Abstract
Study Objectives:
To examine developmental changes in the human sleep electroencephalogram (EEG) during late adolescence.
Setting:
A 4-bed sleep laboratory.
Participants:
Fourteen adolescents (5 boys) were studied at ages 15 or 16 (initial) and again at ages 17 to 19 (follow-up).
Interventions:
N/A
Measurements and Results:
All-night polysomnography was recorded at each assessment and scored according to the criteria of Rechtschaffen and Kales. A 27% decline in duration of slow wave sleep, and a 22% increase of stage 2 sleep was observed from the initial to the follow-up session. All-night spectral analysis of 2 central and 2 occipital leads revealed a significant decline of NREM and REM sleep EEG power with increasing age across frequencies in both states. Time-frequency analysis revealed that the decline in power was consistent across the night for all bands except the delta band. The decreases in power were most pronounced over the left central (C3/A2) and right occipital (O2/A1) derivations.
Conclusions:
Using longitudinal data, we show that the developmental changes to the sleeping EEG that begin in early adolescence continue into late adolescence. As with early adolescents, we observed hemispheric asymmetry in the decline of sleep EEG power. This decline was state and frequency nonspecific, suggesting that it may be due to the pruning of synapses known to occur during adolescence.
Citation:
Tarokh L; Van Reen E; LeBourgeois M; Seifer R; Carskadon MA. Sleep EEG provides evidence that cortical changes persist into late adolescence. SLEEP 2011;34(10):1385–1393.
Keywords: Sleep EEG, development, adolescence, spectral analysis
INTRODUCTION
The human brain enters a new phase in late childhood involving significant cortical restructuring. That this brain reorganization is reflected in the sleep EEG is known for early adolescents but less well examined with longitudinal data from older adolescents. The brain—that had until now been building synaptic connections—begins to prune them. Postmortem studies indicate that pruning of cortical synapses in the healthy human brain across the second decade is expansive throughout the cortex.1 Some have proposed that this process is reflected in MRI structural scans as reduced cortical gray matter. Thus, a number of cross-sectional2–4 and longitudinal5–7 MRI studies show that cortical gray matter declines between the ages of about 9 to 25 years. Furthermore, the age at which this decline in gray matter begins appears to differ among cortical regions.3,5–7 One study using a combined longitudinal and cross-sectional approach, for example, found that the gray matter decline begins as early as 6.8 years in the left occipital pole and as late as 18.1 years in the right temporal insula.7
The pruning of synapses across adolescence may be reflected in other measures of neural activity, including positron emission tomography (PET)8 and the electroencephalogram (EEG).4,9,10 One cross-sectional study bridged the gap between MRI and waking EEG measures by recording both in healthy individuals ages 10 to 30 years.4 In this study, 2 min of awake eyes closed EEG was recorded in subjects who had undergone an MRI scan. This study found a decline in EEG power and cortical gray matter with age, and the authors attributed both findings to synaptic pruning. A more recent study confirmed this association by examining the relationship between the sleeping EEG and MRI in 41 healthy children and adolescents ages 8 to 19 years.11 An expected decline in EEG spectral power and gray matter volume was found across age. These authors also reported a significant correlation of delta power (1-4.5 Hz) at derivation C4 and gray matter volume in a number of cortical regions, suggesting that sleep EEG activity is a reliable index of the structural changes in the brain.
Indeed, because the EEG is a measure of synchronous synaptic activity, reduced EEG signal amplitude likely reflects decreased number of synapses. A number of cross-sectional waking EEG studies support this association, showing a diminution of EEG spectral power with increasing age.9,12–14 Furthermore, this decline in EEG spectral power appears for most frequencies, supporting the notion that the EEG reflects a global process, such as synaptic pruning. For example, a cross-sectional study by Boord et al.9 examined waking EEG power in 1831 subjects, ages 6 to 86 years, and found a decline in power with age during adolescence across frequencies in the delta (1.4-3.5 Hz), theta (4-7.5 Hz), alpha (8-13 Hz), and beta (14.5-30 Hz) bands.
As with MRI studies, waking EEG studies have shown regional differences in the timing of the decline of spectral power. One such study examined left and right central and occipital electrodes in 222 healthy males ages 4 to 90 years and showed that power at the occipital derivations in young children (ages 4 to 8) exceeded power at central derivations; by adulthood, however, the occipital-central differences were minimal.14 The combination of crude age categorization (13- to 17-year-olds in one category and 18- to 30-year-olds in another category) and the cross-sectional nature of the study limit the assessment of EEG power changes during adolescence. Another cross-sectional study recorded waking EEG at 8 derivations (F4, F3, C3, C4, Cz, Pz, O2, and O1) across a narrower age range (6 to 17 years) in 158 healthy individuals.12 This study provided evidence for a decline in EEG power that began in posterior electrodes (O1 and O2) and progressed towards the frontal regions with age. In summary, waking EEG studies indicate that the decline in power is frequency nonspecific and shows a posterior to anterior progression of the change.
When the sleep EEG has been examined in cross-sectional and longitudinal samples, similar declines of EEG power with increasing age have been reported. The cross-sectional study of Gaudreau and colleagues15 recorded sleeping EEG in 54 participants, including children (6-10 years), adolescents (14-16 years), young adults (19-29 years), and middle-aged adults (36-60 years). They reported greatest NREM sleep EEG power in all frequency bands examined (delta, theta, alpha, and sigma) for children compared to the older groups, and EEG spectral power for the adolescent group was in between the children and young adult groups. One EEG electrode (left central, C3/A2) was used; therefore, no regional information was available. Another cross-sectional study, using the same electrode derivation, found that prepubertal children (mean age 11.3 years) had less NREM and REM sleep EEG spectral power than mature adolescents (mean age 14.1 years) across a range of frequencies.16 Kurth and colleagues17 recorded high-density sleep EEG (109 derivations) in 55 subjects ages 2.4 to 19.4 years. As in other studies, this cross-sectional analysis showed a frequency-nonspecific spectral power decline in children over age 10 years.
Several groups have reported findings from sleep EEG recorded in longitudinal studies. Feinberg et al.18 examined sleep in 2 cohorts studied at 6-month intervals spanning ages 9 to 18 years, with an overlap at ages 12 to 15 years. The sleep EEG signal was examined for 2 frequency bands (delta = 1-4 Hz and theta = 4-8 Hz) at 5 EEG derivations (Fz, Cz, C3, C4, and O1) during NREM sleep. They found delta and theta power density declined with age in both cohorts; however, the decline began earlier in the theta band. Delta, but not theta, power showed a “back to front” pattern, with O1 as the first place the power decline began and Fz as the last. Furthermore, they report that the rate of decline in delta and theta power slowed after the age of 16.4 years.19 Tarokh and Carskadon20 used longitudinal data to show reduction of NREM and REM sleep EEG power across frequencies from 1 to 16 Hz in children ages 9 or 10 followed up 1.5 to 3 years later. They also examined power at 4 derivations (left/right central and occipital) and showed that the decline in power was asymmetrical, with the greatest change in power occurring at the right occipital and left central derivations for NREM and REM sleep and for most frequencies.
The overall picture emerging from the study of waking and sleep EEG in adolescence is a state-independent, frequency-independent decline in power with age. Furthermore, data from EEG and MRI studies indicate that adolescent cortical maturation begins in posterior cortices and proceeds anteriorly. There are, however, noteworthy deviations from this pattern: MRI data indicate that a majority of the temporal lobe matures after the frontal lobes and sleeping EEG data indicate that the posterior to anterior progression occurs only for the delta band.17,18 With a few exceptions, however, the aforementioned EEG studies were cross-sectional, limiting the interpretation of the results due to large interindividual variability in EEG power.20,21 The aim of the present study was to extend the literature by examining changes to the sleep EEG in late adolescence. To this end, we analyzed all-night sleep EEG in 14 teens who were 15 or 16 years old, and again 2 to 3 years later at ages 17 to 19 years. We examined sleep EEG changes across 4 derivations, the night, and a range of frequencies. If the decline in sleep EEG power reflects structural changes in the brain (e.g., declines in cortical gray matter volume), this decline should be nonspecific for state and frequency. Furthermore, evidence from imaging studies indicates that the extent and timing of the diminution of cortical gray matter is region specific. Therefore, we hypothesized that we would observe regional differences in the reduction of sleep EEG power in late adolescents.
METHODS
Participants
Participants were recruited using flyers, mailings to previous participants, and radio and newspaper advertisements. Exclusion criteria assessed using questionnaires included a current or chronic illness, evidence of learning disability, sleep disorder, or a pattern of insufficient sleep or excessive daytime sleepiness as indicated by sleeping < 8 h nightly and/or taking ≥ 2 naps per week. Additional exclusion criteria included a personal or family history of psychopathology as assessed through the depression, psychosis and alcohol abuse/dependence modules from the Structured Clinical Interview for DSM-IV22 and the Family History Screen.23
Fourteen participants, ages 15 to 16 years (mean 15.8, SD 0.5) at the time of the initial recording and 17 to 19 years (mean 18.3, SD 0.6) at the time of the follow-up recording participated in this study (Table 1). Ten participants were white, 3 were multiracial, and 1 was black. Nine girls and 5 boys participated in this study. The Lifespan Institutional Review Board for the Protection of Human Subjects approved all procedures, informed consent was obtained from the participants and their parents, and participants received monetary compensation.
Table 1.
Individual and mean data for all participants
| Subject | Sex | Age1 | Age2 | NREM Power Change | Spindle 1 (Hz) | Spindle 2 (Hz) |
|---|---|---|---|---|---|---|
| S1 | F | 16.6 | 19.2 | −3.4 | 13.4 | 13.8 |
| S2 | F | 16.6 | 19.3 | −25.7 | 13.4 | 13.4 |
| S3 | F | 15.1 | 17.6 | −26.2 | 13.2 | 13.6 |
| S4 | M | 15.2 | 17.7 | −13.0 | 12.8 | 12.8 |
| S5 | M | 15.3 | 17.9 | −16.2 | 12.8 | 13.0 |
| S6 | F | 15.4 | 17.9 | −48.5 | 13.0 | 14.0 |
| S7 | F | 15.4 | 17.8 | −17.4 | 13.6 | 14.2 |
| S8 | M | 15.6 | 18.1 | −19.9 | 13.0 | 13.2 |
| S9 | F | 15.6 | 18.1 | −14.7 | 12.6 | 13.0 |
| S10 | M | 15.8 | 18.4 | −19.9 | 13.4 | 13.4 |
| S11 | M | 15.9 | 18.1 | −27.7 | 13.0 | 13.0 |
| S12 | F | 16.2 | 18.9 | −17.3 | 13.0 | 14.2 |
| S13 | F | 16.3 | 18.8 | −21.2 | 13.6 | 14.0 |
| S14 | F | 16.4 | 19.1 | −19.9 | 13.6 | 14.2 |
| Mean | N/A | 15.8 | 18.3 | −20.8 | 13.2 | 13.6 |
| Std | N/A | 0.5 | 0.6 | 10.1 | 0.3 | 0.5 |
Age1, Age at the initial assessment (years); Age2, Age at the follow-up assessment (years); NREM Sleep Power Change, Percent change in mean power from the initial to the follow-up assessment from 0.6 to 16 Hz at electrode C3/A2; Spindle 1, Frequency of the peak in the sigma band at the initial assessment; Spindle 2, Frequency of the peak in the sigma band at the follow-up assessment.
Procedures
Participants slept in the lab on 2 separate occasions, initially when they were 15 or 16 years old (referred to as the Initial recording throughout this paper) and again 26 to 33 months (mean 30, SD 1.7) later (referred to as the Follow-up recording throughout this paper). Before the sleep lab assessments, participants slept on a schedule of ≥ 9 h time in bed (TIB) for at least one week, using the participants' school day rise time to anchor scheduled sleep. Scheduled time in bed was the same at both assessments (see Table 2), although the precise bed and rise times may have varied. The in-lab mean bedtime and rise times at the initial session were 21:42 (SD 23 min) and 06:42 (SD 23 min), respectively, while at the follow-up session bedtime was 22:10 (SD 49 min) and rise time was 07:12 (SD 54 min). This schedule was maintained for at least one week prior to the in-lab. Compliance to the sleep schedule was confirmed using sleep diaries, continuous wrist actigraphy, and daily phone calls to the lab's time-stamped answering machine at rise times and bedtimes. On study days, participants were well slept, healthy, and taking no medications. Participants slept in individual darkened bedrooms while polysomnography (PSG) was recorded for 2 nights on each occasion. The first night included a screen for sleep related breathing abnormalities and periodic limb movements using oral/nasal thermocouples, and leg electromyogram (EMG), respectively. No sleep disorders were detected, i.e., participants experienced few apneas or hypopneas (AHI < 5), no sleep onset REM episodes, and no signs of periodic limb movements or parasomnias. The data from the second night are reported here for all participants, except for one (subject 14) in which the adaptation night was used at the follow-up session due to an extended bout (67 min) of wakefulness on the baseline night.
Table 2.
Sleep stage variables
| Sleep Variable | Initial |
Follow-Up |
P-values |
||||
|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | Sex | Time | Sex × Time | |
| Stage 1 | 35 (11) | 28 (7) | 43 (10) | 32 (9) | 0.02 | 0.11 | 0.65 |
| Stage 2 | 226 (35) | 205 (40) | 259 (40) | 261 (18) | 0.51 | 0.004 | 0.42 |
| SWS | 156 (30) | 180 (49) | 115 (28) | 125 (39) | 0.23 | 0.002 | 0.60 |
| REM | 99 (17) | 103 (18) | 107 (44) | 101 (22) | 0.97 | 0.79 | 0.67 |
| WASO | 10 (13) | 7 (6) | 18 (36) | 6 (6) | 0.38 | 0.70 | 0.64 |
| Sleep latency | 7 (4) | 10 (6) | 11 (5) | 6 (5) | 0.67 | 0.86 | 0.07 |
| REM sleep latency | 165 (51) | 161 (36) | 141 (50) | 165 (50) | 0.59 | 0.62 | 0.46 |
| Total sleep time | 516 (11) | 517 (7) | 524 (58) | 520 (11) | 0.88 | 0.67 | 0.85 |
| Total recording Time | 540 (< 1) | 541 (1) | 557 (40) | 540 (< 1) | 0.38 | 0.39 | 0.35 |
Means (standard deviations) in minutes and P-values of sleep stage variables. SWS, slow wave sleep; WASO, wake after sleep onset; Sleep latency, time in minutes to the first 1.5 consecutive minutes of stage 1 or first stage 2; Sex, main effect of sex (male versus female); Time, main effect of recording session (Initial versus Follow-up); Sex × Time, Interaction of sex and time.
Polysomnography (PSG) Recording
Right and left electrooculogram (EOG), electromyogram (EMG; mentalis, submentalis), and electrocardiogram (ECG) along with 2 central (C3/A2 and C4/A1) and 2 occipital (O1/A2 and O2/A1) EEG electrodes placed according to the international 10-20 system24 were recorded. The recordings were performed on 2 separate systems. The initial recordings were performed using the Albert Grass Heritage System (Astromed, Grass, West Warwick, RI) with GAMMA software. The signals were collected and stored at a sampling frequency of at least 100 Hz, filtered with Grass Model 8 amplifiers (high-pass EEG filter, 0.3 Hz; low-pass EEG filter, 35 Hz; notch filter 60 Hz) and digitized online (12 bit AD converter; Butterworth filter, −12 dB/octave; low pass-filter, −6 dB at 35 Hz, time constant 1.0 s). All follow-up recordings were performed on the TWin system (Astromed, Grass, West Warwick, RI) using TWin AS40 bedside amplifiers, from which the signals were collected digitally with a sampling frequency of 400 Hz and filtered offline (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz; notch filter 60 Hz). Electrode impedance values were below 10 KΩ. A known signal was input into both systems simultaneously to assess whether signals from the two systems were comparable. Signals from the 2 systems were in good agreement from 0.2 to 16 Hz; however, small discrepancies emerged at higher frequencies. Therefore, spectral power > 16 Hz was not examined.
Definition of the Sleep Cycles
Sleep cycles were defined using a modified version of the criteria of Feinberg and Floyd.25 A NREM sleep episode was required to last 15 min, beginning with stage 2 and ending with the occurrence of a REM sleep epoch. Furthermore, the definition of Feinberg and Floyd requires that a REM sleep episode (with the exception of the first REM episode) last ≥ 5 minutes. We modified these criteria, such that a REM sleep episode of any length signaled the end of a NREM sleep episode. An accurate assessment of the sleep EEG spectra, however, requires ≥ 3 min; therefore, only REM sleep episodes that were sufficiently long were used.
Children often “skip” their first REM sleep episode, and because a skipped first REM sleep episode affects calculation of the first and second NREM sleep episodes, we applied the criteria of Jenni and Carskadon16 to correct for this. These criteria consider the first REM sleep episode skipped when a continuous episode of stage 1, 2, awake, or movement time lasting ≥ 12 min is followed by stage 3 or 4 (slow wave sleep; SWS) sleep. According to this criterion, the first NREM sleep episode ends with the epoch preceding the interval of non-SWS, and the second NREM sleep episode begins with the first epoch of SWS following this interval, ending with the onset of REM sleep. Applying these criteria, 4 of the 14 participants skipped their first REM sleep episode on the initial recording session, while 3 skipped their first REM sleep episode on the follow-up recording session.
Power Spectral Analysis
The criteria of Rechtschaffen and Kales26 were used to visually score EEG in 30-sec epochs. Interrater and intrarater reliability were ≥ 86%, and epochs with artifacts were visually identified and rejected for spectral analysis. A fast Fourier transform (Matlab, The MathWorks Inc., Natick, MA) was used to calculate power spectra (0.6 to 16 Hz) on each 30-sec artifact-free epoch at all EEG derivations (C3/A2, C4/A1, O2/A1, and O1/A2). A Hanning window was applied to the data before the transform, and averages of six 5-sec epochs were calculated. The frequency resolution was 0.2 Hz, and the lowest 2 frequency bins, 0.2 and 0.4 Hz, were discarded due to the sensitivity of these bins to noise. Spectra were calculated separately for NREM sleep (stages 2, 3, and 4) and REM sleep.
Time-frequency analyses were performed to examine the evolution of the EEG signal across the night. To account for NREM and REM sleep episodes of different durations across participants, the definition of Jenni and Carskadon16 was used to subdivide each NREM sleep episode into 10 equal intervals, and each REM sleep episode into 3 equal intervals. Therefore, all participants had an equal number of intervals over which to average and perform statistical testing. All participants had ≥ 4 NREM sleep and 3 REM sleep episodes; therefore, only these cycles were further analyzed.
Statistics
Statistical analysis of the sleep stage variables was made using a 2 (sex: male versus female) by 2 (recording session: initial versus follow-up) repeated-measures analysis of variance (ANOVA), with α set to 0.05.
The EEG power changes in the all-night spectra in addition to the time-frequency spectra were assessed using a bootstrap test. The bootstrap method makes no assumptions regarding the distribution of the data set; its application to sleep EEG data has been described.20,27 The principle underlying the bootstrap is to generate a random sample, called the bootstrap distribution, by randomly sampling (with replacement) from the original pool of data. Separate bootstrap tests were performed at each frequency and derivation for the all-night spectra; individual bootstrap tests were performed at each frequency, derivation, and time point for the time-frequency data.
The large number of statistical tests raises the issue of multiple comparisons; however, traditional approaches to correct for multiple comparisons such as the Bonferroni correction are too conservative for EEG data because they are based on the assumption that each sample is independent. Furthermore, Maris and Oostenveld29 have shown that such nonparametric tests as the bootstrap applied to EEG and MEG data control the false alarm rate. Nonetheless, we addressed the issue of multiple comparisons in two ways: first, we reported statistically significant effects only when they were present at the same frequency for multiple electrodes or at adjacent frequency bins; second, we reported P-values so that the magnitude of the statistical significance would be apparent.
We also assessed whether the age-related decline in sleep EEG power was consistent across the night. Thus, we averaged spectra within each of the first 4 NREM sleep and first 3 REM sleep episodes and performed an ANOVA at each frequency bin with factors episode (1-4 for NREM sleep, and 1-3 for REM sleep) and assessment (initial versus follow-up). We examined the interaction term to assess whether the developmental decline in power was the same across episodes.
RESULTS
Sleep Stage Variables
As noted in the methods section, total recording time and total sleep time were nearly identical at both assessments (Table 2). In contrast, we found a significant decline in minutes of slow wave sleep (P = 0.002) and a significant increase in minutes of stage 2 sleep (P = 0.004) with maturation. Within-subjects calculations showed a 27% decline in SWS and a 22% increase in stage 2 sleep. In addition, girls had approximately 10 minutes more stage 1 sleep than boys at both recording sessions (P = 0.02).
All-Night Power Spectra
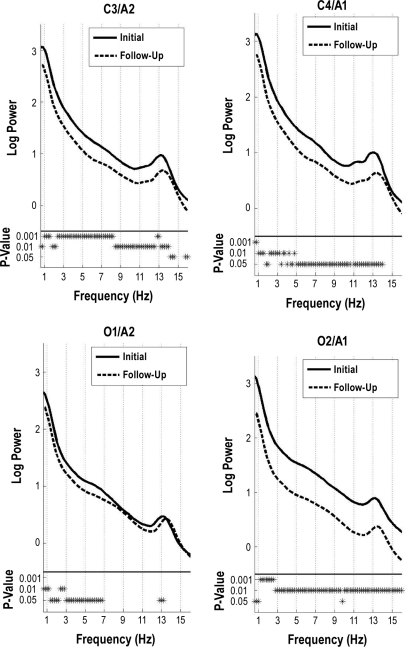
Sleep EEG power decreased from the initial to the follow-up assessments during NREM and REM sleep. For NREM sleep, power in the 2 central derivations (C3/A2 and C4/A1) was diminished at follow-up compared to the initial session at all frequencies except a narrow band in the high sigma range (Figure 1). The decline in power over the occipital channels was asymmetrical, with greater decline in power at the right occipital derivation. Over the right occipital derivation, power at the initial session was greater in all frequency bins. In contrast, over the left occipital derivation this was true only for frequencies < 7 Hz and a few bins in the sigma band.
Figure 1.
All night NREM sleep spectra. Subject average NREM sleep spectra of the log power at 2 central (C3/A2 and C4/A1) and 2 occipital (O2/A1 and O1/A2) derivations. The solid line corresponds to data from the initial session, while the dashed line corresponds to data from the follow-up session. Significance at 3 different levels (P < 0.05, P < 0.01, and P < 0.001) is indicated at the bottom of each plot for each frequency bin by an asterisk. The frequency resolution was 0.2 Hz.
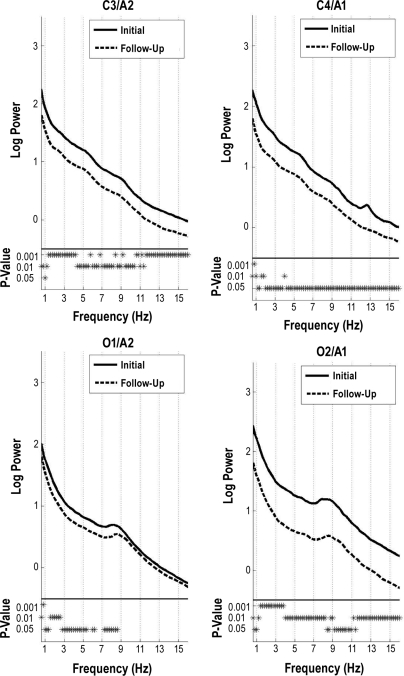
The changes in the NREM sleep spectra were mirrored in the REM sleep spectra (Figure 2). In the central derivations, power decreased with increasing age in all frequency bins. Furthermore, similar to the NREM sleep spectra, the decline in occipital power was larger over the right hemisphere. Power was significantly greater at the initial session in all frequency bins for the right occipital derivation and at most bins below 9 Hz in the left occipital derivations.
Figure 2.
All-night REM sleep spectra. Subject average spectra for REM sleep. For details, see the caption of Figure 1.
Frequency of the Spindle Peak
Visual inspection of the NREM sleep spectra showed the frequency of the peak in the sigma band (11-16 Hz) increased at the follow-up assessment (Figure 1). We tested whether this observation was statistically significant by performing a paired t-test on the frequency of the peak value in the sigma band at the left central (C3/A2) derivation. We found a statistically significant increase in the frequency of the peak (P = 0.002). The frequency of the peak for individuals at both assessments is reported in Table 1.
Time-Frequency Analysis
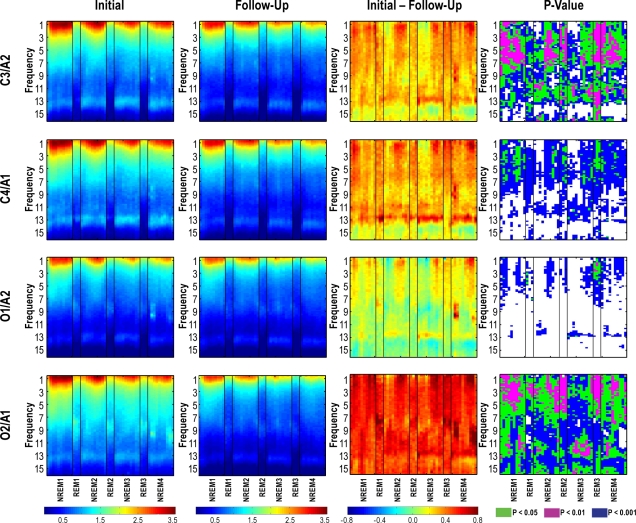
The results from the time-frequency analysis paralleled the all-night findings. The developmental decline in power was most pronounced over the left and right central and right occipital derivations (Figure 3). On the other hand, the left occipital derivation showed the least change in power with age. Notably absent is significance (or low significance in the case of O2/A1) in the high sigma band (∼13.5 to 15 Hz) across the night. This is most likely due to the shift in the frequency of the peak in the spindle band described above. The declines in power were not restricted to one sleep state or portion of the night. Therefore, the findings from the all-night sleep EEG were not driven by one particular sleep cycle.
Figure 3.
Time-Frequency plots averaged over participants. In each plot, 4 NREM sleep episodes (10 data points) interleaved with 3 REM sleep episodes (3 data points) are plotted on the x-axis and frequency is plotted on the y-axis (with a frequency resolution of 0.2 Hz). Each row of the figure represents an EEG derivation. The leftmost column of the figure is the log of the power data from the initial session while the plot in the column to the right is the log of the power data from the follow-up session. The third column from the left is log power at the initial session minus log power at the follow-up session. The far right column of the figure shows color-coded P-values.
The interaction term of the ANOVA with factors cycle and assessment was examined to assess whether the developmental decline in sleep EEG power was consistent across the night. For NREM sleep we found a significant interaction for all derivations in the delta band with α set to 0.05: C3/A2 (significant frequencies = 0.6 to 3.8 Hz; 5 to 5.2 Hz), C4/A1 (significant frequencies = 1.4 to 2.4 Hz), O1/A2 (significant frequencies = 1.8 Hz), and O2/A1 (significant frequencies = 0.6 to 3 Hz). We found no significant interactions for REM sleep.
Comparison with Previously Published Data in Early Adolescents
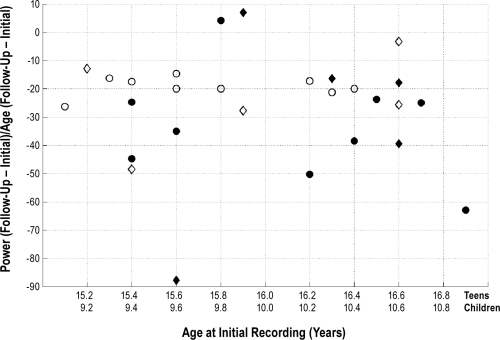
To examine how the sleep EEG changes in the late adolescents reported here compare to our previous longitudinal study in early adolescents (ages 9-10 years at the initial assessment and 11 to 13 years at follow-up)20 we constructed a summary variable to describe the change in power across frequency normalized by the time between assessments. Specifically, we define the rate of decline at C3/A2 as follows:
In this equation, PI and PF are average all-night NREM sleep power from 0.6 to 16 Hz (measured in μV2) at the initial and follow-up session, respectively; and AI and AF are age (measured in decimal years) at the initial and follow-up session, respectively. We plot PΔ for both cohorts in Figure 4. A t-test comparing PΔ in the early adolescent versus the late adolescent group revealed no significant difference between the groups (t26 = −1.62; P = 0.11).
Figure 4.
Rate of power decline as a function of age at the initial recording session for the teens in this study and children in a previous study.20 Teens are plotted with open symbols and children are depicted with filled symbols. Females are shown as diamonds and males as circles.
DISCUSSION
Our longitudinal data show changes to the sleep EEG in late adolescents, perhaps a continuation of a process that emerges in early adolescence. An important feature of this study is the sleep-wake schedule control imposed on adolescents prior to the laboratory sessions. In particular, the duration and timing of sleep before PSG assessments was the same at both recording sessions for each teen, ensuring that such factors as sleep pressure did not affect our findings. Furthermore, the duration of recordings was the same at both sessions for each teen, and recordings were performed under monitored laboratory conditions (lighting, temperature, and noise were controlled).
Under these controlled conditions, we found changes in sleep architecture: less slow wave sleep time and an increase in the minutes of stage 2 sleep. A number of cross-sectional16,29–31 and longitudinal32,33 sleep studies have found such a change in early to mid-adolescence. Two studies estimate about a 40% decline in stage 416 and SWS32 between Tanner stage 1 and Tanner stage 5 adolescents. The changes we observed in sleep stage variables in later adolescence were comparable to those observed during early adolescence (between the mean age of 10.1 and 12.4) in our previous longitudinal study.20 To wit, the decline in SWS and increase in stage 2 sleep in the present study were 27% and 22%, respectively; in our early adolescent sample, the decline in SWS was 29% and the increase in stage 2 sleep was 17%.
We also report here a significant decline of NREM and REM sleep EEG power over a short interval of approximately 2.5 years in late adolescence. We attribute this decline to the synaptic pruning that takes place in the healthy human cortex. Support for this notion includes: (1) the decline in power was state nonspecific (found in NREM and REM sleep), and (2) the decline was consistent across the night with the exception of the delta band. We found that the maturational decline in NREM sleep EEG power was greater at the beginning of the night in the delta band for all derivations. NREM sleep EEG activity in the delta band has been proposed as a marker of sleep homeostasis, and the decrease in this measure over the course of sleep is thought to reflect the dissipation of sleep pressure.34 Thus, our finding may indicate that there is a developmental change in the dissipation of sleep pressure during late adolescence. The analyses reported here, however, cannot address sleep homeostasis since the dissipation of sleep pressure across the night requires additional analyses to fit an exponential decay function to EEG delta activity. Campbell and colleagues35 examined this issue across the ages of 9 to 18 years and found no change in the rate of dissipation of sleep pressure in their sample. Further longitudinal study of this phenomenon, under well-controlled experimental conditions (e.g., control of prior sleep duration and sleeping environment) is necessary to achieve a definitive assessment of whether homeostatic sleep regulation changes across adolescence.
The change in NREM sleep EEG power was comparable in the current late adolescent cohort to that of our early adolescent group (Figure 4).20 This issue, however, needs to be more thoroughly examined with data from extended well-controlled longitudinal studies. Figure 4 also raises an important point regarding individual variability in the decline of sleep EEG power across development. In both data sets, the rate of decay is variable across individuals and unrelated to age at the initial assessment. Individual variability is also apparent in Figure 2 in the data of Campbell and Feinberg,19 showing delta and theta sleep EEG power as a function of age. Furthermore, a study in macaque monkeys also showed large interindividual variability in the synaptic pruning phase of primate development.36 Studies with larger sample sizes are needed to examine what factors lead to the large degree of interindividual variability in the decline of sleep EEG power, which likely is a reflection of synaptic pruning.
Another important feature of our data was hemispheric asymmetry in the decline of sleep EEG power, with greater reduction across more frequencies in the left central and right occipital channels. This finding is similar to the asymmetry found in our previous report of early adolescents.20 A number of MRI studies have shown significant regional differences in the timing and rate of change of cortical thickness across development.3,5–7 Using MRI data, Shaw7 noted that the left frontal cortex is thicker than the right, while the right occipital cortex is thicker than the left in children (∼ age 5 years). Over the course of development this pattern is reversed, and by the age of 20 years cortical thickness is greater over the left posterior cortex than the right. MRI data show that around age 17 years, the left and right cortices are of equal thickness in the posterior lobes. Our data show a similar pattern with greater power at the right occipital derivation than the left occipital derivation at the initial session (ages 15/16 years) and no difference between the derivations at follow-up (age 17.6 to 19.3 years). At the follow-up session, power in the two central derivations was similar, as was power at the two occipital derivations, suggesting that hemispheric asymmetry is diminished or gone towards the end of adolescence. In contrast, a recent study by Feinberg and colleagues18 using a Gompertz function to fit delta and theta sleep EEG power between the ages of 9 and 18 years, did not find a difference between the right and left central derivations in the parameters of the fit. Further longitudinal studies with more dense EEG electrode arrays are necessary to characterize topographic brain changes during adolescence as observed through the sleep EEG.
Several limitations of this study are important to note. First, the sample size was too small to examine effects of sex and other factors (such as pubertal status) on the changes to the sleep EEG. Further longitudinal work with larger samples across broader age ranges are needed to address the role of individual differences. Despite these limitations, we extend the literature by showing substantial changes to sleep EEG power and architecture in late adolescence under conditions where prior sleep was well controlled.
A number of psychiatric illnesses (e.g., anxiety and mood disorders, psychosis, eating disorders, personality disorders, schizophrenia, and substance abuse) emerge during adolescence. One prevailing explanation is that the age of onset may result from a malfunction in the timing and/or magnitude of the structural changes to the cerebral cortex.10 The sleep EEG provides a means to track brain development noninvasively. We hope the normative data presented will help further our understanding of the emergence of psychiatric illness during this period by making aberrations from this trajectory apparent.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Drs. Christine Acebo, Tracy Rupp, Oskar Jenni, Gahan Fallone, and Margaret Borkowski for assistance with recruitment, screening, recording and evaluation of the participants. The authors are grateful to Drs. Elizabeth Forbes and Judith Owens for performing the Tanner staging, William Coon and Henry Arantes for sleep stage scoring. We also thank our research staff, laboratory technicians, and participants. This work was performed at E.P. Bradley Sleep Research Laboratory, Providence, RI. It was supported by the National Institute on Alcohol Abuse and Alcoholism (AA13252 to Dr. Carskadon).
Footnotes
A commentary on this article appears in this issue on page 1287.
REFERENCES
- 1.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Hasan KM, Sankar A, Halphen C, et al. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18:1735–9. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- 3.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28:228–37. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 6.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–8. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 9.Boord PR, Rennie CJ, Williams LM. Integrating “brain” and “body” measures: correlations between EEG and metabolic changes over the human lifespan. J Integr Neurosci. 2007;6:205–18. doi: 10.1142/s0219635207001416. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 11.Buchmann A, Ringli M, Kurth S, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–15. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 12.Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr Clin Neurophysiol. 1988;69:91–9. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- 13.Matousek M, Petersen I. Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalogr Clin Neurophysiol. 1973;35:603–12. doi: 10.1016/0013-4694(73)90213-7. [DOI] [PubMed] [Google Scholar]
- 14.Dustman RE, Shearer DE, Emmerson RY. Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin Neurophysiol. 1999;110:1399–409. doi: 10.1016/s1388-2457(99)00102-9. [DOI] [PubMed] [Google Scholar]
- 15.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res. 2001;10:165–72. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 17.Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–9. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinberg I, de Bie E, Davis NM, Campbell IG. Topographic differences in the adolescent maturation of the slow wave EEG during NREM sleep. Sleep. 34:325–33. doi: 10.1093/sleep/34.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–9. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckelmuller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbons M, Williams JBW. User guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 23.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry. 2000;57:675–82. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 24.Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroenceph Clin Neurophysiol. 1958;10:370–1. [Google Scholar]
- 25.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 27.Tarokh L, Carskadon MA. Sleep electroencephalogram in children with a parental history of alcohol abuse/dependence. J Sleep Res. 2010;19:165–74. doi: 10.1111/j.1365-2869.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–90. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: Findings using standard measurement methods. Sleep. 1984;7:289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- 30.Karacan I, Anch M, Thornby JI, Okawa M, Williams RL. Longitudinal sleep patterns during pubertal growth: four-year follow up. Pediatr Res. 1975;9:842–6. doi: 10.1203/00006450-197511000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Williams RL, Karacan I, Hursch CJ, Davis CE. Sleep patterns of pubertal males. Pediatr Res. 1972;6:643–8. doi: 10.1203/00006450-197208000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Carskadon MA. The second decade. In: Guilleminault C, editor. Sleep and waking disorders: indications and techniques. Menlo Park: Addison Wesley; 1982. pp. 99–125. [Google Scholar]
- 33.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achermann P, Borbely AA. Sleep homeostasis and models of sleep regulation. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. Missouri: Elsevier Saunders; 2010. pp. 431–44. [Google Scholar]
- 35.Campbell IG, Darchia N, Higgins LM, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–20. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]