Abstract
The effects of nerve sparing on the risk of positive surgical margins (PSMs) and biochemical recurrence after radical prostatectomy (RP) remain controversial. We examined data from 1018 men treated by RP between 1988 and 2006 at five centers in the Shared Equal Access Regional Cancer Hospital database. Neither bilateral nor unilateral nerve-sparing techniques were associated with a higher risk of PSM; on multivariate analysis of individual sides, the risk of PSM on either side was not increased by nerve sparing on either side. The risk for biochemical recurrence was not affected by bilateral or unilateral nerve sparing. When used on appropriately selected patients, nerve sparing does not increase the probability of PSM or biochemical recurrence after RP.
Keywords: radical prostatectomy, biochemical recurrence, nerve sparing
Introduction
Nerve-sparing (NS) radical prostatectomy (RP) has been employed with increasing frequency in the past two decades as a means of preserving erectile function in men while adequately excising the prostate. Although sparing the cavernosal nerves as an attempt to preserve potency was first described by Walsh and Donker,1 much of this increase in NS procedures has been made possible by the shift in patient and tumor characteristics caused by the advent of widespread prostate-specific antigen (PSA) testing.2 More patients are presenting with organ-confined disease, and the patients themselves are younger and more likely to desire preservation of sexual potency.
Nevertheless, positive surgical margins (PSMs) remain common, with frequencies ranging from 6 to 41%.3–5 Patients must be carefully selected to reduce the risk of PSMs and disease recurrence. We sought to compare the demographic, clinicopathological data and biochemical recurrence rates between NS and non-NS (NNS) techniques in a large multicenter cohort of well-defined ethnically diverse patients.
Materials and methods
Study population
After institutional review board approval for data abstraction was obtained from each institution, data from patients treated with RP from 1988 to 2006 at the Veterans Affairs Medical Centers in West Los Angeles, Palo Alto and San Francisco, CA; Augusta, GA; and Durham, NC, were combined into the Shared Equal Access Regional Cancer Hospital (SEARCH) database.6 A total of 1771 patients, excluding patients treated with preoperative androgen deprivation or radiation therapy, underwent RP at the five centers enrolled in the SEARCH database. Operative reports did not clarify if NS was performed in 753 patients, who were excluded from the study, leaving a population of 1018 patients. The decision for NS was based on preoperative clinical and biopsy characteristics and intraoperative observation and palpation by the operating surgeon. Intraoperative frozen sections of prostate margins were not used to determine the need for nerve resection. Prostatectomy specimens were sectioned according to each institutional protocol. Patients were followed to determine PSA recurrence. Recurrence was defined as a single PSA of greater than 0.2 ng ml−1 or two values of 0.2 ng ml−1 or secondary treatment for an elevated postoperative PSA. Median follow-up was 35 months (range 1–192). A total of 20 patients with no follow-up data were included in analysis for evaluating differences in preoperative and pathological characteristics but not biochemical recurrence.
Statistical analysis
Patients were separated into three groups based on surgical technique, including bilateral nerve sparing (BNS), unilateral nerve sparing (UNS) and nerve sparing (NNS). For the purpose of analysis, techniques referred to in operative notes as ‘partially NS’ were coded as NS on the respective side. The distribution of clinicopathological characteristics was compared among the groups using analysis of variance (ANOVA) for continuous variables and the χ2-test for categorical variables. Age at RP, preoperative PSA, body mass index (BMI) and the percent of biopsy cores with cancer were examined as continuous variables. Gleason sum (7 or >7 vs 2–6), clinical stage (T2b or >T2b vs <T2b) and race (black, nonblack–nonwhite vs white) were examined as categorical variables. The type of prostatectomy (retropubic vs perineal or laparoscopic) was not available in our database and therefore could not be included in our analyses.
The odds ratios of PSMs were estimated for the different surgery groups using a multivariate logistic regression model. We adjusted for age at RP, race, BMI, percent of cores positive at biopsy, Gleason score, clinical stage, preoperative PSA, year of surgery and the center at which surgery was performed. A separate logistic regression was then performed investigating for risk factors for positive margins for each side of the prostate. These models adjusted for the same clinicopathological factors, but compared the risks of left NS and right NS to neither for PSMs on each side. In the UNS group, the rates of positive margins ipsilateral and contralateral to the side of NS were compared by chi-square analysis.
A Cox proportional hazard model7 was used to compare the groups to each other as well as to determine the pathological and clinical features that were significant predictors of time to biochemical recurrence.
Survival curves were estimated using the Kaplan–Meier technique. All clinical (age, clinical stage, PSA and biopsy Gleason score) and pathological (surgical Gleason score, pathological stage, extracapsular extension and surgical margin status, and seminal vesicle invasion) variables were similar between the centers and, therefore, data were combined for analysis. Statistical analyses were performed using STATA 9.2 (Stata Corporation, College Station, TX, USA).
Results
Patient demographics
BNS and UNS groups had significantly lower percent positive biopsy cores (P = 0.037), BMI (P<0.001) and PSA (P<0.001) than the NNS group. NS groups also had fewer high grade or high stage tumors (P<0.001). The BNS group had a higher proportion of black patients (P<0.001) than the other groups. Although the ages of the groups did not differ significantly when analyzed as three groups, the NS groups combined had a lower mean age than NNS (P<0.001). Table 1 shows the comparison of all preoperative characteristics and pathological findings used in our analyses.
Table 1.
Clinical and pathological features in men undergoing radical prostatectomy within the SEARCH database stratified by nerve sparing-technique
| Nerve-sparing technique
|
P-value* | |||
|---|---|---|---|---|
| BNS | UNS | NNS | ||
| Number of patients | 579 | 136 | 303 | |
| Age at surgery, mean (years) | 59.977a | 63.62 | <0.001† | |
| Body mass index (kg m−2) | 27.92 | 28.99 | 28.04 | <0.001† |
| Race (%) | <0.001 | |||
| White | 290 (50) | 88 (65) | 186 (62) | |
| Black | 243 (42) | 43 (32) | 87 (29) | |
| Other | 42 (7) | 5 (4) | 27 (9) | |
| Biopsy Gleason score (%) | <0.001 | |||
| <7 | 404 (70) | 84 (62) | 165 (52) | |
| 7 | 145 (25) | 35 (26) | 92 (31) | |
| >7 | 27 (5) | 16 (12) | 43 (14) | |
| Clinical stage (%) | <0.001 | |||
| <T2b | 492 (87) | 112 (82) | 222 (74) | |
| T2b | 37 (7) | 17 (13) | 50 (17) | |
| >T2b | 39 (7) | 7 (5) | 27 (9) | |
| Positive biopsy cores (%) | 0.33 | 0.36 | 0.44 | 0.037† |
| PSA | 8.28 | 10.51 | 11.74 | 0.001† |
| Pathological Gleason score (%) | <0.001 | |||
| <7 | 309 (54) | 56 (42) | 108 (37) | |
| 7 | 231 (40) | 66 (49) | 133 (45) | |
| >7 | 36 (6) | 12 (9) | 54 (18) | |
| PSM | 217 (38) | 53 (40) | 124 (43) | 0.40 |
| Capsular penetration (%) | 135 (20) | 67 (39) | 28 (61) | <0.001 |
| Seminal vesicle involvement (%) | 38 (7) | 10 (8) | 43 (15) | <0.001 |
| Positive lymph nodes (%) | 6 (1) | 3 (3) | 8 (3) | 0.25 |
| Apical PSM | 77 (19) | 12 (18) | 27 (10) | 0.40 |
| Bladder neck PSM | 17 (3) | 3 (2) | 20 (7) | 0.007 |
| Recurrence | 128 (22) | 33 (25) | 119 (40) | <0.001 |
Abbreviations: BNS, bilateral nerve sparing; NNS, non-NS; PSA, prostate-specific antigen; PSM, positive surgical margin; UNS, unilateral nerve sparing.
P-value by chi-square test except where noted.
P-value by analysis of variance.
BNS and UNS groups combined to compare age to NNS groups.
On pathology, NS groups had lower Gleason score (P<0.001) and incidence of extracapsular extension (P<0.001) and seminal vesicle invasion (P<0.001). Univariate analysis revealed a lower overall recurrence rate for the NS groups relative to the NNS group (Table 1).
Nerve sparing and positive surgical margins
Preoperative factors associated with a higher risk of PSM included PSA (P<0.001), BMI (P = 0.007), percent positive biopsy cores (P<0.001), Gleason score (P = 0.02) and clinical stage (P = 0.05). Neither BNS nor UNS groups had a higher risk of PSM (Table 2). On unilateral analysis, the risk of PSM on either side was not increased by NS on either side. That is, neither the left nor the right side had a higher risk of positive margins based on NS on either side. The odds ratios of PSMs on the left side based on NS on the left and right sides were 0.61, P = 0.32, and 1.58, P = 0.26, respectively. For the right side, the odds ratios were 0.94, P = 0.87, and 0.79, P = 0.56 for left NS and right NS, respectively. Analysis of the UNS group revealed no difference between the rates of positive margins ipsilateral and contralateral to the side of NS (19.1 vs 26.5%, P = 0.15).
Table 2.
Odds ratios for positive surgical margins in multivariate logistic regression
| Odds ratio | 95% CI | P-value | ||
|---|---|---|---|---|
| Age | 1.00 | 0.97 | 1.02 | 0.70 |
| PSA | 1.04 | 1.02 | 1.07 | <0.001* |
| BMI | 1.04 | 1.01 | 1.08 | 0.007* |
| Positive cores (%) | 3.61 | 1.85 | 7.04 | <0.001* |
| Black race | 0.78 | 0.56 | 1.09 | 0.14 |
| Other race | 0.70 | 0.36 | 1.35 | 0.29 |
| Gleason<7 | 1.00 | Referent | ||
| Gleason = 7 | 1.52 | 1.06 | 2.19 | 0.022* |
| Gleason>7 | 1.20 | 0.67 | 2.16 | 0.53 |
| Clinical stage <T2b | 1.00 | Referent | ||
| Clinical stage = T2b | 1.77 | 1.01 | 3.10 | 0.047* |
| Clinical stage >T2b | 0.47 | 0.24 | 0.94 | 0.032* |
| NNS | 1.00 | Referent | ||
| BNS | 0.95 | 0.63 | 1.45 | 0.82 |
| UNS | 0.99 | 0.59 | 1.66 | 0.97 |
Abbreviations: BMI, body mass index; BNS, bilateral nerve sparing; NNS, non-NS; PSA, prostate-specific antigen; UNS, unilateral nerve sparing.
Significant to P<0.05.
Nerve sparing and biochemical recurrence
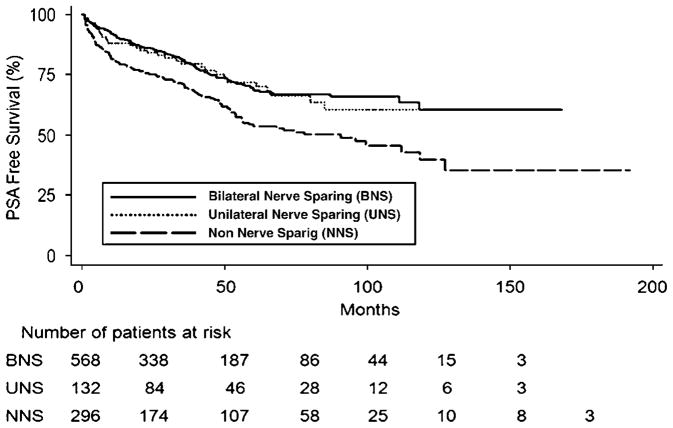
NS groups had a lower incidence of biochemical recurrence than the NNS group. On a Kaplan–Meier curve, the UNS and BNS groups are virtually identical and separate immediately from the NNS group (Figure 1). This difference is significant on univariate analysis (P<0.001).
Figure 1.
Kaplan–Meier curve showing prostate-specific antigen (PSA)-free survival by radical prostatectomy (RP) technique.
In the Cox proportional hazard analysis, preoperative PSA (P = 0.002), percent positive cores (P = 0.01), Gleason score (P<0.001) and BMI (P = 0.04) were significant independent predictors of biochemical recurrence (Table 3). The risk for biochemical recurrence was not significantly increased by BNS (hazard ratio, HR 0.61, P = 0.006) or UNS (HR 0.70, P = 0.13) as the HR is less than 1 for both modalities when compared to the NNS group.
Table 3.
Hazard ratios for positive biochemical recurrence from multivariate Cox proportional hazards regression
| Hazard ratio | 95% CI | P-Value | ||
|---|---|---|---|---|
| Age | 1.02 | 0.99 | 1.04 | 0.14 |
| PSA | 1.02 | 1.01 | 1.03 | 0.002* |
| BMI | 1.03 | 1.001 | 1.06 | 0.041* |
| Positive cores (%) | 2.10 | 1.18 | 3.72 | 0.012* |
| Black race | 1.22 | 0.91 | 1.64 | 0.19 |
| Other race | 0.97 | 0.54 | 1.72 | 0.91 |
| Gleason<7 | 1.00 | Referent | ||
| Gleason = 7 | 1.79 | 1.30 | 2.45 | <0.001* |
| Gleason>7 | 1.96 | 1.22 | 3.15 | 0.006* |
| Clinical stage <T2b | 1.00 | Referent | ||
| Clinical stage = T2b | 1.24 | 0.80 | 1.91 | 0.34 |
| Clinical stage >T2b | 1.47 | 0.91 | 2.36 | 0.11 |
| NNS | 1.00 | Referent | ||
| BNS | 0.61 | 0.43 | 0.87 | 0.006* |
| UNS | 0.70 | 0.45 | 1.11 | 0.13 |
Abbreviations: BMI, body mass index; BNS, bilateral nerve sparing; NNS, non-NS; PSA, prostate-specific antigen; UNS, unilateral nerve sparing.
Significant to P<0.05.
Discussion
The decision to perform RP with neurovascular bundle preservation must be individualized to the patient’s clinical features. The decision is a balance between minimizing postoperative erectile dysfunction and maximizing surgical removal of the prostate and the surrounding soft tissue. Sparing the neurovascular bundle has the assumed potential to transect prostate, or cause a PSM in a region of extracapsular disease, due to a close dissection plane. The importance of having a negative surgical margin during RP has been demonstrated in several studies that show that a positive margin is an independent risk factor for biochemical recurrence.8–10 In a study of almost 1000 patients who underwent RP by a single surgeon, the rate of biochemical recurrence after PSMs was 19%, compared to 7% in patients with negative margins.11 A multicenter study from the SEARCH database showed that patients with PSM and organ-confined disease had recurrence rates similar to patients with extracapsular extension.12 Surgeons must consider many clinicopathological characteristics, because biopsy results alone have been questioned when determining the location and extent of tumor in order to predict extracapsular disease. Studies have revealed that up to 74% of prostatectomy specimens with unilaterally positive biopsies have tumor in the contralateral side, with PSMs rates on the biopsy-shown ‘benign’ side from 24–31%.13,14 Despite these concerns, single-institution studies have not shown NS RP to be an independent risk factor for PSMs or biochemical recurrence.15–18
Our findings from a large multicenter cohort of well-defined ethnically diverse patients support the continued use of NS RP. We found no increase in the risk of either PSMs or biochemical recurrence in our multivariate models. Moreover, comparing the risk of positive margins on individual sides based on the side of NS revealed no significant increased risk of positive margins. By stratifying the margins by side, we were able to investigate if NS approach puts the surgical margins at risk either ipsilateral or contralateral to the spared nerve. These data suggest that we are able to appropriately select patients for NS procedures based on their preoperative clinicopathological characteristics.
The differences between the groups in preoperative clinicopathological characteristics highlight the decision-making process in selecting a patient for NS vs NNS procedure. The men selected for NS procedures were typically younger with lower PSA, biopsy Gleason score, clinical stage and percent positive biopsy cores. Nearly all of these characteristics were significant predictors for PSMs, biochemical recurrence or both. One notable exception was clinical stage, which in contrast to previous studies was not a significant predictor of recurrence. This appears to be due to colinearity between percent positive cores and clinical stage. When percent positive cores was left out of the Cox proportional hazard analysis, the effect of clinical stage became significant, with a HR of 1.38 (P = 0.10) for T2b disease and 1.74 (P = 0.01) for >T2b disease relative to low-risk (<T2b) disease. Moreover, an ANOVA comparing the mean percent positive cores between the clinical stage categories revealed a significant association between percent positive cores and stage (P<0.001). Thus, ‘percent positive biopsy cores’ was a stronger predictor of recurrence in our model than clinical stage.
The NS groups also had more favorable pathological characteristics than the NNS group, with lower pathological Gleason score and a lower incidence of capsular penetration and seminal vesicle involvement on univariate analysis.
The impact of NS on recurrence has been investigated before,15,16 with similar findings. However, our analysis differed in several fundamental ways. The previous studies were single-institution case series, whereas we included patients from five different centers and many different surgeons. More importantly, our statistical models differed significantly. The previous studies compared the risks of NS and NNS technique when adjusting for pathological risks for recurrence including extracapsular extension, pathological Gleason, surgical margin status, and SV involvement15 or margins, pathological Gleason, SV involvement, clinical stage, tumor volume, capsular involvement, and extraprostatic extension.16 In contrast, we adjusted for known pre-operative risks for recurrence, including recently identified risk factors such as BMI and percent positive biopsy cores. Therefore, our results should not be affected by any interaction between NS technique and pathological characteristics such as PSMs. Additionally, by including data available prior to the time of surgery, this approach addressed the question of whether patients are being appropriately selected for NS prostatectomy.
There are several limitations to this study. This is a retrospective study, allowing for selection bias, with the most favorable cases undergoing NS procedures. We accounted for this by using a multivariate model incorporating clinical and biopsy data available that is known to correlate with either extracapsular extension at pathology or biochemical recurrence. In addition, a rather large portion of our study population was excluded because it was unknown whether an attempt was made to preserve their cavernosal nerves. When these patients were included in the analysis as a separate ‘UNKNOWN’ group, their clinicopathological characteristics most closely resembled those of the NNS group. Inclusion as either a separate UNKNOWN group or as NNS did not affect the outcome of the analyses. The relatively short median follow-up of 35 months is also a limitation; however, most biochemical recurrences do occur within 3 years of surgery and this follow-up interval is similar in other single-institution series. Another important limitation is that there are varying degrees on the margins taken even between NS procedures. Our definition of NS did not include any confirmation of postprocedural potency, but was taken purely from the operative description and classified dichotomously as either NS or not. In addition, some cases are described in operative reports as ‘partially NS.’ Our analysis classified these as NS. The inclusion of multiple surgeons at multiple institutions also raises the possibility of significant variability in both operative procedure and pathological examination. The results of single-surgeon and single-institution studies have previously been published, confirming that in the hands of an experienced surgeon with standardized procedures, NS does not increase the risk of biochemical recurrence or PSMs. While it is true that our study subjects the data to more variability in the method of both surgery and pathological sectioning, the advantage of a large, multi-center study with multiple surgeons and techniques is that it allows generalization of these results. In addition, the center at which a prostatectomy was performed was included in our analyses as an independent variable. Finally, it is possible that some patients had intraoperative factors, such as tactile adherence, that helped surgeons appropriately select for NS. We did not have information on intraoperative decision-making, deviation from NS plans or the impact of individual surgeons on outcomes.
Conclusions
When used on appropriately selected patients, UNS and BNS do not increase the probability of PSMs or biochemical recurrence after RP. NS remains a safe means of preserving potency on well-selected patients undergoing RP.
Acknowledgments
This work was supported by the Department of Veterans Affairs, National Institute of Health T32 Grant DK07790-01 (JLN, CJK), National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), the Department of Defense, Prostate Cancer Research Program, (SJF), and the American Urological Association Foundation Astellas Rising Star in Urology Award (SJF).
Footnotes
Conflicts of interest/Disclosure
Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 2.Smith DS, Catalona WJ, Herschman JD. Longitudinal screening for prostate cancer with prostate-specific antigen. JAMA. 1996;276:1309–1315. [PubMed] [Google Scholar]
- 3.Ohori M, Scardino PT. Localized prostate cancer. Curr Probl Surg. 2002;39:833–957. doi: 10.1067/msg.2002.126335. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI. Pathologic assessment of the surgical specimen. Urol Clin North Am. 2001;28:567–594. doi: 10.1016/s0094-0143(05)70164-6. [DOI] [PubMed] [Google Scholar]
- 5.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy: the 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Amling CL, Dorey F, Kane CJ, Presti JC, Jr, Terris MK, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670–674. doi: 10.1016/s0090-4295(02)01847-2. [DOI] [PubMed] [Google Scholar]
- 7.Cox DR. Regression models and life tables. J Roy Stat Soc. 1972;34:187–220. [Google Scholar]
- 8.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Schnall M, Tomaszewski JE, et al. A multivariate analysis of clinical and pathological factors that predict for prostate specific antigen failure after radical prostatectomy for prostate cancer. J Urol. 1995;154:131–138. [PubMed] [Google Scholar]
- 9.Swindle P, Eastham JA, Ohori M, Kattan MW, Wheeler T, Maru N, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–907. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 10.Blute ML, Bostwick DG, Bergstralh EJ, Slezak JM, Martin SK, Amling CL, et al. Anatomic site-specific positive margins in organ-confined prostate cancer and its impact on outcome after radical prostatectomy. Urology. 1997;50:733–739. doi: 10.1016/S0090-4295(97)00450-0. [DOI] [PubMed] [Google Scholar]
- 11.Simon MA, Kim S, Soloway MS. Prostate specific antigen recurrence rates are low after radical retropubic prostatectomy and positive margins. J Urol. 2006;175:140–145. doi: 10.1016/S0022-5347(05)00050-9. [DOI] [PubMed] [Google Scholar]
- 12.Freedland SJ, Aronson WJ, Presti JC, Kane CJ, Terris MK, Elashoff D, et al. Should a positive surgical margin following radical prostatectomy be pathological stage T2 or T3? Results from the SEARCH database. J Urol. 2003;169:2142–2146. doi: 10.1097/01.ju.0000061760.23169.be. [DOI] [PubMed] [Google Scholar]
- 13.Connolly SS, O’Malley KJ, O’Brien A, Kelly DG, Mulvin DW, Quinlan DM. Can prostate biopsies predict suitability for nerve-sparing radical prostatectomy? Scand J Urol Nephrol. 2004;38:216–220. doi: 10.1080/00365590310006237. [DOI] [PubMed] [Google Scholar]
- 14.Obek C, Louis P, Civantos F, Soloway MS. Comparison of digital rectal examination and biopsy results with the radical prostatectomy specimen. J Urol. 1999;161:494–499. [PubMed] [Google Scholar]
- 15.Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, Blute ML. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004;172:1328–1332. doi: 10.1097/01.ju.0000138681.64035.dc. [DOI] [PubMed] [Google Scholar]
- 16.Sofer M, Hamilton-Nelson KL, Schlesselman JJ, Soloway MS. Risk of positive margins and biochemical recurrence in relation to nerve-sparing radical prostatectomy. J Clin Oncol. 2002;20:1853–1858. doi: 10.1200/JCO.2002.07.069. [DOI] [PubMed] [Google Scholar]
- 17.Palisaar RJ, Noldus J, Graefen M, Erbersdobler A, Haese A, Huland H. Influence of nerve-sparing (NS) procedure during radical prostatectomy (RP) on margin status and biochemical failure. Eur Urol. 2005;47:176–184. doi: 10.1016/j.eururo.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Katz R, Salomon L, Hoznek A, de la Taille A, Antiphon P, Abbou CC. Positive surgical margins in laparoscopic radical prostatectomy: the impact of apical dissection, bladder neck remodeling and nerve preservation. J Urol. 2003;169:2049–2052. doi: 10.1097/01.ju.0000065822.15012.b7. [DOI] [PubMed] [Google Scholar]