Abstract
Study Objectives:
To evaluate the efficacy and safety of doxepin (DXP) 3 mg and 6 mg in adults diagnosed with primary insomnia.
Design and Methods:
The study was a randomized, double-blind, parallel-group, placebo-controlled trial. Patients meeting DSM-IV-TR criteria for primary insomnia were randomized to 35 days of nightly treatment with DXP 3 mg (n = 75), DXP 6 mg (n = 73), or placebo (PBO; n = 73), followed by 2 nights of single-blind PBO to evaluate discontinuation (DC) effects. Efficacy was assessed using polysomnography (PSG) and patient reports. Efficacy data were examined for Night (N) 1, N15, and N29. Safety assessments were conducted throughout the study.
Results:
Compared with PBO, DXP 3 and 6 mg significantly improved wake time after sleep onset (WASO) on N1 (3 mg and 6 mg; P < 0.0001), N15 (3 mg P = 0.0025; 6 mg P = 0.0009), and N29 (3 mg P = 0.0248; 6 mg P = 0.0009), latency to persistent sleep (LPS) on N1 (3 mg P = 0.0047; 6 mg P = 0.0007), and total sleep time (TST) on N1 (3 mg and 6 mg P < 0.0001), N15 (6 mg P = 0.0035), and N29 (3 mg P = 0.0261; 6 mg P < 0.0001). In terms of early morning awakenings, DXP 3 and 6 mg demonstrated significant improvements in SE in the final quarter of the night on N1, N15, and N29, with the exception of 3 mg on N29 (P = 0.0691). Rates of discontinuation were low, and the safety profiles were comparable across the 3 treatment groups. There were no significant next-day residual effects, and there were no spontaneous reports of memory impairment, complex sleep behaviors, anticholinergic effects, weight gain, or increased appetite. Additionally, there was no evidence of rebound insomnia after DXP discontinuation.
Conclusions:
Five weeks of nightly administration of DXP 3 mg and 6 mg to adults with chronic primary insomnia resulted in significant and sustained improvements in sleep maintenance and early morning awakenings (with the exception of SE in the final quarter of the night on N29 for 3 mg [P = 0.0691]). These sleep improvements were not accompanied by next-day residual effects or followed by rebound insomnia or withdrawal effects upon discontinuation. These findings confirm the unique profile of sleep maintenance efficacy and safety of DXP observed in prior studies.
Citation:
Krystal AD; Lankford A; Durrence HH; Ludington E; Jochelson P; Rogowski R; Roth T. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. SLEEP 2011;34(10):1433–1442.
Keywords: Chronic insomnia, sleep maintenance insomnia, early morning awakenings, doxepin, WASO, TST, WTDS
INTRODUCTION
Insomnia, the most common sleep disorder, has an estimated prevalence of 10% to 16%, and a median duration of 4 years, with more than one-third of patients reporting chronicity in excess of 10 years.1–4 This disorder is characterized by difficulty with sleep onset, sleep maintenance, waking too early, and/or non-restorative sleep despite adequate opportunity to sleep that is associated with daytime impairment or distress.5–7 Affected individuals may experience more than one type of sleep disturbance, and the type of sleep disturbance—problems with onset, maintenance, and/or restoration—may change with time.8
Problems with sleep onset and maintenance have been the subject of far more research than problems with restorative sleep and, of these, sleep maintenance problems—the inability to stay asleep throughout the night or the propensity for early morning awakenings—is the most common, reported by over 70%.9,10 Yet sleep maintenance problems, particularly early morning awakening, are difficult to treat with medications without causing daytime impairment.
The clinical management of insomnia has been dominated since the 1960s by agents that facilitate γ-aminobutyric acid (GABA) mediated inhibition of key arousal systems in the brain. As a result, GABAergic agents including barbiturates, benzodiazepines, and non-benzodiazepines, have served as the pharmacological model for the effects of insomnia agents.11 According to this model, the pharmacokinetics of an agent determine the nature of its effects on sleep, in that the degree of sedative/hypnotic effect is proportional to plasma drug levels.12,13 As a result, agents which have sleep-enhancing effects in the latter part of the night are likely to have next-day residual effects, particularly soon after waking, when plasma drug levels will only be incrementally lower than they are towards the end of the sleep period. However, this may not be the case for medications which enhance sleep by mechanisms other than enhancing GABAergic inhibition. A dissociation of pharmacokinetics and clinical effects and a greater specificity of effects appears to be particularly likely for agents that block the effects of one of the wake-promoting systems whose activity levels depend upon behaviors, environment, and circadian rhythm.14,15 As a result, agents which block these wake promoting systems have the potential to have an improved risk-benefit profile in the treatment of particular subgroups of insomnia patients.
One such agent is doxepin, a compound with potent H1 histamine blocking activity that has demonstrated significant sleep promoting effects in both comorbid16 and primary insomnia17–19 populations at doses ranging from 25 mg to 150 mg. Adverse effects data from these trials, however, indicate that DXP at doses ≥ 25 mg is associated with undesirable side effects, including anticholinergic and antiadrenergic effects, which may occur because of blockade of receptors other than H1.16,17 However, at dosages ≤ 6 mg, it appears that the pharmacologic effects are primarily due to histamine antagonism, such that it is possible to achieve potent blockade of the wake-promoting effects of histamine via H1 antagonism without significant effects on other receptors.20–23 Consistent with this, DXP at these low doses has demonstrated significant sleep maintenance effects in the last quarter of the night while manifesting no evidence of morning impairment in objective and subjective assessments of next-day function in chronic insomnia patients.20–22
The current study seeks to confirm the efficacy and safety profile of DXP at doses of 3 mg and 6 mg in a 5-week trial of adults with chronic primary insomnia. It is the first parallel-group study of low-dose DXP in adults, and, as such, allowed an assessment of longer duration of treatment than prior studies in this population. Additionally, this study was designed to allow the evaluation of the potential for rebound insomnia and withdrawal effects following discontinuation after 35 consecutive days of nightly administration. This trial is intended to better define the characteristics of DXP 3 mg and 6 mg as therapies for individuals with chronic sleep maintenance difficulties.
METHODS
Study Design
This randomized, double-blind, placebo-controlled, parallel-group study was conducted in 22 sleep centers in the United States. An institutional review board approved the protocol and informed consent form for each study site, and the study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonisation of Good Clinical Practices. All patients signed an informed consent form prior to screening procedures. Patients were compensated for their participation.
Patients
Men and women between the ages of 18 and 64 (inclusive) years with a DSM-IV-TR diagnosis of primary insomnia who reported sleep maintenance difficulty were eligible for inclusion in the study. Patient screening for general eligibility and sleep history was conducted during an initial clinic visit and involved a medical, sleep, and psychiatric history; physical examination; vital sign measurements; clinical laboratory tests; and an electrocardiogram. Patients meeting initial screening criteria were asked to record their sleep patterns onto a daily sleep diary prior to PSG screening (≥ 7 days of assessment). The initial screening results and sleep diary data were used to verify a DSM-IV-TR diagnosis of insomnia for at least the last 3 months.
Primary reasons for patient exclusion from the study were as follows: (1) excessive use of alcohol, nicotine, or caffeinated beverages; (2) intentional napping more than twice per week; (3) having a variation in bedtime > 2 h on 5 of 7 nights; or (4) use of a hypnotic or any other medication known to affect sleep.
Patients meeting eligibility criteria began 2 weeks of single-blind placebo dosing. Patients spent the first 2 nights in a sleep laboratory to determine whether they met PSG screening criteria. Patients were required to meet the following polysomnographic entry criteria in order to be eligible for randomization: latency to persistent sleep (LPS) > 10 min on both PSG screening nights; mean wake time during sleep (WTDS) ≥ 60 min on both PSG screening nights, with no night < 45 min; and TST > 240 and ≤ 400 min on both screening nights. Patients were excluded from the study if they had ≥ 10 apnea/hypopnea events or periodic leg movements with arousals/h of sleep. Patients who remained eligible continued to take single-blind placebo for 5 consecutive nights at home.
Procedure
After completing the 5 nights of single-blind placebo, patients participated in 2 consecutive nights of 8-h continuous PSG recordings in a sleep laboratory. Eligible patients were randomly assigned to one of 3 treatment groups (DXP 3 mg, 6 mg, or placebo) in a 1:1:1 ratio. The placebo ingredients were identical to the 3 mg and 6 mg capsules, with all being opaque white capsules. Patients continued to take single-blind PBO for 5 consecutive nights at home, until the start of double-blind treatment. Subsequently, patients began 35 consecutive nights of treatment which included supervised administration of study drug in a sleep laboratory and nightly unsupervised administration of study medication at home between visits. At each study visit during the double-blind treatment period, patients had 2 nights of 8-h PSG recording (N1 and N2; N15 and N16; and N29 and N30). During the discontinuation period (N36 and N37), patients received single-blind PBO and had 2 final nights of 8-h PSG recording.
Patients were instructed to arrive at the study center 2 h prior to their median habitual bedtime on sleep laboratory nights for 8 h of continuous PSG recording. Study drug was administered approximately 30 min before the patient's bedtime (lights-out). The following morning, approximately 1 h after completion of PSG recording, patients filled out a questionnaire assessing sleep characteristics and completed tests evaluating next-day residual effects. Safety assessments were performed pre- and post-dose.
Study Assessments
The PSG recordings were conducted in accordance with the Rechtschaffen and Kales manual,24 and were scored by a PSG technologist at a central PSG facility in a blinded fashion. The prospectively defined primary efficacy endpoint was WASO on N1. Other prospectively defined PSG efficacy variables included WASO at other time points, LPS, number of awakenings after sleep onset (NAASO), TST, SE, and wake time after sleep ([WTAS] time from the last epoch of sleep until the end of the 8-h recording period). SE was further analyzed prospectively by quarter of the night and by hour. Sleep architecture included the percentages and duration (in min) of stage 1, 2, and 3/4 sleep, REM sleep, and latency to REM sleep. Patients also completed a morning questionnaire to provide their assessments of treatment. Patient-reported measures included latency to sleep onset (LSO), WASO (sWASO), TST (sTST), NAASO (sNAASO), and sleep quality (scale from −3 to 3; −3 = extremely poor, −2 = very poor, −1 = poor, 0 = fair, 1 = good, 2 = very good, 3 = excellent). All efficacy measures were assessed for up to 4 weeks; results are provided for efficacy assessments on N1, N15, and N29.
Next-day residual effects were assessed objectively with the Digit Symbol Substitution Test (DSST)25 and Symbol Copying Test (SCT),25 and subjectively with a 100-mm visual analog scale (VAS) for sleepiness. Safety was also assessed by monitoring adverse events (AEs) and by examining the changes from baseline in laboratory test values (hematology, serum chemistry, and urinalysis), vital signs, 12-lead electrocardiograms, and physical examinations.
Rebound insomnia was evaluated primarily as the change in WASO from baseline to N36 and N37 (individually), and also defined as the percentage of patients with ≥ 35-min increase in WASO compared to baseline. Withdrawal symptoms were assessed during the DC period and included Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ) scores, vital signs, and spontaneously reported AEs. The BWSQ consists of 20 items or questions designed to assess symptoms experienced by patients at any time during administration of, or withdrawal from, a benzodiazepine or benzodiazepine-related compound.26 Withdrawal was defined as the emergence of ≥ 3 new symptoms or the worsening of previous symptoms during the DC period on the BWSQ. AEs occurring during the DC period were reviewed for events that might signal withdrawal and/or rebound insomnia.
Treatment adherence was assessed in 2 ways. While in the sleep laboratory, a mouth check was performed. For study drug self-administered at home, an adherence check was performed by counting the capsules returned at each study visit. Additionally, patients were asked if they had taken study drug for the 5 nights prior to each visit. When subjects returned to the study center for a scheduled or unscheduled visit, they were instructed to bring their study drug supplies for accountability procedures.
Statistical Analysis
Efficacy analyses were conducted using the intent-to-treat analysis set. This dataset included all randomized patients who received ≥ 1 dose of double-blind study drug. Analysis of covariance (ANCOVA) methods were used to compare mean WASO values from N1 between placebo, DXP 3 mg, or DXP 6 mg, using a model that included main effects for treatment and study center with the baseline WASO as a covariate. The same methods were used to analyze all other continuous efficacy variables on N1, N15, and N29, and also for WASO on N15 and N29. For several of the key variables, the average across the double-blind period was calculated using N1, N15, and N29. For LPS, latency to REM sleep, latency to stage 2 sleep, and LSO data were analyzed using log-transformed values (natural log). In order to determine if treatment benefit was maintained across time, a one-sample (paired) t-test was used to assess whether the change from Night 1 to Night 29 was significantly different from zero for WASO, TST, and LPS. Standardized effect sizes for all PSG variables (Cohen's d) were calculated by computing differences from placebo for each active condition and dividing by the pooled standard deviation.30
The patient-reported sleep maintenance and sleep duration endpoints, sWASO and sTST, were further analyzed post hoc with a mixed-effect model repeated measures (MMRM) approach. Both sWASO and sTST were analyzed using the MMRM method that included fixed effects for treatment group, time (as a discrete factor), the treatment-by-time interaction, and the baseline value of the endpoint. In order to avoid the potential sensitivity of tests and estimators to the choice of an arbitrary covariance structure, each analysis used an unstructured covariance matrix for the repeated observations within each subject.
The mean changes from Night 1 to the average of the D2 and D3 DSST, SCT, and VAS values were compared among treatments using an ANCOVA model with terms for treatment and center, and the Night 1 value as a covariate. Pairwise comparisons of each active treatment versus placebo using the Dunnett test were performed. For the DSST, SCT, and VAS, the average of the data from the 2 mornings after are presented (average of D2 and D3, D16 and D17, and D30 and D31).
The analysis set for discontinuation effects included all patients who received single-blind PBO during the DC period and had PSG data at baseline and on N36. The analysis set for withdrawal effects on the BWSQ included all patients who completed assessments at N36 and the final study visit. Descriptive statistics are presented for the variables assessed during the DC period.
Funding for this study was provided by Somaxon Pharmaceuticals, Inc. Data and statistical analyses for this study were fully available to all authors. A representative of the sponsor ensured that the procedures set out a priori in the protocol were followed and that all FDA regulations were adhered to. All authors were involved with the preparation of the manuscript and vouch for the completeness and accuracy of the data and analyses presented.
RESULTS
Study Population
A total of 1082 patients were screened for participation. Of the 229 patients randomized, 203 patients (89%) completed both double-blind treatment and discontinuation periods. Of the 26 patients (11%) who discontinued the study, 8 patients (3.5%) discontinued after randomization (baseline), but before receiving a dose of double-blind study drug (these patients were not included in ITT or safety analyses data sets), and 18 patients (11%) discontinued during double-blind treatment, with no patients discontinuing during the DC period. Early discontinuation rates and baseline characteristics were comparable across treatment groups (Table 1).
Table 1.
Study disposition and demographics
| Parameter | Placebo (n = 76) | Doxepin 3 mg (n = 77) | Doxepin 6 mg (n = 76) | Total (n = 229) |
|---|---|---|---|---|
| Completed study | 88% | 88% | 89% | 89% |
| Discontinued from study | 12% | 12% | 11% | 11% |
| Reason: Adverse Event | 1% | 3% | 4% | 3% |
| Reason: Consent Withdrawn | 4% | 3% | 0% | 2% |
| Reason: Protocol Violation | 0% | 1% | 0% | < 1% |
| Reason: Noncompliance | 3% | 3% | 3% | 3% |
| Reason: Other | 4% | 3% | 4% | 4% |
| Age (years) | (n = 73) | (n = 75) | (n = 73) | (n = 221)1 |
| Mean (SD) | 43.6 (12.3) | 45.5 (10.6) | 44.2 (11.1) | 44.5 (11.3) |
| Range | 18–64 | 20–64 | 19–63 | 18–64 |
| BMI (kg/m2) | (n = 73) | (n = 75) | (n = 73) | (n = 221)1 |
| Mean (SD) | 26.4 (4.5) | 27.8 (4.9) | 27.4 (4.1) | 27.2 (4.6) |
| Range | 18.2–40.6 | 19.1–41.5 | 19.2–38.0 | 18.2–41.5 |
| Gender | (n = 73) | (n = 75) | (n = 73) | (n = 221)1 |
| Female | 70% | 77% | 71% | 73% |
| Male | 30% | 23% | 29% | 27% |
| Race/Ethnicity | (n = 73) | (n = 75) | (n = 73) | (n = 221)1 |
| Caucasian | 48% | 44% | 53% | 48% |
| African American | 34% | 35% | 29% | 33% |
| Hispanic | 15% | 20% | 14% | 16% |
| Other | 2% | 1% | 4% | 3% |
Eight patients who did not receive study drug are not included in these data sets (N = 221).
Efficacy
PSG measures
Sleep maintenance and duration:
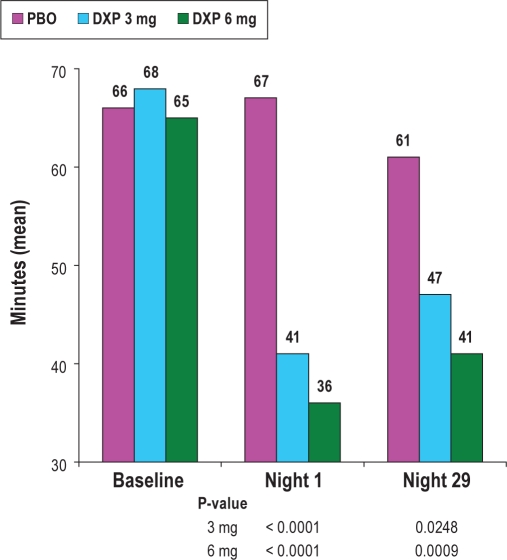
Unless otherwise noted, only significant results are presented. All of the following comparisons were versus placebo unless otherwise noted. WASO was significantly improved on N1 (primary efficacy endpoint; P < 0.0001), N15 (P = 0.0053), and N29 (P = 0.0299; Figure 1) for DXP 3 mg, and on N1 (primary efficacy endpoint; P < 0.0001), N15 (P = 0.0023), and N29 (P = 0.0012) for DXP 6 mg. To determine if there were any effects based on ethnicity, summary statistics were further examined for WASO on N1 separately for African Americans and Caucasians, the 2 largest ethnic groups. Overall, the improvement in WASO on N1 for DXP 3 mg and 6 mg treatments compared with the placebo group did not differ across the ethnic subgroups, indicating doxepin was effective independent of race. Mean WASO (SD) in African Americans on N1 were: PBO = 71.2 (61.6); DXP 3 mg = 39.9 (27.8); DXP 6 mg = 30.8 (22.3). Mean WASO in Caucasians on N1 were: PBO = 70.5 (46.1); DXP 3 mg = 42.4 (34.4); DXP 6 mg = 36.0 (24.1). To determine if there were any efficacy differences between the 2 active doses, a 2-sample t-test was used to compare the 2 dose groups; there were no significant differences between groups on WASO.
Figure 1.
Effects of doxepin 3 mg and 6 mg on wake after sleep onset (WASO). Data included in this Figure are derived from the ITT analysis set.
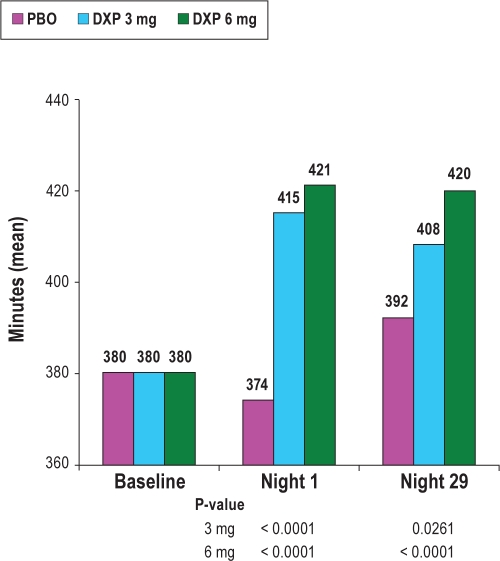
TST and consequently SE were also significantly improved on N1 (P < 0.0001) and N29 (P = 0.0262; Figure 2) for DXP 3 mg, and on N1 (P < 0.0001), N15 (P = 0.0157), and N29 (P = 0.0003) for DXP 6 mg. There were no significant differences in NAASO for any dose at any time point. The PSG variables (at each time point and overall) and the associated effect sizes (overall only) appear in Table 2.
Figure 2.
Effects of doxepin 3 mg and 6 mg on total sleep time (TST).
Table 2.
Effect of doxepin and placebo on PSG sleep onset, sleep maintenance, and early morning awakening parameters
| Measure | Baseline |
Night 1 |
Night 15 |
Night 29 |
Double-Blind Average* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Effect size (d) | |
| WASO (min) | |||||||||||
| Placebo | 65.7 | (36.8) | 66.8 | (49.9) | 60.5 | (51.9) | 60.5 | (38.8) | 62.5 | (37.7) | N/A |
| DXP 3 mg | 67.8 | (33.6) | 41.4*** | (31.5) | 44.7** | (29.2) | 47.2* | (43.5) | 44.4*** | (26.6) | 0.58 |
| DXP 6 mg | 65.0 | (33.2) | 36.3*** | (26.1) | 41.7** | (29.4) | 40.7** | (37.3) | 39.5*** | (26.1) | 0.72 |
| TST (min) | |||||||||||
| Placebo | 380.2 | (44.4) | 373.9 | (71.7) | 389.2 | (62.8) | 391.5 | (48.9) | 385.0 | (53.0) | N/A |
| DXP 3 mg | 380.3 | (46.1) | 415.3*** | (41.7) | 402.1 | (50.4) | 408.0* | (53.5) | 408.5*** | (43.0) | 0.48 |
| DXP 6 mg | 380.3 | (43.1) | 420.5*** | (37.1) | 411.4** | (50.4) | 419.5*** | (44.2) | 417.1*** | (36.8) | 0.72 |
| SE Last Quarter of Night (%) | |||||||||||
| Placebo | 78.3 | (14.6) | 79.9 | (20.4) | 81.2 | (19.1) | 80.7 | (16.7) | 80.8 | (13.9) | N/A |
| DXP 3 mg | 79.1 | (15.5) | 88.3** | (13.8) | 86.6* | (13.6) | 85.1 | (14.1) | 86.8*** | (9.9) | 0.50 |
| DXP 6 mg | 79.8 | (15.0) | 89.8*** | (9.4) | 87.4* | (12.5) | 87.8** | (14.0) | 88.4*** | (9.1) | 0.65 |
| LPS (min) | |||||||||||
| Placebo | 37.9 | (28.4) | 44.8 | (54.6) | 34.0 | (39.0) | 32.0 | (35.3) | 36.9 | (34.1) | N/A |
| DXP 3 mg | 35.9 | (29.8) | 26.7** | (23.4) | 38.0 | (39.6) | 28.5 | (26.0) | 31.0 | (24.9) | 0.20 |
| DXP 6 mg | 39.1 | (34.1) | 27.1** | (25.4) | 31.7 | (35.9) | 24.6 | (21.1) | 27.8 | (21.6) | 0.32 |
Double-blind Average includes Nights 1, 15 and 29;
SD, standard deviation; DXP, doxepin; WASO, wake after sleep onset; TST, total sleep time; SE, sleep efficiency; LPS, latency to persistent sleep;
P < 0.05 vs placebo; data included in this Table are from the ITT analysis set;
P < 0.01;
P ≤ 0.0001.
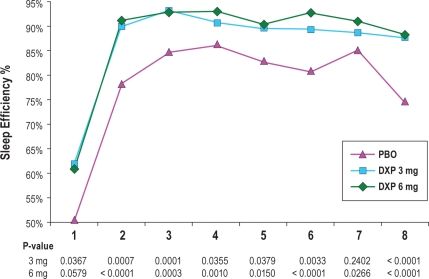
In terms of early morning awakenings, SE in the last quarter of the night was significantly improved on N1 (P = 0.0008) and N15 (P = 0.0220) for DXP 3 mg, and on N1 (P < 0.0001), N15 (P = 0.0239), and N29 (P = 0.0029) for DXP 6 mg. SE in Hour 8 was significantly improved on N1 (P < 0.0001; Figure 3) and N29 (P = 0.0315) for DXP 3 mg, and on N1 (P < 0.0001), N15 (P = 0.0162), and N29 (P = 0.0020) for DXP 6 mg. WTAS was significantly improved on N1 (P = 0.0001) for DXP 3 mg, and also on N1 (P = 0.0016) for DXP 6 mg.
Figure 3.
Effects of doxepin 3 mg and 6 mg across the night: sleep efficiency % by hour on Night 1.
Sleep onset:
LPS was significantly improved on N1 (P = 0.0047) for DXP 3 mg, and on N1 (P = 0.0007) for DXP 6 mg.
Patient-reported measures
In terms of patient-reported sleep maintenance data, there were significant improvements in sWASO for both doses of DXP on N1 (3 mg P = 0.0003; 6 mg P = 0.0004), and also on the double-blind average across N1, N15, and N29 (3 mg P = 0.0354; 6 mg P = 0.0178). There were also significant improvements in sTST for both doses of DXP at N1 (3 mg P = 0.0088; 6 mg P = 0.0135). Sleep quality was significantly improved for both doses at N1 (3 mg P = 0.0068; 6 mg P < 0. 0001), and for the 6-mg dose for double-blind average (P = 0.0028). Subjective LSO was significantly improved on N1 at DXP 6-mg dose (P = 0.0492).
Results from the MMRM approach using sWASO indicated that there was a main effect for treatment, and that the treatment-by-time interaction was not significant. Both treatment effects were significantly different from PBO (DXP 3 mg P = 0.0213; DXP 6 mg P = 0.0014) across the entire treatment period. The estimated difference in sWASO between DXP 3 mg and PBO was −10.2 min (SE = 4.41). The estimated difference in sWASO between DXP 6 mg and PBO was −14.2 min (SE = 4.41). Similar to the sWASO results, the MMRM approach using sTST indicated that there was a main effect for treatment, and that the treatment-by-time interaction was not significant. Both treatment effects were significantly different from PBO across the entire treatment period (DXP 3 mg P = 0.0469; DXP 6 mg P = 0.0042). The estimated difference in sTST between DXP 3 mg and PBO was 11.9 min (SE = 5.97). The estimated difference in sTST between DXP 6 mg and PBO was 17.3 min (SE = 5.96).
Tolerance to sedative effects
As mentioned previously, WASO was significantly improved on N1, N15, and N29 for DXP 3 mg and 6 mg, suggesting no evidence of tolerance to the sleep maintenance effects. TST and consequently SE were both significantly improved on N1, and N29 for DXP 3 mg, and on N1, N15 (P = 0.0023), and N29 (P = 0.0012) for DXP 6 mg. For sleep onset, LPS was significantly improved on N1, but not on N15 or N29 for DXP 3 mg and 6 mg, suggesting the development of tolerance for the sleep onset effects.
The t-test test to examine WASO efficacy across time determined that N1 data was not significantly different from N29 data for all 3 groups. Mean changes from N1 to N29 (P-values in parentheses) were: PBO = −6.2 (0.33); DXP 3 mg = 6.2 (0.28); DXP 6 mg = 4.6 (0.27).
The t-test test to examine TST efficacy across time determined that N1 data was not significantly different from N29 data for DXP 3 mg and DXP 6 mg. The effect in the PBO group was significantly different. Mean change from N1 to N29 (P-values in parentheses) were: PBO = 18.5 (0.02); DXP 3 mg = −7.7 (0.17); DXP 6 mg = −1.5 (0.76).
The t-test test to examine LPS efficacy across time determined that N1 data was not significantly different from N29 data for DXP 3 mg and DXP 6 mg. The effect in the PBO group was significantly different. Mean change from N1 to N29 (P-values in parentheses) are: PBO = −14.1 (0.04); DXP 3 mg = 1.8 (0.55); DXP 6 mg = −2.2 (0.49).
Safety
Sleep architecture
Across the trial, there were increases in the duration of stage 2 sleep for both doses of DXP, which were significant at most time points. There were no significant differences between the 2 DXP groups (individually) versus placebo in minutes of stage 1 sleep, stage 3/4 sleep, or REM sleep. With regards to percentages within each sleep stage, there were small (< 3.5%), significant DXP effects compared with placebo on % of stage 1 sleep (significantly less), % of stage 2 (significantly more), and % of REM sleep (significantly less in 6 mg group) across the trial versus PBO. However, there were no differences between doxepin and placebo with respect to REM latency with either dose at any time point.
Rebound insomnia and withdrawal effects
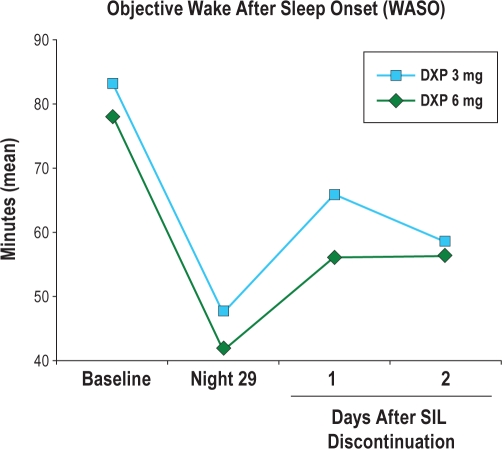
Mean WASO (Figure 4) remained improved relative to baseline for DXP 3 mg and 6 mg on N36, the first DC night, with sustained improvement on N37, the second DC night. Additionally, the percentage of patients meeting PSG-defined rebound insomnia criteria based on PSG data was similar across groups. Across the 2 nights, rebound insomnia (based on WASO criteria) was experienced by 1% of the PBO group, 1% of the DXP 3 mg group, and 4% of the DXP 6 mg group.
Figure 4.
Effects of doxepin 3 mg and 6 mg after doxepin discontinuation: mean WASO across the Trial; *P < 0.05. Days 1 and 2 of doxepin discontinuation took place on nights 36 and 37.
To evaluate the occurrence of withdrawal effects, AEs occurring during the DC period were reviewed for events that might signal withdrawal and/or rebound insomnia. Overall, there was a low incidence of AEs reported during the DC period. Approximately 8% of patients in each of the 3 groups experienced an AE during the DC period. A review of these AEs revealed no evidence of physical dependence, withdrawal syndrome, or worsening insomnia. Additionally, BWSQ data indicated no evidence of withdrawal syndrome. There were only 2 patients (1 in PBO group, 1 in DXP 3 mg group) who experienced ≥ 3 new symptoms on the BWSQ, the predetermined withdrawal criteria. The mean BWSQ total scores from Day 38 were very low overall (PBO = 0.6, DXP 3 mg = 0.8, DXP 6 mg = 0.4), and similar across the treatment groups.
Residual sedation
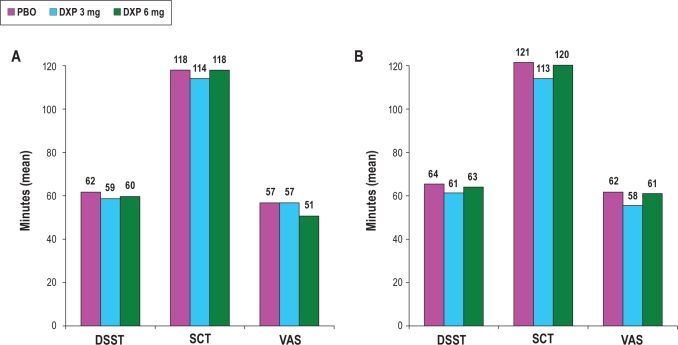
There were no significant differences between PBO and any dose of DXP on any of the measures assessing either psychomotor function (DSST and SCT; Figure 5) or next-day alertness (VAS; Figure 5) at any time point.
Figure 5.
Effects of doxepin 3 mg and 6 mg on next-day residual sedation parameters following Night 1 (A) and Night 29 (B); VAS data are inverted for consistency with DSST and SCT results. No comparisons between conditions were statistically significant.
Adverse events and laboratory results
Overall, there was a low incidence of AEs reported during the study. Twenty patients (27%) experienced at least one AE in the PBO group, 26 patients (35%) in the DXP 3 mg group, and 23 (32%) in the DXP 6 mg group. The most common AEs reported were headache, somnolence/sedation, and nausea. Table 3 summarizes all adverse events occurring in > 2% of patients.
Table 3.
Summary of adverse events reported by more than 2% of patients in any treatment group
| Parameter | Placebo (n = 73) | Doxepin 3 mg (n = 75) | Doxepin 6 mg (n = 73) |
|---|---|---|---|
| Patients with any adverse event | 27% | 35% | 32% |
| Headache | 10% | 5% | 0% |
| Somnolence/sedation | 5% | 9% | 8% |
| Diarrhea | 3% | 0% | 0% |
| Upper respiratory tract infection | 1% | 3% | 1% |
| Vomiting | 1% | 0% | 3% |
| Blood glucose increased | 0% | 3% | 0% |
| Nausea | 0% | 4% | 5% |
Rates of study discontinuation were similar in the 2 DXP groups and placebo (placebo = 12%; DXP 3 mg = 12%; DXP 6 mg = 11%). Additionally, the number of patients who withdrew from the study due to an AE was comparable across all 3 groups (placebo = 1%; DXP 3 mg = 3%; DXP 6 mg = 4%).
There were no reports of complex sleep behaviors, memory impairment, or cognitive disorder in any DXP-treated subject. There were no reports of adverse events potentially associated with anticholinergic effects (e.g., dry mouth, blurred vision).
No clinically meaningful changes were observed in mean laboratory values, ECGs, vital sign measurements, body weight, or physical examination findings. Overall, DXP was well-tolerated, with no apparent dose-related effects on safety and a comparable overall rate of AEs and study discontinuations as placebo.
DISCUSSION
The results of this randomized, placebo-controlled five-week study of adults with chronic primary insomnia suggest that DXP 3 mg and 6 mg improves sleep maintenance, including in the final hours of the night, in adults without any next-day residual effects, rebound insomnia, or substantive increase in adverse effects compared with placebo. Improvement in patient-reported sleep maintenance was also noted and paralleled the PSG findings. Improvements in sleep maintenance were immediate, occurring on the first night of treatment, and were sustained across all four weeks of study treatment. Additionally, there was no evidence of decreased response across time and minimal evidence of differential efficacy between the 3 mg and 6 mg doses, with standardized effect sizes ranging from medium to large. These improvements do not appear to have occurred at the expense of next-day residual sedation or safety issues. There were no significant differences between PBO and either DXP group in next-day residual sedation, and both doses of DXP were well tolerated with a low incidence of adverse events, essentially comparable to that observed in the PBO group. There were no reports of memory impairment, complex sleep behaviors, anticholinergic effects, weight gain, or increased appetite. In addition, there was no substantive evidence of an effect of DXP on REM or stage 3/4 sleep versus PBO.
Withdrawal symptoms, rebound insomnia, and other drug discontinuation effects are concerns associated with insomnia medication use. Unlike prior studies of low-dose DXP, this trial allowed for the assessment of whether this treatment is associated with these phenomena. There was no indication in this study that DXP 3 mg or 6 mg was associated with rebound insomnia, withdrawal symptoms, or any other discontinuation effects. The significant improvements on N1 in sleep maintenance endpoints were maintained on N29, suggesting no pharmacologic tolerance to the sleep maintenance effects across the trial.
This study also appears to provide confirmation that low-dose DXP (≤ 6 mg/day) has a greater specificity of pharmacologic effects than higher doses of DXP. Evidence for the relative absence of effects other than H1 antagonism includes the absence of AEs commonly associated with higher doses of DXP (e.g., anticholinergic effects, increased appetite, weight gain, orthostatic hypotension)16,17 and the absence of significant REM suppression, confirming the findings of previous trials.20–23 Also as in previous trials of chronic insomnia patients, low-dose DXP improves sleep towards the end of the night without leading to next-day residual sedation after awakening.20–22 This was particularly evident with the 6 mg dose, where sleep was significantly improved in the last quarter of the night versus PBO as well as in the eighth and final hour of the night compared with PBO at all time points assessed in the study. Yet no significant next-day residual effects were found with DSST, SCT, and VAS alertness ratings assessed shortly after awakening (Hour 9) for either the 3 mg or 6 mg DXP dosages.
With GABAergic agents, where clinical effects are proportional to serum level of drug, a potent effect in the final hour of the night would likely be accompanied by a comparable effect shortly after waking as manifested in significant next-day residual impairment. In this regard, it is important to note that GABAergic agents with sleep maintenance efficacy, eszopiclone and zolpidem CR, do not have maintenance efficacy that persists through the last hour of the night.27,28
In a study of comparable design carried out with zolpidem CR, no therapeutic effect in the eighth hour of the night was observed, yet somnolence as an adverse event was still reported by 14.7% of subjects vs 1.8% with placebo.27 Similarly, in a study of eszopiclone of comparable design, no effect on sleep was noted in the eighth hour of the night, and daytime somnolence was reported by 8% of eszopiclone-treated subjects compared with 3% receiving placebo.28 In contrast, despite having a half-life of 15.3 hours and a Tmax of 3.5 hours, we found that DXP 6 mg had its most robust effect in the eighth hour of the night. Further, the relative increase in somnolence being reported as an adverse event with this agent was smaller than with either eszopiclone or zolpidem CR (8% DXP 6 mg vs 5% placebo).
This contrast between the findings of the current study and the characteristics of agents which enhance GABAergic inhibition speaks to a fundamental mechanistic difference between low-dose DXP and these agents. Unlike agents which enhance GABAergic inhibition, low-dose DXP is characterized by a dissociation between blood level and clinical effects. Towards the end of the night the clinical effects of low-dose DXP are relatively augmented, as higher blood levels occur earlier in the night (Tmax is 3.5 h) with less clinical effect (for example, see Figure 3). We hypothesize that this reflects that, as noted in the hypothalamus in animal studies, histamine release in humans may be relatively higher towards the end of the night, providing more histamine to be blocked with H1 antagonism and leading to a greater clinical difference between DXP and placebo.29 Further, the clinical effects of DXP are relatively diminished compared to what would be expected on the basis of blood levels shortly after waking. Were this not the case, we would see evidence of residual sedation shortly after waking given that blood levels are only incrementally lower in the hour after waking than in the final hour of the night (Hour 8).
The fact that the greatest clinical effect is observed in Hour 8 with essentially no effects occurring after waking shortly thereafter is evidence of a dissociation of clinical effects and blood level with DXP, which distinguishes DXP from agents which enhance GABAergic inhibition. Although the reason that the clinical effects of low-dose DXP are so markedly diminished shortly after waking remains unknown, possibilities include that a substantial increase in histamine release might occur after waking thus overwhelming the H1 antagonism of low-dose DXP. Additionally, there may be an increase in activity in other parallel wake-promoting systems that could sustain wakefulness and functional capacity, such as orexin or norepinephrine, that are not blocked by DXP, which appears to block only H1 mediated histamine effects.
There are a number of limitations of this study. Although we saw no evidence of dependence phenomena such as tolerance and withdrawal, we cannot rule out that such phenomena may not occur with longer-term nightly use given that the duration of the double-blind period was 5 weeks. In addition, the population of subjects included in this study was limited to primary insomnia patients, so it is possible that the findings might not apply to the treatment of patients with insomnia occurring with comorbid conditions. Though studying primary insomnia patients has advantages for determining drug efficacy in terms of lack of complicating conditions and medications, the majority of patients seen in clinical practice have comorbid conditions, and future studies will be needed to determine the efficacy and safety of doxepin 3 and 6 mg in these populations of insomnia patients. Additionally, the battery of tests used to assess residual impairment was limited and the study would have been strengthened by inclusion of assessments to test balance and sustained attention, or measures of hyperarousal such as body temperature and metabolic rate.
Although further work is needed to determine the mechanism for the unique effects observed with low-dose DXP, there are clear clinical ramifications. Low-dose DXP appears well-suited as a treatment for chronic insomnia patients with sleep maintenance difficulty, particularly when that includes problems in the latter part of the night, often referred to as “early morning awakening.” In terms of early morning awakenings, DXP at these doses appears to be capable of addressing this type of sleep difficulty without substantively increasing residual sedation and impairment.
The current study also builds on the prior studies with low-dose DXP in providing evidence of sleep maintenance efficacy,20–23 while also providing evidence for the absence of discontinuation effects (rebound insomnia, other withdrawal symptoms) across five weeks of nightly treatment in adults. Together these studies provide the best evidence available that a medication can effectively treat both sleep maintenance and early morning awakenings across a variety of chronic insomnia populations without evidence of residual effects the following morning.20–22 Further, there was no evidence of tolerance to the sleep maintenance effects of low-dose DXP in either this trial (3 mg and 6 mg) or in a previous 3-month trial (3 mg).22 Thus, DXP 3 mg and 6 mg have promise as a treatment for chronic insomnia patients with sleep maintenance difficulties and may have unique potential to be an effective treatment for insomnia patients who experience early morning awakenings.
DISCLOSURE STATEMENT
This study (No. SP-0501) was fully funded and supported by Somaxon Pharmaceuticals, Inc., San Diego, California. Dr. Krystal has received grants from the NIH, Sanofi-Aventis, Cephalon, GlaxoSmithKline, Merck, Neurocrine, Pfizer, Sepracor, Somaxon, Takeda, Transcept, Respironics, Neurogen, Evotec, and Astellas. Dr. Krystal is also currently or previously a consultant for Abbott, Actelion, Arena, Astellas, Axiom, AstraZeneca, BMS, Cephalon, Eli Lilly, GlaxoSmithKline, Jazz, Johnson and Johnson, King, Merck, Neurocrine, Neurogen, Neuronetics, Novartis, Organon, Ortho-McNeil-Janssen, Pfizer, Respironics, Roche, Sanofi-Aventis, Sepracor, Somaxon, Takeda, Transcept, Astellas, Research Triangle Institute, Kingsdown Inc., CHDI. Dr. Lankford has received grant support from Actelion, ApniCure Inc., Arena, Cephalon, Evotec, Fisher Paykel, GlaxoSmithKline, Lilly, Merck, Neurim, Neurocrine, Neurogen, Organon, Pfizer, Respironics, Sanofi-Aventis, Schering-Plough, Sepracor, Somaxon Pharmaceuticals, Inc., Sunovion, Takeda, Transcept, UBC, Ventus, and Vanda. He has served as a consultant or on the advisory board of Actelion, ApniCure Inc., Cephalon, Pfizer, and Somaxon Pharmaceuticals, Inc., and has served on the speakers bureau of Jazz Pharmaceuticals and Sanofi-Aventis. Dr. Roth has received grants from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport. He has served as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, élan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Ceullular, Jazz, Johnson and Johnson, King, Lundbeck McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Additionally, Dr. Roth has served as a speaker for Cephalon, Sanofi, and Takeda. Drs. Durrence and Rogowski are employees of Somaxon Pharmaceuticals, Inc. Dr. Ludington was an employee of Somaxon Pharmaceuticals, Inc., during the course of this study through October 2010. Dr. Jochelson was an employee of Somaxon Pharmaceuticals, Inc. from April 2005 to March 2009 and then as a consultant in 2009.
REFERENCES
- 1.Lichstein KL, Durrence HH, Taylor DJ, et al. Epidemiology of sleep: age, gender, and ethnicity. Mahwah, NJ: Erlbam; 2004. [Google Scholar]
- 2.Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 2004;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31:333–46. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 4.Buysse D, Reynolds CF, III, Kupfer DJ, et al. Effects of diagnosis on treatment recommendations in chronic insomnia-a report from the APA/NIMH DSM-IV field trial. Sleep. 1997;20:542–52. doi: 10.1093/sleep/20.7.542. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Association; 1997. text revision (DSM-IV-TR) [Google Scholar]
- 6.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 7.Edinger JD, Bonnet M, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report on an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 8.Hohagen F, Kappler C, Schramm E, et al. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening – temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17:551–4. [PubMed] [Google Scholar]
- 9.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–41. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–755. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. J Clin Sleep Med. 2006;2:S19–S26. [PubMed] [Google Scholar]
- 12.Ebert B, Wafford KA, Deacon S. Treating insomnia: current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–29. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Patat A, Paty I, Hindmarch I. Pharmacodynamic profile of zaleplon, a new non-benzodiazepine hypnotic agent. Hum Psychopharmacol. 2001;16:369–92. doi: 10.1002/hup.310. [DOI] [PubMed] [Google Scholar]
- 14.McQuade R, Creton D, Stanford SC. Effect of novel environmental stimuli on rat behaviour and central noradrenaline function measured by in vivo microdialysis. Psychopharmacology (Berl) 1999;145:393–400. doi: 10.1007/s002130051073. [DOI] [PubMed] [Google Scholar]
- 15.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth T, Zorick F, Wittig R, et al. The effects of doxepin HCl on sleep and depression. J Clin Psychiatry. 1982;43:366–8. [PubMed] [Google Scholar]
- 17.Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: A placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry. 2001;62:453–63. doi: 10.4088/jcp.v62n0609. [DOI] [PubMed] [Google Scholar]
- 18.Hajak G, Rodenbeck A, Adler L, et al. Nocturnal melatonin secretion and sleep after doxepin administration in chronic primary insomnia. Pharmacopsychiatry. 1996;29:187–92. doi: 10.1055/s-2007-979569. [DOI] [PubMed] [Google Scholar]
- 19.Rodenbeck A, Cohrs S, Jordan W, et al. The sleep-improving effects of doxepin are paralleled by a normalized plasma cortisol secretion in primary insomnia. Psychopharmacology. 2003;170:423–8. doi: 10.1007/s00213-003-1565-0. [DOI] [PubMed] [Google Scholar]
- 20.Roth T, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1, 3 and 6 mg in adults with primary insomnia. Sleep. 2007;30:1555–61. doi: 10.1093/sleep/30.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1, 3 and 6 mg in elderly patients with primary insomnia. J Clin Psych. 2008;69:1557–64. doi: 10.4088/jcp.v69n1005. [DOI] [PubMed] [Google Scholar]
- 22.Krystal A, Durrence H, Scharf M, et al. Long-term efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33:1553–61. doi: 10.1093/sleep/33.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth T, Durrence H, Jochelson P, et al. Efficacy and safety of doxepin 6 mg in a model of transient insomnia. Sleep Med. 2010;11:843–7. doi: 10.1016/j.sleep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A, editors. Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, UCLA; 1968. [Google Scholar]
- 25.Wechsler DA. Manual for the Wechsler Adult Intelligence Scale. New York: The Psychological Corporation; 1955. [Google Scholar]
- 26.Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 27.Roth T, Soubrane C, Titeux L, Walsh JK Zoladult Study Group. Efficacy and safety of zolpidem-MR: a double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. 2006;7:397–406. doi: 10.1016/j.sleep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Zammit GK, McNabb LJ, Caron J, Amato DA, Roth T. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979–91. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]
- 29.Tuomisto L, Tuomisto J. Diurnal variations in brain and pituitary histamine and histamine-N-methyltransferase in the rat and guinea pig. Med Biol. 1982;60:204–9. [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]