Abstract
Cobalamin deficiency is relatively common, but the great majority of cases in epidemiologic surveys have subclinical cobalamin deficiency (SCCD), not classical clinical deficiency. Because SCCD has no known clinical expression, its diagnosis depends solely on biochemical biomarkers, whose optimal application becomes crucial yet remains unsettled. This review critically examines the current diagnostic concepts, tools, and interpretations. Their exploration begins with understanding that SCCD differs from clinical deficiency not just in degree of deficiency but in fundamental pathophysiology, causes, likelihood and rate of progression, and known health risks (the causation of which by SCCD awaits proof by randomized clinical trials). Conclusions from SCCD data, therefore, often may not apply to clinical deficiency and vice versa. Although many investigators view cobalamin testing as unreliable, cobalamin, like all diagnostic biomarkers, performs satisfactorily in clinical deficiency but less well in SCCD. The lack of a diagnostic gold standard limits the ability to weigh the performance characteristics of metabolic biomarkers such as methylmalonic acid (MMA) and holotranscobalamin II, whose specificities remain incompletely defined outside their relations to each other. Variable cutoff selections affect diagnostic conclusions heavily and need to be much better rationalized. The maximization of reliability and specificity of diagnosis is far more important today than the identification of ever-earlier stages of SCCD. The limitations of all current biomarkers make the combination of ≥2 test result abnormalities, such as cobalamin and MMA, the most reliable approach to diagnosing deficiency in the research setting; reliance on one test alone courts frequent misdiagnosis. Much work remains to be done.
INTRODUCTION
The time appears ripe, 2 decades into the era of population studies of metabolic cobalamin status, such as NHANES, to critically review the concepts and the performances of the diagnostic biomarkers. Such a review must consider the diverse perspectives of epidemiologic information users, whether investigators who mine and reanalyze the data or clinicians who try to adapt relevant findings to medical care.
The current state of uncertainty about cobalamin deficiency and about its reliable diagnosis especially requires critical thinking. This uncertainty is most obvious in the clinical arena, but its ramifications extend to and must be understood in the public health arena. Even though the public health focus is almost entirely subsumed by subclinical cobalamin deficiency (SCCD) and not clinical deficiency, understanding the complicated subject in either arena begins with the clinical perspective.
Clinicians have become uncertain about what to do with the many asymptomatic patients with mild biochemical abnormalities who increasingly displace ill patients as the face of cobalamin deficiency in medical practice, as important distinctions between clinical disease and biochemical changes blur (1). This incompletely understood SCCD raises quandaries: should such patients be treated, monitored, or perhaps ignored? And, if treated, for how long, with what doses, and to what endpoints? The use of oral cobalamin without a precise diagnosis dominates the landscape and confuses patients and physicians. Moreover, diagnostic abilities have atrophied, most obviously in the disastrous loss of ability to test cobalamin absorption status, which had been crucial to informed prognosis and management. Deficiency that arises from intrinsic factor (IF)–related malabsorption usually progresses inexorably (and was once lethal), whereas nonmalabsorptive or minimally malabsorptive deficiency progresses very slowly or not at all (2).
In the public health arena, the emphasis on frequencies and finding the earliest possible cases made insufficient room for the important distinctions between the cobalamin deficiencies, their causes, and their outcomes. The proper balance between the pursuit of ultimate sensitivity of biochemical detection and overdiagnosis awaits rigorous determination of the effect that the diagnosis of SCCD actually has on health and the benefits of intervention.
THE EVOLUTION OF DIAGNOSTIC TESTING OF COBALAMIN DEFICIENCY
The early history of testing
From 1949 to the late 1980s cobalamin testing consisted almost exclusively of serum cobalamin measurement. The chief goal was to diagnose or rule out the uncommon but very serious deficiency state caused in 94% of cases by pernicious anemia (the irreversible malabsorptive disease defined by loss of gastric IF secretion) and intestinal diseases that prevent IF uptake (3). The clinical hallmark of cobalamin deficiency was megaloblastic anemia (>70% of cases) and/or, somewhat less often, neurologic dysfunction (4).
Readers accustomed to current views of cobalamin as an insensitive test may be surprised to learn that sensitivities were in fact 95–97% in medical encounters (probably because clinical deficiency appears only after its underlying severe malabsorption had sufficiently depleted cobalamin stores). Specificity was always viewed as the greater problem because unexplained low cobalamin concentrations seemed frequent. Not only did folate deficiency regularly depress serum cobalamin, but pregnancy does so even more often (4). Many other low cobalamin concentrations without clinical signs or symptoms had no obvious explanations.
The first useful functional metabolic tests appeared in the 1960s. Urine methylmalonic acid (MMA) became measurable (5) but was relatively insensitive. A highly sensitive tool identified cobalamin and folate deficiencies by their impairment of deoxyuridine suppression of thymidine uptake by cultured marrow cells (6). Demand for these cumbersome tests was limited.
Metabolic markers and subclinical deficiency
Two developments in the mid-1980s changed the scope of cobalamin deficiency and its determination. One was the first documentation of minimal, often “subclinical,” deficiency in a series of studies from my laboratory in patients who lacked anemia, macrocytosis, or deficiency symptoms (7–12). We used the deoxyuridine suppression test, which has proven to be as sensitive as MMA and homocysteine testing (13). Equally important, the deficiencies only rarely arose from disruption of IF-mediated absorption, such as pernicious anemia (8, 10, 14). Our discovery of SCCD indicated that many unexplained low cobalamin concentrations described in the earlier clinical literature were neither spurious nor artifactual.
The second, nearly concurrent, development was the introduction of assays that reliably detect minute elevations of MMA and homocysteine, the known substrates of cobalamin-mediated reactions (15–18). These assays facilitated large surveys that proved that SCCD was much more common than classical clinical deficiency. The criteria and the performance characteristics of the diagnostic markers that define SCCD and clinical deficiency are contrasted in Table 1.
TABLE 1.
Performance characteristics of the criteria and biomarkers that define clinical and subclinical cobalamin deficiency (SCCD)1
| Criteria | Clinical deficiency | SCCD |
| Serum cobalamin <148 pmol/L | Sensitivity: 95–97%Specificity: not determined formally but probably <80% | Sensitivity: 38–39% (55–84% with higher cutoffs)2Specificity: not determined (appears to decline with higher cutoffs)2 Positive predictive value: 58–78% |
| Elevated serum MMA or plasma total homocysteine3 | Sensitivity: >95% for either metaboliteSpecificity: not determined formally (MMA appears to be superior to homocysteine) | Sensitivity: not determinable because abnormality of one or both is required for SCCD diagnosisSpecificity: not determinable because no gold standard exists for comparison (MMA appears to be superior to homocysteine) |
| Low serum holo-TC II | Sensitivity and specificity: presumably similar to those of cobalamin | Sensitivity and specificity: 2–6% higher than those of cobalamin |
| Macrocytosis or macrocytic anemia with megaloblastic changes such as neutrophil hypersegmentation4 | Present in 70–80% of cases; may be hard to recognize in mild casesSpecificity of macrocytosis or macrocytic anemia without megaloblastic changes is low5 | SCCD, by definition, cannot display megaloblastic anemia, which indicates clinical deficiencyNormocytic anemia is almost never attributable to cobalamin deficiency5 |
| Clinical signs of myelopathy, neuropathy, or cognitive dysfunction | Present less often than anemia, but data are imprecise (estimate: 50%)Findings are often stereotypic but are not specific for cobalamin deficiency | SCCD cannot be accompanied by clinical signs, which indicate clinical cobalamin deficiency6 |
MMA, methylmalonic acid; holo-TC II, holotranscobalamin II.
If cobalamin cutoffs are 200–300 pmol/L, instead of 148 pmol/L, sensitivity increases but specificity declines considerably.
Neither test is necessary in clinical deficiency if the clinical signs are typical and serum cobalamin is low. The tests are always necessary in SCCD because they are key for its diagnosis.
Normocytic anemias, whether in clinical deficiency or SCCD, should almost never be attributed to cobalamin deficiency. The only exception is microcytosis caused by iron deficiency or thalassemia that coexist with macrocytosis in 5–10% of cases of pernicious anemia; the combination of the 2 often produces a normal mean corpuscular volume.
If macrocytosis or macrocytic anemia exists but is not clearly megaloblastic, the likely diagnosis is either atypical clinical cobalamin deficiency or macrocytosis unrelated to cobalamin, such as alcohol abuse (the most common cause of macrocytosis), myelodysplastic syndrome, or copper deficiency.
Studies have shown mild electrophysiologic changes in SCCD (11). These changes can improve after therapy, but controlled clinical trials will need to determine their importance. The same applies to statistical associations with cognitive changes. Whether deficiency with isolated electrophysiologic abnormalities requires reclassification as clinical deficiency is not clear.
SCCD as a public health issue
Because of SCCD's high frequency, cobalamin deficiency emerged as a potential public health issue. Research focused on determining the prevalence of biochemical deficiency in populations, especially the elderly. Cross-sectional studies also explored whether health risks accompanied the subclinical state. Serious suspicions have been raised in relation to neurologic and cognitive risks, but the mixed record is not detailed here. The most critical gap remains the absence of clinical trials to determine whether any of the statistical links reflect causation.
In all this activity, investigators have lost sight of important distinctions between clinical deficiency and SCCD (1, 19, 20), which are not merely sequential stations along a continuum of depletion. The sharp disparities begin with pathogenesis, which is clear in clinical deficiency but uncertain in most cases of SCCD. The causes usually dictate whether the deficiency is permanent and progressive (eg, pernicious anemia) or transient and ephemeral. The belief that SCCD must inevitably progress to clinical deficiency misappropriated the behavior of pernicious anemia and continues to color misperceptions about SCCD. The several courses proposed for SCCD (Figure 1) originate from scattered published observations but the natural histories of SCCD remain inadequately studied. Long-term longitudinal studies (21) have been rare.
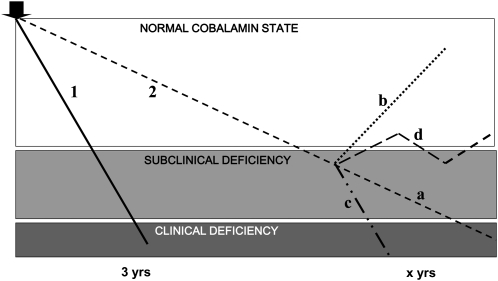
FIGURE 1.
Schematic illustration of the diverse courses that cobalamin deficiency states may follow, depending on their underlying causes. The fields represent, from top to bottom, the normal cobalamin state, subclinical deficiency (mild metabolic abnormalities without clinical signs or symptoms), and clinical deficiency (mild and then progressively more severe hematologic and/or neurologic signs and symptoms). The thick arrow (upper left) marks the onset of gradual cobalamin depletion whose progressions are arbitrarily represented as linear. Line 1: the depletion produced by severe, permanent malabsorption typified by pernicious anemia. Line 2: the less complete, less inexorable disruption of cobalamin balance (eg, dietary insufficiency or a malabsorption limited to food-bound cobalamin). Based on various published direct or indirect (but nonsystematic) observations, the diagram posits a slower course of unknown duration that also increases the time spent transiting through subclinical cobalamin deficiency (SCCD), which may explain why SCCD is more common. At some point, this course may (a) eventually progress sufficiently to produce clinical, symptomatic deficiency, (b) remit completely for reasons that may or may not be known, (c) accelerate and reach clinical deficiency more quickly (eg, chronic gastritis transforms into pernicious anemia as intrinsic factor secretion disappears), or (d) fluctuate indefinitely between normal and mildly subclinical deficiency states. Modified and expanded from reference 19.
SCCD usually lacks the severe IF-related malabsorption that leads inexorably to clinical deficiency. Absorption is normal in most cases, except for a mild and occasionally antibiotic-responsive malabsorption limited to food-bound cobalamin in 30–50% of cases (8, 10, 11, 14, 19). Claims of higher malabsorption rates (22) used dubious diagnostic criteria (fictitiously named “Carmel's criteria”) instead of testing absorption (2). The limited role of malabsorption (14) may help explain the long latency and frequent spontaneous metabolic remissions in SCCD.
These differences illustrate why the facts and therapeutic imperatives of clinical deficiency do not apply automatically to SCCD. Conversely, population surveys generate data relevant mostly to SCCD that may translate poorly to clinical patients; the frequency of cobalamin-related biochemical abnormalities is ≈5–15% in the elderly (23–25), whereas the rate of pernicious anemia is only 1.9% (26). The increasingly routine reliance on metabolic testing in clinical medicine is an example of often wasteful translation from epidemiologic research (1). Macrocytosis, the hallmark and forerunner of the anemia of clinical cobalamin deficiency, is by definition irrelevant to SCCD and frequently nonspecific (27, 28); but population surveys often misattribute normocytic anemia to cobalamin deficiency (29–31).
Methodologic and interpretive concerns in SCCD
Achievement of the highest sensitivity and earliest detection of SCCD has captured substantial diagnostic attention. The advantages are uncertain, and the attempt increases risks of overdiagnosis because the much-needed diagnostic “gold” standard has remained elusive. Another concern for epidemiologic research is that relevant cobalamin deficiency exists only in very small subsets within large populations and applying broad statistical brushstrokes can obscure the critical minorities. Analyses that identify (and distinguish between) neglected subsets, such as persons who have clinical deficiency or malabsorption, may mitigate distortion and add valuable depth to the flat landscape of broad population analyses.
DIAGNOSTIC MARKERS
The preeminence of serum cobalamin as a diagnostic tool has come under challenge because of its reduced sensitivity for SCCD. This decrease is not surprising because most biomarkers lose sensitivity when applied to mild or marginal conditions. The lack of a gold standard complicates diagnostic evaluations and decisions, especially when diagnosis depends completely on biomarkers, as it does in SCCD. (Reliance on biomarkers is lower in the clinical arena, where clinical manifestations provide a diagnostic anchor and serum cobalamin often serves only a confirmatory function.) Because all biomarkers lack sufficient sensitivity and specificity to be reliable in SCCD, reliance on any single diagnostic test is risky in epidemiologic research and public health surveys. Which tests to use and how many results must be abnormal to provide reasonable diagnostic certainty of deficiency depend on many circumstances, and opinions vary.
All the biomarkers and their assays have idiosyncratic requirements and confounders, but some influences seem universal. Renal dysfunction elevates all biomarker concentrations; MMA and homocysteine correlate with even minor changes in creatinine and other indexes of glomerular filtration (32, 33), whereas cobalamin and holotranscobalamin II (holo-TC II) seem to rise significantly only with advanced renal dysfunction and by uncertain mechanisms (32, 34). Genetic influences on serum concentrations, transport, and use are becoming increasingly apparent. However, cutoff selection remains the most important external influence on biomarker data and requires careful, agenda-free science.
SERUM COBALAMIN MEASUREMENT
Methodologic notes
Cobalamin assays require extraction of cobalamin from its binding proteins in plasma and conversion of the native cobalamins to cyanocobalamin, the nonphysiologic form that serves as the assay standard. Quantitation, initially based on the growth-promoting function of cobalamin on microbes, was replaced by radioisotopic competitive binding techniques using IF as the cobalamin-binding protein. Transcobalamin (TC) I, also called haptocorrin, whose affinity for nonfunctional corrin analogs produces spuriously high “cobalamin” values in sera that contain substantial amounts of such analogs, must not contaminate the IF (35).
Although a few reference laboratories prefer microbiologic assays, immunoenzymatic luminescence methods that rely on competitive binding by IF have now replaced isotopic assays. The newer methods need attention. They involve proprietary secrets, and their manufacturers do not always observe transparency (36) or track assay performance closely. A laboratory survey reported diagnostic misclassifications (37). Unfortunately, monitoring and proficiency testing are currently suboptimal because they do not use optimal low-cobalamin control specimens.
The most worrisome immunoenzymatic assay failures have been occasional systematic overestimates that seem limited to samples from overtly cobalamin-deficient patients, often with pernicious anemia, and without commensurate errors in normal sera (38–40). These errors have involved many manufacturers' assays at various times; in one report, 3 of 5 commercial assays failed to give the expected low cobalamin results in one or both of 2 patients (40). Sometimes the error is demonstrably due to the kit's failure to denature the sample's anti-IF antibody, which competes with the kit's anti-IF antibody. An error that affected 73% of cobalamin-deficient specimens was undetected for months (38). A clinical study attributed an unprecedented 86% rate of falsely normal cobalamin results to insensitivity of cobalamin testing (41), but its use of the previously mentioned assay suggests assay artifact instead (42). The error is hard to detect because it does not appear to affect normal sera. Control samples that contain anti-IF antibody may be needed. Cobalamin is stable on storage (43), but spuriously high results can result in blood samples drawn into certain phlebotomy tubes that contain gel (44).
Performance characteristics
Aside from the mentioned assay artifacts, falsely normal cobalamin concentrations are infrequent in patients with clinically expressed deficiency. Indeed, the sensitivity of cobalamin concentrations <148 pmol/L (<200 ng/L) usually exceeded 95% in patients with megaloblastic anemia (15, 17, 18) and was 90% in a clinical survey that used a >50% decline in MMA concentration after therapy as a gold standard (45). However, sensitivity fell to 38–39% in nonclinical population surveys that compared against MMA or homocysteine endpoints (24, 46). These surveys did not determine the specificity of cobalamin <148 pmol/L, but positive predictive values were 58–78% in metabolic surveys in which SCCD predominated (23, 24, 46, 47). One nonclinical population survey, which used highly stringent MMA criteria (>450 and >750 nmol/L), reported specificities of 72% and 75%, respectively, for cobalamin concentrations <200 pmol/L, along with sensitivities of 55% and 66%, respectively (25).
The sensitivities and specificities of biomarkers are difficult to define in SCCD because the only possible comparators are other biomarkers, which have their own sensitivity and/or specificity limitations. In the examples above, MMA was elevated more often than cobalamin, and investigators usually deferred to the abnormal MMA results. A normal MMA result can serve as evidence against cobalamin deficiency, but distinguishing between a “falsely normal” cobalamin result and a “falsely abnormal” MMA result is difficult, especially if abnormalities are mild and clinical arbiters are lacking. Combined biomarkers (eg, MMA plus homocysteine) tended to perform better than either one alone (23, 24), as did profound degrees of abnormality.
MMA and homocysteine response to therapy can be diagnostically helpful, whereas cobalamin and holo-TC II responses are uninformative because concentrations rise as cobalamin enters the bloodstream irrespective of antecedent status. However, tests of biochemical response are not practical in most population surveys, and “improvement” can also represent regression to the mean.
Influence of cutoff selection
Cobalamin studies in the 1950s reported impossibly low cutoffs, ≈<90 pmol/L, perhaps because definitions were based in part on very severe deficiency (4). Most subsequent studies have used cutoffs ≈<148 pmol/L (<200 ng/L).
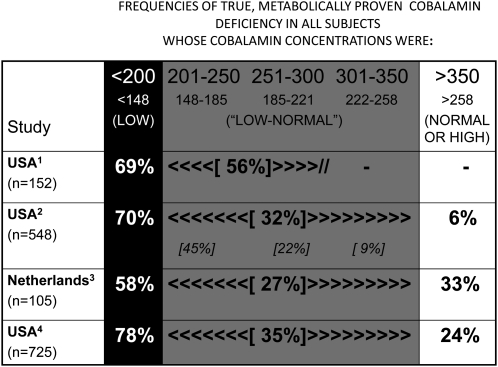
When metabolic testing in SCCD uncovered the insensitivity of serum cobalamin relative to MMA, a marker of incompletely defined specificity, some investigators suggested that the cutoff be moved from <148 to <221 or <258 pmol/L (0.738 pmol/L = 1 ng/L) to capture more abnormalities (23, 47) and emphasized that 2.9–5.2% of patients with clinical deficiency also had cobalamin concentrations >148 pmol/L (ie, as excellent a 95–97% sensitivity as that of homocysteine and MMA). The cutoff changes implied 2 untested premises: that all SCCD requires treatment and that “early detection” justifies mislabeling many nondeficient persons. The frequency of falsely low cobalamin concentrations by metabolic criteria jumped from 22–42% at cutoffs of <148 pmol/L (<200 ng/L) to 44–73% at cutoffs of <201–350 pmol/L (23, 24, 46, 47), as shown in Figure 2. In other words, only a minority of the new cases even had SCCD (and extremely few had clinical deficiency). Moreover, MMA and homocysteine abnormalities continued to be found, even at high-normal serum cobalamin concentrations (>350 ng/L) (Figure 2). Despite the issues, many investigators and clinical laboratories quickly adopted the new cutoffs.
FIGURE 2.
The influence of higher cutoffs for serum cobalamin concentrations on the frequency of true deficiency in the cases labeled as “deficient” by each cutoff. True deficiency was defined by each study's abnormal methylmalonic acid and homocysteine results. Results of 4 surveys with metabolic data that provided the frequencies (or permitted their calculations) are shown here. This figure summarizes the cobalamin concentrations and cutoffs in ng/L ranges in the top line and in pmol/L in smaller font in the second line (1 ng = 0.738 pmol), which closely approximate the different points in all 4 studies. The arrowheads that bracket the frequency rates of metabolically defined deficiency in the center box (201–350 ng/L) delineate the relevant ranges of cobalamin values. The further data breakdowns available in reference 23 provide more discrete subset rates (italicized in smaller font). 1Reference 47, 2Reference 23, 3Reference 46, 4Reference 24.
The major impact that such cutoff choices have on the frequency of deficiency (23–25, 46–51) is quantified in Table 2. Studies that used cutoffs of 248 pmol/L (300 ng/L) labeled 34–50% of their populations as cobalamin deficient, compared with 5–12% at traditional cutoffs. This impact of cutoff manipulation calls attention to the difficulties that surround the choices.
TABLE 2.
Influence of diagnostic cutoff selection on the frequency of “abnormal” serum cobalamin concentrations in epidemiologic surveys of the elderly1
| Source location, year (reference) | Study population | n | Compared cobalamin cutoffs | Frequencies of “abnormal” cobalamin that resulted |
| pmol/L | % | |||
| Denver, 1992 (47) | Elderly outpatients | 152 | <148 vs <221 | 8.5 vs 25.0 |
| Framingham, 1994 (23) | Elderly town dwellers | 548 | <148 vs <258 | 5.3 vs 40.5 |
| Netherlands, 1998 (46) | Elderly town dwellers | 105 | <150 vs <260 | 24.8 vs 60.1 |
| Oklahoma City, 1998 (48) | Elderly outpatients | 303 | <148 vs <221 | 6.3 vs 16.2 |
| United States, 1999 (49) | Disabled elderly town-dwelling women | 762 | <148 vs <258 | 6.2 vs 33.5 |
| Los Angeles, 1999 (24) | Elderly town dwellers and outpatients | 591 | <140 vs <258 | 11.8 vs 50.4 |
| United Kingdom, 2007 (25) | Elderly town dwellers in medical registries | 2403 | <150 vs <200 vs <300 | 8.6 vs 29.3 vs 71.7 |
| Norway, 2009 (50) | Adults aged 47–49 y | 3684 | <150 vs <200 vs <400 | 0.4 vs 3.1 vs 64.5 |
| Norway, 2009 (50) | Adults aged 71–74 y | 3262 | <150 vs <200 vs <400 | 3.1 vs 6.7 vs 67.6 |
| Georgia, 2010 (51) | Octogenarians | 79 | <148 vs <185 vs <258 | 7.6 vs 11.4 vs 38.0 |
| Georgia, 2010 (51) | Centenarians | 215 | <148 vs <185 vs <258 | 11.6 vs 22.8 vs 39.1 |
All of the surveys focused on elderly subjects, but the Norwegian survey (50) also included middle-aged adults.
Other interpretive issues
Spuriously low cobalamin concentrations in pregnancy and folate deficiency limit cobalamin specificity (4). Variations in plasma cobalamin-binding proteins can produce low or elevated cobalamin concentrations because TC I normally carries >80% of plasma cobalamin and often determines cobalamin concentrations (52). TC I deficiency may account for as much as 15% of low cobalamin concentrations (53); if so, it may cause more low cobalamin concentrations than does pernicious anemia. TC I deficiency often has a genetic origin (54); mutations in both alleles cause cobalamin concentrations of <100 pmol/L, whereas the more common heterozygous state causes mildly low or borderline cobalamin concentrations (53, 54).
Spuriously high cobalamin concentrations often accompany elevated TC I levels. The best known causes of dramatic elevations, such as chronic myelogenous leukemia and some cancers, are rare (4). Equally high cobalamin concentrations result from antibodies to TC II (often after hydroxocobalamin injections) or TC I (55–57), which may account for 8% of high cobalamin concentrations (58). More widespread are the more moderate cobalamin and TC I elevations found in renal failure (34), in blacks compared with other ethnic groups (59), and in association with homozygosity for α,1,2-fucosyltransferase gene polymorphisms (60).
SERUM MMA MEASUREMENT
The metabolic dependence of isomerization of l-methylmalonyl coenzyme A by methylmalonyl coenzyme A mutase with 5′-deoxyadenosylcobalamin as a cofactor, and its independence from folate metabolism, have made MMA an attractive biomarker of cobalamin insufficiency.
Methodologic notes
Gas chromatographic assay of MMA in urine has been available since the late 1950s (5) and a more sensitive gas chromatography/mass spectrometry method appeared in 1979 (61). Sensitive capillary gas chromatography/mass spectrometry assays with various modifications soon made it possible to measure small concentrations in serum accurately and precisely (15–18). As automation progressed, serum MMA determination became more widely available.
Performance characteristics
The sensitivity of serum MMA elevation for cobalamin deficiency in patients with overt pernicious anemia was usually >95% (15, 17, 18). Most MMA elevations were large, often in the thousands of nmol/L (15). In these and similar (62) clinical settings, MMA, like homocysteine and holo-TC II (62), performed only marginally better than serum cobalamin. All biomarkers appear to be less sensitive in SCCD, but no biochemical gold standard exists against which to compare them reliably. MMA values are usually only borderline or mildly abnormal in SCCD and, unlike in clinical deficiency, they are rarely >800 nmol/L.
MMA elevation is consistently more frequent than low cobalamin concentrations in population surveys (23, 24, 30, 50). Cutoff selections determine the frequency, but this disparity with cobalamin suggests MMA's superior sensitivity for cobalamin deficiency (indeed, a normal MMA value supports the likelihood of normal cobalamin status even when cobalamin concentration is low). Nevertheless, at least some of the isolated MMA elevations reflect limitations in MMA specificity, which has been difficult to determine reliably. A large Norwegian survey identified creatinine, cobalamin, age, and sex as the major identifiable influences on serum MMA, but these explained only 16% of MMA variation (50). A smaller study showed that cobalamin predicted only 2% of the serum MMA value, whereas cystatin C, an index of glomerular filtration, predicted 8% (33).
Additional influences on MMA surely exist and require systematic study. Some authors have suggested, but not documented, a role for plasma volume contraction in some unexplained MMA elevations (15). Antibiotics sometimes lower MMA concentrations, which suggests influences from active propionate metabolism by intestinal bacteria (18, 63). Neither heterozygosity for genetic methylmalonic aciduria (64) nor MMA-related polymorphisms seem, thus far, to elevate serum MMA significantly. The observation of elevated MMA concentrations during the first year of life (65) remains a puzzle. The values return to normal spontaneously, but statistical association with lower cobalamin and higher homocysteine values (both still normal) and reduction of MMA concentrations by cobalamin therapy raise the possibility of mild cobalamin deficiency (66).
Given its undetermined specificity, MMA cannot be the gold standard for cobalamin deficiency (eg, as used in the holo-TC II literature). Moreover, a follow-up of 432 untreated, asymptomatic patients with MMA >280 nmol/L showed that MMA fluctuated over 1–3.9 y (CV: 34%) (21). MMA elevation progressed in only 16% of cases, compared with spontaneous decline in 44% and no change in the remainder. As these findings suggest, minor changes, whether spontaneous or after treatment (41), should not be overinterpreted (42).
The influence of cutoff selection
Published MMA cutoffs have ranged from 210 to 480 nmol/L, sometimes within the same laboratory. Frequencies of MMA abnormality, and therefore by extension often of SCCD, vary inversely with the selected cutoff. The Hordaland data (50) aptly show the effects of cutoff migration (Table 3). As another example, the MMA cutoffs in the 4 surveys in Figure 2 (23, 24, 46, 47) ranged from 240 to 370 nmol/L, and their 5–44% frequencies of MMA abnormality diagnoses bore inverse relations to the cutoffs.
TABLE 3.
Influence of diagnostic cutoff selection on frequency of “abnormal” serum methylmalonic acid concentrations, as illustrated in middle-aged and elderly subjects in the Hordaland survey1
| Frequency of “abnormal” MMA that resulted from selected cutoffs2 |
||
| Selected MMA cutoff | 3684 adults aged 47–49 y | 3262 adults aged 71–74 y |
| % | ||
| >210 nmol/L | 14.7 | 36.7 |
| >260 nmol/L | 5.3 | 17.7 |
| >370 nmol/L | 1.2 | 5.2 |
| >750 nmol/L3 | 0.1 | 0.9 |
This survey showed lower rates of cobalamin deficiency than most population surveys to date. Data are from reference 50. MMA, methylmalonic acid.
The frequencies of “abnormal” results with the use of these cutoffs may be higher in other populations.
This highly stringent cutoff is also likely to select a much higher proportion of subjects with clinical deficiency that arises from malabsorption (eg, pernicious anemia) and a lower proportion of subjects with subclinical deficiency than do lower cutoffs.
The most commonly applied MMA cutoff is ≈270 nmol/L. Many laboratories defined cutoffs by 3 or 2 SD from the mean (≈>370 or >270 nmol/L, respectively). Others made physiologic choices (eg, by demonstration or projection of MMA concentrations in cobalamin-repleted subjects) as low as 210 nmol/L (67). The maximally suppressed MMA concentration might represent true normality but it is not necessarily practical or desirable in clinical or epidemiologic settings. Such MMA response can help, as it helps when assessing therapeutic effectiveness (17), but abnormal values can also regress to the mean. It is also possible that some improvements of mildly elevated or even normal MMA merely signify fuller saturation of the methylmalonyl coenzyme A mutase by its cobalamin cofactor. On balance, when studies use MMA alone to define cobalamin deficiency, unusually low MMA cutoffs such as 210 nmol/L (30, 31) compound the risk of overdiagnosis (R Carmel, unpublished observations, 2010). A potential data-based approach to defining biomarker cutoffs may be to construct 2-phase regression models to determine where their slopes change in relation to other biomarkers (68).
HOLOTRANSCOBALAMIN II
TC I and TC II (those who call TC I haptocorrin refer to TC II as TC) carry cobalamin in the bloodstream. Holo-TC II, the TC II fraction with attached cobalamin, forms at the ileal cell and delivers its cobalamin to tissues so rapidly that <20–30% of plasma cobalamin normally exists as holo-TC II at any time. The remaining cobalamin circulates in holo-TC I, which turns over slowly with no discernible delivery to cells and is considered metabolically unavailable.
Two subtly different and, in fact, competing diagnostic claims about holo-TC II (69) are that low concentrations either indicate impaired holo-TC II transfer into the bloodstream from the ileal cell (ie, a marker of cobalamin malabsorption) or denote inadequate availability to tissues (ie, metabolic insufficiency). Both claims may be partially true (70), an ambiguity that ultimately renders the test nonspecific for either claim. Malabsorption and deficiency can and often do exist without each other (69). Transient (eg, drug-induced) malabsorption might exist for only a few weeks or months and produce low holo-TC II concentrations without ever creating cobalamin deficiency, which requires years of storage depletion. To complicate holo-TC II status further, the gut may not be the sole contributor to circulating holo-TC II (69).
Some authors view holo-TC II abnormality as the earliest possible marker of cobalamin deficiency because it occurs in patients with normal concentrations of all other biomarkers. Current research cannot prove or disprove such conclusions, which court tautology. The full range of alternative influences on holo-TC II concentrations has not been adequately explored.
Methodologic notes
Lindemans et al (71) suggested in 1983 that measurement of holo-TC II, whose cobalamin is available to cells, may provide a truer snapshot of cobalamin status than total cobalamin. Documentation was limited for many years because holo-TC II concentrations are very small and early methods were crude and often indirect (69). Reliable study became possible with the reports of an accurate enzyme-linked immunosorbent assay and radioimmunoassay in 2002 (72, 73). An automated 2-step sandwich microparticle enzyme immunoassay centered on an antibody to holo-TC II (74) replaced the radioimmunoassay in 2008. Reference intervals are similar but not identical in the 3 methods; plasma samples might also yield slightly higher values than do serum samples.
Performance characteristics
Careful clinical assessment has been limited, in part because cobalamin absorption testing became unavailable after the late 1990s. Reliable studies must resolve what holo-TC II reflects and explore potential confounders of results.
Performance assessment has largely relied on statistical comparisons in populations that did not include well-characterized patients. Studies compared sensitivity and specificity with those of cobalamin and often used MMA as the benchmark despite its undefined specificity. Most receiver operator characteristics–based comparisons and similar analyses of sensitivity and specificity have shown that holo-TC II modestly outperforms total cobalamin, with areas under the curves of 0.75–0.90 and 0.72–0.85, respectively (25, 62, 75, 76). One study (25) concluded that neither test is suitable for the screening of asymptomatic populations (ie, SCCD) because false-positive results outnumber true-positive ones. A clinical study in 49 patients with early but clinically expressed deficiency concluded that neither metabolic biomarkers nor holo-TC II outperformed cobalamin as a predictor of response to cobalamin therapy (62).
The clinical specificity of holo-TC II, as opposed to its comparability against MMA, is unclear. Holo-TC II rises in renal failure (34), but modest renal insufficiency does not affect holo-TC II or total cobalamin as much as it affects MMA and total homocysteine concentrations (32). Holo-TC II becomes normal in patients with pernicious anemia after correction of their cobalamin deficiency but continues to be mildly but significantly lower than in control subjects (70), which supports absorption status as an independent influence on holo-TC II values. Whether prolonged fasting can similarly affect holo-TC II concentrations is unknown. A frequent genetic variant, the substitution of proline for arginine in TCN2 codon 259, may impair holo-TC II function; its effect on serum holo-TC II concentrations is unsettled (77–79). Suggested but unexplored clinical confounders of holo-TC II include oral contraceptive use, myelodysplasia and other hematologic conditions, histiocytic disorders, folate disorders, and alcoholism (76, 80, 81). A proposed diagnostic advantage for holo-TC II over cobalamin in pregnancy (82) is inconclusive: holo-TC II was not measured before pregnancy but its concentrations rose postpartum, which suggests that an unrecognized holo-TC II decline had, in fact, occurred during pregnancy.
The influence of cutoff choices
Laboratories have used holo-TC II cutoffs as high as 50 pmol/L. A multicenter evaluation of laboratory performance noted cutoffs between 11 and 41 pmol/L in 8 studies, and the authors favored the higher values as more representative of normality (76). However, the automated assay that dominates the commercial market identified a 95% CI cutoff of 19 pmol/L (74), which was even lower than the 24 pmol/L cutoff in its predecessor assay (72). The influence of cutoffs on outcomes awaits formal study. Only one group has suggested a sex difference in values, with a lower cutoff for women aged <45 y (83).
Other interpretive issues
Miller et al (75) reported that holo-TC II and total cobalamin results agreed in 93% of cases (Table 4, groups 1 and 4). The crux to understanding how the 2 biomarkers' performances differ lies within the remaining 7%, of whom only 1 in the 2 markers indicated cobalamin deficiency. Clinical, metabolic, and absorptive investigation of this subset would be invaluable.
TABLE 4.
Comparison of frequencies of agreement and disagreement between cobalamin and holo-transcobalamin II (holo-TC II) results in 607 subjects in the Sacramento Area Latino Study of Aging 1
| Group | Cobalamin results | Holo-TC II results | n | Frequency in study population |
| % | ||||
| 1 | Low | Low | 32 | 5.3 |
| 2 | Normal | Low | 28 | 4.6 |
| 3 | Low | Normal | 14 | 2.3 |
| 4 | Normal | Normal | 533 | 87.8 |
The cutoff used to differentiate low from normal cobalamin results was 148 pmol/L. The cutoff used to differentiate low from normal holo-TC II results was 35 pmol/L. Data are from reference 75.
PLASMA TOTAL HOMOCYSTEINE
Plasma homocysteine has largely comparable sensitivity to MMA for cobalamin deficiency and is also excellent for monitoring response to cobalamin therapy (17). However, homocysteine is too limited by its confounders to serve as a cobalamin status biomarker and I will not discuss it in detail. Its equal susceptibility to folate changes prevents its use to distinguish between cobalamin and folate deficiencies in epidemiologic surveys such as the National Health and Nutrition Examination Survey. Although folic acid fortification may have left cobalamin deficiency as the chief influence on homocysteine concentrations among the elderly in the United States (84), folate status explains 15% of homocysteine variation, compared with 4% for cobalamin status in European countries without fortification (33). Homocysteine is also the most susceptible of the 4 biomarkers to preanalytic influences of sample collection and processing, and it is the only one with sex-related differences in reference intervals that require stratified analyses. A review of expert opinion provides further discussion of the homocysteine assay and its interpretations (85).
CONCLUSIONS AND RECOMMENDATIONS
Issues of concepts and goals
Both public health experts and clinicians must consider all the implications of SCCD's heavy predominance in population surveys in contrast to medical encounters. SCCD differs fundamentally, not just in degree, from clinical deficiency. That SCCD rarely arises from IF-related malabsorption may explain its unlikely or delayed progression to clinical deficiency. The differences make unfiltered equations of findings and expectations between the public health and clinical sectors potentially confusing for both sectors. Epidemiologists have reported potentially important associations between SCCD and neurocognitive changes, but only prospective clinical trials can transform statistical associations into causative health risks and prove that intervention brings benefits. Randomized trials may be unethical in clinical cobalamin deficiency, but they are ethical, and indeed imperative, in SCCD.
More comprehensive efforts must be marshalled to satisfy the US Food and Drug Administration's charge that researchers monitor and clarify folic acid fortification's effect on cobalamin deficiency. Despite some intrinsic limitations, only large surveys can identify enough potential subjects. However, proper focus on the population subsets at greatest risk may require means to differentiate between nonmalabsorptive SCCD and incipient stages of pernicious anemia (28); more diverse and sensitive neurologic and cognitive tests are also needed.
Epidemiologic focus should extend to cobalamin status in young children (MMA is often high in the first year of life), persons with Helicobacter pylori infection, and chronic users of drugs that suppress gastric acid secretion. Attention to cobalamin excess and its effects may also become necessary. Daily 1000–2000 μg cyanocobalamin self-supplementation has grown, and this nonphysiologic cobalamin can accumulate substantially in cells under such circumstances (86). Red cells could become a valuable research resource.
Methodologic and interpretive issues
The greatest intrinsic source of diagnostic uncertainty in cobalamin status assessment is the absence of a gold standard for deficiency. This barrier and the limitations of all markers dictate that researchers no longer rely on any single test. A model for combining tests in all future research, preferably with the use of markers of different cobalamin properties and nonoverlapping confounder influences, is presented in Table 5. Studies can combine a marker of cobalamin content, such as serum cobalamin or holo-TC II, with a metabolic function indicator, such as serum MMA. Despite minor statistical advantages over cobalamin, holo-TC II awaits systematic study of subjects defined by relevant characteristics that include absorption status, not just by their MMA status, and of confounding influences on holo-TC II results. When considering everything, the best diagnostic combination currently appears to be cobalamin and MMA. Reliable diagnosis of deficiency requires abnormal results in both chosen tests. Basing diagnoses on an abnormality of either test alone compounds their nonspecificities and guarantees overdiagnosis of deficiency. Research must also better assess the specificity of MMA elevation in SCCD.
TABLE 5.
Suggested principles for optimal diagnostic testing for cobalamin deficiency in epidemiologic research in the absence of a diagnostic gold standard1
| Biomarker results |
||
| Cobalamin2 | MMA | Diagnostic interpretation |
| Abnormal | Abnormal | Cobalamin deficiency |
| Normal | Abnormal | No deficiency3 |
| Abnormal | Normal | No deficiency4 |
| Normal | Normal | No deficiency |
Principles and assumptions are as follows: 1) Reliance on one biomarker alone, whether the result is normal or abnormal, is inadequate for reliable biochemical diagnosis when clinical assessment is unavailable or clinical deficiency is absent. 2) The use of all 4 biomarkers, although informative sometimes, is excessive and multiplies the likelihood of diagnostic categorization disagreements. 3) The choice should include at least one indicator of vitamin amount [total cobalamin or holo-transcobalamin II (holo-TC II)] and at least one indicator of metabolic function [methylmalonic acid (MMA) or homocysteine]. 4) Plasma homocysteine, although reasonably sensitive, has disabling specificity problems that prevent its selection as one of the key biomarkers. 5) Although holo-TC II has slightly better sensitivity and specificity than total cobalamin, the sparse information on alternative influences on holo-TC II (ie, diagnostic confounding and specificity) makes cobalamin a superior choice. 6) This schema is less applicable to clinical cobalamin deficiency than to subclinical cobalamin deficiency because clinical findings can modify some of the interpretations. The diagnostic interpretations shown are consistent with the principle that biochemical diagnosis of deficiency can be certain only when more than one biochemical abnormality is evident. In some cases, this rule might miss a diagnosis.
Serum cobalamin is preferable to holo-TC II because research has better characterized it and its shortcomings. If holo-TC II testing is substituted for cobalamin testing in this column, the diagnostic interpretations in the right-hand column will be similar.
The normal cobalamin result in the face of an abnormal MMA result suggests either metabolic cobalamin deficiency with a falsely normal serum cobalamin concentration or nondeficiency with a falsely elevated MMA concentration. The odds may favor the former formulation but the uncertainty mandates against a diagnosis of deficiency in a research study. (In the clinical setting, other information on the patient can bear on the diagnostic interpretation; in a research study, the possibility of doing so depends on the study design.)
Falsely low cobalamin: suspect transcobalamin I deficiency or folate deficiency if the subject is not pregnant. (If holo-TC II was tested instead of cobalamin, the diagnostic possibilities include a falsely low holo-TC II, whose causes remain poorly defined, and cobalamin malabsorption without cobalamin deficiency.)
The greatest extrinsic source of diagnostic uncertainty (and noncomparability of studies) for all biomarkers is the highly variable use of cutoffs. Experts should develop guidelines for appropriate criteria and methods that laboratories can use when defining cutoffs.
The current cobalamin assay methods also require attention. The limited transparency of commercial sources and possibly frequent malfunctions of many immunoenzymatic methods that disproportionately affect samples from subjects with cobalamin deficiency and can easily escape detection are disquieting. The inclusion of control samples that contain anti-IF antibody may improve detection of the error. The microbiological cobalamin assay may be a viable alternative for large reference laboratories.
Planning for future capabilities
The ability to identify IF-related malabsorption, primarily pernicious anemia, would greatly improve the categorization of cobalamin deficiency in surveys. Such information is likely to be as relevant to deficiency as the biochemical expression of deficiency, and may also hold the key to understanding the putative neurologic complications when folic acid intake is high (28). Inclusion of the anti-IF antibody assay, despite its low sensitivity, seems the best available option after the disappearance of reliable absorption tests. The antibody test has near-100% specificity for pernicious anemia and requires only a serum sample, and laboratories may measure it post hoc in samples with low cobalamin concentrations. An improved capability for measurement of nonfunctional analogs of cobalamin, which can accumulate in some subjects and may have adverse implications, may also be useful to future surveys.
Acknowledgments
The author had responsibility for all parts of the manuscript. He has submitted a patent application for work related to transcobalamin I deficiency and its genetic aspects. The author did not declare a conflict of interest.
REFERENCES
- 1.Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood 2008;112:2214–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmel R. The disappearance of cobalamin absorption testing: a critical diagnostic loss. J Nutr 2007;137:2481–4 [DOI] [PubMed] [Google Scholar]
- 3.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994;96:239–46 [DOI] [PubMed] [Google Scholar]
- 4.Chanarin I. The megaloblastic anaemias, 2nd ed. Oxford, United Kingdom: Blackwell Scientific Publishing, 1979 [Google Scholar]
- 5.Cox EV, White AM. Methylmalonic acid excretion: index of vitamin B12 deficiency. Lancet 1962;2:853–6 [DOI] [PubMed] [Google Scholar]
- 6.Metz J, Kelly A, Swett VC, Waxman S, Herbert V. Deranged DNA synthesis by bone marrow from vitamin B12-deficient humans. Br J Haematol 1968;14:575–92 [DOI] [PubMed] [Google Scholar]
- 7.Carmel R, Karnaze DS. The deoxyuridine suppression test identifies subtle cobalamin deficiency in patients without typical megaloblastic anemia. JAMA 1985;253:1284–7 [PubMed] [Google Scholar]
- 8.Carmel R, Sinow RM, Karnaze DS. Atypical cobalamin deficiency: subtle biochemical evidence of deficiency is commonly demonstrable in patients without megaloblastic anemia and is often associated with protein-bound cobalamin malabsorption. J Lab Clin Med 1987;109:454–63 [PubMed] [Google Scholar]
- 9.Karnaze DS, Carmel R. Low serum cobalamin levels in primary degenerative dementia: Do some patients harbor atypical cobalamin deficiency states? Arch Intern Med 1987;147:429–31 [PubMed] [Google Scholar]
- 10.Carmel R, Sinow RM, Siegel ME, Samloff IM. Food cobalamin malabsorption occurs frequently in patients with unexplained low serum cobalamin levels. Arch Intern Med 1988;148:1715–9 [PubMed] [Google Scholar]
- 11.Carmel R. Subtle and atypical cobalamin deficiency states. Am J Hematol 1990;34:108–14 [DOI] [PubMed] [Google Scholar]
- 12.Carmel R. Reversal by cobalamin therapy of minimal defects in the deoxyuridine suppression test in patients without anemia: further evidence for a subtle metabolic cobalamin deficiency. J Lab Clin Med 1992;119:240–4 [PubMed] [Google Scholar]
- 13.Carmel R, Rasmussen K, Jacobsen DW, Green R. Comparison of the deoxyuridine suppression test with serum levels of methylmalonic acid and homocysteine in mild cobalamin deficiency. Br J Haematol 1996;93:311–8 [DOI] [PubMed] [Google Scholar]
- 14.Carmel R. Malabsorption of food cobalamin. Baillieres Clin Haematol 1995;8:639–55 [DOI] [PubMed] [Google Scholar]
- 15.Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Assay of methylmalonic acid in the serum of patients with cobalamin deficiency using capillary gas chromatography-mass spectrometry. J Clin Invest 1986;77:1606–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen K. Solid-phase sample extraction for rapid determination of methylmalonic acid in serum and urine by a stable-isotope-dilution method. Clin Chem 1989;35:260–4 [PubMed] [Google Scholar]
- 17.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency. I. Usefulness of serum methylmalonic acid and total homocysteine levels. Am J Hematol 1990;34:90–8 [DOI] [PubMed] [Google Scholar]
- 18.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency. II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol 1990;34:99–107 [DOI] [PubMed] [Google Scholar]
- 19.Carmel R. Cobalamin, the stomach, and aging. Am J Clin Nutr 1997;66:750–9 [DOI] [PubMed] [Google Scholar]
- 20.Carmel R, Sarrai M. Diagnosis and management of clinical and subclinical cobalamin deficiency: advances and controversies. Curr Hematol Rep 2006;5:23–33 [DOI] [PubMed] [Google Scholar]
- 21.Hvas AM, Ellegaard J, Nexo E. Increased plasma methylmalonic acid level does not predict clinical manifestations of vitamin B12 deficiency. Arch Intern Med 2001;161:1534–41 [DOI] [PubMed] [Google Scholar]
- 22.Andres E, Kurtz JE, Perrin AE, et al. Oral cobalamin therapy for the treatment of patients with food cobalamin malabsorption. Am J Med 2001;111:126–9 [DOI] [PubMed] [Google Scholar]
- 23.Lindenbaum J, Rosenberg IH, Wilson PWF, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly. Am J Clin Nutr 1994;60:2–11 [DOI] [PubMed] [Google Scholar]
- 24.Carmel R, Green R, Jacobsen DW, Rasmussen K, Florea M, Azen C. Serum cobalamin, homocysteine and methylmalonic acid concentrations in a multiethnic elderly population: ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr 1999;70:904–10 [DOI] [PubMed] [Google Scholar]
- 25.Clarke R, Sherliker P, Hin H, et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin Chem 2007;53:963–70 [DOI] [PubMed] [Google Scholar]
- 26.Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med 1996;156:1097–100 [PubMed] [Google Scholar]
- 27.Carmel R. Mean corpuscular volume and other concerns in the study of vitamin B-12 deficiency: epidemiology with physiology. Am J Clin Nutr 2008;87:1962–3 [DOI] [PubMed] [Google Scholar]
- 28.Carmel R. Does high folic acid intake affect unrecognized cobalamin deficiency, and how will we know it if we see it? Am J Clin Nutr 2009;90:1449–50 [DOI] [PubMed] [Google Scholar]
- 29.Brouwer I, Verhoef P. Folic acid fortification: is masking of vitamin B12 deficiency what we should really worry about? Am J Clin Nutr 2007;86:897–8 [DOI] [PubMed] [Google Scholar]
- 30.Selhub J, Morris MS, Jacques PF, Rosenberg IH. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr 2009;89(suppl):702S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr 2010;91:1733–44 [DOI] [PubMed] [Google Scholar]
- 32.Loikas S, Koskinen P, Irjala K, et al. Renal impairment compromises the use of total homocysteine and methylmalonic acid but not total vitamin B12 and holotranscobalamin in screening for vitamin B12 deficiency in the aged. Clin Chem Lab Med 2007;45:197–201 [DOI] [PubMed] [Google Scholar]
- 33.Lewerin C, Ljungman S, Nilsson-Ehle H. Glomerular filtration rate as measured by serum cystatin C is an important determinant of plasma homocysteine and serum methylmalonic acid in the elderly. J Intern Med 2007;261:65–73 [DOI] [PubMed] [Google Scholar]
- 34.Carmel R, Vasireddy H, Aurangzeb I, George K. High serum cobalamin levels in the clinical setting: clinical associations and holo-transcobalamin changes. Clin Lab Haematol 2001;23:365–71 [DOI] [PubMed] [Google Scholar]
- 35.Kolhouse JF, Kondo H, Allen NC, Podell E, Allen RH. Cobalamin analogues are present in human plasma and can mask cobalamin deficiency because current radioisotope dilution assays are not specific for true cobalamin. N Engl J Med 1978;299:785–92 [DOI] [PubMed] [Google Scholar]
- 36.Emancipator K, Mansbach L, Robert T, Waskiewicz D. Failure of assay to identify low cobalamin concentration. Representatives of Bayer Diagnostics respond. Clin Chem 2000;46:2018–9 [PubMed] [Google Scholar]
- 37.Vogeser M, Lorenzl S. Comparison of automated assays for the determination of vitamin B12 in serum. Clin Biochem 2007;40:1342–5 [DOI] [PubMed] [Google Scholar]
- 38.Carmel R, Brar S, Agrawal A, Penha PD. Failure of assay to identify low cobalamin concentrations. Clin Chem 2000;46:2017–8 [PubMed] [Google Scholar]
- 39.Vlasveld LT, van't Wout JW, Meeuwissen P, Castel A. High measured cobalamin (vitamin B12) concentration attributable to an analytical problem in testing serum from a patient with pernicious anemia. Clin Chem 2006;52:157–8 [DOI] [PubMed] [Google Scholar]
- 40.Hamilton MS, Blackmore S, Lee A. Possible cause of false normal B-12 assays. BMJ 2006;333:654–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon LR. Cobalamin-responsive disorders in the ambulatory care setting: unreliability of cobalamin, methylmalonic acid, and homocysteine testing. Blood 2005;105:978–85 [DOI] [PubMed] [Google Scholar]
- 42.Carmel R. Is testing for clinical cobalamin deficiency truly unreliable? Blood 2005;106:1136–7 [DOI] [PubMed] [Google Scholar]
- 43.Drammeh BS, Schleicher RL, Pfeiffer CM, Jain RB, Zhang M, Nguyen PH. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem 2008;54:1883–91 [DOI] [PubMed] [Google Scholar]
- 44.Lowrey I, Smith G. Elevated results in a vitamin B12 assay when using serum separator blood collection tubes. Ann Clin Biochem 2003;40:560–2 [DOI] [PubMed] [Google Scholar]
- 45.Bolann BJ, Solli JD, Schneede J, et al. Evaluation of indicators of cobalamin deficiency defined as cobalamin-induced reduction in increased serum methylmalonic acid. Clin Chem 2000;46:1744–50 [PubMed] [Google Scholar]
- 46.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc 1992;40:1197–204 [PubMed] [Google Scholar]
- 47.van Asselt DZB, de Groot LCPGM, van Staveren WA, et al. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am J Clin Nutr 1998;68:328–34 [DOI] [PubMed] [Google Scholar]
- 48.Bernard MA, Nakonezny PA, Kashner TM. The effect of vitamin B12 deficiency on older veterans and its relationship to health. J Am Geriatr Soc 1998;46:1199–206 [DOI] [PubMed] [Google Scholar]
- 49.Stabler SP, Allen RH, Fried LP, et al. Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am J Clin Nutr 1999;70:911–9 [DOI] [PubMed] [Google Scholar]
- 50.Vogiatzoglou A, Oulhaj A, Smith AD, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem 2009;55:2198–206 [DOI] [PubMed] [Google Scholar]
- 51.Johnson MA, Hausman DB, Davey A, Poon LW, Allen RH, Stabler SP. Vitamin B12 deficiency in African American and white octogenarians and centenarians in Georgia. J Nutr Health Aging 2010;14:339–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carmel R, Brar S, Frouhar Z. Plasma total transcobalamin. I. Ethnic/racial patterns and comparison with lactoferrin. Am J Clin Pathol 2001;116:576–80 [DOI] [PubMed] [Google Scholar]
- 53.Carmel R. Mild transcobalamin I (haptocorrin) deficiency and low serum cobalamin concentrations. Clin Chem 2003;49:1367–74 [DOI] [PubMed] [Google Scholar]
- 54.Carmel R, Parker J, Kelman Z. Genomic mutations associated with mild and severe deficiencies of transcobalamin I (haptocorrin) that cause mildly and severely low serum cobalamin levels. Br J Haematol 2009;147:386–91 [DOI] [PubMed] [Google Scholar]
- 55.Carmel R, Baril L. Circulating immunoglobulin-transcobalamin I (R binder) complexes. J Lab Clin Med 1978;91:769–79 [PubMed] [Google Scholar]
- 56.Carmel R, Tatsis B, Baril L. Circulating antibody to transcobalamin II causing retention of vitamin B12 in the blood. Blood 1977;49:987–1000 [PubMed] [Google Scholar]
- 57.Skouby AP, Hippe E, Olesen H. Antibody to transcobalamin II and B12 binding capacity in patients treated with hydroxocobalamin. Blood 1971;38:769–74 [PubMed] [Google Scholar]
- 58.Jeffery J, Millar H, MacKenzie P, Fahie-Wilson M, Hamilton M, Ayling RM. An Ig complexed form of vitamin B12 is a common cause of elevated serum concentrations. Clin Biochem 2010;43:82–8 [DOI] [PubMed] [Google Scholar]
- 59.Carmel R. Ethnic and racial factors in cobalamin metabolism and its disorders. Semin Hematol 1999;36:88–100 [PubMed] [Google Scholar]
- 60.Hazra A, Kraft P, Selhub J, et al. Common variants of FUT2 are associated with plasma vitamin B12 levels. Nat Genet 2008;40:1160–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norman EJ, Berry HK, Denton MD. Identification and quantitation of urinary dicarboxylic acids as their dicyclohexyl esters in diseasee states by gas chromatography-mass spectrometry. Biomed Mass Spectrom 1979;6:546–53 [DOI] [PubMed] [Google Scholar]
- 62.Goringe A, Ellis R, McDowell I, et al. The limited value of methylmalonic acid, homocysteine and holotranscobalamin in the diagnosis of early B12 deficency. Haematologica 2006;91:231–4 [PubMed] [Google Scholar]
- 63.Sentongo TA, Azzam R, Charrow J. Vitamin B12 status, methylmalonic acidemia, and bacterial overgrowth in short bowel syndrome. J Pediatr Gastroenterol Nutr 2009;48:495–7 [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen K, Nathan E. The clinical evaluation of cobalamin deficiency by determination of methylmalonic acid in serum or urine is not invalidated by the presence of heterozygous methylmalonic-acidaemia. J Clin Chem Clin Biochem 1990;28:419–21 [DOI] [PubMed] [Google Scholar]
- 65.Monsen AL, Refsum H, Markestad T, Ueland PM. Cobalamin status and its biochemical markers methylmalonic acid and homocysteine in different age groups from 4 days to 19 years. Clin Chem 2003;49:2067–75 [DOI] [PubMed] [Google Scholar]
- 66.Bjørke-Monsen AL, Torsvik I, Saetran H, Markestad T, Ueland PM. Common metabolic profile in infants indicating impaired cobalamin status responds to cobalamin supplementation. Pediatrics 2008;122:83–91 [DOI] [PubMed] [Google Scholar]
- 67.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr 2005;82:442–50 [DOI] [PubMed] [Google Scholar]
- 68.Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G. The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food Nutr Bull 2008;29:S67–73 [DOI] [PubMed] [Google Scholar]
- 69.Carmel R. Measuring and interpreting holo-transcobalamin (holo-transcobalamin II). Clin Chem 2002;48:407–9 [PubMed] [Google Scholar]
- 70.Chen X, Remacha AF, Sarda MP, Carmel R. Influence of cobalamin deficiency compared with that of cobalamin absorption on serum holotranscobalamin II. Am J Clin Nutr 2005;81:110–4 [DOI] [PubMed] [Google Scholar]
- 71.Lindemans J, Schoester M, van Kapel J. Application of a simple immunoadsorption assay for the measurement of saturated and unsaturated transcobalamin II and R-binders. Clin Chim Acta 1983;132:53–61 [DOI] [PubMed] [Google Scholar]
- 72.Ulleland M, Eilertsen I, Quadros EV, et al. Direct assay for cobalamin bound to transcobalamin (holo-transcobalamin) in serum. Clin Chem 2002;48:526–32 [PubMed] [Google Scholar]
- 73.Nexo E, Christensen AL, Hvas AM, Petersen TE, Fedosov S. Quantification of holo-transcobalamin, a marker of vitamin B12 deficiency. Clin Chem 2002;48:561–2 [PubMed] [Google Scholar]
- 74.Brady J, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem 2008;54:567–73 [DOI] [PubMed] [Google Scholar]
- 75.Miller JW, Garrod MG, Rockwood AL, et al. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem 2006;52:278–85 [DOI] [PubMed] [Google Scholar]
- 76.Morkbak AL, Heimdal RM, Emmens K, et al. Evaluation of the technical performance of novel holotranscobalamin (holoTC) assays in a multicenter European demonstration project. Clin Chem Lab Med 2005;43:1058–64 [DOI] [PubMed] [Google Scholar]
- 77.Namour F, Olivier JL, Abdelmoutalleb I, et al. Transcobalamin codon 259 polymorphism in HT-29 and Caco-2 cells and in Caucasians: relation to transcobalamin and homocysteine concentration in blood. Blood 2001;97:1092–8 [DOI] [PubMed] [Google Scholar]
- 78.Miller JW, Ramos MI, Garrod MG, Flynn MA, Green R. Transcobalamin II 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood 2002;100:718–20 [DOI] [PubMed] [Google Scholar]
- 79.Zetterberg H, Nexo E, Regland B, et al. The transcobalamin (TC) codon 259 genetic polymorphism influences holo-TC concentration in cerebrospinal fluid from patients with Alzheimer disease. Clin Chem 2003;49:1195–8 [DOI] [PubMed] [Google Scholar]
- 80.Carmel R. The distribution of endogenous cobalamin among cobalamin-binding proteins in the blood in normal and abnormal states. Am J Clin Nutr 1985;41:713–9 [DOI] [PubMed] [Google Scholar]
- 81.Wickramasinghe SN, Ratnayaka ID. Limited value of serum holo-transcobalamin II measurements in the differential diagnosis of macrocytosis. J Clin Pathol 1996;49:755–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morkbak AL, Hvas AM, Milman N, Nexo E. Holotranscobalamin remains unchanged during pregnancy. Longitudinal changes of cobalamins and their binding proteins during pregnancy and postpartum. Haematologica 2007;92:1711–2 [DOI] [PubMed] [Google Scholar]
- 83.Refsum H, Johnston C, Guttormsen AB, Nexo E. Holotranscobalamin and total transcobalamin in human plasma: determination, determinants, and reference values in healthy adults. Clin Chem 2006;52:129–37 [DOI] [PubMed] [Google Scholar]
- 84.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med 2005;43:1048–51 [DOI] [PubMed] [Google Scholar]
- 85.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004;50:3–32 [DOI] [PubMed] [Google Scholar]
- 86.Gimsing P, Hippe E, Helleberg-Rasmussen I, et al. Cobalamin forms in plasma and tissue during treatment of vitamin B12 deficiency. Scand J Haematol 1982;29:311–8 [DOI] [PubMed] [Google Scholar]