Abstract
It is well-known fact that various pathogens, including bacteria, virus, and protozoa, induce abortion in humans and animals. However the mechanisms of infectious abortion are little known. In this study, we demonstrated that Listeria monocytogenes infection in trophoblast giant cells decreased heme oxygenase (HO)-1 and B-cell lymphoma-extra large (Bcl-XL) expression, and that their overexpression inhibited cell death induced by the infection. Furthermore, HO-1 and Bcl-XL expression levels were also decreased by L. monocytogenes in pregnant mice. Treatment with cobalt protoporphyrin, which is known to induce HO-1, inhibited infectious abortion. Taken together, our study indicates that L. monocytogenes infection decreases HO-1 and Bcl-XL expression and induces cell death in placenta, leading to infectious abortion.
Introduction
Listeriosis is caused by gram-positive Listeria monocytogenes. In humans, this pathogen has the ability to cross the intestinal, placental, and blood-brain barriers, leading to gastroenteritis, maternofetal infections, and meningoencephalitis, respectively. A key feature of the virulence of L. monocytogenes is its ability to avoid the killing mechanisms of professional and non- professional phagocytic host cells [1]–[4]. L. monocytogenes infections in humans are caused mainly by injection of contaminated food, such as daily products, raw vegetables, fish, poultry, processed chicken, and beef [5].
L. monocytogenes induces cell death in vitro and in vivo in various cell types including hepatocytes [6], lymphocytes [7], and dendritic cells [8]. Cell death induced by L. monocytogenes is associated with listeriolysin O, a pore-forming toxin that allows bacteria to lyse the phagosomal membrane and escape into the cytosol.
In a previous study, we investigated abortion induced by brucella infections and demonstrated that it was associated with cell death of placental immune cells, the trophoblast giant (TG) cells. Furthermore, we found that heme oxygenase (HO)-1 expression inhibited infectious abortions in vivo and cell death in vitro [9]. HO-1 plays a key role in cytoprotection, anti-oxidation, and anti-inflammation. Most of the physiological functions of HO-1 are associated with its enzymatic activity in heme catabolism [10], [11]. In humans, HO-1 deficiency is associated with susceptibility to oxidative stress and an increased pro-inflammatory state, leading to severe endothelial damage [12]. Mice lacking HO-1 develop progressive inflammatory disease [13] and show enhanced lipopolysaccharide-induced toxemia [14]. Although the protective properties of HO-1 have been studied using various inflammatory models [15]–[20], the molecular mechanisms, timing, and mode of HO-1 function during disease remains largely unknown. HO-1 expression is known to be associated with B-cell lymphoma-extra large (Bcl-XL) expression [21]. Bcl-XL is one of the several anti-apoptotic proteins that are members of the Bcl-2 family [22].
L. monocytogenes infection causes abortion in pregnant mice [23]. However, the factors involved in abortion induced by L. monocytogenes infection in these animals remain unknown. In the present study, we investigated the roles of the anti-apoptotic factors, HO-1 and Bcl-XL, in abortion induced by L. monocytogenes infection. HO-1 and Bcl-XL expression was down-regulated by L. monocytogenes infection or interferon (IFN)-γ treatment, leading to infectious abortion. HO-1 and Bcl-XL overexpression suppressed this infectious abortion. These results suggest that HO-1 and Bcl-XL play a critical role in the control of infectious abortion induced by L. monocytogenes.
Results
L. monocytogenes infection decreased HO-1 and Bcl-XL expression in TG cells
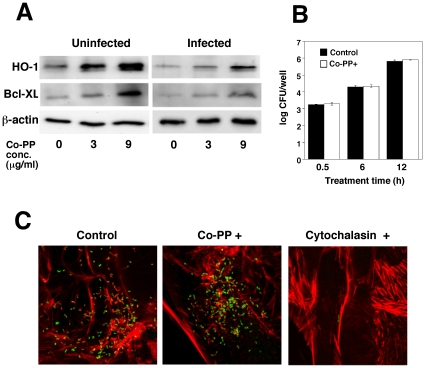
L. monocytogenes has been shown to infect the placenta and induce cell death in vitro and in vivo [24]–[26]. TG cells are placental immune cells existing in maternal-fetal interface and these cells are important for maintaining pregnancy [27]. In a previous study, we demonstrated that HO-1 plays a role in inhibiting cell death induced by Brucella abortus infection. To investigate the mechanisms through which L. monocytogenes induces cell death in placenta, we measured HO-1 expression in TG cells. HO-1 was expressed in TG cells, but its expression decreased on L. monocytogenes infection (Fig. 1A). Furthermore, HO-1 expression was enhanced by the HO-1 inducer cobalt protoporphyrin (Co-PP), in a concentration-dependent manner (Fig. 1A). No significant difference was observed in intracellular growth of bacteria between Co-PP-treated and non-treated TG cells (Fig. 1B, C). These results indicate that L. monocytogenes infection decreases HO-1 expression. To investigate the mechanism of HO-1, Bcl-XL expression was analyzed (Fig. 1A). Bcl-XL, an anti-apoptotic protein induced by HO-1, belongs to the Bcl-2 family [28], [29]. Bcl-XL expression was enhanced by the HO-1 inducer Co-PP and decreased by L. monocytogenes infection as well as HO-1. Furthermore, we showed that this reduction in expression was recovered by Co-PP.
Figure 1. Decreased HO-1 and Bcl-XL expression in TG cells infected with L. monocytogenes.
(A) TG cells were first treated with Co-PP and then infected with L. monocytogenes. The infected cells were cultured in 50 µg/ml of gentamicin. After 6 h, HO-1 and Bcl-XL expression was analyzed by immunoblotting. A representative immunoblot of three independent experiments is shown. (B) TG cells were treated with Co-PP and then infected with L. monocytogenes. The infected cells were cultured in 50 µg/ml of gentamicin. After 0.5, 2, and 6 h incubation, the infected cells were washed with PBS and lysed with cold distilled water. CFU were determined by serial dilution on BHI agar plates. (C) L. monocytogenes was deposited on TG cells by centrifugation at 150×g for 10 min at room temperature, incubated for 6 h, fixed, and stained. The figure shows FITC-labeled bacteria (green) and Alexa Fluor 594-labeled actin filaments (red) merged images. The left-hand panel shows untreated cells, the center panel Co-PP (9 µg/ml)-treated cells, and the right-hand panel, cytochalasin D-treated cells.
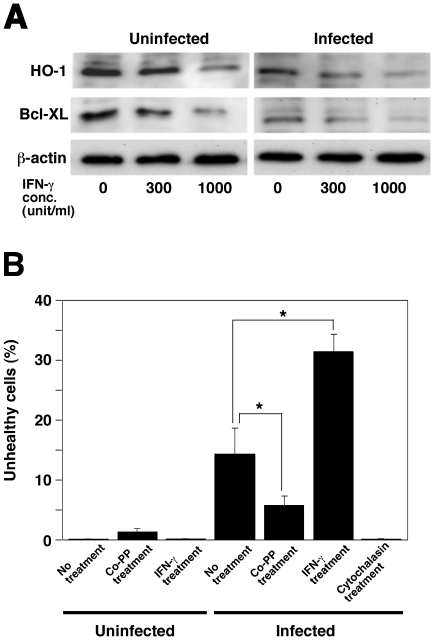
Since an increase in IFN-γ due to L. monocytogenes infection was observed to promote abortion in pregnant mice [30], we investigated the effect of IFN-γ treatment on HO-1 and Bcl-XL expression in TG cells. HO-1 and Bcl-XL expression in TG cells decreased significantly in a concentration-dependent manner on treatment with IFN-γ, with the down-regulation being enhanced further by L. monocytogenes infection (Fig. 2A).
Figure 2. Induction of cell death by L. monocytogenes infection.
(A) TG cells were treated with IFN-γ (0, 300, and 1,000 units/ml) for 24 h and infected with L. monocytogenes for 6 h. HO-1 and Bcl-XL expression in TG cells was analyzed by immunoblotting. A representative immunoblot of three independent experiments is shown. (B) Cell death was determined using the JC-1 Mitochondrial Membrane Potential Assay Kit. One hundred TG cells per coverslip were examined to determine the total number of live or dead cells. All values represent the average and the standard deviation of three identical experiments. Statistically significant differences compared with the control are indicated by asterisks (*, P<0.05).
HO-1 and Bcl-XL protect against cell death induced by L. monocytogenes infection
To examine whether HO-1 and Bcl-XL inhibited cell death, TG cells were infected with L. monocytogenes with or without Co-PP treatment and the rate of cell death was determined measuring mitochondrial membrane potential. Mitochondrial membrane potential has been used as an indicator of cell death. In this experimental system, cell death induced cells with low mitochondrial membrane potential were detected as unhealthy cells (Fig. 2B). Treatment with Co-PP inhibited cell death induced by L. monocytogenes infection in TG cells as compared with untreated TG cell. In contrast, cell death induced by L. monocytogenes infection in IFN-γ-treated TG cells was enhanced compared to that in untreated TG cells (Fig. 2B). Treatment with cytochalasin D, which is known to inhibit L. monocytogenes internalization, was found to inhibit the death of TG cells by L. monocytogenes infection compared with non-treated TG cells (Fig. 2B). These results indicate that internalization of L. monocytogenes decreases HO-1 and Bcl-XL expression leading to enhancement of cell death.
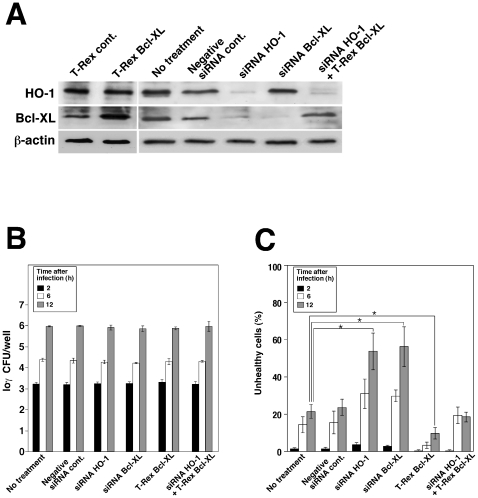
To confirm the effect of HO-1 and Bcl-XL on TG cell death following infection with L. monocytogenes, we reduced the amount of endogenous HO-1 and Bcl-XL by transfecting HO-1-specific small interfering RNA (siRNA) duplexes into TG cells. After 48 h of transfection with HO-1-specific siRNA, HO-1 and Bcl-XL expression levels were no longer detectable, but were not affected by transfection with β-actin or control siRNA (Fig. 3A). HO-1 or Bcl-XL knockdown did not induce cell death in TG cells (Fig. 3C). While L. monocytogenes infection resulted in a slight induction of cell death in TG cells, HO-1 or Bcl-XL knockdown enhanced cell death in infected TG cells (Fig. 3C). Bcl-XL overexpression in the T-Rex system inhibited cell death compared to cells not expressing the protein (Fig. 3C). There was no significant difference in bacterial growth between transfected and non-transfected TG cells (Fig. 3B). These results suggest that HO-1 and Bcl-XL play critical roles in the inhibition of cell death induced by L. monocytogenes infections.
Figure 3. Prevention of cell death by HO-1 and Bcl-XL expression.
(A) TG cells were treated for 48 h with either siRNA targeting HO-1, Bcl-XL, or control siRNA (QIAGEN AllStars Negative Control). Bcl-XL overexpression was achieved by transfecting the cells with pcDNA4/TO-Bcl-XL. HO-1 and Bcl-XL expression was monitored by immunoblotting. β-actin was used as an internal control. A representative immunoblot of three independent experiments is shown. (B) TG cells were infected with L. monocytogenes. The infected cells were cultured with media containing 50 µg/ml gentamicin for 2, 6, and 12 h. The cells were then washed with PBS and lysed with cold distilled water. CFU was determined by serial dilution on BHI agar plates. All values represent the average and the standard deviation of three identical experiments. (C) Cell death was determined using the JC-1 Mitochondrial Membrane Potential Assay Kit. One hundred TG cells per coverslip were examined to determine the total number of live or dead cells. All values represent the average and the standard deviation of three identical experiments. Statistically significant differences compared with the control are indicated by asterisks (*, P<0.05).
Abortion induced by L. monocytogenes infection is dependent on HO-1 and Bcl-XL expression in the placenta
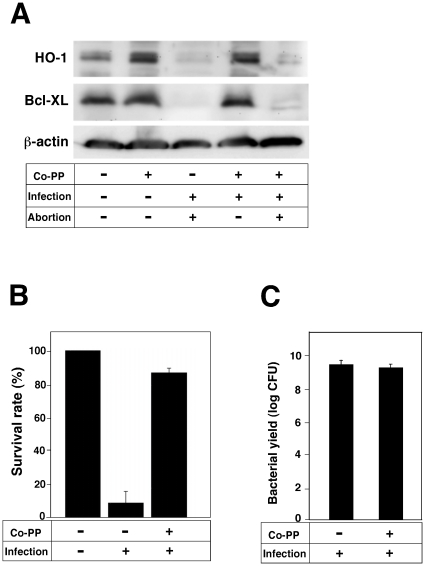
Previous studies have reported the presence of HO-1 in the mammalian placenta and postulated that it has a protective role during pregnancy [31]–[33]. We assume that the inhibitory action of HO-1 and Bcl-XL on cell death leads to a successful pregnancy. To examine whether HO-1 and Bcl-XL actually block abortion induced by L. monocytogenes infection, we measured HO-1 and Bcl-XL expression levels in the placenta of L. monocytogenes-infected mice. Both HO-1 and Bcl-XL were expressed in the placenta of mice, with levels being decreased by L. monocytogenes infection (Fig. 4A). Moreover, injection of L. monocytogenes with Co-PP restored HO-1 and Bcl-XL expression levels (Fig. 4A). We next investigated the role of HO-1 and Bcl-XL expression on abortion induced by L. monocytogenes. Infection of L. monocytogenes induced abortion in pregnant mice (Fig. 4B). HO-1 and Bcl-XL expression induced by Co-PP injection blocked abortion in L. monocytogenes-infected mice (Fig. 4B). There was no significant difference in the growth of bacteria in livers (Fig. 4C) and placenta (data not shown) between Co-PP-treated and untreated mice. These results suggest that abortion induced by L. monocytogenes infection is dependent on HO-1 and Bcl-XL expression in the placenta.
Figure 4. Prevention of infectious abortion by HO-1 and Bcl-XL expression.
(A) Pregnant mice were infected with 105 cells of L. monocytogenes in 0.1 ml of saline at day 13.5 of pregnancy with or without Co-PP treatment (5 mg/kg). At day 16.5, the placentas, fetuses, and livers were removed. HO-1 and Bcl-XL expression in the placenta was analyzed by immunoblotting. A representative immunoblot of three independent experiments is shown. (B) Survival rates were determined by the presence or absence of a heartbeat in the fetuses. (C) Livers were homogenized in saline and diluted with PBS. CFU was determined by plating the diluted samples on BHI agar plate.
Discussion
Heme oxygenases (HOs) are heme catabolic enzymes. Heme is degraded to carbon monoxide, biliverdin, and ferrous ion. Biliverdin is converted to bilirubin, which is believed to be a potent anti-oxidant. Three isoforms of HOs have been identified. HO-1 is an inducible isoform produced in response to various types of stress, such as oxidative stress, heat stress, endotoxin stress, hypoxia, heavy metal stress, and cytokine stress [34]. Furthermore, HO-1 plays a role in cytoprotection, anti-oxidation, anti-inflammation, and graft acceptance [35]–[37]. HO-1 is also down-regulated at the fetal maternal interface during spontaneous abortion in both humans and mice [33], [38]–[40]. Up-regulation of HO-1 by Co-PP prevents abortion, while down-regulation by zinc protoporphyrin increases the chances of abortion [31]. It has been reported that during pregnancy, all placental cell types are positive for HOs and that different types of trophoblast cells are important sources of these enzymes [32], [38], [40], [41]. As we anticipated, HO-1 was associated with infectious abortion. It is well known that various pathogens, such as Brucella spp., L monocytogenes, Leptospira spp., Buniyavirus, and Toxoplasma gondii, cause infectious abortion. However, the mechanisms responsible for infectious abortion remain unclear. Previously, we reported that HO-1 was associated with abortion induced by B. abortus infection. B. abortus are gram-negative, intracellular, and zoonotic bacteria that cause down-regulation of HO-1 in the placenta leading to abortion. However, it remains unclear whether HO-1 is a common regulator for abortion induced by various pathogens. In this study, we used gram-positive, intracellular, and zoonotic L. monocytogenes to examine this possibility.
In order to investigate the detailed mechanisms of infectious abortion induced by L. monocytogenes, we studied TG cells in vitro. TG cells are immunocompetent cells present in the placenta [42]–[44] and play a critical role in implantation and pregnancy [27], [42]. HO-1 expression in TG cells was decreased by L. monocytogenes infection (Fig. 1A) and treatment with IFN-γ (Fig. 2A). Furthermore, it is well known that IFN-γ is induced by L. monocytogenes infection in mice [30] and there is evidence that Th1 cytokines, such as IFN-γ, inhibit HO-1 expression resulting in allograft rejection [37]. These results indicate that Th1 cytokines induced by L. monocytogenes infection control HO-1 expression.
Although HO-1 appears to play a critical role in the control of infectious abortion, the mechanisms of this control remain unclear. We focused on Bcl-XL since HO-1 enhances the expression of this anti-apoptotic protein [21]. We found that Bcl-XL expression was enhanced by a HO-1 inducer, Co-PP (Figs. 1A, 4A) and furthermore that Bcl-XL overexpression prevented cell death induced by L. monocytogenes infection (Fig. 3C). These results suggest that Bcl-XL is a key factor that protects placenta cells from injury induced by L. monocytogenes infection, thereγy resulting in successful pregnancy.
We also observed that HO-1 and Bcl-XL expression was down-regulated in the placenta of pregnant mice by L. monocytogenes infection (Fig. 4A), while it was up-regulated by Co-PP and inhibited infectious abortion (Fig. 4B). These results suggest that HO-1 and Bcl-XL have critical roles against infectious abortion induced by L. monocytogenes.
Although HO-1 and Bcl-XL play an important role to protect cells from cell death, it is still unknown how HO-1 and Bcl-XL inhibit cell death induced by L. monocytogenes infection. Caspase-9 is an apoptotic protein and its activation is inhibited by Bcl-XL [45]. In TG cells, however, L. monocytogenes infection failed to induce caspase-9 activation (data not shown). These results may indicate that L. monocytogenes induces cell death trough alternative pathways involved with Bcl-XL.
In humans, it was reported that L. monocytogenes infects extravillous trophoblasts (EVTs), and spreads across maternal-fetal barrier [46]. However, there is less information about molecular mechanisms by which L. monocytogenes passes maternal-fetus barrier. Since trophoblast cells such as EVTs in human or TG cells in mouse exists in maternal-fetal interface, down regulation of HO-1 and Bcl-XL leading to enhancement of cell death may be a key event for L. monocytogenes to spread across the barrier.
In conclusion, our results indicate that down-regulation of HO-1 induced by various pathogens may be a key event in infectious abortion. Antimicrobial drugs are usually used in the treatment of listeriosis. However, an increasing number of multidrug-resistant L. monocytogenes have been reported [47], [48]. It is noteworthy that the HO-1 inducer Co-PP suppressed abortion induced by L. monocytogenes. Therefore, HO-1 has potential as a putative therapeutic target in infectious abortion.
Methods
Bacterial strains
L. monocytogenes EGD was maintained as a frozen glycerol stock and cultured in brain heart infusion (BHI) broth (Becton Dickinson) or on BHI broth containing 1.5% agar.
Cell culture
Mouse trophoblast stem (TS) cell line was gifted from Dr. Tanaka [44], [49]. TS cells were cultured in TS medium in the presence of fibroblast growth factor-4, heparin, and mouse embryonic fibroblast-conditioned medium as described previously [49]. The TS medium was prepared by adding 20% fetal bovine serum, 1 mM sodium pyruvate, 100 µM β-mercaptoethanol, and 2 mM L-glutamine to RPMI 1640. To induce differentiation to TG cells, the cells were cultured in TS medium alone for 3 days at 37°C in a CO2 incubator. The cells were then seeded in a 48-well (1–2×105 per well) or a 12-well (4–8×105 per well) tissue culture plate.
Immunoblotting
The protein samples were separated on a 15% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane, which was incubated for 16 h at 4°C with anti-HO-1 rabbit polyclonal antibody (Stressgen) or anti-Bcl-XL rabbit polyclonal antibody (Cell Signaling Technology) at a dilution of 1∶5000 or 1∶1000 in 5% skim milk. The membrane was washed three times in Tris-buffered saline with 0.02% Tween 20, incubated for 30 min with 0.01 µg/ml horseradish peroxidase-conjugated secondary antibody, and washed again. The immunoreactions were visualized using the enhanced chemiluminescence detection system (GE Healthcare Life Science). The β-actin antibody was purchased from Sigma.
Efficiency of bacterial replication within cultured cells
L. monocytogenes strains were deposited onto TG cells at a multiplicity of infection (MOI) of 10 by centrifugation at 150×g for 10 min at room temperature. To measure the intracellular replication efficiency, the infected cells were incubated at 37°C for 30 min, washed once with TS medium, and then incubated in TS medium containing gentamicin (50 µg/ml) for 0.5, 2, 6, and 12 h. The cells were washed three times with phosphate-buffered saline (PBS) and lysed with cold distilled water. Colony forming unit (CFU) was determined by serial dilution on BHI agar plates. Cytochalasin D (Wako), recombinant IFN-γ (Cedarlane Laboratories) or Co-PP was added to the TS medium at the indicated concentrations 2, 16, and 24 h before infection.
Immunofluorescence microscopy
Bacteria were deposited onto TG cells grown on coverslips by centrifugation at 150×g for 5 min at room temperature and were then incubated at 37°C for 30 min. The samples were washed twice with PBS and fixed with 4% paraformaldehyde in PBS for 30 min at room temperature, washed three times with PBS, and incubated successively three times for 5 min in blocking buffer (5% bovine serum albumin in PBS) at room temperature. The samples were permeabilized in 0.2% Triton X-100 and washed three times with PBS, followed by treatment with 5 µg/ml anti-L. monocytogenes polyclonal rabbit antibody (Viro Stat) diluted in blocking buffer to identify intracellular bacteria. After incubation for 1 h at 37°C, the samples were washed three times for 5 min with blocking buffer, stained with FITC-labeled goat anti-rabbit IgG (0.01 µg/ml, Chemicon) in blocking buffer, and incubated for 1 h at 37°C. Fluorescent images were obtained using a FluoView FV100 confocal laser scanning microscope (Olympus).
Expression of recombinant protein
Total RNA was isolated from TG cells using the RNA Purification Kit (Qiagen), and the purified RNA samples were stored at −30°C until use. RNA was quantified by absorption at 260 nm using the SmartSpec 3000 spectrophotometer (Bio-Rad). RT-PCR was performed using Superscript II Kit (Invitrogen). The primers used for mouse Bcl-XL amplification were 5′- ATGTCTCAGAGCAACCGGG AG -3′ and 5′- TCACTTCCGACTGAAGAGTGA -3′. To express Bcl-XL in TG cells, amplified DNA encoding Bcl-XL from TG cells in RT-PCR was cloned into the pcDNA4/TO vector of the T-Rex System (Invitrogen). pcDNA4/TO-Bcl-XL was transfected into TG cells using the FuGENE 6 Transfection Reagent (Roche) at a final concentration of 1.2 µg/ml.
siRNA experiment
siRNA duplexes used for silencing mouse HO-1 (target sequence: CAGCCACACAGCACTATGTAA) and Bcl-XL (target sequence: AAAGTGCAGTTCAGTAATAAA) and AllStars Negative Control siRNA were purchased from QIAGEN. TG cells were transfected transiently using the X-tremeGENE siRNA Transfection Reagent (Roche) with or without a final concentration of 10 nM for siRNAs.
Determination of cell death
Cell death was determined using the JC-1 Mitochondrial Membrane Potential Assay Kit (Cayman Chemical) according to the manufacturer's instructions. Mitochondrial membrane potential, DJm, an important parameter of mitochondrial function, is used as an indicator of cell health. Healthy cells have a high mitochondrial DJm and red fluorescence, while apoptotic or unhealthy cells have a low DJm and green fluorescence [50].
Mice
Six to 10-week-old BALB/c female mice were mated individually to 6- to 10-week-old BALB/c male mice. The parent mice were obtained from Kyudo Co., Ltd.. Vaginal plug was observed at day 0.5 of gestation. The normal gestational time for these mice is 19 days.
Virulence in pregnant mice
Groups of five pregnant mice were infected intraperitoneally at 13.5 days of gestation with approximately 105 cells of L. monocytogenes in 0.1 ml saline with or without Co-PP (5 mg/kg, Sigma). On day 16.5 of gestation, their livers were removed and homogenized in saline. The tissue homogenates were serially diluted with PBS and plated on BHI agar plates to estimate the number of CFU. Fetuses were classified as alive if there was a heartbeat and as dead if there was no heartbeat. The animal experiments were approved by the Animal Research Committee of Yamaguchi University (permit number: 141).
Statistical analyses
Statistical analyses were performed using Student's t test. Statistically significant differences compared with the controls are indicated by asterisks (*, P<0.05). Data are expressed as the mean of triplicate samples from three identical experiments, and the error bars represent the standard deviations.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported partially by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (C), 22580333. Website: http://www.mext.go.jp/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1.Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, et al. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorber B. Listeriosis. Clin Infect Dis. 1997;24:1–9. doi: 10.1093/clinids/24.1.1. quiz 10–11. [DOI] [PubMed] [Google Scholar]
- 6.Rogers HW, Callery MP, Deck B, Unanue ER. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 7.Merrick JC, Edelson BT, Bhardwaj V, Swanson PE, Unanue ER. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol. 1997;151:785–792. [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman CA, Domann E, Rohde M, Bruder D, Darji A, et al. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 9.Tachibana M, Watanabe K, Yamasaki Y, Suzuki H, Watarai M. Expression of heme oxygenase-1 is associated with abortion caused by Brucella abortus infection in pregnant mice. Microb Pathog. 2008;45:105–109. doi: 10.1016/j.micpath.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, et al. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med. 2005;202:1703–1713. doi: 10.1084/jem.20051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, et al. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 17.Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 18.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 19.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 20.Zwerina J, Tzima S, Hayer S, Redlich K, Hoffmann O, et al. Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone resorption. Faseb J. 2005;19:2011–2013. doi: 10.1096/fj.05-4278fje. [DOI] [PubMed] [Google Scholar]
- 21.Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruger AL, Peterson SJ, Schwartzman ML, Fusco H, McClung JA, et al. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 23.Abram M, Schluter D, Vuckovic D, Waber B, Doric M, et al. Effects of pregnancy-associated Listeria monocytogenes infection: necrotizing hepatitis due to impaired maternal immune response and significantly increased abortion rate. Virchows Arch. 2002;441:368–379. doi: 10.1007/s00428-002-0649-2. [DOI] [PubMed] [Google Scholar]
- 24.Abram M, Schluter D, Vuckovic D, Wraber B, Doric M, et al. Murine model of pregnancy-associated Listeria monocytogenes infection. FEMS Immunol Med Microbiol. 2003;35:177–182. doi: 10.1016/S0928-8244(02)00449-2. [DOI] [PubMed] [Google Scholar]
- 25.Irvin EA, Williams D, Voss KA, Smith MA. Listeria monocytogenes infection in pregnant guinea pigs is associated with maternal liver necrosis, a decrease in maternal serum TNF-alpha concentrations, and an increase in placental apoptosis. Reprod Toxicol. 2008;26:123–129. doi: 10.1016/j.reprotox.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Le Monnier A, Join-Lambert OF, Jaubert F, Berche P, Kayal S. Invasion of the placenta during murine listeriosis. Infect Immun. 2006;74:663–672. doi: 10.1128/IAI.74.1.663-672.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albieri A, Hoshida MS, Gagioti SM, Leanza EC, Abrahamsohn I, et al. Interferon-gamma alters the phagocytic activity of the mouse trophoblast. Reprod Biol Endocrinol. 2005;3:34. doi: 10.1186/1477-7827-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 30.Hara H, Kawamura I, Nomura T, Tominaga T, Tsuchiya K, et al. Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement. Infect Immun. 2007;75:3791–3801. doi: 10.1128/IAI.01779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sollwedel A, Bertoja AZ, Zenclussen ML, Gerlof K, Lisewski U, et al. Protection from abortion by heme oxygenase-1 up-regulation is associated with increased levels of Bag-1 and neuropilin-1 at the fetal-maternal interface. J Immunol. 2005;175:4875–4885. doi: 10.4049/jimmunol.175.8.4875. [DOI] [PubMed] [Google Scholar]
- 32.Zenclussen AC, Sollwedel A, Bertoja AZ, Gerlof K, Zenclussen ML, et al. Heme oxygenase as a therapeutic target in immunological pregnancy complications. Int Immunopharmacol. 2005;5:41–51. doi: 10.1016/j.intimp.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, et al. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol. 2006;69:35–52. doi: 10.1016/j.jri.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez P, Guillen MI, Gomar F, Alcaraz MJ. Expression of heme oxygenase-1 and regulation by cytokines in human osteoarthritic chondrocytes. Biochem Pharmacol. 2003;66:2049–2052. doi: 10.1016/s0006-2952(03)00543-4. [DOI] [PubMed] [Google Scholar]
- 35.Soares MP, Lin Y, Sato K, Stuhlmeier KM, Bach FH. Accommodation. Immunol Today. 1999;20:434–437. doi: 10.1016/s0167-5699(99)01530-3. [DOI] [PubMed] [Google Scholar]
- 36.Tullius SG, Nieminen-Kelha M, Buelow R, Reutzel-Selke A, Martins PN, et al. Inhibition of ischemia/reperfusion injury and chronic graft deterioration by a single-donor treatment with cobalt-protoporphyrin for the induction of heme oxygenase-1. Transplantation. 2002;74:591–598. doi: 10.1097/00007890-200209150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Woo J, Iyer S, Mori N, Buelow R. Alleviation of graft-versus-host disease after conditioning with cobalt-protoporphyrin, an inducer of heme oxygenase-1. Transplantation. 2000;69:623–633. doi: 10.1097/00007890-200002270-00026. [DOI] [PubMed] [Google Scholar]
- 38.Barber A, Robson SC, Myatt L, Bulmer JN, Lyall F. Heme oxygenase expression in human placenta and placental bed: reduced expression of placenta endothelial HO-2 in preeclampsia and fetal growth restriction. Faseb J. 2001;15:1158–1168. doi: 10.1096/fj.00-0376com. [DOI] [PubMed] [Google Scholar]
- 39.Zenclussen AC, Joachim R, Hagen E, Peiser C, Klapp BF, et al. Heme oxygenase is downregulated in stress-triggered and interleukin-12-mediated murine abortion. Scand J Immunol. 2002;55:560–569. doi: 10.1046/j.1365-3083.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 40.Zenclussen AC, Lim E, Knoeller S, Knackstedt M, Hertwig K, et al. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. Am J Reprod Immunol. 2003;50:66–76. doi: 10.1034/j.1600-0897.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 41.Ihara N, Akagi R, Ejiri K, Kudo T, Furuyama K, et al. Developmental changes of gene expression in heme metabolic enzymes in rat placenta. FEBS Lett. 1998;439:163–167. doi: 10.1016/s0014-5793(98)01324-6. [DOI] [PubMed] [Google Scholar]
- 42.Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- 43.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe K, Tachibana M, Tanaka S, Furuoka H, Horiuchi M, et al. Heat shock cognate protein 70 contributes to Brucella invasion into trophoblast giant cells that cause infectious abortion. BMC Microbiol. 2008;8:212. doi: 10.1186/1471-2180-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci U S A. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeldovich VB, Robbins JR, Kapidzic M, Lauer P, Bakardjiev AI. Invasive extravillous trophoblasts restrict intracellular growth and spread of listeria monocytogenes. PLoS Pathog. 2011;7:e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charpentier E, Courvalin P. Antibiotic resistance in Listeria spp. Antimicrob Agents Chemother. 1999;43:2103–2108. doi: 10.1128/aac.43.9.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poros-Gluchowska J, Markiewicz Z. Antimicrobial resistance of Listeria monocytogenes. Acta Microbiol Pol. 2003;52:113–129. [PubMed] [Google Scholar]
- 49.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 50.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]