Abstract
Study Objectives:
To investigate differences in cortical thickness in narcolepsy patients with cataplexy and control subjects.
Design:
Cortical thickness was measured using a 3-D surface-based method that enables more accurate measurement in deep sulci and localized regional mapping.
Setting:
University hospital.
Patients and Participants:
We enrolled 28 patients with narcolepsy and cataplexy and 33 age-and sex-matched control subjects.
Interventions:
Cortical thickness was measured using a direct method for calculating the distance between corresponding vertices from inner and outer cortical surfaces.
Measurements and Results:
We normalized cortical surfaces using 2-D surface registration and performed diffusion smoothing to reduce the variability of folding patterns and to increase the power of the statistical analysis. Localized cortical thinning in narcolepsy patients with cataplexy was found in orbitofrontal gyri, dorsolateral and medial prefrontal cortexes, insula, cingulate gyri, middle and inferior temporal gyri, and inferior parietal lobule of the right and left hemispheres at the level of a false discovery rate P < 0.05. No significant local increases in cortical thickness were observed in narcolepsy patients. A significant negative correlation was observed between the narcolepsy patients' scores on the Epworth Sleepiness Scale and the cortical thickness of the left supramarginal gyrus.
Conclusions:
Cortical thinning in narcolepsy patients with cataplexy in localized anatomic brain regions may serve as a possible neuroanatomic mechanism of the disturbances in attention, memory, emotion, and sleepiness.
Citation:
Joo EY; Jeon S; Lee M; Kim ST; Yoon U; Koo DL; Lee JM; Hong SB. Analysis of cortical thickness in narcolepsy patients with cataplexy. SLEEP 2011;34(10):1357-1364.
Keywords: Narcolepsy, cataplexy, MRI, cortical thickness
INTRODUCTION
Narcolepsy is characterized by excessive daytime sleepiness (EDS), disruptions of sleep-wake behavior, cataplexies (sudden losses of muscle tone provoked by emotional stimuli), and other rapid eye movement (REM) sleep phenomena, such as sleep paralysis and hypnagogic hallucinations.1
Numerous neuroimaging studies have been performed to characterize the pathophysiology of narcolepsy. Previously, we performed functional imaging studies using 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)2 and 99mTc-ethyl-cysteinate dimer single-photon emission computed tomography (SPECT)3 methods in patients with narcolepsy to better characterize their brain functioning. The results revealed dysfunction of the hypothalamus-thalamus-orbitofrontal pathway, the orbitofrontal cortex, and the inferior parietal lobule.2,3
In the field of brain magnetic resonance imaging (MRI), voxel-based morphometry (VBM) studies in patients with narcolepsy have produced controversial results. One distinct VBM study showed reduced gray matter concentrations (GMCs) in the hypothalamus and nucleus accumbens,4 but a subsequent study failed to find any changes in GMCs in the hypothalamus.5 Another study found GMC reductions in the bilateral inferior temporal and frontal regions.6 Recently, we reported that narcolepsy patients with cataplexy showed reduced GMCs in the hypothalamus, nucleus accumbens, and thalamus bilaterally.7 However, the VBM methods can be inaccurate in representing gray matter morphology, and localization in the sulcal regions where the fine details of the anatomy are often obscured by a partial volume effect. Measuring cortical thickness using the cortical surface has been suggested in studies of gray matter morphometry as a strategy for overcoming the limitation of volumetric analyses.8,9 A cortical-thickness analysis performed at the nodes of a 3-dimensional (3-D) polygonal mesh has the advantage of providing a direct quantitative index of cortical morphology.10 In contrast with GMC or gray matter volumetric analyses, cortical thickness measured from the cortical surfaces differentiates between cortexes of opposing sulcal walls within the same sulcal bed, enabling more precise measurement in deep sulci and analysis of the morphology as a cortical sheet.10
To the best of our knowledge, there have been no studies of cortical thickness in the field of narcolepsy. The aim of this study was to investigate the differences in cortical thickness between narcolepsy patients with cataplexy and healthy control subjects using an advanced surface-based method that allows for more precise thickness measurements of the complex cerebral cortical structure and localized regional mapping.
METHODS
Patients and Control Subjects
We consecutively recruited 32 drug-naïive narcolepsy patients with cataplexy who had no history of taking central nervous system stimulant or cataplexy medication when they visited the sleep center of the university hospital. The diagnosis of narcolepsy with cataplexy was made according to the revised International Classification of Sleep Disorders.11 The presence of cataplexy was determined according to the following criteria suggested by Mignot et al.12: (a) loss of muscle tone, which has a visible effect or involves other muscle groups in addition to the leg muscles; (b) cataplexy occurring more than once a month; (c) the duration of cataplexy is often or always less than 10 minutes; and (d) cataplexy is often or always associated with a normal state of consciousness.
A standard polysomnographic study comprising 1 overnight recording followed by Multiple Sleep Latency Test (MSLT) was also performed. The MSLT consisted of 5 naps scheduled at 2-hour intervals starting around 09:00. Patients were invited to lie down on a bed in a dark sound-proofed room and were instructed to try to fall asleep. Sleep latency was defined as the time that elapsed from the start of the test (lights out) to the first 30-second epoch scored as sleep. Each sleep latency test was ended 20 minutes after the onset of sleep or after 20 minutes of wakefulness. A sleep-onset rapid eye movement (REM) period (SOREMP) was defined as 1 or more epochs of rapid eye movement (REM) sleep occurring within 15 minutes of the first 30-second epoch that was scored as sleep.
Subjects with a mean sleep latency of 8 minutes or less and 2 or more SOREMPs on the MSLT were evaluated for human leukocyte antigen (HLA)-DQB1*0602 and DRB1*1501, which are the best genetic predictors of narcolepsy in humans.13,14 Other information—including the presence of sleep attacks, hypnagogic hallucinations, and sleep paralysis, as well as a positive family history of narcolepsy—was obtained from the patients and their families. Four of the patients were excluded because 3 had concomitant mild to moderate obstructive sleep apnea-hypopnea syndrome and 1 patient had negative HLA typing. Finally, 28 patients with narcolepsy with cataplexy were included in the study.
Thirty-three control subjects from a local community were recruited through an advertisement. Each candidate had a detailed clinical interview, a sleep questionnaire, and an overnight polysomnogram, and the results were evaluated and interpreted by 2 sleep medicine specialists (Joo EY, Hong SB). If a control subject had an apnea-hypopnea index of 5 or greater or had evidence for having another sleep disorder, such as periodic limb movement disorder, on polysomnography, he or she was excluded from further participation.
Control subjects, as well as patients with narcolepsy, were excluded if they exhibited any of the following: (a) mean daily sleep time less than 7 hours, (b) abnormal sleep-wake rhythms, (c) other sleep disorders, (d) heart or respiratory disease, (e) history of cerebrovascular disease, (f) other neurologic or psychiatric diseases, (g) alcohol or illicit drug abuse or current intake of psychoactive medications, and (h) a structural lesion on brain MRI.
All narcolepsy patients and control subjects gave written informed consent before the study. The Institutional Review Board at Samsung Medical Center authorized the informed consent form and the study protocol, which included an MRI scan.
HLA Typing
The HLA plays a key role in the etiology of autoimmune disease. As one component of the trimolecular complex (major histocompatibility complex–peptide–T-cell receptor), the presence of specific HLA alleles determines the repertoire of peptide epitopes that can be present, restricting the specificity of reactive T cells.13 The HLA class II region genes, DQB1*0602 and DRB1*1501, are the best genetic predictors of narcolepsy in humans.13,14
Sequence-specific primers and a BigDye Terminator v. 3.1 cycle Sequencing Kit (Applied Biosystems, Foster City, CA) were used for HLA-DQB1*0602 and DRB1*1501 genotyping according to the manufacturer's instructions (Applied Biosystems, USA).
Emotional Tests
To examine the emotional state of the patients with narcolepsy and control subjects, the Beck Depression Inventory (BDI) was administered on the day of the sleep study. The BDI consists of 21 items, falling within 4 factors: cognitive, affective, motivational, and somatic. We used the BDI scores from 2 subscales: general depressive symptoms and somatic symptoms subscale (impaired sleep, somatic symptoms, fatigue, loss of appetite, weight loss, loss of libido).15
Brain MRI Acquisition
MRI scanning was performed using a Signa 1.5 Tesla scanner (GE Medical Systems, Milwaukee, WI). T1-weighted spoiled gradient-recalled coronal images were obtained using the following scanning variables: 1.6 mm thickness, no gap, 124 slices, repetition time/echo time (TR/TE) = 30/7 msec, flip angle (FA) = 45°, number of excitations (NEX) = 1, matrix = 256 × 256, and field of view (FOV) = 22 × 22 cm. The investigators performing the MRI were blinded to the status of subjects (patients vs control subjects).
Cortical Surface Extraction and Measurement of Cortical Thickness
All T1-weighted MR images were submitted to the CIVET pipeline developed by the Montreal Neurological Institute (MNI) for measurement of cortical thickness. An overview of the processing pipeline is described in Figure 1. First, intensity and nonuniformity artifacts were corrected using N3 algorithms.16 Non-brain tissues were removed using a BET algorithm, and brain images were registered into ICBM-152 stereotaxic space using affine linear transformation. After the normalization, brain images were segmented into gray matter, white matter, cerebrospinal fluid, and background using an artificial neural network-based method.17,18
Figure 1.
An outline of the cortical thickness measurement pipeline. (A) Data are registered into MNI (Montreal Neurological Institute)152 stereotaxic space after nonuniformity artifact correction. (B) Data are classified into 3 tissues (white matter, gray matter, or cerebrospinal fluid) and background. (C) Inner-brain mask for 3-D surface deformable model. (D) Reconstructed white matter surface. (E) The gray matter surface, which is expanded out from the white matter surface by a Laplacian map. (F) The cortical thickness was measured by the t-link method between corresponding vertexes of the gray matter and white matter surface. Thickness was smoothed using 20-mm surface-based kernels, mapped on a common template.
The white matter surfaces for each hemisphere were extracted using the deformable model, which started with a relatively small number of polyhedral shape vertexes. Over the iterations, vertexes were increased by a subdividing method until the surfaces had a relatively high resolution, which consisted of 81,920 polygons of discrete triangular elements. Then, the gray matter surfaces were extracted using a constrained Laplacian-based automated segmentation with proximities (CLASP) algorithm,19 which expanded the boundary between gray matter and cerebrospinal fluid along the Laplacian map. The CLASP algorithm was validated by comparing the results with the simulated20 and manually drawn data.9
The extracted white matter and gray matter surfaces were normalized and interpolated for corresponding vertexes between individual subjects using surface-based registration.21 Finally, surfaces were inversely transformed back into the native space and the cortical thickness was calculated using the t-link method, which measured the Euclidean distances between the corresponding vertexes of the gray matter and white matter surfaces.22
Statistical Analysis
The measured cortical thicknesses were smoothed using the surface-based diffusion smoothing method21 with 20-mm full-width half-maximum kernels. Previous studies have shown that the 20-mm kernel size could bring out the maximal statistical power while minimizing the false positive errors.23
The statistical analysis was performed using Surfstat toolbox24 and SPSS 16.0 (Chicago, IL). To estimate the neuroanatomic alterations between patients with narcolepsy and control subjects, we used a general linear model that controlled for age, sex, and intracranial volume (ICV) at each vertex and mean cortical thickness respectively.10 The multiple-comparisons problem was corrected for by the false discovery rate (FDR, P < 0.05, 2-tailed). Both hemispheres were tested together according to the equation,
where Y is the cortical thickness, b0 is the Y intercept, b1∼4 are the regression coefficients, and ε is the residual error.
For the analysis of the correlation of cortical thickness and the clinical scores, only the patients with narcolepsy were selected to avoid confounding the clinical state. Cortical thickness was regressed against the clinical scores at each vertex using age, sex, and ICV as covariance parameters. To explore significant clusters, a permutation test was performed (uncorrected P < 0.001)25 according to the following equation,
where Y is the cortical thickness, b0 is the Y intercept, b1∼4 are the regression coefficients, and ε is the residual error.
RESULTS
Clinical Characteristics
All subjects were right-handed. The age of onset for EDS in patients was 16.5 ± 5.9 years (range, 6.2–32.5 y) and that of cataplexy was 21.6 ± 6.0 years (range, 12–37.2 y). The mean duration of EDS was 11.0 ± 6.9 years and that of cataplexy was 6.3 ± 5.4 years in patients. Twenty-one patients (76%) had hypnagogic or hypnapompic hallucinations, and 18 patients (67%) had a history of sleep paralysis. The mean Epworth Sleepiness Scale score was 16.7 ± 5.9 in patients versus 4.3 ± 1.3 in control subjects (t test, P < 0.01). All patients showed positive HLA typing (DR2 and DQB1*0602). Further characteristics and the sleep-study findings in all subjects are summarized in Table 1 (control subjects underwent only overnight polysomnography and not the MSLT). Patients with narcolepsy reported feeling significantly more depressed than did control subjects (BDI scores 13.5 ± 4.2 vs 6.3 ± 3.1, P = 0.022) and experiencing general depressive symptoms (10.8 ± 4.3 vs 4.1 ± 2.0, P = 0.035) but not somatic symptoms on the BDI subscale (3.5 ± 1.8 vs 2.2 ± 1.6, P = 0.281). No patients had taken antidepressants or had been previously diagnosed with major depressive disorder.
Table 1.
Sleep study findings in patients with narcolepsy and control subjects
| Patients with arcolepsy (n = 28) | Control subjects (n = 33) | P value | |
|---|---|---|---|
| Men:women, no. | 10:18 | 15:18 | 0.441 |
| Age, y | 26.9 ± 7.9 (19-44) | 30.1 ± 11.1 (20-44) | 0.212 |
| Overnight polysomnography | |||
| Sleep latency, min | 4.1 ± 4.2 (0-21) | 12.8 ± 1.5 (10 - 15) | < 0.001a |
| REM sleep latency, min | 49.1 ± 55.6 (0-203) | 95.5 ± 14.3 (75-149) | 0.015a |
| AHI, no. of events/h | 2.0 ± 2.1 (0-7.3) | 3.7 ± 2.0 (0-6.5) | 0.546 |
| AI, no. of events/h | 15.4 ± 6.3 (5.2-32.1) | 15.6 ± 5.2 (5.2-28.0) | 0.630 |
| Multiple Sleep Latency Test | |||
| Mean sleep latency, min | 2.4 ± 1.9 (0.2-5.6) | ||
| SOREMP, no. | 3.8 ± 1.2 (2-5) | ||
| Mean REM sleep latency, min | 3.8 ± 2.9 (0.2-10.7) |
Data are presented as mean value ± standard deviation (range). REM, rapid eye movement; AHI, apnea-hypopnea index; AI, arousal index; SOREMP; sleep-onset rapid eye movement period.
Independent t test, P < 0.05.
All patients and control subjects underwent a brain MRI with the same protocol, which revealed no gross abnormal findings on visual inspection.
Cortical Thickness Analysis
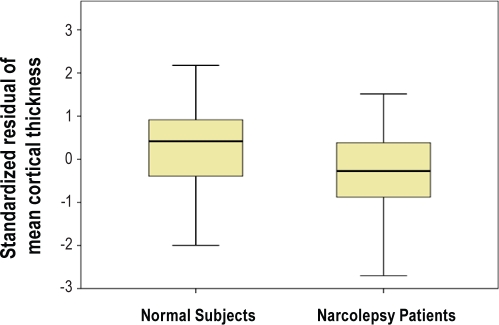
Compared with the cortical thickness of control subjects, the mean cortical thickness of narcolepsy patients with cataplexy was significantly thinner, after controlling for age, sex, and ICV (t = 2.349, P = 0.022; Figure 2).
Figure 2.
Comparison of mean cortical thickness. Standardized residuals of mean cortical thickness, after controlling for age, sex, and intracranial volume, show significant differences between patients with narcolepsy and control subjects (P < 0.05, 2-tailed).
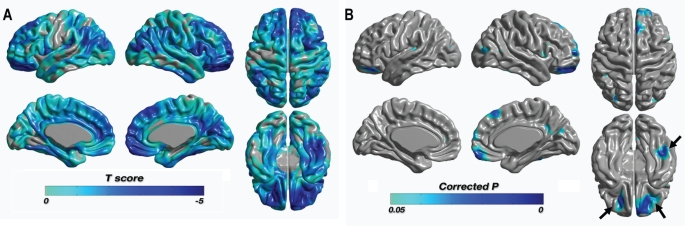
A significant localized thinning of cortical thickness in patients was found in the right orbitorectal gyri, the right dorsolateral (superior/middle/inferior) frontal gyri, the right medial frontal gyrus, the left dorsolateral (middle/inferior) frontal gyri, the right cingulate gyrus, the right and left insular cortexes, the right middle and inferior temporal gyri, the left posterior parietal lobule, the right precuneus, and the left middle occipital gyrus at the level of a FDR P < 0.05 (see Table 2, Figure 3). There were no brain regions that showed increased cortical thickness in patients.
Table 2.
Brain regions showing significant decrease in the cortical thickness using a 3-D surface-based method in narcolepsy patients with cataplexy, compared with control subjects
| Location | BA | Side | MNI Coordinates (mm) |
t value at peak vertex | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Orbital Gyrus | 11 | R | 3.8113 | 54.8979 | −23.6106 | 3.8251 |
| Rectal Gyrus | 11 | R | 10.1794 | 46.0596 | −27.5407 | 3.5836 |
| Superior Frontal Gyrus | 8 | R | 3.6216 | 29.6032 | 53.1333 | 3.9518 |
| 10 | R | 26.3899 | 62.3889 | 6.2533 | 3.9573 | |
| 11 | R | 21.5543 | 45.6995 | −18.4682 | 4.4662 | |
| Middle Frontal Gyrus | 10 | R | 28.4052 | 60.4533 | 9.3403 | 3.9015 |
| 11 | R | 16.937 | 46.7533 | −20.2598 | 4.3043 | |
| 11 | L | −21.8889 | 31.969 | −20.1297 | 3.8823 | |
| Inferior Frontal Gyrus | 11 | R | 22.4247 | 37.3808 | −20.6315 | 4.5868 |
| 11 | L | −18.773 | 40.0731 | −20.3163 | 4.5688 | |
| Medial Frontal Gyrus | 8 | R | 3.7983 | 27.9762 | 49.4819 | 3.6868 |
| 11 | R | 8.1522 | 63.3977 | −16.8404 | 4.3218 | |
| Cingulate Gyrus | 31 | R | 8.8418 | −54.7904 | 27.9109 | 3.4456 |
| Insula | 13 | R | 46.2955 | 10.1583 | 0.4361 | 3.2705 |
| 44 | R | 40.6132 | 7.3173 | 7.7049 | 3.3362 | |
| 13 | L | −39.5428 | −29.6356 | 13.6789 | 3.3094 | |
| Middle Temporal Gyrus | 39 | R | 50.2511 | −58.9136 | 9.2086 | 3.4188 |
| Inferior Temporal Gyrus | 37 | R | 47.5631 | −40.9257 | −21.4293 | 4.6071 |
| Inferior Parietal Lobule | 40 | L | −45.5707 | −44.0952 | 42.355 | 3.2668 |
| Precuneus | 23 | R | 4.6614 | −62.6397 | 19.9941 | 3.2644 |
| Middle Occipital Gyrus | 19 | L | −28.4317 | −81.9936 | 17.0961 | 4.1555 |
Note that all reporting peak vertexes are survived at the level of a false discovery rate P < 0.05. MNI, Montreal Neurological Institute; BA, Brodmann area; B, bilateral; L, left; R, right.
Figure 3.
Statistical maps of differences in cortical thickness between narcolepsy patients with cataplexy and control subjects. (A) Statistical t-map with t-value range of −4.607 to 2.239 and positive values truncated. Most of the cortical area was thinner than in healthy control subjects. (B) There were no significantly thicker regions than in the healthy control subjects. Only thinner regions were significant (marked as blue) at the level of false discovery rate corrected P < 0.05. Clusters of bilateral inferior frontal gyrus and right inferior temporal gyrus (arrows) were also significant with a permutation test (P < 0.001). The left-hand side of the images represent the left hemisphere of the brain.
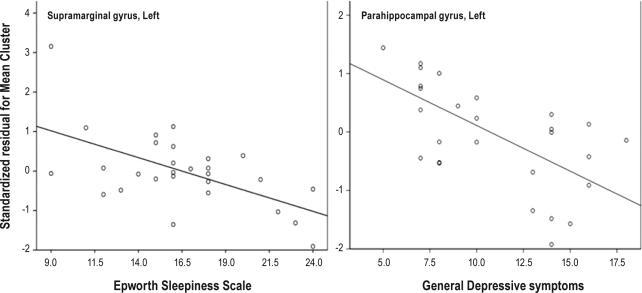
In patients, correlation analyses with the confounders of age, sex, and ICV showed that cortical thickness in the left supramarginal gyrus was negatively correlated with their score on the Epworth Sleepiness Scale (r = −0.698, P = 0.018) (Figure 4). The BDI scores of general depressive symptoms were negatively correlated with the cortical thickness in the left parahippocampal gyrus (r = −0.672, P = 0.016) but not somatic scores (Figure 4). There was no significant association between cortical thickness and duration of EDS or cataplexy, as well as other clinical factors or polysomnography parameters in the patients.
Figure 4.
Correlation analysis between cortical thickness and scores on the Epworth Sleepiness Scale and Beck Depression Inventory. With the confounders of age, sex, and intracranial volume controlled, negative correlation was observed between cortical thickness in the left supramarginal gyrus and the score on the Epworth Sleepiness Scale (r = −0.698, P = 0.018) (left side) and between the cortical thickness in the left parahippocampal gyrus and the scores of general depressive symptoms on the Beck Depression Inventory (r = −0.672, P = 0.016) (right side).
DISCUSSION
In the present study, a cortical thickness analysis was performed on brain MRI studies to identify cerebral structural abnormalities in narcolepsy patients with cataplexy, compared with age- and sex-matched control subjects.
Cortical Thinning of the Medial and Dorsolateral Prefrontal and Inferior Parietal Lobules
The hypocretin neurons play an essential role in driving arousal and in maintaining normal wakefulness.26 A lack of hypocretin neurotransmission produces a chronic state of hypoarousal that is characterized by EDS, frequent transitions between wake and sleep, and episodes of cataplexy.27 More than 90% of narcolepsy patients with cataplexy have very low or undetectable hypocretin levels in their CSF.28 The medial prefrontal cortex is known to be one of several loci that receive excitatory projections from hypocretin neurons through the paraventricular thalamic nucleus,29 and this region is associated with motivation, attention, and the processes of working memory.30 Many patients with narcolepsy have attention deficit or memory decline, with as many as 50% reporting recent memory disturbances.31,32 Attention and memory are closely related, and, thus, a person with attention impairment frequently complains of memory impairment rather than difficulties with attention. The dorsolateral prefrontal cortex and the parietal cortex, which are referred to as part of the executive attention network, are implicated in directing attention, according to the requirements of a task in a PET study.33 Our findings showed significant cortical thinning in the medial and dorsolateral prefrontal cortex and in the inferior parietal lobule in narcolepsy patients with cataplexy, and these regions are related to the executive attention network and working memory processes. Moreover, we found that the greater the EDS of patients, the greater the cortical thinning in the left supramaginal gyrus. Functional MRI study revealed that stronger linear responses to a Baddeley's logical reasoning task were found after total sleep deprivation in several brain regions, including bilateral inferior parietal lobes and left dorsolateral prefrontal cortex.34 During conditions of sustained attention in functional MRI tasks, bilateral activation of the prefrontal and parietal cortexes were found in patients with narcolepsy.35 The critical role of the inferior parietal cortex in mediating reflexive shifts of attention within and between sensory modalities has been duplicated in a transcranial magnetic stimulation study.36 Well-designed neuropsychological examinations have found deficits in divided and flexible attention in narcolepsy.37 Thus, cortical thinning in the dorsolateral/medial prefrontal and inferior parietal lobules of the patients may account for attention deficits and memory decrements and may play a role in the EDS of narcolepsy patients with cataplexy.
Cortical Thinning of the Orbitofrontal, Insula, and Cingulate Regions
Depressive and neurotic symptoms are considered to be common in narcolepsy. More than 30% of patients with narcolepsy report having a depressive mood.38 An H215O-PET study of patients with familial depressive disorders showed reduced cerebral activity and cortical volume loss in the frontal cortex ventral to the genu of the corpus callosum.39 Cortical thinning of the orbitofrontal gyri in this study also may explain the presence of a depressive tendency in patients with narcolepsy. Our patients reported more depressive feelings on the BDI, although there was no one who had taken antidepressants or who had been previously diagnosed with major depressive disorder. The insular cortex has numerous connections with the cerebral cortex, basal ganglia, and limbic structures. Patients with mesial temporal lobe epilepsy and emotional symptoms have been shown to have hypometabolism in the anterior insula.40 The anterior cingulate and the ventromedial prefrontal cortex, including the orbitorectal gyri, participate in awareness and emotional processing.41,42 A functional MRI study has shown that emotional pictures result in increased blood flow in the cingulate gyrus and anterior temporal regions, including the amygdala.43 Our previous SPECT study revealed that cerebral perfusion was increased in the bilateral premotor cortexes, the cingulate gyri, the sensorimotor cortexes, and the right insula during cataplexy, compared with during the awake non-catapletic period.44 These findings may imply that the insula, cingulate, and fronto-temporal areas are inactive or in a dysfunctional state during the asymptomatic state, but they become overactivated in response to emotional changes that may trigger a cataplectic pathway. All patients involved in this study had cataplexy, which was evoked by emotional stimuli. Scores of general depressive symptoms in the BDI were negatively correlated with the cortical thickness of left parahippocampal gyrus in the patients but somatic scores in BDI were not. In depressed patients, significantly greater activation in the parahippocampal gyrus was found during the working memory task in a functional MRI study45 and a lower baseline cerebral metabolism was found in the parahippocampal gyrus as well as the anterior cingulate and insula regions.46 Considering the functional connectivity of parahippocampal gyrus with limbic cortexes, the irritability and emotional lability of patients with narcolepsy might represent an intrinsic biochemical deficit of the condition rather than secondary phenomenon related to symptom adaptation.47
Therefore, the significant cortical thinning of the insula, the cingulate, the orbitofrontal, and the anterior temporal cortexes that was found in this study may suggest that these structural abnormalities in parts of the limbic circuit might be related to depressive mood and emotional instability frequently observed in narcolepsy patients with cataplexy.
Comparison of the Cortical-thickness Analysis and Voxel-based Morphometry Results in Narcolepsy
MRI morphometric analyses of the brain have become a widely used approach to investigate neuroanatomic correlates of neurologic disorders. A commonly used method for performing voxel-based comparisons of gray matter is known as VBM.48 On the contrary, the estimation of cortical thickness based on T1-weighted images represents a viable methodologic alternative to volumetric measurements for the assessment of subtle cortical changes in the human brain.49 Previous VBM studies of narcolepsy have produced somewhat different results.4–7 Even though these studies used the same VBM method, the detailed processes used (e.g., the SPM version, modulated or unmodulated, grand mean scaling, absolute or relative thresholding and study subjects) differed,50 and thus, the VBM results may be inconsistent. The results of a cortical-thickness analysis may be less subject to variation between laboratories because thickness-measurement procedures are quite consistent.51 Interestingly, the spatial distribution of cortical thinning over the brain in this study and of the reduced GMCs in our previous VBM study were very similar even though the population of patients and control subjects and the study periods were different between the 2 studies. Cortical thinning of the dorsolateral prefrontal, orbitofrontal, and temporal cortexes is consistent with the areas in which we observed significant reduced GMCs in patients with narcolepsy.7 The only exception was that GMCs in the bilateral nuclei accumbens, hypothalami, and thalami were definitely reduced in the VBM analysis. The regions of interest in the cortical-thickness analysis did not involve subcortical areas such as the thalamus or hypothalamus. The similarities between our VBM and cortical thickness studies support the results of the present study.
In conclusion, this work presents a pattern of regional decrease in the cortical thickness in narcolepsy patients with cataplexy. It was not clear whether decreases in cortical thickness in multiple brain cortexes of narcoleptic brains are developmental or acquired findings because this study was conducted as a cross-sectional analysis and there was no significant correlation between cortical thickness and duration of EDS or cataplexy. But, the cortical thinning of the dorsolateral and medial prefrontal, orbitofrontal, inferior parietal lobules, insula, and cingulate in patients may serve as a possible neuroanatomic explanation of the disturbances in attention, memory, emotion, and sleepiness of narcolepsy patients with cataplexy.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by a Grant (2010K00817) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science, and Technology, and by a Grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A110097).
REFERENCES
- 1.Guilleminault C, Dement WC. 235 cases of excessive daytime sleepiness. Diagnosis and tentative classification. J Neurol Sci. 1977;31:13–27. doi: 10.1016/0022-510x(77)90003-x. [DOI] [PubMed] [Google Scholar]
- 2.Joo EY, Tae WS, Kim JH, Kim BT, Hong SB. Glucose hypometabolism of hypothalamus and thalamus in narcolepsy. Ann Neurol. 2004;56:437–40. doi: 10.1002/ana.20212. [DOI] [PubMed] [Google Scholar]
- 3.Joo EY, Hong SB, Tae WS, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005;28:410–16. doi: 10.1016/j.neuroimage.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Draganski B, Geisler P, Hajak G, et al. Hypothalamic gray matter changes in narcoleptic patients. Nat Med. 2002;8:1186–8. doi: 10.1038/nm1102-1186. [DOI] [PubMed] [Google Scholar]
- 5.Overeem S, Steens SC, Good CD, et al. Voxel-based morphometry in hypocretin-deficient narcolepsy. Sleep. 2003;26:44–6. [PubMed] [Google Scholar]
- 6.Kaufmann C, Schuld A, Pollmacher T, Auer DP. Reduced cortical gray matter in narcolepsy: preliminary findings with voxel-based morphometry. Neurology. 2002;58:1852–5. doi: 10.1212/wnl.58.12.1852. [DOI] [PubMed] [Google Scholar]
- 7.Joo EY, Tae WS, Kim ST, Hong SB. Gray matter concentration abnormality in brains of narcolepsy patients. Korean J Radiol. 2009;10:552–8. doi: 10.3348/kjr.2009.10.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13:375–80. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- 10.Im K, Lee JM, Lee J, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31:31–8. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Sleep Medicine. Diagnostic & Coding Manual. 2nd ed. Westchester, IL: The American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders. [Google Scholar]
- 12.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–20. [PubMed] [Google Scholar]
- 13.Doherty DG, Penzotti JE, Koelle DM, et al. Structural basis of specificity and degeneracy of T cell recognition: pluriallelic restriction of T cell responses to a peptide antigen involves both specific and promiscuous interactions between the T cell receptor, peptide, and HLA-DR. J Immunol. 1998;161:3527–35. [PubMed] [Google Scholar]
- 14.Chabas D, Taheri S, Renier C, Mignot E. The genetics of narcolepsy. Annu Rev Genomics Hum Genet. 2003;4:459–83. doi: 10.1146/annurev.genom.4.070802.110432. [DOI] [PubMed] [Google Scholar]
- 15.Lee YH, Song JY. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Korean J Clin Psychol. 1991;10:98–113. [Google Scholar]
- 16.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 17.Zijdenbos A, Forghani R, Evans A. Automatic quantification of MS lesions in 3D MRI brain data sets: validation of INSECT. In: Wells WM, Colchester A, Delp S, editors. Medical Image Computing and Computer-Assisted Interventation (MICCAI98) Cambridge, MA: Springer-Verlag; 1998. pp. 439–48. [Google Scholar]
- 18.Zijdenbos AP, Evans AC, Riahi F, Sled JG, Chui J, Kollakian V. Automatic quantification of multiple sclerosis lesion volume using stereotaxic space. Lecture Notes in Computer Science; Proceedings of the 4th International Conference on Visualization in BioMed Computing VBC; 1996. pp. 439–48. [Google Scholar]
- 19.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–21. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Lee JK, Lee JM, Kim JS, Kim IY, Evans AC, Kim SI. A novel quantitative cross-validation of different cortical surface reconstruction algorithms using MRI phantom. Neuroimage. 2006;31:572–84. doi: 10.1016/j.neuroimage.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 21.Robbins S, Evans AC, Collins DL, Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 2004;8:311–23. doi: 10.1016/j.media.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–56. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 23.Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cereb Cortex. 2005;15:995–1001. doi: 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- 24.Worsley KJ, Taylor JE, Carbonell F, et al. SurfStat: a Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. NeuroImage. 2009;47:S102. [Google Scholar]
- 25.Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- 27.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 28.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 29.Bubser M, Deutch AY. Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res. 1998;787:304–10. doi: 10.1016/s0006-8993(97)01373-5. [DOI] [PubMed] [Google Scholar]
- 30.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 31.Aguirre M, Broughton R, Stuss D. Does memory impairment exist in narcolepsy-cataplexy? J Clin Exp Neuropsychol. 1985;7:14–24. doi: 10.1080/01688638508401239. [DOI] [PubMed] [Google Scholar]
- 32.Schulz H, Wilde-Frenz J, Grabietz-Kurfurst U. Cognitive deficits in patients with daytime sleepiness. Acta Neurol Belg. 1997;97:108–12. [PubMed] [Google Scholar]
- 33.Gazzaniga MS, Ivry RB, Mangun GR. Cognitive Neuroscience: The Biology of the Mind. New York, NY: W.W. Norton & Company; 2002. pp. 244–300. [Google Scholar]
- 34.Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27:445–51. [PubMed] [Google Scholar]
- 35.Thomas RJ. Fatigue in the executive cortical network demonstrated in narcoleptics using functional magnetic resonance imaging—a preliminary study. Sleep Med. 2005;6:399–406. doi: 10.1016/j.sleep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Chambers CD, Payne JM, Mattingley JB. Parietal disruption impairs reflexive spatial attention within and between sensory modalities. Neuropsychologia. 2007;45:1715–24. doi: 10.1016/j.neuropsychologia.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Rieger M, Mayer G, Gauggel S. Attention deficits in patients with narcolepsy. Sleep. 2003;26:36–43. [PubMed] [Google Scholar]
- 38.Vandeputte M, de Weerd A. Sleep disorders and depressive feelings: a global survey with the Beck depression scale. Sleep Med. 2003;4:343–5. doi: 10.1016/s1389-9457(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 39.Drevets WC, Price JL, Simpson JR, Jr., et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 40.Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol. 2002;51:202–8. doi: 10.1002/ana.10087. [DOI] [PubMed] [Google Scholar]
- 41.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–74. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Park KY, Seo SW, et al. Reversible Verbal and Visual Memory Deficits after Left Retrosplenial Infarction. J Clin Neurol. 2007;3:62–66. doi: 10.3988/jcn.2007.3.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee GP, Meador KJ, Loring DW, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol. 2004;17:9–17. doi: 10.1097/00146965-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Hong SB, Tae WS, Joo EY. Cerebral perfusion changes during cataplexy in narcolepsy patients. Neurology. 2006;66:1747–9. doi: 10.1212/01.wnl.0000218205.72668.ab. [DOI] [PubMed] [Google Scholar]
- 45.Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry. 2011;168:173–82. doi: 10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- 46.Smith GS, Workman CI, Kramer E, et al. The relationship between the acute cerebral metabolic response to citalopram and chronic citalopram treatment outcome. Am J Geriatr Psychiatry. 2011;19:53–63. doi: 10.1097/jgp.0b013e3181eafde4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise MS. Narcolepsy and other disorders of excessive sleepiness. Med Clin North Am. 2004;88:597–610. vii–viii. doi: 10.1016/j.mcna.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 49.Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–80. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 51.Lin JJ, Salamon N, Lee AD, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]