Abstract
Previous studies in our laboratory demonstrated that blueberry (BB) extract exhibited antitumor activity against MDA-MB-231 triple negative breast cancer (TNBC) cells and decreased metastatic potential in vitro. The current study tested 2 doses of whole BB powder, 5 and 10% (wt:wt) in the diet, against MDA-MB-231 tumor growth in female nude mice. In this study, tumor volume was 75% lower in mice fed the 5% BB diet and 60% lower in mice fed the 10% BB diet than in control mice (P ≤ 0.05). Tumor cell proliferation (Ki-67) was lower in the 5 and 10% BB-fed mice and cell death (Caspase 3) was greater in the 10% BB-fed mice compared to control mice (P ≤ 0.05). Gene analysis of tumor tissues from the 5% BB-fed mice revealed significantly altered expression of genes important to inflammation, cancer, and metastasis, specifically, Wnt signaling, thrombospondin-2, IL-13, and IFNγ. To confirm effects on Wnt signaling, analysis of tumor tissues from 5% BB-fed mice revealed lower β-catenin expression and glycogen synthase kinase-3β phosphorylation with greater expression of the β-catenin inhibitory protein adenomatous polyposis coli compared to controls. A second study tested the ability of the 5% BB diet to inhibit MDA-MB-231-luc-D3H2LN metastasis in vivo. In this study, 5% BB-fed mice developed 70% fewer liver metastases (P = 0.04) and 25% fewer lymph node metastases (P = 0.09) compared to control mice. This study demonstrates the oral antitumor and metastasis activity of whole BB powder against TNBC in mice.
Introduction
TNBC4 is so named due to lack of expression of the ER, PR, and the HER2 protein (1). This type of cancer comprises ~10–15% of all breast cancers (2) and has a poorer outcome than those breast tumors expressing ER/PR or the HER2 protein (3, 4). Additionally, patients with TNBC have shorter relapse-free and overall survival (5), a higher likelihood of distant metastasis within the first 5 y of diagnosis, larger tumors (>2 cm), and a greater percentage of positive LN than patients with other subtypes of breast cancer (6). TNBC are also characterized by an aggressive clinical history with poor disease-free and overall survival (7, 8). Currently, there is no defined, standard treatment strategy for prevention of recurrence for this disease other than traditional chemotherapy, to which it is highly resistant. Due to the lack of ER, PR, and HER2 expression, traditional targeted therapies are not an option. A limited amount of data exists on which to base targeted preventive measures for occurrence and recurrence of this disease. Thus, there is an urgent need to identify both vulnerable targets in these breast cancers and safe, effective dietary strategies for the prevention of primary disease, metastasis, and recurrence.
BB are rich in bioactive substances such as flavonoids (anthocyanins, flavan-3-ols, flavones, and flavonols) and proanthocyanidins and exhibit inhibitory effects such as induction of apoptosis, inhibition of cell proliferation, modulation of cell signaling, metastasis and effects on gene expression in a variety of cancer cell lines (9–12). In addition, BB possess potent antioxidant potential (13, 14), which can be beneficial to the prevention of carcinogenesis and metastasis.
Tumor invasion and metastasis is a complex, multi-step process influenced by many factors such as the expression of key proteases, their inhibitory proteins, and the signaling pathways that control them. The bioactive substances in berries inhibit different components of this system. Proanthocyanidins inhibit β-glucuronidase, elastase, and hyaluronidase (15, 16), which are enzymes important to degradation of the extracellular matrix. Anthocyanidins and proanthocyanidins have been shown to stabilize the extracellular matrix by stabilizing collagen, increasing collagen synthesis, and inhibiting collagenase (17). Studies have also shown that luteolin and quercetin inhibit MMP-2 and MMP-9 secretion (18) and cell migration (19).
Preliminary data from our laboratory showed that whole BB juice had an inhibitory effect on the proliferation and survival of TNBC cells in vitro. In addition, treatment with BB juice decreased the metastatic potential of MDA-MB-231 TNBC cells in vitro through reduced activity of MMP-9 and urokinase-type plasminogen activator while increasing the expression of plasminogen activator inhibitor-1 (12). In vivo, whole BB juice decreased MDA-MB-231 tumor size and cell proliferation and increased apoptosis. Immunohistochemical analysis of tumor tissues from BB-fed mice showed lower activation of the cell signaling proteins phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), and nuclear factor-κB (NF-κB), which are important to cancer cell survival and metastasis. To build on these previous findings, the current study objective was to determine the effect of whole BB powder on the metastasis of TNBC cells in vivo and further explore the mechanisms through microarray analysis.
Materials and Methods
Mice and diets.
Female, 6- to 7-wk-old, intact, BALB/c Nu-Nu, athymic mice were purchased from Charles River Laboratories. The mice were housed and maintained under standard conditions in the City of Hope Animal Facility. All experimental protocols were in accordance with the NIH guidelines for the ethical treatment of animals and approved by the City of Hope Research Animal Care Committee. Mice were randomly assigned to cohorts (n = 10 mice/cohort) that received 1 of 3 pelleted diets for the duration of the experiment. Whole BB powder was used in this study to avoid the trauma of daily gavage required when using BB juice. The control diet (AIN-93G) and the AIN-93G diet modified to contain 5 or 10% freeze-dried whole BB powder (wt:wt) were produced and purchased from Research Diets. Sucrose and cellulose were decreased in the BB diets to adjust for the addition of the BB powder (Table 1). The nutrient content and total phenolic and anthocyanin content of the BB powder is provided in Supplemental Table 1. In addition, diets were isocaloric and nutritionally balanced for fat, protein, and carbohydrate content. The BB doses were based on previous studies by the Stoner (20) laboratory with black raspberry and a study by Wu et al. (21) using BB powder. The U.S. Highbush Blueberry Council provided the powder in sealed aluminum cans, which remained unopened until it was added to the diets. All mice consumed food and water ad libitum. The amount of food consumed and body weight were measured weekly to monitor differences between groups and the overall health of the mice.
TABLE 1.
Formulations and nutrient composition of experimental diets
| Diet1 |
|||
| Nutrient | D10012G | D09092001 | D09092002 |
| AIN-93G | 5% BB2 | 10% BB | |
| Total energy, kcal/kg | 4030 | 3990 | 3960 |
| g/kg diet | |||
| Protein | 203.0 | 201.0 | 199.0 |
| Carbohydrate | 647.0 | 641.0 | 636.0 |
| Fat | 70.0 | 69.0 | 69.0 |
| Fiber | 50.0 | 50.0 | 49.0 |
| Casein | 200.0 | 198.3 | 196.5 |
| l-Cystine | 3.0 | 3.0 | 3.0 |
| Corn starch | 404.6 | 404.6 | 404.6 |
| Maltodextrin 10 | 132.0 | 132.0 | 132.0 |
| Sucrose | 100.0 | 70.3 | 40.03 |
| Cellulose, BW200 | 50.0 | 40.6 | 31.0 |
| Soybean oil | 70.0 | 70.0 | 70.0 |
| t-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Mineral mix S10022C3 | 3.5 | 3.5 | 3.5 |
| Vitamin mix V100374 | 10.0 | 10.0 | 10.0 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| BB powder | 0 | 50.45 | 101.82 |
Cell culture.
MDA-MB-231 cells were obtained from American Type Culture Collection. Cells were cultured in RPMI-1640 containing 10% FBS in the presence of 0.10 U/L penicillin and 0.1 g/L streptomycin and incubated at 37°C with 95% air and 5% carbon dioxide and used in experiments during the linear phase of growth. MDA-MB-231-luc-D3H2LN luciferase-expressing cells were purchased from Caliper Life Sciences. This cell line was cultured in Eagle’s MEM with 10% FBS in the presence of 1 × 105 U/L penicillin and 0.1 g/L streptomycin. MDA-MB-231-luc-D3H2LN cells will metastasize to the LN, lungs, and liver upon orthotopic injection into immune-deficient mice, according to the manufacturer.
Experimental protocol (microarray).
After 2 wk of receiving the experimental diets, mice were s.c. injected in the hind flank with MDA-MB-231 cells mixed with an equal volume of Matrigel (BD Biosciences) at a concentration of 2 × 106 cells/injection site. Body weights were monitored weekly as an indicator of overall health and diet intake was measured to monitor differences between groups. Tumor size was measured weekly using calipers and volume calculated by the formula [(4/3π r12 × r2) (0.125)], where r1 is the smaller radius and r2 is the larger radius. Six weeks postinjection, mice were euthanized and tumors were removed, weighed, and sent for hematoxylin and eosin, Ki-67, and Caspase 3 histological staining through the City of Hope Pathology Department Core Facility. RNA for microarray and RT-PCR analysis was extracted from 3 tumors of the control and 5% BB groups using the Trizol method following the procedures reported in our recent paper (22). Ki-67 and Caspase 3 staining was quantified from 6 random fields; stained and unstained cells were counted and divided by the total number of cells counted to generate the percentage of positive cells in each group.
Microarray analysis.
Synthesis and labeling of cRNA targets, hybridization of GeneChips, and signal detection were carried out by the Microarray Core Facility at the City of Hope using the Affymetrix GeneChip Human Gene1.0 ST array (Affymetrix) as previously reported (22).
Real-time PCR.
TriZol reagent (Invitrogen) was used for total RNA isolation. The same RNA was used in this assay as that used in the microarray analysis to ensure consistency in the data. The 2 most upregulated genes in the microarray analysis were verified, because the changes between the control and 5% BB groups were the most evident. An iScript cDNA Synthesis kit (Bio-Rad) and SYBR Green Supermix were used for cDNA preparation and to carry out the PCR reaction. PCR primers for LYZ were as follows: 5′CTCATTGTTCTGGGGCTTGT3′ and TGCTTCTGTCTCCAGCATTG3′ thrombospondin 2 (THBS): 5′TCGTGCGCTTTGACTACATC3′ and TTGGAGACGATCTCGAACTG3′ and human β-actin (used as an internal control): 5′AGAAGGAGATCACTGCCCTGGCACC3′ and 5′CCTGCTTCGTGATCCACATCTGCTG3′. Reactions were run in triplicate on the iCycler iQ5 Real-Time PCR Detection system (Bio-Rad) and the results were analyzed with the software provided.
In vivo metastasis assay.
The study was carried out using 6- to 7-wk-old intact, female, BALB/c Nu-Nu, athymic mice. Mice were divided into 2 experimental groups of 10 mice. Two weeks prior to injection with tumor cells, mice were started on 1 of 2 diets: control (AIN-96G) or AIN-96G + 5% BB powder (wt:wt). We chose the 5% BB diet for further study, because the 10% BB diet did not confer any advantage in decreasing tumor volume. After 2 wk of receiving the experimental diets, luciferase-expressing MDA-MB-231-luc-D3H2LN cells suspended in matrigel (2 × 106 cells/injection site) were injected into the mammary fat pad of the mice. Mice were imaged just prior to cell injection to obtain a baseline reading and then weekly starting at wk 2 posttumor cell injection using the IVIS imaging system. For imaging, mice were anesthetized using isoflurane and then injected with D-Luciferin Firefly potassium salt (Caliper Life Sciences) at a concentration of 150 mg/kg. Three weeks postinjection, imaging included shielding the primary tumor with black fabric to minimize the bioluminescence from the primary tumor so that metastatic regions could be observed. The front limbs were secured with tape to expose the axillary/brachial LN areas. Five weeks postinjection, mice were euthanized via CO2 asphyxiation and tumors were removed, weighed, and sent for β-catenin histological staining at the City of Hope Pathology Department Core Facility. β-Catenin staining intensity was graded as 1 (weak), 2 (moderate), or 3 (strong). A total of 15 fields (3 tumors, 5 fields/tumor) were analyzed per group. To confirm metastases, organs (liver, lungs, and axillary/brachial LN) were removed, placed in Petri dishes containing 0.30 μg/L luciferin, and imaged for luminescent signals.
Western blotting.
Protein was isolated from tumor specimens from 3 separate mice per experimental group (control and 5% BB) using T-PER Tissue Protein Extraction Reagent (Thermo Scientific) according to the manufacturer’s protocol. Briefly, tumor specimens were weighed and homogenized in T-PER reagent containing protease and phosphatase inhibitor cocktail (Sigma-Aldrich) using the T25 Basic tissue homogenizer (IKA Works). Samples were centrifuged at 10,000 × g for 5 min and the supernatant was run on a 10% acrylamide gel, transferred to a nitrocellulose membrane, and probed with antibodies to phospho-GSK-3β, APC, or β-actin (as a loading control) (Cell Signaling Technology). Bands were visualized via chemiluminescence using HRP-conjugated secondary antibodies and quantified using Biorad Quantity One software.
Statistical analysis.
Statistical analysis of data (with the exception of microarray data) was done using either Student’s t test to compare 2 means, 1-way ANOVA plus Dunnett’s Multiple Comparison Test to compare more than one mean to a control, or 2-way ANOVA plus Bonferroni post test to compare multiple means over time; metastasis frequency was analyzed as a contingency table by using GraphPad Prism 4 software. Differences were considered significant at P ≤ 0.05.
Results
MDA-MB-231 tumor volume.
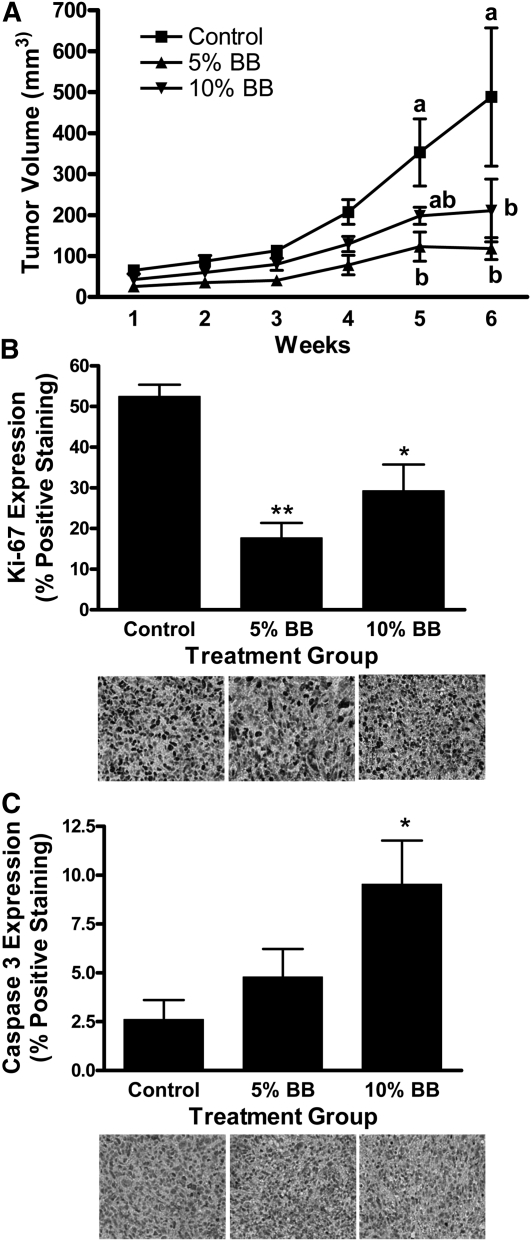
Food intake and weight gain did not differ between experimental groups (data not shown). Tumor volume was affected by diet (P = 0.0001) and time (P = 0.0001). It was lower than in controls in the 5% BB-fed group at wk 5 and 6 and in the 10% BB-fed group at wk 6 (Fig. 1A). Cell proliferation (Ki-67 expression) was lower in the 5% (P ≤ 0.01) and 10% (P ≤ 0.05) BB-fed groups (Fig. 1B) and apoptosis (Caspase 3 expression) was greater in the 10% BB group compared to control mice (P ≤ 0.05) (Fig. 1C).
FIGURE 1.
MDA-MB-231 tumor volume (A), proliferation (B), and apoptosis (C) in female nude mice fed control, 5% BB powder, or 10% BB powder diet for 8 wk. Data are means ± SEM, n = 6. In A, labeled means at a time without a common letter differ, P ≤ 0.05. In B and C, asterisks indicate different from control: *P ≤ 0.05, **P ≤ 0.01. BB, blueberry.
Validation of microarray results by real-time PCR.
Microarray analysis results were validated by real-time PCR using primers for the 2 most highly upregulated genes: THBS2 and LYZ in tumor samples. Expression of both genes was greater in tumors from 5% BB-fed mice compared to controls (P ≤ 0.05), validating the microarray analysis results (Supplemental Fig. 1).
Analysis of BB-responsive genes.
The most relevant gene networks significantly altered by 5% BB powder ingestion identified by IPA in our data set are shown in Table 2. The top networks affected by BB ingestion in this study include: inflammatory disease, cancer, cell morphology (28 focus genes), reproductive system development and function, developmental disorder, genetic disorder (25 focus genes), inflammatory response, cell-to-cell signaling and interaction, hematological system development and function (22 focus genes), cell cycle, cancer, gastrointestinal disease (20 focus genes) and inflammatory response, cellular movement, and hematological system development and function (14 focus genes). IL-13 and IFNγ, both upregulated, are 2 central molecules in the top network regulated by 5% BB ingestion. The proteins encoded by these genes play important roles in tumor growth.
TABLE 2.
Gene networks regulated by 5% blueberry ingestion in MDA-MB-231-derived tumors in female nude mice1–3
| Network 1. Score: 43, focus genes: 28. Top functions: inflammatory disease, cancer, cell morphology |
| Symbol (Entrez Gene ID, log ratio) ALOX15 (246, −0.70); ALOX5AP (241, 0.815); ARG1 (383, 0.922); Arginase (group); Collagen Alpha 1 (group); CYBB (1536, 1.262); Eotaxin (group); ESM1 (11082, 0.837); F13A1 (2162, 1.111); Fc Gamma Receptor (group); FCGR1A (2209, −1.044); GBP6 (16335, 1.217); GNRH1 (2796, −0.678); GPRC5B (51704, −0.626); HBA1 (3039, 0.932); HBD (3045, 0.645); HBG1 (3047, 1.208); Hemoglobin (complex); HLA-DOA (3111, −0.755); IFIT5 (24138, 0.707); IFNG (3458, 0.787); IL13 (3596, 0.986); IL13RA2 (3598, −0.674); INHBA (3624, 0.611); KCNJ15 (3772, 0.626); KIR2DS1 (3806, 0.678); LTB4R (1241, −1.118); MHC Class II (complex); NPY2R (4887, −0.748); PKC ALPHA/BETA (family); PLEK (5341, 1.159); QRFP (347148, −0.744); SEPP1 (6414, 1.369); SLAMF1 (6504, −0.773); UBD (10537, −0.813). |
| Network 3. Score: 30, focus genes: 22. Top functions: inflammatory response, cell-to-cell signaling and interaction, hematological system development and function. |
| Symbol (Entrez Gene ID, log ratio) C3AR1 (719, 0.856); CD86 (942, 0.964); CD180 (4064, 0.707); CLEC7A (64581, 0.912); CLIP2 (7461, −0.599);CORO1A (11151, 0.844); Ifn (group); IFN Beta (group); Ifn gamma (complex); IFN TYPE1 (family); IFNA17 (3451, -0.689); IFN Alpha/Beta (family); Iga (complex); IL23 (complex); IL12 (complex); Il12 family (group); Il12 receptor (complex); IL23R (149233, −0.978); LRRFIP1 (9208, −0.641); LSP1 (4046, 0.995) MUC2 (4583, −0.029); NFKB (complex); NFKBID (84807, −0.955); PRF1 (5551, −0.610); PTAFR (5724, −0.768); STAT4 (6775, 0.774); TH2 cytokine (group); TLE6 (79816, −0.888); Tlr (group); TLR7 (51284, 0.910); TNFSF4 (7292, −0.779); UMOD (7369, −0.734); VSNL1 (7447, −0.857); XAF1 (54739, −0.692); XCL1 (6375, −1.209). |
| Network 5. Score: 15, focus genes: 14. Top functions: inflammatory response, cellular movement, hematological system development and function. |
| Symbol (Entrez Gene ID, log ratio) APOBEC3B (9582, 0.220); AZU1 (566, −0.274); BDKRB1 (623, −0.861); CD276 (80381, −0.145); CDA (978, −0.762); CEACAM8 (1088, −0.771); CEACAM3 (1084, −0.158); CFTR (1080, 0.845); CHST4 (10164, 1.238); CLTCL1 (8218, −0.544); CTSF (8722, −0.695); GALNTL4 (8693, 0.087); IL8 (3576, 0.568); IL17B (27190, 0.025); IL17C (27189, −0.094); IL1F6 (27179, −0.416); IL1F9 (56300, −0.575); Importin alpha (group); LPA (4018, −0.611); LPAR2 (9170, −0.614); LPAR3 (23566, −0.247); NANOG (79923, −0.341); NPIP (9284, −1.177); NXF5 (55998, −0.915); NXT1 (29107, 0.067); PDX1 (3651, −0.136); PILRB (29990, −0.704); PMP2 (5375, −0.675); POT1 (25913, 0.702); SNX4 (8723, 0.602); STEAP4 (79689, 0.671); SVIL (6840, −0.470); TNF (7124, 0.565); TNIP3 (79931, 0.284). |
Values are based on the log2 ratio (a value of 1 equals a 2-fold change).
Positive values are increases and negative values are decreases.
Gene ID: see Reference (60).
Genes were also grouped by biological function. Of the biological functions modulated by 5% BB ingestion, several are of prime interest to this investigation (Supplemental Table 2). Under diseases and disorders, those genes important to inflammatory response (58 genes) and inflammatory disease (69 genes) were altered by 5% BB. Under molecular and cellular functions, those genes important to cell-to-cell signaling and interaction (69 genes), cellular movement (66 genes) were altered. Under physiological system development and function, immune cell trafficking (48 genes) and humoral immune response (23 genes) were altered by 5% BB. These groupings show several important biological systems important to cancer that were affected by BB ingestion in our study.
Specific genes of interest that were significantly upregulated in BB-fed mice included: wingless-type MMTV integration site family member 7B (WNT7B) (2.2-fold; P ≤ 0.02) and secreted frizzled-related protein 4 (SFRP4) (3.5-fold; P ≤ 0.007), both of which are involved in the Wnt signaling pathway. THBS2, which functions as an inhibitor of tumor growth and angiogenesis along with mediating cell-cell and cell-matrix interaction, was upregulated 13.3-fold (P ≤ 0.08). Alternatively, the leukotriene B4 receptor (LTB4R) gene, which is important to signal transduction and metastasis, was downregulated (2.2-fold; P ≤ 0.001) by BB ingestion (Supplemental Table 2).
#x03B2-Catenin signaling pathway in orthotopic MDA-MB-231 mammary tumors.
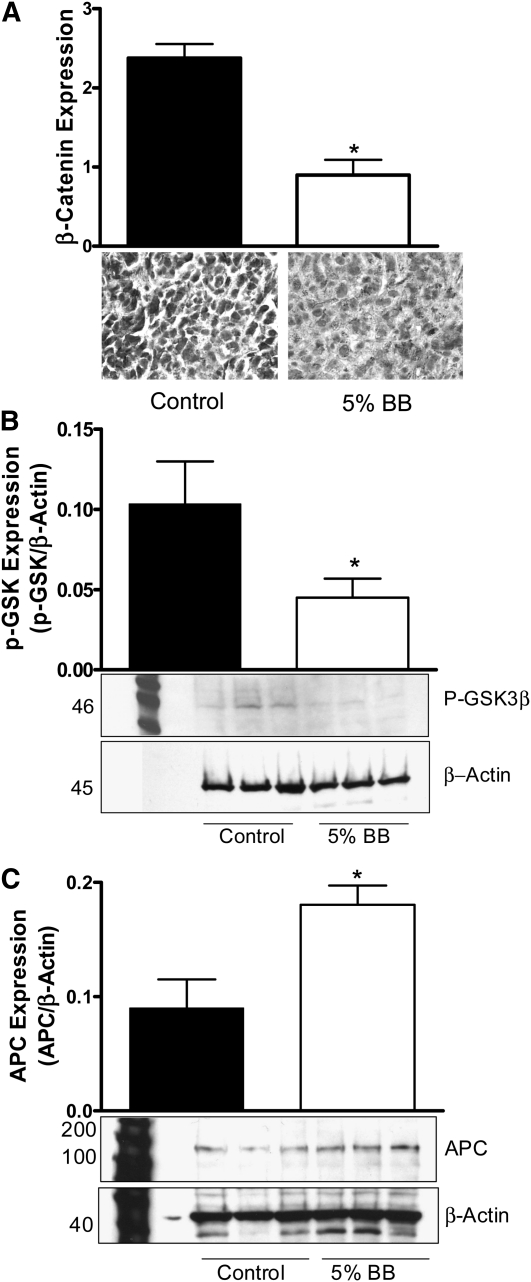
Based on the microarray analysis results, we investigated the expression/activation of proteins integral to the β-catenin signaling pathway in MDA-MB-231–derived tumors. β-Catenin expression in mice fed the 5% BB diet was lower compared to control mice (P ≤ 0.001) (Fig. 2A). GSK-3β phosphorylation was significantly lower and APC protein expression was significantly greater compared to controls (Fig. 2B,C), indicating inhibition of β-catenin.
FIGURE 2.
β-Catenin (A), pGSK-3β (ΒB), and APC (C) expression in orthotopic MDA-MB-231 mammary tumors of female nude mice fed control or 5% BB diet for 7 wk. (A) Immunostaining staining intensity was graded as 1 (weak), 2 (moderate), or 3 (strong). (B,C) Three separate Western blots were quantified using Quantity One Software. Data are mean ± SEM, n = 15 (A) or 3 (means of triplicates) (B,C). Different from control, *P ≤ 0.05. APC, adenomatous polyposis coli; BB, blueberry; p-GSK, phospho-glycogen synthase kinase.
MDA-MB-231-luc-D3H2LN tumor metastasis in nude mice.
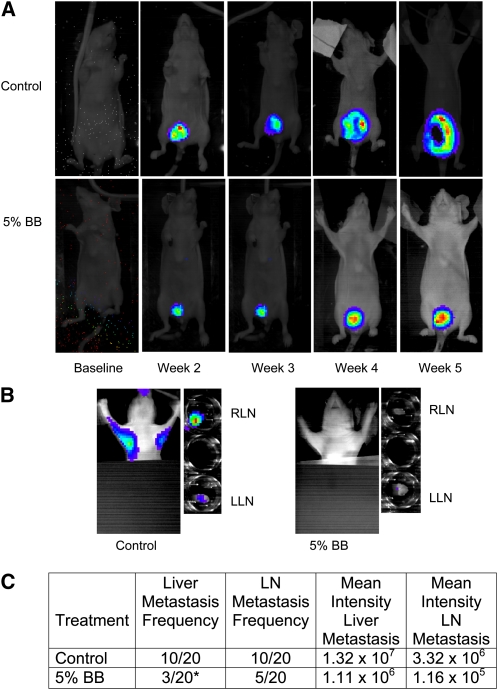
To test the inhibition of metastasis vivo, we monitored the primary growth (Fig. 3A) and metastasis of MDA-MB-231-luc-D3H2LN (luciferase-expressing) tumors in female nude mice using real-time imaging. Mice were maintained on the 5% BB diet or control diet and metastasis was monitored weekly. LN metastasis tended to be 25% lower (P = 0.09) (Fig. 3B) and liver metastasis was 70% lower (P ≤ 0.04) in the 5% BB-fed mice compared to control mice (Fig. 3C).
FIGURE 3.
In situ tumor growth and metastasis monitored by Xenogen IVIS imaging at different time points after MDA-MB-231 tumor implantation in female nude mice fed control or 5% BB diet for 7 wk. (A) Orthotopic breast tumor growth from baseline to wk 5 postimplantation. (B) In situ and ex-vivo imaging of RLN and LLN lymph node metastasis at wk 5 postimplantation. (C) Frequency of metastasis and mean intensity of liver and LN metastases analyzed by contingency table. Intensity data are mean ± SEM for the number of mice with metastases. *Different from control, P ≤ 0.05. BB, blueberry; LLN, left lymph node; RLN, right lymph node.
Discussion
This study illustrated that BB powder intake at 5 and 10% of the diet was nontoxic and did not affect food intake, weight, or overall health of the mice. In addition, the decreased tumor volume in the BB groups showed that BB intake suppressed the growth of triple negative breast tumors in mice. Interestingly, tumor volume was lower in the 5% BB diet than the 10% BB diet, although this difference was not significant (no dose response observed). This is consistent with a study by Wu et al. (21), who found that a 5% BB diet fed to pregnant Sprague-Dawley rats had significant, positive effects on breast maturation markers in progeny, whereas the 10% diet did not. This suggests that there may be an optimal level of BB intake.
The decrease in Ki-67 in both BB groups compared to controls and the significant increase in apoptosis (Caspase 3) in the 10% BB group suggests that the decreased tumor volume was likely due to decreased proliferation of the tumor cells in both BB groups and an increase in apoptosis in the 10% group. These data are in agreement with previous studies in our laboratory using BB extract in the same xenograft model (12).
For microarray analysis, we utilized tumor specimens from mice fed the 5% BB diet, because there was no apparent advantage to 10% BB intake with regard to tumor volume reduction. The results suggest several mechanisms for the effects of BB intake on TNBC growth. BB intake affected gene networks important to the inflammatory response, which is not surprising given the established antioxidant action of BB phytochemicals. The oxidation radical absorbance capacity value of whole BB is noteworthy at 6552 μmol TE/100 g (23). Inflammation is an important mediator of the promotion and progression of cancer and proinflammatory conditions within tumor tissues are related to malignant progression and metastasis (24). Inflammatory cells in primary tumors can also induce angiogenesis, contributing to blood vessel growth and subsequent metastasis; nonsteroidal, antiinflammatory drugs inhibit this process (25). Therefore, the antiinflammatory effects of BB ingestion are likely involved in its observed antitumorigenic and antimetastatic activity.
In addition, BB intake affected specific gene networks involved in cell-to-cell signaling and interaction, which are important to both cell survival and migratory behavior. BB also modulated genes involved in the cell cycle and cell movement, cancer, cell morphology, and cell-to-cell interaction. These results are in agreement with our previously published in vitro results (12).
The number one gene network affected by BB ingestion was inflammatory disease, cancer and cell morphology, and the 2 central molecules in this network are IL-13 and IFNγ. IFNγ is an immunostimulatory cytokine that also displays antiproliferative and tumoricidal properties (26–30). IL-13 is a cytokine that inhibits the production of proinflammatory cytokines and regulates cell proliferation, apoptosis, and differentiation (31, 32), specifically in breast tumor cell lines (33, 34). LTB4R was also included in this network. This gene was significantly downregulated in BB-fed mice compared to controls. This receptor is involved in eicosanoid signaling (35), inflammatory response (36), signal transduction (37), and cell adhesion and movement (38). Interestingly, LTB4R upregulates MMP 2 and 9 (39) and MMP9 activity was decreased by BB treatment in our previous in vitro experiments. LTB4R also affects metastasis through the activation of NF-κB signaling, which was reduced by BB treatment in our previous study (40). Therefore, a likely mechanism for the reduction of metastatic potential by BB is through a decrease in the expression of LTB4R. These genes and the network to which they belong are essential to the mechanisms we suspect are behind the effects of BB on the growth and metastasis of breast cancer.
THBS2 was the top upregulated gene in the 5% BB group. THBS2 mediates cell-to-cell and cell-to-matrix interactions (41–43). This protein functions as a potent inhibitor of tumor growth, migration, and angiogenesis (44–47). THBS2 modulates the activity of MMP2 (48), PI3K, and ERK (49), all proteins that are integral to cancer cell survival and metastasis. This result supports our previous in vitro data showing lower MMP2 and PI3K activity in BB-treated cells (50).
SFRP4 was significantly upregulated in the 5% BB-treated mice. The protein encoded by this gene is involved in the regulation of apoptosis, cell growth, and differentiation (51) and is commonly downregulated in breast cancer (52) and missing in the MCF-7 breast cancer cell line (52). SFRP4 proteins are inhibitors of Wnt/β-catenin signaling (53), which is involved in metastasis signaling, neoplasia, and cancer. β-Catenin signaling results in the expression of target genes that induce cancer cell proliferation and metastasis, such as Cyclin D-1 and MMP-9.
In the absence of Wnt proteins, β-catenin is bound within an inhibitory complex composed of APC, Axin, GSK-3β, and casein kinase Iα. GSK-3β and casein kinase Iα phosphorylate β-catenin, which is subsequently ubiquitinated and degraded by the proteasome. In response to the presence of Wnt proteins, GSK-3β is phosphorylated by AKT. The phosphorylated form of GSK-3β is inactive and therefore cannot inhibit β-catenin.
β-Catenin expression and GSK-3β phosphorylation was lower and APC expression was greater in tumors from BB-fed mice compared to controls. Because GSK-3β is deactivated by AKT, this is consistent with our previous data that showed BB decreased AKT activity in vitro and in vivo. Other groups have reported the inhibitory activity of phytochemicals on Wnt signaling in breast tissues. Genistein reduced Wnt signaling in mammary tumors of rats (54), curcumin inhibited the expression of β-catenin in MCF-7 and MDA-MB-231 cells (55), EGCG blocked Wnt signaling and the invasion of breast cancer cell lines in vitro (56), and BB punch (a mixture of food extracts that included blueberries) reduced GSK-3β in prostate xenograft tumors in mice (57).
Through real-time imaging, we tracked primary tumor growth and metastasis in mice over the duration of the study. The frequency of liver and LN metastasis in BB-fed mice was lower compared to control mice. Although the decreased metastasis to the LN was not significant (P = 0.09), the study did show a tendency for inhibition. The frequencies of liver compared to LN metastasis did not differ in the control mice; therefore, the differential inhibition by BB is interesting. This may be because the liver is a more distant site from the primary tumor than the LN. However, metastasis does not require circulation through the lymph system. Primary tumors may spread through blood vessels or through lymph vessels independently, and positive LN are regularly detected in the absence of hematogenous metastasis. Different patterns of metastasis may be due to the separate mechanisms behind hemangiogenesis and lymphangiongenesis (25). Therefore, BB may inhibit hematogenous metastasis to the liver more effectively than lymphatic metastasis to the LN. It is also possible that because the liver processes BB phytochemicals, it was exposed to a more concentrated dose of antitumor phytochemicals. Further studies into these hypotheses are required to answer these questions.
The major active ingredients in BB are thought to be the anthocyanins. A recent study using an 8% BB diet analyzed the absorption, metabolism, and tissue distribution of anthocyanins in Sprague-Dawley rats. The authors of this study reported that anthocyanins from blueberries are bioavailable based on their detection in urine and feces of rats after 4 and 8 wk of ingestion. In addition, anthocyanins were metabolized by the intestinal microflora, primarily into phenolic acids. Hippuric acid, the main product of anthocyanin metabolism, was detected in the urine of rats after 4 wk of the BB diet. Therefore, hippuric acid is a possible biomarker of anthocyanin intake in animal and human trials (58).
This study demonstrates that the oral intake of whole BB powder effectively decreased MDA-MB-231–derived triple negative breast tumor growth and metastasis in mice. Taken together with our previous data, we conclude that BB ingestion affects tumor growth and metastasis through the mediation of key processes such as inflammation, cell signaling, survival, and migration through modulation of signaling pathways such as PI3K, AKT, NF-κB and β-catenin.
Importantly, the dosage used in these studies was nontoxic and may be considered physiologic, because the human equivalent dose of the 5% BB diet (based on body surface area) (59) is 300 g (10.6 oz.) of fresh BB (~ 2 cups/d). Future clinical trials using whole BB powder are planned that will aid in the determination of a suitable human dose. It is our hope that the knowledge gained from this study may aid in the design of future dietary strategies for the prevention of TNBC.
Supplementary Material
Acknowledgments
L.A. and S.C. designed research; L.A., N.K., and S.P. conducted research; Z.L. analyzed microarray data; and L.A. wrote the manuscript and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by U.S. Highbush Blueberry Council and NIH grant ES08258.
Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: APC, adenomatous polyposis coli; BB, blueberry; ER, estrogen receptor; GSK, glycogen synthase kinase; LN, lymph node; LYZ, lysozyme; MMP, matrix mettaloproteinase; PR, progesterone receptor; TNBC, triple negative breast cancer.
Literature Cited
- 1.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–18 [DOI] [PubMed] [Google Scholar]
- 2.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–44 [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JF, Macmillan D, Blamey RW, Ellis IO. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer. 2005;116:340–50 [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52 [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34 [DOI] [PubMed] [Google Scholar]
- 7.van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M, Kochli OR, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161:1991–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, Begin LR, Hamel N, Goffin JR, Wong N, Trudel M, et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004;64:830–5 [DOI] [PubMed] [Google Scholar]
- 9.Wedge DE, Meepagala KM, Magee JB, Smith SH, Huang G, Larcom LL. Anticarcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells. J Med Food. 2001;4:49–51 [DOI] [PubMed] [Google Scholar]
- 10.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–39 [DOI] [PubMed] [Google Scholar]
- 11.Boivin D, Blanchette M, Barrette S, Moghrabi A, Beliveau R. Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 2007;27:937–48 [PubMed] [Google Scholar]
- 12.Adams LS, Phung S, Yee N, Seeram NP, Li L, Chen S. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010;70:3594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN. Effect of polyphenol-rich blueberry extract on cognitive performance of mice, on brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198:352–8 [DOI] [PubMed] [Google Scholar]
- 14.Dulebohn RV, Yi W, Srivastava A, Akoh CC, Krewer G, Fischer JG. Effects of blueberry (Vaccinium ashei) on DNA damage, lipid peroxidation, and phase II enzyme activities in rats. J Agric Food Chem. 2008;56:11700–6 [DOI] [PubMed] [Google Scholar]
- 15.Kuppusamy UR, Khoo HE, Das NP. Structure-activity studies of flavonoids as inhibitors of hyaluronidase. Biochem Pharmacol. 1990;40:397–401 [DOI] [PubMed] [Google Scholar]
- 16.Maffei Facino R, Carini M, Aldini G, Bombardelli E, Morazzoni P, Morelli R. Free radicals scavenging action and anti-enzyme activities of procyanidines from Vitis vinifera. A mechanism for their capillary protective action. Arzneimittelforschung. 1994;44:592–601 [PubMed] [Google Scholar]
- 17.Monboisse JC, Braquet P, Randoux A, Borel JP. Non-enzymatic degradation of acid-soluble calf skin collagen by superoxide ion: protective effect of flavonoids. Biochem Pharmacol. 1983;32:53–8 [DOI] [PubMed] [Google Scholar]
- 18.Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, Kandaswami C, Middleton E, Jr, Lee MT. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999;128:999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascolo N, Pinto A, Capasso F. Flavonoids, leucocyte migration and eicosanoids. J Pharm Pharmacol. 1988;40:293–5 [DOI] [PubMed] [Google Scholar]
- 20.Lechner JF, Reen RK, Dombkowski AA, Cukovic D, Salagrama S, Wang LS, Stoner GD. Effects of a black raspberry diet on gene expression in the rat esophagus. Nutr Cancer. 2008;60 Suppl 1:61–9 [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Rahal O, Kang J, Till SR, Prior RL, Simmen RC. In utero and lactational exposure to blueberry via maternal diet promotes mammary epithelial differentiation in prepubescent female rats. Nutr Res. 2009;29:802–11 [DOI] [PubMed] [Google Scholar]
- 22.Adams LS, Phung S, Wu X, Ki L, Chen S. White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. Nutr Cancer. 2008;60:744–56 [DOI] [PubMed] [Google Scholar]
- 23.Cho MJ. Howard LR, Prior RL, Clark JR. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J Sci Food Agric. 2005;85:2149–58 [Google Scholar]
- 24.Mouta C, Heroult M. Inflammatory triggers of lymphangiogenesis. Lymphat Res Biol. 2003;1:201–18 [DOI] [PubMed] [Google Scholar]
- 25.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–43 [DOI] [PubMed] [Google Scholar]
- 26.Suk K, Chang I, Kim YH, Kim S, Kim JY, Kim H, Lee MS. Interferon gamma (IFNgamma) and tumor necrosis factor alpha synergism in ME-180 cervical cancer cell apoptosis and necrosis. IFNgamma inhibits cytoprotective NF-kappa B through STAT1/IRF-1 pathways. J Biol Chem. 2001;276:13153–9 [DOI] [PubMed] [Google Scholar]
- 27.Buszello H. Antiproliferative effects of four different cytokines on renal carcinoma cell lines. Anticancer Res. 1995;15:735–8 [PubMed] [Google Scholar]
- 28.Wu AJ, Chen ZJ, Tsokos M, O'Connell BC, Ambudkar IS, Baum BJ. Interferon-gamma induced cell death in a cultured human salivary gland cell line. J Cell Physiol. 1996;167:297–304 [DOI] [PubMed] [Google Scholar]
- 29.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130 [DOI] [PubMed] [Google Scholar]
- 30.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95 [DOI] [PubMed] [Google Scholar]
- 31.Aversa G, Punnonen J, Cocks BG, de Waal Malefyt R, Vega F, Jr, Zurawski SM, Zurawski G, de Vries JE. An interleukin 4 (IL-4) mutant protein inhibits both IL-4 or IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: support for a common component shared by IL-4 and IL-13 receptors. J Exp Med. 1993;178:2213–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50 [DOI] [PubMed] [Google Scholar]
- 33.Blais Y, Gingras S, Haagensen DE, Labrie F, Simard J. Interleukin-4 and interleukin-13 inhibit estrogen-induced breast cancer cell proliferation and stimulate GCDFP-15 expression in human breast cancer cells. Mol Cell Endocrinol. 1996;121:11–8 [DOI] [PubMed] [Google Scholar]
- 34.Serve H, Oelmann E, Herweg A, Oberberg D, Serve S, Reufi B, Mucke C, Minty A, Thiel E, Berdel WE. Inhibition of proliferation and clonal growth of human breast cancer cells by interleukin 13. Cancer Res. 1996;56:3583–8 [PubMed] [Google Scholar]
- 35.Miyahara N, Miyahara S, Takeda K, Gelfand EW. Role of the LTB4/BLT1 pathway in allergen-induced airway hyperresponsiveness and inflammation. Allergol Int. 2006;55:91–7 [DOI] [PubMed] [Google Scholar]
- 36.Yokomizo T, Uozumi N, Takahashi T, Kume K, Izumi T, Shimizu T. Leukotriene A4 hydrolase and leukotriene B4 metabolism. J Lipid Mediat Cell Signal. 1995;12:321–32 [DOI] [PubMed] [Google Scholar]
- 37.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32 [DOI] [PubMed] [Google Scholar]
- 38.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, Shen D, Cruz AR, Aikawa M, Luster AD, et al. Inhibited aortic aneurysm formation in BLT1-deficient mice. J Immunol. 2007;179:691–7 [DOI] [PubMed] [Google Scholar]
- 40.Kim EY, Seo JM, Cho KJ, Kim JH. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene. 2010;29:1167–78 [DOI] [PubMed] [Google Scholar]
- 41.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clezardin P, Serre CM, Trzeciak MC, Drouin J, Delmas PD. Thrombospondin binds to the surface of human osteosarcoma cells and mediates platelet-osteosarcoma cell interaction. Cancer Res. 1991;51:2621–7 [PubMed] [Google Scholar]
- 43.Dardik R, Lahav J. Multiple domains are involved in the interaction of endothelial cell thrombospondin with fibronectin. Eur J Biochem. 1989;185:581–8 [DOI] [PubMed] [Google Scholar]
- 44.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA. 1999;96:14888–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streit M, Stephen AE, Hawighorst T, Matsuda K, Lange-Asschenfeldt B, Brown LF, Vacanti JP, Detmar M. Systemic inhibition of tumor growth and angiogenesis by thrombospondin-2 using cell-based antiangiogenic gene therapy. Cancer Res. 2002;62:2004–12 [PubMed] [Google Scholar]
- 46.Noh YH, Matsuda K, Hong YK, Kunstfeld R, Riccardi L, Koch M, Oura H, Dadras SS, Streit M, Detmar M. An N-terminal 80 kDa recombinant fragment of human thrombospondin-2 inhibits vascular endothelial growth factor induced endothelial cell migration in vitro and tumor growth and angiogenesis in vivo. J Invest Dermatol. 2003;121:1536–43 [DOI] [PubMed] [Google Scholar]
- 47.Chijiwa T, Abe Y, Ikoma N, Yamazaki H, Tsukamoto H, Suemizu H, Kawai K, Wakui M, Nishime C, Matsumoto H, et al. Thrombospondin 2 inhibits metastasis of human malignant melanoma through microenvironment-modification in NOD/SCID/gammaCnull (NOG) mice. Int J Oncol. 2009;34:5–13 [PubMed] [Google Scholar]
- 48.Fears CY, Grammer JR, Stewart JE, Jr, Annis DS, Mosher DF, Bornstein P, Gladson CL. Low-density lipoprotein receptor-related protein contributes to the antiangiogenic activity of thrombospondin-2 in a murine glioma model. Cancer Res. 2005;65:9338–46 [DOI] [PubMed] [Google Scholar]
- 49.Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem. 2002;277:20453–60 [DOI] [PubMed] [Google Scholar]
- 50.Adams LS, Phung S, Yee N, Seeram NP, Li L, Chen S. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010;70:3594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He B, Lee AY, Dadfarmay S, You L, Xu Z, Reguart N, Mazieres J, Mikami I, McCormick F, Jablons DM. Secreted frizzled-related protein 4 is silenced by hypermethylation and induces apoptosis in beta-catenin-deficient human mesothelioma cells. Cancer Res. 2005;65:743–8 [PubMed] [Google Scholar]
- 52.Lee AY, He B, You L, Dadfarmay S, Xu Z, Mazieres J, Mikami I, McCormick F, Jablons DM. Expression of the secreted frizzled-related protein gene family is downregulated in human mesothelioma. Oncogene. 2004;23:6672–6 [DOI] [PubMed] [Google Scholar]
- 53.Turashvili G, Bouchal J, Burkadze G, Kolar Z. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006;73:213–23 [DOI] [PubMed] [Google Scholar]
- 54.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16 [DOI] [PubMed] [Google Scholar]
- 55.Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling. Chem Biol Interact. 2009;181:263–71 [DOI] [PubMed] [Google Scholar]
- 56.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–75 [DOI] [PubMed] [Google Scholar]
- 57.Singh J, Xie C, Yao M, Hua S, Vignarajan S, Jardine G, Hambly BD, Sved P, Dong Q. Food extracts consumed in Mediterranean countries and East Asia reduce protein concentrations of androgen receptor, phospho-protein kinase B, and phospho-cytosolic phospholipase A(2)alpha in human prostate cancer cells. J Nutr. 2010;140:786–91 [DOI] [PubMed] [Google Scholar]
- 58.Del Bo C, Ciappellano S, Klimis-Zacas D, Martini D, Gardana C, Riso P, Porrini M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the Sprague-Dawley rat. J Agric Food Chem. 2010;58:2491–7 [DOI] [PubMed] [Google Scholar]
- 59.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61 [DOI] [PubMed] [Google Scholar]
- 60.Entrez Gene [cited 2011] Available from: http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene.
- 61.Reeves PG. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J Nutr. 1997;127:S838–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.