Abstract
Persistent organic pollutants such as halogenated aromatic hydrocarbons (HAHs) biomagnify in food webs and accumulate to high concentrations in top predators like odontocete cetaceans (toothed whales). The most toxic HAHs are the 2,3,7,8-substituted halogenated dibenzo-p-dioxins and furans, and non-ortho-substituted polychlorinated biphenyls (PCBs), which exert their effects via the aryl hydrocarbon receptor (AHR). Understanding the impact of HAHs in wildlife is limited by the lack of taxon-specific information about the relative potencies of toxicologically important congeners. To assess whether Toxic Equivalency Factors (TEFs) determined in rodents are predictive of HAH relative potencies in a cetacean, we used beluga and mouse AHRs expressed in vitro from cloned cDNAs to measure the relative AHR-binding affinities of ten HAHs from five different structural classes. The rank order of mean IC50s for competitive binding to beluga AHR was: TCDD<TCDF<PCB-126< PCB-169< PCB-77< PCB-81≪< PCB-156~PCB-128< PCB-105< PCB-118. The rank order of mean IC50s for binding to the mouse AHR was TCDD<TCDF< PCB-126< PCB-169< PCB-81< PCB-77< PCB-156≪ PCB-128~PCB-105~PCB-118. Ki values for binding of HAHs to beluga and mouse AHRs were highly correlated (r2= 0.96). Comparison of Ki values suggested that the beluga AHR had a higher affinity than the mouse AHR for most of the HAHs tested, consistent with the ~2-fold higher [3H]TCDD-binding affinity determined previously. These results are consistent with the World Health Organization mammalian TEFs for non- and mono-ortho PCB congeners. The comparatively high HAH binding affinities of the beluga AHR relative to those of an AHR from a dioxin-responsive mouse suggests that beluga, and perhaps cetaceans in general, may be particularly sensitive to the toxic effects of AHR agonists. Further study is warranted in order to more fully address this important question affecting protected and endangered species.
Keywords: AHR, TCDD, PCB, dioxin, risk assessment, structure-activity relationships, relative potencies, species-specific, beluga, cetacean
1. Introduction
Halogenated aromatic hydrocarbons (HAHs) and other persistent pollutants are ubiquitous in the marine environment. As top predators, cetaceans are exposed to particularly high levels of these lipophilic chemicals as they biomagnify in the food web (AMAP 1998). Reports have documented substantial levels of chemical burdens in tissues coincident with adverse health status in certain populations of cetaceans, including St. Lawrence beluga (De Guise et al. 1995), Puget Sound killer whales (Ross et al. 2000), the Mediterranean striped dolphin (Borrell et al. 1996) and the coastal Southeast and Gulf of Mexico bottlenose dolphin (Finklea et al. 2000). This epidemiological evidence, combined with some experimental evidence (Ross 2000) and the presence of high concentrations of polychlorinated biphenyls (PCBs) and other HAHs in marine mammals in general, has raised concern about the possible impact of these contaminants on marine mammal health (MMC 1999). However, attempts to assess the impact of HAHs on marine mammals are complicated by the inability to obtain direct experimental evidence pertaining to their sensitivity to chemicals of concern.
HAHs that can achieve a planar conformation exert their toxic effects via the aryl hydrocarbon receptor (AHR) (Schmidt and Bradfield 1996). Mechanistic studies of AHR-dependent toxicity have focused predominantly on 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most potent AHR agonist. However, TCDD is not the most prevalent HAH in the environment. HAHs found in environmental samples include the polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and PCBs, among others. Hundreds of HAH isomers and congeners exist, and these differ in toxic potency by orders of magnitude. Consequently, assessing the risk associated with exposure to mixtures of HAH is complicated by the need to account for these varying toxic potencies.
Toxic Equivalency is a means of integrating the toxicity estimates for mixtures of HAHs that bind to and cause toxicity via the AHR. The potency of AHR-binding compounds can be expressed relative to that of TCDD. In single experiments, these values are referred to as Relative Potencies (REPs). REP values from multiple experiments and endpoints are integrated to produce Toxic Equivalency Factors (TEFs) (van den Berg et al. 1998; van den Berg et al. 2006). TEFs are order of magnitude estimates of a compound’s toxic potency relative to the toxic potency of TCDD, and are derived from a large database of information on toxic and biochemical effects. TEFs are used to convert measured concentrations of HAHs to an expression of TCDD equivalents, or TEQ (Safe 1990). Because this has become an important and widely applied concept in environmental risk assessment, the World Health Organization (WHO) and affiliates have derived TEFs of AHR agonists for humans and mammals, and significant effort has been made to update and refine these to include the best available data on relative potencies (Ahlborg et al. 1994; van den Berg et al. 1998; van den Berg et al. 2006) combined with expert judgment that incorporates other mechanistic information. However, the TEFs for wildlife have not been updated since 1997 (van den Berg et al. 1998).
REPs contribute to the derivation of TEFs according to the type of endpoint measured and its assessed relevance to whole-animal toxicity. In general, REPs generated by measuring AHR-dependent, non-toxic endpoints tend to correlate with REPs for toxic endpoints, thus supporting the TEQ concept (Safe 1990). Several different endpoints can used to determine REPs. While in vivo toxicity is generally favored for use in the derivation of TEFs, in vitro endpoints, including AHR binding affinity, are also used, especially when information from in vivo effects is not available (Safe 1990; Haws et al. 2006; van den Berg et al., 2006; USEPA, 2008).
To circumvent the practical and ethical limitations on toxicological research in marine mammals, indirect approaches such as extrapolation of results from laboratory rodents have been proposed (Ross 2000). One approach to assessing the risk of HAHs in marine mammals involves the use of TEQs to express the cumulative toxic potential of HAH mixtures. The use of the TEQ approach to express marine mammal contaminant burdens is widespread (Berggrena et al. 1999; Jones et al. 1999; Minh et al. 2000; Ross et al. 2000; Watanabe et al. 2000; Wilson et al. 2007).
An important uncertainty is inherent in the use of TEFs derived from studies in laboratory rodents to calculate TEQs in marine mammal tissues. TEF values can vary among species, sometimes dramatically (Walker and Peterson 1991; Kennedy et al. 1996; USEPA 2001). There have been very few determinations of REPs for HAH congeners in wildlife (Walker and Peterson 1991; Kennedy et al. 1996), and AHR-dependent REPs have not been determined for any marine mammal. TEFs for mammals (including humans) have been derived almost exclusively from studies in laboratory rodents (Haws et al. 2006). Application of these TEF values to marine mammals assumes that structure-activity relationships for AHR agonists are similar between rodents and marine mammals, but this assumption has not yet been tested. If TEFs are to be a part of the standard language for assessing risk to cetacean populations, it would be desirable, at minimum, to have relative potency data that assess the validity of using “mammalian” (i.e. rodent) TEFs for cetaceans. However, in vivo studies of dose-response relationships for HAH toxicity or enzyme induction are not possible in cetaceans. Because of this, assessment of cetacean TEFs must rely exclusively on in vitro approaches.
The aim of this study was to determine REPs for HAHs binding to the beluga AHR. Although specific binding of [3H]TCDD to beluga AHR can be measured using beluga hepatic cytosol, the lability of AHR expressed in beluga tissue even under the best possible conditions of tissue preparation make tissue cytosols unreliable for determining binding constants (Jensen and Hahn 2001). We therefore utilized an approach involving in vitro expression from cloned cDNA. A competitive binding assay was optimized for beluga and mouse AHRs expressed in vitro from cDNAs (Burbach et al. 1992; Jensen and Hahn 2001) and this assay was used to determine the binding affinities (expressed as Ki) of ten representative HAHs relative to TCDD. The Ki values were used to infer the REPs for HAH binding to beluga and mouse AHRs. This is the first determination of a set of REPs for a marine mammal. These data support the use of mammalian TEFs for assessing HAH impacts in cetaceans.
2. Materials and Methods
2.1. Chemicals
2,3,7,8-Tetrachloro[1,6-3H]dibenzo-p-dioxin ([3H]TCDD) (specific activity 33.3 Ci/mmol, purity >97%) was purchased from Chemsyn Science Laboratories (Lenexa, KS). Unlabeled TCDD, TCDF and PCBs (IUPAC numbers 126, 169, 77, 81, 105, 118, 156, and 128) were purchased from Ultra Scientific (North Kingstown, RI).
The purity of each PCB congener as stated by the manufacturer was ≥99%. However, to determine whether the mono-ortho PCBs possessed traces of the highly potent AHR agonist PCB-126, the congeners were analyzed by comprehensive two-dimensional gas chromatography with flame ionization detection (GC×GC-FID) and mass spectral detection (GC×GC-MS). Compared to one-dimensional high-resolution gas chromatography, which often lacks the resolving power for separating mixtures of PCBs (Frame 1997), GC×GC separates and resolves at least an order of magnitude more compounds, has a much larger signal to noise ratio, and sorts compounds based on their chemical class. It therefore can provide highly refined inventories of PCBs and other organochlorines and pollutants in environmental samples (Reddy et al. 2002; Frysinger et al. 2003; Marriot et al. 2003; Korytár et al. 2006). For example, GC×GC analysis of PCBs is capable of separating non-ortho and mono-ortho PCBs from PCBs fully halogenated in the ortho position (Korytár et al. 2002). We analyzed the purity of the individual compounds (PCB-105, PCB-118, PCB-126, and PCB-156) with a particular interest in contamination by PCB-126. Detailed instrument settings and sample results are shown in the Appendix. Based on these analyses, the congeners employed in this study did not contain any other PCBs. Serial dilutions of the TCDD, TCDF, and PCBs were made in dimethylsulfoxide (DMSO) as described previously (Hahn et al. 1996).
2.2. In vitro expression of AHR
The mouse AHR expression construct (pSPORTmAHR, Ahb−1 allele) and human AHR expression construct (pSPORThAHR) were gifts from Dr. Christopher Bradfield (Burbach et al. 1992; Dolwick et al. 1993). The beluga AHR expression vector (pSP64belAHR) was described previously (Jensen and Hahn 2001).
AHR proteins were expressed using the SP6 Quick Transcription/Translation system (Promega) per the supplier’s instructions, with 2 μg DNA/reaction. A TNT reaction programmed with empty pSP64polyA vector (“unprogrammed lysate” or UPL) was used to determine non-specific binding (Jensen and Hahn 2001). For competitive binding assays, the reaction volumes were diluted with 8 parts MEEMDG buffer (35mM MOPS, 1mM EDTA, 5 mM EGTA, 0.02% NaN3, 20mM Na2MoO4, 10% (v:v) glycerol, 1mM DTT, pH 7.5 containing protease inhibitors (Jensen and Hahn 2001)).
2.3. Competitive binding assay
The hydroxyapatite (HAP) adsorption assay is a well-established method for measuring AHR binding in rodent liver cytosols (Gasiewicz and Neal 1982; Petrulis and Bunce 2000) and has also been used with AHR proteins expressed by in vitro transcription and translation (Coumailleau et al. 1995; Kim and Hahn 2002; Karchner et al. 2006). We optimized the HAP assay for use with TNT-expressed beluga and mouse AHR proteins (Jensen 2000). Optimization was attempted for the TNT-expressed human AHR as well, but detectable levels of specific binding were very low under these conditions, so the comparison with human AHR was not possible.
Briefly, diluted TNT reactions (150 μl) were added to 16×100-mm glass tubes containing [3H]TCDD (1–2 nM nominal concentration) and one of 10 concentrations of unlabeled HAH competitor (0–133 μM, 1% DMSO final) in duplicate. Incubations were vortexed thoroughly and kept on ice for 8–16 hours. For these experiments, 1–2 nM [3H]TCDD was chosen because it was at least two times the Kd for both beluga and mouse AHRs. In each experiment, the actual concentration of radioligand was determined by liquid scintillation counting of an aliquot of each incubation mixture; actual concentrations are listed in table and figure legends.
Following the incubation on ice, tubes were vortexed again, and a 25 μl aliquot of each incubation mixture was taken for liquid scintillation counting. Aliquots (150 μl) of 10% DNA grade HAP (Bio-Rad) in MEEDMG (maintained in suspension by stirring) were distributed to 13×100-mm glass culture tubes and 50 μl from each incubation tube were added to duplicate HAP tubes and mixed by gently vortexing until the HAP pellet was fully resuspended. Tubes were placed on ice for 30 min, with resuspension by vortexing every 10 minutes. After adding 500 μl of wash solution (MEEDMG with 0.1% Tween 20), the tube was vortexed and spun at 1000 rpm (approx. 300 g) for 5 min at room temperature. The supernatant was removed by aspiration, and the pellet was washed two more times as above. After the last wash, the pellet was resuspended in 500 μl of 95% ethanol and transferred to a 7ml scintillation vial. Two ml of Scintiverse II was added, and the radioactivity in dpm was measured on a Beckman LS5000TD liquid scintillation counter.
Competitive binding curves were obtained for ten HAHs: 2,3,7,8-TCDD, 2,3,7,8-TCDF, PCB-126, PCB-169, PCB-77, PCB-81, PCB-105, PCB-118, PCB-156, and PCB-128. Three sets of competitive inhibition curves were generated for each compound representing independent experiments and separate fresh batches of translated AHR proteins.
2.4. Binding curve analysis
Specific binding (SB) was defined as the difference between total binding (incubations containing [3H]TCDD) and nonspecific binding. Non-specific binding (NSB) was measured by incubation of unprogrammed TNT lysate with [3H]TCDD and treatment with HAP as above. The binding detected using UPL was the same as the minimum levels of binding detected when 200-fold excess unlabeled TCDF is used, as described earlier (Jensen and Hahn 2001). Specific binding of [3H]TCDD in the presence of competitor was expressed as fractional specific binding:
| (1) |
where SBx is SB in the presence of a given concentration of compound x and SBmax is the SB of [3H]TCDD in the absence of competitor. The fractional SB data were analyzed using the following model for one-site competition:
| (2) |
Top is the fraction of [3H]TCDD specific binding in the absence of competitor, Bottom is the fraction of [3H]TCDD binding observed when specific binding sites are occupied with unlabeled competitor, X is the log of the concentration of competitor in nM, and Y is the fractional SB at each competitor concentration. Data were fit by unweighted non-linear regression using Prism version 2.0 for the Macintosh (GraphPad Software, San Diego).
Analyses were carried out both by “fixing” the top and bottom of the curves to 1.0 and 0 fractional SB, respectively, as well as by allowing the program to determine the maxima and minima from provided data. The rationale for fixing the top and bottom of the curves is as follows. Because data points are expressed as fractional specific binding, the only way that a one-site competition model can be correct is if the actual minimum of the curve is zero (no specific binding, full displacement of [3H]TCDD by competitor). In practice, however, the actual concentration of competitor needed to displace >95% of [3H]TCDD specific binding may be greater than the highest concentrations tested, and perhaps beyond the limits of solubility. By allowing the model to fit the data points assuming that 100% inhibition is possible, the equation is able to predict an IC50 that is based on the data points yet is not underestimated because of solubility limitations and the resulting false minima. Comparing the IC50 values for each compound determined by both methods showed that, in general, the IC50s derived from both sets of curve fitting parameters were in close agreement for compounds that compete well for AHR binding (e.g. TCDD, 126, 81). For compounds that did not compete as well for [3H]TCDD binding (e.g. 105, 118) the fitted curve minimum was far from 0%, and the IC50s calculated from the “fixed” fits tended to be higher than those estimated from the “unfixed” fits (Jensen 2000). In order to best compare the binding of HAH compounds, the data from the “fixed” analysis were used throughout.
The Ki for each competitor was calculated from the IC50 and the Kd for [3H]TCDD binding using the method of Cheng and Prusoff (1973):
| (3) |
The Kd values used for the expressed beluga, and mouse AHRs were those reported previously: 0.43 nM ± 0.16 nM (beluga) and 0.68 ± 0.23 nM (mouse) (Jensen and Hahn 2001).
The one-site competition model (equation (2)) assumes a Hill slope of 1. To test the sensitivity of our results to this assumption, we also fit the data using a sigmoid curve with variable slope. Ki values obtained from these fits were almost identical to those obtained from the one-site competition model.
3. Results and discussion
In order to begin to establish an experimental basis for the application of the TEQ concept to cetaceans and to further characterize the beluga AHR, we performed competitive inhibition studies to measure the relative affinities and infer ligand-binding REPs for HAH binding to the beluga AHR. For comparison, a high affinity AHR from a strain of mouse that is sensitive to the toxic effects of AHR agonists (mouse Ahb−1 allele) was included in the analysis. This is the first determination of HAH REPs for a marine mammal, and the most comprehensive set of REPs derived for in vitro HAH binding to mouse AHR.
3.1. Competitive binding of beluga and mouse AHRs to various HAH
To compare the HAH structure-AHR binding relationships of beluga and mouse AHRs, the HAP assay was used to measure the relative abilities of HAHs to compete for binding to in vitro expressed AHRs from these two species. Five different structural classes of HAH were represented: 2,3,7,8-TCDD (PCDD), 2,3,7,8-TCDF (PCDF), PCB-126, -169, -77, and -81 (non-ortho PCBs), PCB-105, -118, and -156 (mono-ortho PCBs), and PCB-128 (di-ortho PCBs). All of these HAH classes are found in the environment and detected in marine mammal tissues, including beluga (Martineau et al. 1987; Vorkamp et al. 2004).
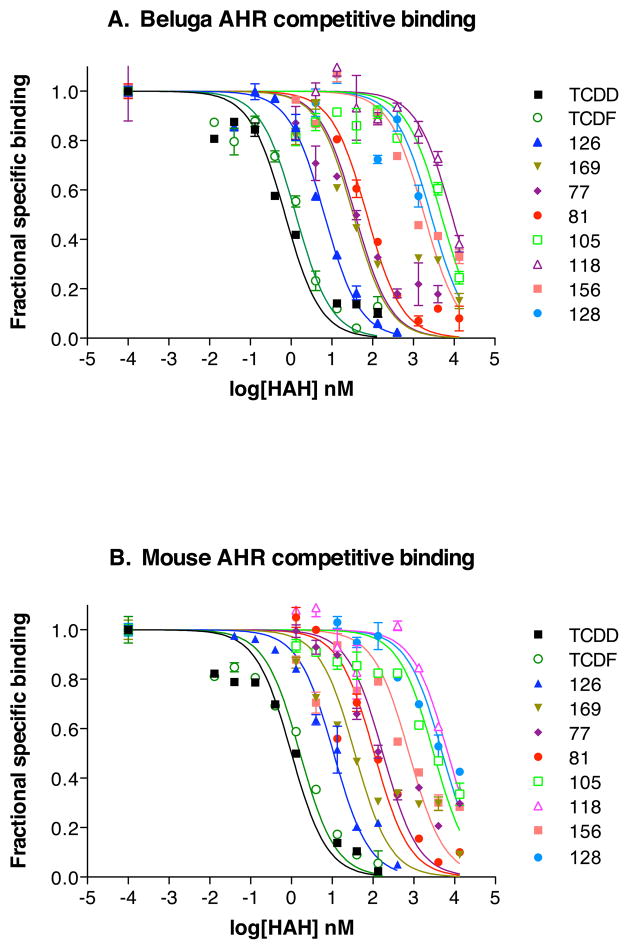
Representative inhibition curves generated for these compounds for beluga and mouse AHRs are shown in Figure 1. All of the HAHs tested were able to displace [3H]TCDD from the beluga AHR (Figure 1). The data were fit to a one-site competition model (Equation 2) as described in Methods. The IC50 values spanned 5 orders of magnitude (Table 1). The rank order of mean IC50s for inhibition of [3H]TCDD binding to beluga AHR was: TCDD<TCDF<PCB-126< PCB-169< PCB-77< PCB-81≪< PCB-156~PCB-128< PCB-105< PCB-118. The rank order of mean IC50s for inhibition of [3H]TCDD binding to the mouse AHR was TCDD<TCDF< PCB-126< PCB-169< PCB-81< PCB-77< PCB-156≪ PCB-128~PCB-105~PCB-118 (Table 1).
Figure 1. Competitive binding curves for mouse and beluga AHR for various HAH.
Inhibition of binding of [3H]TCDD (2 nM nominal; 1.6 nM measured) to (A) beluga and (B) mouse AHRs by various HAH was determined with the HAP assay. Curves were fit to a one-site competition model. IC50s were calculated for ten HAHs: 2,3,7,8-TCDD, 2,3,7,8-TCDF, PCB-126, PCB-169, PCB-77, PCB-81, PCB-105, PCB-118, PCB-156, and PCB-128. Bars represent 1 standard deviation.
Table 1.
Summary of IC50s, Ki, and relative potencies determined for beluga and mouse AHRs.
| HAH | Chlorination pattern | Beluga | Mouse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean IC50 | Mean Ki | 95% confidence interval for Ki | Relative potency (from mean Ki) | Mean IC50 (nM) | Mean Ki (nM) | 95% confidence interval for Ki | Relative potency (from mean Ki) | ||
| TCDD | 2,3,7,8 | 0.47 | 0.10 | 0.05–0.22 | 1.00 | 1.21 | 0.36 | 0.25–0.51 | 1.00 |
| TCDF | 2,3,7,8 | 1.35 | 0.33 | 0.24–0.46 | 0.31 | 1.62 | 1.1 | 0.21–5.8 | 0.32 |
| 126 | 3,3′,4,4′,5 | 8.1 | 1.6 | 1.3–2.1 | 0.063 | 12.6 | 4.6 | 3.1–6.8 | 0.078 |
| 169 | 3,3′,4,4′,5,5′ | 25.1 | 7.5 | 3.9–14.7 | 0.014 | 63.1 | 33 | 8.3–132 | 0.011 |
| 77 | 3,3′,4,4′ | 35.5 | 7.8 | 7.3–8.3 | 0.013 | 224 | 94 | 47.6–185 | 0.0038 |
| 81 | 3,4,4′,5 | 89.1 | 19 | 12.9–26.8 | 0.0055 | 112 | 34 | 30–38 | 0.011 |
| 105 | 2,3,3′,4,4′ | 3550 | 567 | 292–1100 | 0.00018 | 5010 | 1620 | 927–2830 | 0.00022 |
| 118 | 2,3′,4,4′,5 | 6310 | 1440 | 930–2220 | 0.000071 | 6310 | 3190 | 930–10900 | 0.00011 |
| 156 | 2,3,3′,4,4′,5 | 1590 | 309 | 196–485 | 0.00033 | 1000 | 284 | 186–433 | 0.0013 |
| 128 | 2,2′,3,3′,4,4′ | 2240 | 384 | 246–600 | 0.00027 | 6310 | 1420 | 826–2450 | 0.00025 |
Mean IC50 was determined from the two experiments conducted at 2 nM [3H]TCDD (nominal concentration; measured concentration was 1.6 nM for both experiments).
Mean Ki was determined from all three data sets, including two experiments at 2 nM [3H]TCDD (1.6 nM measured) and one experiment at 1 nM [3H]TCDD (0.9 nM measured). The 95% confidence intervals for Ki values were calculated from the mean and SD of all three experiments.
Inhibition constants (Ki) were calculated based on the IC50s, the Kd values for binding of [3H]TCDD to the beluga and mouse AHRs (Jensen and Hahn 2001), and the measured [3H]TCDD concentration in the incubations (Equation 3). Ki values are specific for the receptor and competitor, and should be independent of radioligand incubation concentrations. Therefore, Ki values can be compared across experiments conducted under similar conditions. The Ki values were not sensitive to the choice of model (constant or variable slope) used to fit the data (see Methods).
The mean Ki values for TCDD were 0.10 nM (95% confidence interval: 0.05–0.22 nM) for the beluga AHR and 0.36 nM (95% confidence interval: 0.25–0.51 nM) for the mouse AHR (Table 1). These are in reasonable agreement with the Kd values determined for TCDD binding directly using a saturation binding assay, 0.43 nM for beluga AHR, and 0.68 nM for mouse AHR (Jensen and Hahn 2001). 2,3,7,8-TCDF also had a very high affinity for both AHRs. All of the non-ortho PCBs had binding affinities that were less than those of TCDD and TCDF, but greater than those of the mono or di-ortho PCBs. Of these four non-ortho PCBs, PCB-126 clearly had the highest affinity for both AHRs. The Ki values for PCB 77, 169, and 81 were very similar to each other for both beluga and mouse AHR.
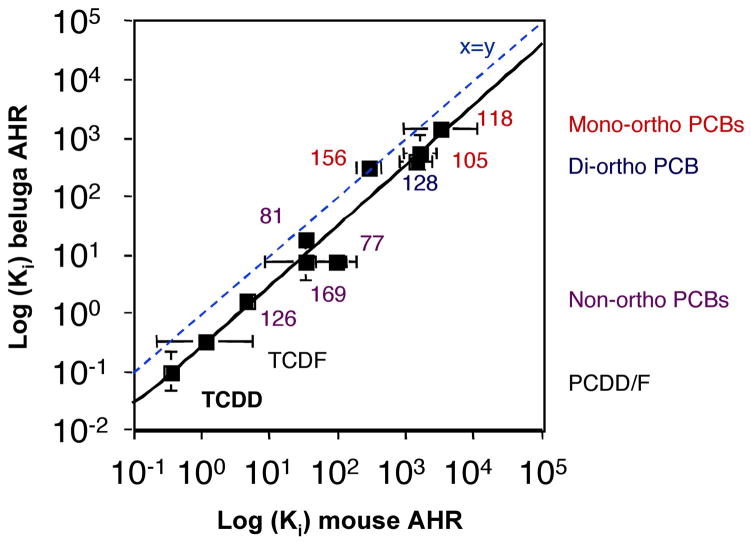
The Ki values calculated for HAH inhibition of [3H]TCDD binding to beluga and mouse AHRs shared a very similar rank order overall and within HAH structure groups (Table 1, Figure 2). These in turn were very similar to the rank order of IC50 values determined for inhibition of [3H]TCDD binding in rat hepatic cytosol (Bandiera et al. 1982). There was a strong correlation between log Ki values obtained for beluga and mouse AHRs (r2= 0.96)(Figure 2). The similarity of the structure-binding relationships of the beluga AHR compared with those of rodent AHRs suggests that the structural requirements for high affinity ligand binding are very similar among these mammalian AHRs.
Figure 2. Log plot of beluga and mouse AHR Ki for various HAH.
Log[Ki] derived from competitive binding of [3H]TCDD to beluga and mouse AHR are plotted for each HAH examined. The equation of the line for log mouse Ki vs log beluga Ki is y = 1.03x - 0.54. The dashed line indicates y=x. Bars represent 95% confidence interval.
In addition to illustrating the similar structure-binding relationships for beluga and mouse AHRs, Fig. 2 shows that the Ki values for the beluga AHR were consistently lower than those for the mouse AHR (all points but one fall below the line of equal Ki values); this is unlikely to have occurred by chance (p~0.01, binomial test). To determine whether this difference was merely a reflection of the species-specific differences in the [3H]TCDD Kd values used in equation 3 (0.43 nM and 0.68 nM for beluga and mouse AHRs, respectively), we repeated the Ki calculation using the mean of the beluga and mouse Kd values (0.55 nM) rather than the species-specific Kd values. Ki values for the beluga AHR remained consistently lower than those for the mouse AHR. Although it will require additional experiments to confirm, this result suggests that, as compared to the mouse AHR, the beluga AHR may exhibit a somewhat greater affinity for a variety of AHR ligands.
For the beluga and mouse AHRs, the three mono-ortho and the single di-ortho PCBs examined had similar affinities that were much lower than the affinities of the coplanar HAHs. The di-ortho PCB 128 had a binding affinity that was similar to or greater than some of the mono-ortho PCBs. Binding studies with rat cytosols have shown that di-ortho PCBs tend to have IC50s that are one to two orders of magnitude greater than the mono-ortho congeners (Bandiera et al. 1982). In this study, the ability of PCB 128 to inhibit [3H]TCDD binding was not an artifact of curve fitting to few points, because the PCB 128 curves reached 90% inhibition for beluga AHR and 80% for mouse AHR. Di-ortho PCBs are known to act as partial agonists or antagonists of the AHR and can exert toxic effects via non-AHR pathways; because of this, they have been eliminated from the TEF database (van den Berg et al. 2006).
The HAP competitive binding assay described here was also used in attempts to derive HAH IC50 values for the in vitro-expressed human AHR. Despite several experiments under various conditions, binding of [3H]TCDD to the human AHR was not sufficiently stable in this assay to allow determination of [3H]TCDD SB or its displacement by competitors (Jensen 2000). This is consistent with a previous report that the HAP assay was not effective in measuring [3H]TCDD binding to AHR from human placenta (Nakai and Bunce 1995). Specific binding of [3H]TCDD to in vitro-expressed human AHR can be measured using other assays (Ema et al. 1994; Jensen and Hahn 2001; Fan et al. 2009), suggesting that the difficulty lies in the association of the human AHR with HAP or in the stability of the AHR-[3H]TCDD complex to buffer washes, rather than instability of the human AHR protein under these conditions. Similar results have been seen with AHRs from common tern (Karchner et al. 2006). Additional studies to assess the relevance of rodent-derived TEFs for humans are warranted.
3.2. Relating beluga relative potencies to mammalian Toxic Equivalency Factors
We used competitive binding assays to infer structure-activity relationships for HAHs acting through the beluga AHR. Relative potencies for AHR binding were determined from the mean Ki values for each HAH (Table 1). The REPs span 5 orders of magnitude in the beluga (1.0 to 0.00007), and 4 orders of magnitude for the mouse (1.0 to 0.0002). The compound with the lowest REP for both beluga and mouse was mono-ortho PCB-118.
It is informative to compare the REPs derived from competitive binding of HAHs to beluga, mouse, and rat AHRs (Table 2) to WHO-06 TEFs. For the most potent AHR agonists (TCDD, TCDF, 126,169), REPs derived from AHR binding reflect TEFs derived from broad toxicity assessments quite well. For non-ortho PCBs 77 and 81, and mono- and di-ortho PCBs, however, the binding REPs vary across species and do not always correspond as well to the suggested mammalian TEF. For example, PCB 81 has higher binding REP in beluga (14-fold) and mouse (28-fold) compared to rat, so it is possible that the TEF might also be an order of magnitude higher in these species. On the other hand, PCB 118 has a much lower REP in beluga (15-fold) and mouse (10-fold) compared to rat. These species differences underscore the importance of continuing to generate cetacean-specific REPs with a variety of molecular endpoints.
Table 2.
Relative potencies (REPs) for AHR binding and human Toxic Equivalency Factors (TEFs).
| HAH | Chlorination pattern | Beluga REPs for AHR binding | Mouse REPs for AHR binding | Rat REPs for AHR bindinga | 2006 WHO TEFs for humans b |
|---|---|---|---|---|---|
| TCDD | 2, 3, 7, 8, | 1.00 | 1.00 | 1.0 | 1 |
| TCDF | 2, 3, 7, 8, | 0.31 | 0.32 | Not done | 0.1 |
| 126 | 3, 3′, 4, 4′, 5 | 0.063 | 0.078 | 0.08 | 0.1 |
| 169 | 3, 3′, 4, 4′, 5, 5′ | 0.014 | 0.011 | Not done | 0.03 |
| 77 | 3, 3′, 4, 4′ | 0.013 | 0.0038 | 0.01 | 0.0001 |
| 81 | 3, 4, 4′, 5 | 0.0055 | 0.011 | 0.0004 | 0.0003 |
| 105 | 2, 3, 3′, 4, 4′ | 0.00018 | 0.00022 | 0.0023 | 0.00003 |
| 118 | 2, 3′, 4, 4′, 5 | 0.000071 | 0.00011 | 0.0011 | 0.00003 |
| 156 | 2, 3, 3′, 4, 4′, 5 | 0.00033 | 0.0013 | 0.0014 | 0.00003 |
| 128 | 2, 2′, 3, 3′, 4, 4′ | 0.00027 | 0.00025 | Not done | no TEF |
calculated from IC50 reported in Bandiera et al., 1982
reported in van den Berg et al., 2006
3.3 Limitations of using competitive binding affinities to infer relative potencies
Relative potencies inferred from measures of relative AHR binding affinities have long been included among the endpoints that can contribute to the estimation of TEFs, especially when information from in vivo effects is not available (Safe 1990; Haws et al. 2006; van den Berg et al., 2006; USEPA, 2008). A limitation of using relative AHR-binding affinities to infer REPs is that competitive binding assays do not distinguish full agonists from partial agonists or antagonists. For example, in rodents, di-ortho PCBs bind to the AHR with low affinity but generally do not elicit in vivo toxic or biochemical effects (Goldstein and Safe 1989). More recent studies have shown that di-ortho PCBs can antagonize downstream responses of more potent AHR agonists (Petrulis and Bunce 1999; Hestermann et al. 2000). PCB-128, the di-ortho PCB used in our study, is among the congeners shown to act as a competitive antagonist (Aarts et al. 1995; Hestermann et al. 2000; Suh et al. 2003). Thus, although we can calculate an AHR-binding REP for this compound (Table 2), it would be inappropriate to assign a TEF based on these data. Assignment of TEFs must incorporate all available information, including mechanistic understanding obtained from studies demonstrating antagonistic effects. In addition to the caveats about di-ortho PCBs, some mono-ortho PCBs have partial AHR agonist activity, which also results in antagonistic effects at high ratios of partial agonist to full agonist (Hestermann et al. 2000; Howard et al. 2010). The latest WHO panel acknowledged the potential importance of this factor in the determining the risk of mixtures (van den Berg et al. 2006).
An additional limitation of our data, and of all data used to infer relative potencies, is that the single REP values determined for each compound do not reflect the uncertainties inherent in the measurements of the endpoints (Starr et al. 1999). We might consider the variation around our Ki values as an indication of the uncertainties associated with the calculated REPs. The coefficients of variation associated with the beluga Ki values averaged 35% with a range of 6–59%. With this degree of variability, our REP values can be considered estimates within an order-of-magnitude or less of the actual relative potencies inferred from this endpoint.
3.4 Implications for sensitivity of beluga to HAHs
Despite the important limitation noted above, the similarity in the structure-activity relationships observed for HAH binding to the beluga and mouse AHRs suggests that the mechanisms of toxicity observed in rodent models, and TEF values determined in those models, are relevant to beluga and likely to other cetaceans as well. The Ki values and REPs calculated for the binding of HAHs to the beluga AHR suggest that some of these compounds can activate the beluga AHR and cause downstream responses such as CYP1A induction and toxicity. Consistent with this, in a study of stranded beluga, hepatic CYP1A protein levels correlated well with the concentrations of non-ortho and mono-ortho PCBs in blubber (White et al. 1994). In addition, several studies have shown relative depletion of coplanar HAHs among the suite of HAHs detected in cetacean tissues, suggesting a high, possibly induced, capacity for CYP1A-dependent metabolism in cetaceans (Tanabe et al. 1988; Boon et al. 1997). This and other evidence for CYP1A inducibility in cetaceans (Godard et al 2004; Garrick et al 2006), along with the demonstration of high affinity AHR binding of various HAHs shown here, confirms the presence of a complete AHR signaling cascade in cetaceans.
An important factor that can be easily overlooked in discussions of species-specific relative potencies is absolute differences in sensitivity. Beyond structure-activity relationships, another potentially important observation from our results is that the absolute binding affinities of HAH for the beluga AHR were consistently 2- to 3-fold higher than those for the mouse AHR (Figure 2, Table 1). This is especially true for TCDD and PCBs 126, 77, 81, and 128, for which the 95% confidence intervals for the Ki values do not overlap those of mouse AHR. (An exception to this pattern was PCB 156, which had nearly identical Ki values in both species.) Thus, the beluga AHR has a higher affinity than mouse AHR for most of the HAHs tested; this is consistent with the ~2-fold higher binding affinity of [3H]TCDD for the beluga AHR vs. the mouse AHR as determined by saturation binding (Jensen and Hahn 2001). Such differences in AHR affinity for TCDD may predict species- or strain-differences in sensitivity to TCDD toxicity. For example, differences in absolute TCDD binding affinities among mouse AHR alleles in vitro correspond with differences between strains in sensitivity to TCDD effects (Poland et al. 1994). Similarly, a 7-fold difference between bird species in AHR binding affinity for TCDD corresponds to an 80- to 250-fold difference in sensitivity to toxicity in vivo (Karchner et al. 2006). Thus, for two species that differ in overall sensitivity to AHR ligands, the same mixture containing a particular level of TEQ might be more toxic (potent) in one species (e.g. beluga) compared to the other (e.g. mouse), even if TEF values do not differ substantially between the two species. One caveat concerning this conclusion is that although low affinity AHRs are always associated with reduced sensitivity, possession of a high-affinity AHR does not guarantee high sensitivity. Additional studies will be needed to explore other factors that may influence the sensitivity of beluga and other cetaceans to these chemicals.
Our studies utilized AHR proteins expressed by in vitro transcription and translation to measure AHR-binding affinities and infer relative potencies. More detailed receptor-binding experiments might be possible using systems for expressing higher levels of AHR (Ramadoss and Perdew 2004; Fan et al. 2009). In addition, transfection assays to measure transcriptional activation by ligand-bound AHR might be used to obtain a more direct assessment of agonist activity (Karchner et al. 2006). Finally, the assessment of ligand potency in cell cultures may be useful for species such as cetaceans (Carvan et al. 1994; Garrick et al. 2006) and humans (Silkworth et al. 2005) that cannot be studied in vivo.
4. Conclusion
These results support the application of the WHO mammalian TEFs for coplanar and mono-ortho HAHs to belugas and likely other cetaceans. Together with available field and in vitro data, the relatively high binding affinity of the beluga AHR for HAHs suggests that beluga and perhaps cetaceans in general may have greater sensitivity to HAHs than that predicted by extrapolation from experiments in rodents. Studies that examine other endpoints in cetacean-specific cell culture and in vitro systems, such as those described by Godard et al. (2004) and Garrick et al. (2006), will be important for further addressing questions of relative sensitivity and for refining the application of TEFs to cetaceans. Finally, these results demonstrate the value of molecular and in vitro approaches for addressing toxicological questions in protected species for which direct testing is impossible.
Supplementary Material
Acknowledgments
This project was funded in part by the NOAA National Sea Grant College Program Office, Department of Commerce, under Grant No. NA46RG0470, Woods Hole Oceanographic Institution (WHOI) Sea Grant Project No. R/B-137 and Grant No. NA86RG0075, WHOI Sea Grant Project No. R/B-151, and by NIH grant R01ES006272.
We thank Dr. C. Bradfield for providing mouse and human AHR clones and two anonymous reviewers for helpful comments. This research was supported in part by the NOAA National Sea Grant College Program Office, Department of Commerce, under Grant No. NA46RG0470, Woods Hole Oceanographic Institution (WHOI) Sea Grant Project No. R/B-137 and Grant No. NA86RG0075, WHOI Sea Grant Project No. R/B-151 and by NIH grant R01ES006272. The U.S. Government is authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon.
Abbreviations used
- AHR
aryl hydrocarbon (Ah) receptor
- ARNT
AHR nuclear translocator
- bHLH
basic-helix-loop-helix
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TCDF
tetrachlorodibenzofuran
- PCB
polychlorinated biphenyl
- TEF
Toxic Equivalency Factor
- TEQ
TCDD Equivalent
- REP
Relative Potency
- HAH
Halogenated Aromatic Hydrocarbon
Footnotes
The authors declare they have no competing financial interests.
References
- Aarts JM, Denison MS, Cox MA, Schalk MA, Garrison PM, Tullis K, de Haan LH, Brouwer A. Species-specific antagonism of Ah receptor action by 2,2′,5,5′-tetrachloro- and 2,2′,3,3′4,4′-hexachlorobiphenyl. Eur J Pharmacol. 1995;293:463–474. doi: 10.1016/0926-6917(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Ahlborg UG, Becking GC, Birnbaum L, Brouwer A, Derks H, Feeley M, Golor G, Hanberg A, Larsen JC, Liem AK, Safe S, Schlatter C, Waern F, Younes M, Yrjanheikki E. Toxic equivalency factors for dioxin-like PCBs. Chemosphere. 1994;28:1049–1067. [Google Scholar]
- AMAP. AMAP Assessment Report: Arctic Pollution Issues. Oslo, Norway: 1998. [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Berggrena P, Ishaq R, Zebuhr YCN, Bandh C, Broman D. Patterns and levels of organochlorines (DDTs, PCBs, non-ortho PCBs and PCDD/Fs) in male harbor porpoises (Phocoena phocoena) from the Baltic Sea, the Kattegat-Skagerrak Seas and the West coast of Norway. Mar Pollut Bull. 1999;38:1070–1084. [Google Scholar]
- Boon JP, Vandermeer J, Allchin CR, Law RJ, Klunsoyr J, Leonards PEG, Spliid H, Storrhansen E, McKenzie C, Wells DE. Concentration-dependent changes of PCB patterns in fish-eating mammals: Structural evidence for induction of cytochrome P450. Environ Contam Toxicol. 1997;33:298–311. doi: 10.1007/s002449900257. [DOI] [PubMed] [Google Scholar]
- Borrell A, Aguilar A, Corsolini S, Focardi S. Evaluation of toxicity and sex-related variation of PCB levels in Mediterranean striped dolphins affected by an epizootic. Chemosphere. 1996;32:2359–2369. doi: 10.1016/0045-6535(96)00143-9. [DOI] [PubMed] [Google Scholar]
- Burbach KM, Poland A, Bradfield CA. Cloning of the Ah receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvan MJ, III, Santostefano M, Safe S, Busbee DL. Characterization of a bottlenose dolphin (Tursiops truncatus) kidney epithelial cell line. Mar Mammal Sci. 1994;10:52–69. [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Coumailleau P, Poellinger L, Gustafsson JA, Whitelaw ML. Definition of a minimal domain of the dioxin receptor that is associated with Hsp90 and maintains wild type ligand binding affinity and specificity. J Biol Chem. 1995;270:25291–25300. doi: 10.1074/jbc.270.42.25291. [DOI] [PubMed] [Google Scholar]
- De Guise S, Martineau D, Beland P, Fournier M. Possible mechanisms of action of environmental contaminants on St. Lawrence beluga whales (Delphinapterus leucas) Environ Health Perspect. 1995;103(Suppl 4):73–77. doi: 10.1289/ehp.95103s473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993;44:911–917. [PubMed] [Google Scholar]
- Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- Fan MQ, Bell AR, Bell DR, Clode S, Fernandes A, Foster PM, Fry JR, Jiang T, Loizou G, MacNicoll A, Miller BG, Rose M, Shaikh-Omar O, Tran L, White S. Recombinant expression of aryl hydrocarbon receptor for quantitative ligand-binding analysis. Anal Biochem. 2009;384:279–287. doi: 10.1016/j.ab.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finklea B, Miller G, Busbee D. Polychlorinated biphenyl residues in blubber of male Atlantic bottlenose dolphins (Tursiops truncatus) that stranded and died at Matagorda Bay. Bull Environ Contam Toxicol. 2000;64:323–332. doi: 10.1007/s001280000003. [DOI] [PubMed] [Google Scholar]
- Frame G. A collaborative study of 209 PCB congeners and 6 Aroclors on 20 different HRGC columns. 1 Retention and coelution database Fresenius. J Anal Chem. 1997;357:701–713. [Google Scholar]
- Frysinger GS, Gaines RB, Xu L, Reddy CM. Resolving the unresolved complex mixture in petroleum-contaminated sediments. Environ Sci Technol. 2003;37:1653–1662. doi: 10.1021/es020742n. [DOI] [PubMed] [Google Scholar]
- Garrick RA, Woodin BR, Wilson JY, Middlebrooks BL, Stegeman JJ. Cytochrome P4501A is induced in endothelial cell lines from the kidney and lung of the bottlenose dolphin, Tursiops truncatus. Aquat Toxicol. 2006;76:295–305. doi: 10.1016/j.aquatox.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Neal RA. The examination and quantitation of tissue cytosolic receptors for 2,3,7,8-tetrachlorodibenzo-p-dioxin using hydroxylapatite. Anal Biochem. 1982;124:1–11. doi: 10.1016/0003-2697(82)90212-3. [DOI] [PubMed] [Google Scholar]
- Godard C, Smolowitz R, Wilson J, Payne R, Stegeman J. Induction of cetacean cytochrome P4501A1 by β-naphthoflavone exposure of skin biopsy slices. Toxicol Sci. 2004;80:268–275. doi: 10.1093/toxsci/kfh124. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, Safe S. Mechanism of action and structure-activity relationships for the chlorinated dibenzo-p-dioxins and related compounds. In: Kimbrough RD, Jensen AA, editors. Halogenated biphenyls, terphenyls, napthalenes, dibenzodioxins, and related products. Elsevier Science Publishers, Biomedical Division; Amsterdam: 1989. pp. 239–293. [Google Scholar]
- Hahn ME, Woodward BL, Stegeman J, Kennedy SW. Rapid assessment of induced cytochrome P4501A (CYP1A) protein and catalytic activity in fish hepatoma cells grown in multi-well plates. Environ Toxicol Chem. 1996;15:582–591. [Google Scholar]
- Haws LC, Su SH, Harris M, Devito MJ, Walker NJ, Farland WH, Finley B, Birnbaum LS. Development of a refined database of mammalian relative potency estimates for dioxin-like compounds. Toxicol Sci. 2006;89:4–30. doi: 10.1093/toxsci/kfi294. [DOI] [PubMed] [Google Scholar]
- Hestermann EV, Stegeman JJ, Hahn ME. Relative contributions of affinity and intrinsic efficacy to aryl hydrocarbon receptor ligand potency. Toxicol Appl Pharmacol. 2000;168:160–172. doi: 10.1006/taap.2000.9026. [DOI] [PubMed] [Google Scholar]
- Howard GJ, Schlezinger JJ, Hahn ME, Webster TF. Generalized Concentration Addition predicts joint effects of aryl hydrocarbon receptor agonists with partial agonists and competitive antagonists. Environ Health Persp. 2010;118:666–672. doi: 10.1289/ehp.0901312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BA. Biology, vol PhD. Woods Hole Oceanographic Institution/Massachusetts Institute of Technology; Woods Hole, MA/Boston, MA: 2000. Ph.D. Thesis: Characterization of an aryl hydrocarbon receptor from a cetacean: an approach for assessing contaminant susceptibility in protected species. [Google Scholar]
- Jensen BA, Hahn ME. cDNA cloning and characterization of a high affinity aryl hydrocarbon receptor in a cetacean, the beluga, Delphinapterus leucas. Toxicol Sci. 2001;64:41–56. doi: 10.1093/toxsci/64.1.41. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hannah DJ, Buckland SJ, van Maanen R, Leathem SV, Dawson S, Slooten E, van Helden A, Donoghue M. Polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls in New Zealand cetaceans. J Cetacean Res Manag. 1999;1(special issue):157–167. [Google Scholar]
- Karchner SI, Franks DG, Kennedy SW, Hahn ME. The molecular basis for differential dioxin sensitivity in birds: role of the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2006;103:6252–6257. doi: 10.1073/pnas.0509950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SW, Lorenzen A, Jones SP, Hahn ME, Stegeman JJ. Cytochrome P4501A induction in avian hepatocyte cultures: a promising approach for predicting the sensitivity of avian species to toxic effects of halogenated aromatic hydrocarbons. Toxicol Appl Pharmacol. 1996;141:214–230. doi: 10.1006/taap.1996.0278. [DOI] [PubMed] [Google Scholar]
- Kim EY, Hahn ME. cDNA cloning and characterization of an aryl hydrocarbon receptor from the harbor seal (Phoca vitulina): A biomarker of dioxin susceptibility? Aquat Toxicol. 2002;58:57–73. doi: 10.1016/s0166-445x(01)00221-1. [DOI] [PubMed] [Google Scholar]
- Korytár P, Haglund P, de Boer J, Brinkman U. Comprehensive two-dimensional gas chromatography for the analysis of organohalogenated micro-contaminants. Trends Anal Chem. 2006;25:373–396. [Google Scholar]
- Korytár P, Leonards P, De Boer P, Brinkman U. High-resolution separation of polychlorinated biphenyls by comprehensive two-dimensional gas chromatography. J Chromatogr. 2002;958:203–218. doi: 10.1016/s0021-9673(02)00327-8. [DOI] [PubMed] [Google Scholar]
- Marriot P, Haglund P, Ong R. A review of environmental toxicant analysis by using multidimensional gas chromatography and comprehensive GC. Clin Chim Acta. 2003;328:1–19. doi: 10.1016/s0009-8981(02)00382-0. [DOI] [PubMed] [Google Scholar]
- Martineau D, Beland P, Desjardins C, Lagace A. Levels of organochlorine chemicals in tissues of beluga whales (Delphinapterus leucas) from the St. Lawrence Estuary, Quebec, Canada. Arch Environ Contam Toxicol. 1987;16:137–147. [Google Scholar]
- Minh TB, Prudente M, Watanabe M, Tanabe S, Nakata H, Miyazaki N, Jefferson TA, Subramanian A. Recent contamination of persistent chlorinated endocrine disrupters in cetaceans from the North Pacific and Asian coastal waters. Water Sci Technol. 2000;42:231–240. [Google Scholar]
- MMC. Marine Mammals and Persistent Ocean Contaminants: Proceedings of the Marine Mammal Commission Workshop; Keystone, CO. 1999. [Google Scholar]
- Nakai JS, Bunce NJ. Characterization of the Ah receptor from human placental tissue. J Biochem Toxicol. 1995;10:151–159. doi: 10.1002/jbt.2570100306. [DOI] [PubMed] [Google Scholar]
- Petrulis JR, Bunce NJ. Competitive inhibition by inducer as a confounding factor in the use of the ethoxyresorufin-O-deethylase (EROD) assay to estimate exposure to dioxin-like compounds. Toxicol Lett. 1999;105:251–260. doi: 10.1016/s0378-4274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- Petrulis JR, Bunce NJ. Competitive behavior in the interactive toxicology of halogenated aromatic compounds. J Biochem Mol Toxicol. 2000;14:73–81. doi: 10.1002/(sici)1099-0461(2000)14:2<73::aid-jbt2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol. 1994;46:915–921. [PubMed] [Google Scholar]
- Ramadoss P, Perdew GH. Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- Reddy CM, Xu L, Eglinton TI, Boon JP, Faulkner DJ. Radiocarbon content of synthetic and natural semi-volatile halogenated organic compounds. Environ Pollut. 2002;120:163–168. doi: 10.1016/s0269-7491(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Ross PS, Ellis GM, Ikonomou MG, Barrett-Lennard LG, Addison RF. High PCB concentrations in free-ranging Pacific killer whales, Orcinus orca: effects of age, sex and dietary preference. Mar Poll Bull. 2000;40:504–515. [Google Scholar]
- Ross PS. Marine mammals as sentinels in ecological risk assessment. Hum Ecol Risk Assess. 2000;6:29–46. [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit Rev Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Silkworth JB, Koganti A, Illouz K, Possolo A, Zhao M, Hamilton SB. Comparison of TCDD and PCB CYP1A induction sensitivities in fresh hepatocytes from human donors, Sprague-Dawley rats, and Rhesus monkeys and HepG2 Cells. Toxicol Sci. 2005;87:508–519. doi: 10.1093/toxsci/kfi261. [DOI] [PubMed] [Google Scholar]
- Starr TB, Greenlee WF, Neal RA, Poland A, Sutter TR. The trouble with TEFs [letter] Environ Health Persp. 1999;107:A492–493. doi: 10.1289/ehp.107-1566591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Kang JS, Yang KH, Kaminski NE. Antagonism of aryl hydrocarbon receptor-dependent induction of CYP1A1 and inhibition of IgM expression by di-ortho-substituted polychlorinated biphenyls. Toxicol Appl Pharmacol. 2003;187:11–21. doi: 10.1016/s0041-008x(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Watanabe M, Kan H, Tatsukawa R. Capacity and mode of PCB metabolism in small cetaceans. Mar Mam Sci. 1988;4:103–124. [Google Scholar]
- U.S. Environmental Protection Agency. Risk Assessment Forum, EPA/630/R-01/002. U.S. Environmental Protection Agency; Washington, DC: 2001. Workshop Report on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife. [Google Scholar]
- U.S. Environmental Protection Agency. Framework for Application of the Toxicity Equivalence Methodology for Polychlorinated Dioxins, Furans, and Biphenyls in Ecological Risk Assessment, EPA/100/R-08/004. Washington, DC: 2008. [Google Scholar]
- van den Berg M, Birnbaum L, Bosveld AT, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkamp K, Riget F, Glasius M, Pecseli M, Lebeuf M, Muir D. Chlorobenzenes, chlorinated pesticides, coplanar chlorobiphenyls and other organochlorine compounds in Greenland biota. Sci Total Environ. 2004;331:157–175. doi: 10.1016/j.scitotenv.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Walker M, Peterson RE. Potencies of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners, relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin, for producing early life stage mortality in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 1991;21:219–238. [Google Scholar]
- Watanabe M, Kannan K, Takahashi A, Loganathan B, Odell D, Tanabe S, Giesy JP. Polychlorinated biphenyls, organochlorine pesticides, tris(4-chlorophenyl)methane, and tri(4-chlorophenyl)methanol in livers of small cetaceans stranded along Florida coastal waters, USA. Environ Toxicol Chem. 2000;19:1566–1574. [Google Scholar]
- White RD, Hahn ME, Lockhart WL, Stegeman JJ. Catalytic and immunochemical characterization of hepatic microsomal cytochromes P450 in beluga whale (Delphinapterus leucas) Toxicol Appl Pharmacol. 1994;126:45–57. doi: 10.1006/taap.1994.1089. [DOI] [PubMed] [Google Scholar]
- Wilson JY, Wells R, Aguilar A, Borrell A, Tornero V, Reijnders P, Moore M, Stegeman JJ. Correlates of Cytochrome P450 1A1 expression in bottlenose dolphin (Tursiops truncatus) integument biopsies. Toxicol Sci. 2007;97:111–119. doi: 10.1093/toxsci/kfm031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.