action-W User's Guide, Version 2.0. Ardsley NY: Ambulatory Monitoring, Inc; [Google Scholar]
- 26.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 27.Jean-Louis G, Kripke DF, Mason WJ, Elliot JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell T, Redline S, Ancoli-Israeil S, Stone KL. Comparison of total sleep time from actigraphy and polysomnography in older men. The MrOS Sleep Study. Sleep. 2007;30:A346–7. [Google Scholar]
- 29.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 32.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 33.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 34.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–4. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 35.Lewis RF, Rennick PM. Manual for the repeatable cognitive-perceptual-motor battery. Grosse Point Park, MI: Axon Publishing; 1979. [Google Scholar]
- 36.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh J, Yesavage J. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 38.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and Evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 40.Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital – Health Statistics-series 1: Programs – collection procedures. 1987;21:1–115. [PubMed] [Google Scholar]
- 41.Pincus T, Summey JA, Soraci SA, Jr, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 42.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 43.Bédard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP) J Clin Exp Neuropsychol. 1993;15:330–41. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- 44.Naëgelé B, Thouvard V, Pépin JL, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- 45.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]