Abstract
Electrophysiological and neuroimaging studies suggest that the integrity of ipsilesional and inter‐hemispheric motor circuits is important for motor recovery after stroke. However, the extent to which each of these tracts contributes to the variance in outcome remains unclear. We examined whether diffusion tensor imaging (DTI)‐derived measures of corticospinal and transcallosal tracts predict motor improvement in an experimental neurorehabilitation trial. 15 chronic stroke patients received bihemispheric transcranial direct current stimulation and simultaneous physical/occupational therapy for five consecutive days. Motor impairment was assessed prior to and after the intervention. At baseline, the patients underwent DTI; probabilistic fiber tracking was used to reconstruct the pyramidal tract (PT), alternate descending motor fibers (aMF), and transcallosal fibers connecting primary motor cortices (M1‐M1). Ipsilesional corticospinal tracts (PT, aMF) and M1‐M1 showed significantly decreased fractional anisotropy (FA) and increased directional diffusivities when compared to age‐matched healthy controls. Partial correlations revealed that greater gains in motor function were related to higher FA values and lower directional diffusivities of transcallosal and ipsilesional corticospinal tracts. M1‐M1 diffusivity had the greatest predictive value. An additional slice‐by‐slice analysis of FA values along the corticospinal tracts demonstrated that the more the ipsilesional FA profiles of patients resembled those of healthy controls, the greater their functional improvement. In conclusion, our study shows that DTI‐derived measures can be used to predict functional potential for subsequent motor recovery in chronic stroke patients. Diffusivity parameters of individual tracts and tract combinations may help in assessing a patient's individual recovery potential and in determining optimal neurorehabilitative interventions. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: alternate motor fibers, corticospinal tract, directional diffusivity, fractional anisotropy, inter‐hemispheric interaction, motor impairment, pyramidal tract, transcallosal fibers
INTRODUCTION
Electrophysiological and neuroimaging studies suggest that the integrity of corticospinal motor fibers correlates with persistent motor impairment in the chronic stroke phase [Schaechter et al., 2009; Talelli et al., 2006; Zhu et al., 2010]. Both the pyramidal tract (PT) and alternate motor fibers (aMF), which might comprise a polysynaptic fiber bundle including the cortico‐rubro‐spinal and cortico‐reticulo‐spinal tracts [Fries et al., 1991; Lang and Schieber, 2003], have been suggested to play an important role in motor recovery after stroke [Lindenberg et al., 2010a]. In addition to surrogate markers of actual motor impairment in these cross‐sectional studies, predictors of recovery potential have been described using longitudinal designs: The functional [Swayne et al., 2008] and structural [Jang et al., 2008] integrity of the PT, cortical activation patterns [Marshall et al., 2009], and motor impairment [Prabhakaran et al., 2008] in the acute or subacute phase were used to predict subsequent motor recovery. In the chronic stroke phase, a combination of diffusion tensor imaging (DTI) and transcranial magnetic stimulation (TMS) yielded information on the integrity of the PT that was useful in estimating motor improvement after experimental rehabilitation [Stinear et al., 2007]. However, much of the variance in motor outcome still remains unexplained [Cramer, 2008b].
More accurate estimations of recovery potential might be possible when considering not only the PT but also other descending corticospinal (aMF) and transcallosal motor tracts (M1‐M1). Models of an imbalance in inter‐hemispheric interactions after stroke highlight the important role of transcallosal connections for recovery [Perez and Cohen, 2009]. Similarly, functional imaging studies demonstrated an alteration of inter‐hemispheric connectivity patterns after stroke [Carter et al., 2010; Grefkes et al., 2008]. Furthermore, experimental neurorehabilitation studies with noninvasive brain stimulation techniques revealed that facilitation of motor recovery can be achieved via up‐regulation of intact ipsilesional motor cortex and via down‐regulation of contralesional motor cortex [Lindenberg et al., 2010b; Schlaug et al., 2008]. Thus, there is ample evidence for the importance of inter‐hemispheric interactions in motor recovery after stroke, although the exact role of contralesional primary and nonprimary motor regions remains elusive [Johansen‐Berg et al., 2002; Werhahn et al., 2003].
DTI‐derived parameters such as fractional anisotropy (FA) and directional diffusivity have been found to reliably reflect the microstructural status of white matter in animal [Song et al., 2003; Sun et al., 2008] and human studies [Acosta‐Cabronero et al., 2010; Naismith et al., 2009; Sidaros et al., 2008]. Using DTI, we related diffusivity measures of corticospinal tracts (PT, aMF) and transcallosal motor fibers (M1‐M1) to functional gains in chronic stroke patients participating in an experimental rehabilitation trial. We aimed to identify a pattern of white matter alterations predictive of functional potential for motor recovery.
METHODS
Subjects
Fifteen chronic stroke patients were included in this study. Ten of them participated in a double‐blind, sham‐controlled 5‐day trial of bihemispheric transcranial direct current stimulation (tDCS) with simultaneous physical/occupational therapy (PT/OT), as reported elsewhere [Lindenberg et al., 2010b]. The remaining five were newly recruited patients, who underwent the same 5‐day intervention of bihemispheric tDCS and PT/OT. All patients, the therapist, and the investigators who conducted the motor assessments were blinded as to whether the patients received real or sham stimulation. Here, we only present data of patients who received real stimulation.
Inclusion criteria were: occurrence of first ischemic stroke at least 5 months prior to enrolment; no previous or subsequent cerebral ischemia; Medical Research Council (MRC) strength grade of ≤3/5 in extensor muscles of the affected upper extremity in the acute phase; no additional neurologic or psychiatric disorders; no concurrent use of antidepressants or CNS stimulants. Prior to enrollment, all patients had received standard PT/OT which is common for the subacute post‐stroke rehabilitation period. None of the patients had undergone any other experimental therapy at the time of enrollment. Patients' demographics and imaging data are provided in Table I. Information on the ten healthy control subjects can be found in the table as well. The study was approved by the Institutional Review Board (IRB), and all patients gave written informed consent.
Table I.
Demographical and imaging data
| Patient | Age (Years) | T post (months) | Gender | Hand | Hem | Location | CST‐LL (cc) | M1M1‐LL (cc) |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | 10 | M | R | L | pontine | 0.02 | 0.00 |
| 2 | 69 | 21 | M | R | L | sc | 0.10 | 0.00 |
| 3 | 54 | 31 | M | R | L | c/sc | 0.10 | 0.12 |
| 4 | 25 | 7 | M | R | R | pontine | 0.17 | 0.00 |
| 5 | 68 | 45 | M | R | L | sc | 0.20 | 0.11 |
| 6 | 52 | 5 | M | R | R | c/sc | 0.20 | 0.00 |
| 7 | 71 | 6 | M | R | R | pontine | 0.32 | 0.00 |
| 8 | 76 | 29 | M | R | R | c/sc | 0.33 | 0.50 |
| 9 | 44 | 24 | M | R | L | sc | 0.47 | 0.32 |
| 10 | 47 | 14 | M | R | R | sc | 0.55 | 0.00 |
| 11 | 76 | 10 | F | R | R | sc | 1.15 | 0.00 |
| 12 | 77 | 38 | F | R | R | sc | 2.11 | 0.64 |
| 13 | 34 | 12 | M | R | L | c/sc | 2.73 | 1.77 |
| 14 | 65 | 65 | M | R | L | c/sc | 3.19 | 2.62 |
| 15 | 47 | 170 | M | R | L | c/sc | 3.92 | 3.51 |
| Mean | 57.3 ± 16.0 | 32.5 ± 41.6 | 1.04 ± 1.30 | 0.64 ± 1.10 | ||||
| Control | 59.3 ± 14.0 | 2 F/8 M | 10 R |
Mean values are reported ± standard deviation.
Abbreviations: c, cortical (in patients with cortical involvement, at least part of the motor cortex was spared); CST‐LL, lesion load of the corticospinal tract [Zhu et al., 2010]; F, female; Hand, handedness; Hem, lesional hemisphere; L, left; M, male; M1M1‐LL, lesion load of transcallosal motor fibers; R, right; sc, subcortical; T post, time post‐stroke.
Study Design
Patients underwent noninvasive brain stimulation (30 min) and simultaneous PT/OT (60 min) on five consecutive days. An experienced therapist administered the therapy focusing on a combination of PT and OT techniques including functional motor tasks of the affected arm and hand to promote sensory‐motor integration, coordination of movement and goal‐directed activities of practical relevance for the patient. An MRI study took place at baseline; motor impairment assessments were conducted prior to and after the intervention.
Transcranial Direct Current Stimulation
Direct current was delivered using a Phoresor® II Auto stimulator (Model No. PM850, IOMED, Salt Lake City, UT) through two saline‐soaked surface gel‐sponge electrodes (see [Schlaug et al., 2008] for more details). The stimulation consisted of 30 min of 1.5 mA direct current with the anode placed over the ipsilesional and the cathode over the contralesional motor cortex (C3 and C4 according to the international 10–20 EEG electrode system).
Motor Assessment
Each patient underwent the Wolf Motor Function Test (WMFT) on two different days prior to the intervention to assure measurement stability at baseline [Whitall et al., 2006]. The assessments were repeated 3 and 7 days after the last intervention session. The WMFT consists of 15 time‐based tasks and two tests of strength [Morris et al., 2001]. Similar to previous studies, completion times were log transformed to account for skewed data distribution [Wolf et al., 2006]. The resulting score has a maximum value of 2.08 sec [log] with lower values reflecting better function of the affected arm.
Two‐tailed paired t‐tests revealed no difference in the two baseline assessments [t(14) = 1.18; P = 0.257] and no differences between the two assessments at days 3 and 7 after the intervention [t(14) = 1.35; P = 0.200]; thus, we calculated mean pre and postintervention scores for our analyses. Differences from baseline to postintervention were calculated as proportional change: (post − pre)/pre*100.
Image Acquisition
At baseline, all patients underwent MRI in a 3T GE scanner, which included T1‐weighted images (resolution: 0.94 × 0.94 × 1.5 mm3), DTI (1.87 × 1.87 × 5.0 mm3 with 25 noncollinear diffusion directions with a b‐value of 1,000 s/mm2, and one with a b‐value of 0 s/mm2; 30 axial slices covered the entire brain including the brainstem), and a FLAIR sequence (0.5 × 0.5 × 5 mm3). T1‐weighted images were spatially normalized using SPM5 (Wellcome Department of Neurology, London, UK) before determining lesion size and location (see Fig. 1) as well as lesion load of transcallosal M1‐M1 fibers and the corticospinal tract [Zhu et al., 2010]. An additional group of 10 age‐ and gender‐matched healthy subjects (see Table I) underwent MRI using the same imaging sequences.
Figure 1.
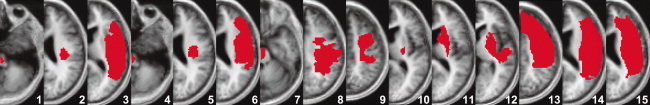
Lesion maps. After spatial normalization to the MNI space using SPM5, individual lesion maps were drawn and superimposed onto a canonical T1‐weighted image. Patients are ordered according to their CST‐lesion load (lowest to highest from left to right). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Preprocessing of Diffusion Tensor Imaging Data
Preprocessing and fiber tracking were performed with FSL (http://www.fmrib.ox.ac.uk/fsl). A 3D affine registration was applied to correct for eddy currents and head motion [Jenkinson and Smith, 2001] followed by brain extraction [Smith, 2002]. A probability distribution of fiber directions was then calculated for each voxel [Behrens et al., 2003], allowing estimates of two directions per voxel [Behrens et al., 2007].
Probabilistic Tractography
For all motor tracts, three regions of interest (ROIs) were drawn on the individual FA images in native diffusion space. In addition, we applied sagittal exclusion masks along the midline for corticospinal tracts and axial exclusion masks caudal to the corpus callosum for the transcallosal tracts.
For the primary motor cortex ROI, the anterior bank of the central sulcus served as the posterior border and a line running laterally from the vertex of the precentral sulcus to the junction of superior frontal and precentral sulci as the anterior border.
To track corticospinal fibers, additional ROIs were drawn in the posterior limb of the internal capsule (PLIC) and in the pons. ROIs in the pons were drawn in two different ways. One ROI included only the basis pontis, resulting in a fiber bundle that corresponded to the PT [Nieuwenhuys et al., 2008]. A different pontine ROI comprised only the posterior part of the pons (tegmentum pontis) to include additional or alternate corticofugal tracts to the spinal cord [Lindenberg et al., 2010a]. Using the pontine ROIs as “seed regions,” we tracked PT and aMF in both hemispheres using ipsilateral PLIC and precentral gyrus ROIs as “waypoints.” ROI sizes did not differ between the hemispheres (all P > 0.3) or across the patient and control groups (all P > 0.35).
To track transcallosal fibers, the contralesional primary motor cortex ROI was used as a seed region and an additional sagittal ROI, which covered the caudal and medial portions of the corpus callosum, as well as the ipsilesional precentral gyrus ROIs served as waypoints.
To determine the anatomical specificity of the corticospinal and transcallosal motor tracts in relation to functional improvements, we used a control tract, the uncinate fasciculus, which does not overlap with our tracts of interest and has not been associated with motor function previously. This hook‐shaped tract that links the rostral temporal lobe with the orbital and inferior frontal gyri was reconstructed according to previous descriptions (e.g., [Kier et al., 2004]).
The resulting probabilistic streamlines were constrained [Heiervang et al., 2006; Schaechter et al., 2009] to voxels with more than 2% of the individual tract‐specific connection probability and binarized to define tract masks (see Fig. 2). We restricted the analysis to voxels within the white matter using individual gray matter masks derived from the T1‐weighted images. The masks were generated with FreeSurfer's automated tools for segmentation [Dale et al., 1999; Fischl et al., 1999]. Errors due to segmentation misclassification, which occurred in the images of several stroke patients (6 of 15), were manually corrected. We binarized the resulting masks and registered them to the b0 image applying a within‐subject, cross‐modal approach using a boundary‐based cost function.
Figure 2.
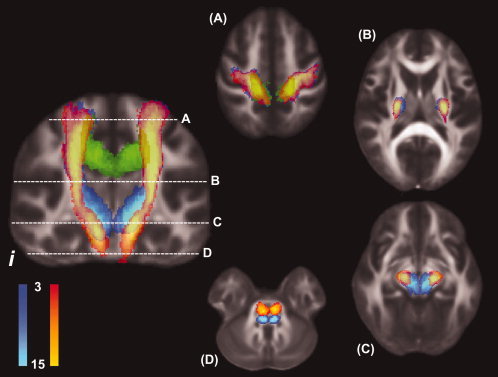
Course of pyramidal tract and alternate motor fibers as they descend from the motor cortex to the pons. Probabilistic tracking results of patients with right‐hemispheric lesions were mirrored along the midline so that all patients' lesions appeared on the same side (i). To generate the canonical image, individual tracts from all patients were normalized, converted to binary images and then summed. Note that both fiber bundles separate before entering the cerebral peduncles. Their positions on the axial slices caudal to the diencephalon suggest that the aMF comprise the cortico‐reticulo‐spinal and cortico‐rubro‐spinal tracts. The coronal slice corresponds to y = −15 in MNI space; the axial slices correspond to (A) z = 55; (B) z = 10; (C) z = −10; and (D) z = −25. Red to yellow: PT; dark to light blue: aMF; green: M1‐M1; i: ipsilesional hemisphere. Colors indicate the degree of voxel‐by‐voxel overlap of the individual normalized tracts.
Tractography‐Based Analysis of Diffusivity Parameters
Directional diffusivities were determined as λ1 > λ2 > λ3, and FA was calculated from these eigenvalues. Axial diffusivity (λ∥)(λ∥) corresponds to λ1 and radial diffusivity (λ⟂)(λ⟂) to (λ2 + λ3)/2 [Basser, 1995; Beaulieu, 2009]. For further analysis, we extracted tract‐specific FA and directional diffusivities (only non‐zero values) in native space. In addition, we normalized individual FA images to the template implemented in FSL (1 × 1 × 1 mm3) using linear and nonlinear algorithms. The resulting matrix was then used to normalize individual tracts to extract diffusivity characteristics on a slice‐by‐slice level for an additional functional data analysis (FDA).
Statistical Analysis
We used two‐tailed two‐sample t‐tests to compare tract‐specific diffusivity values between the patient and control groups. Within the patient group, we performed paired t‐tests to compare values between lesional and contralesional hemispheres. To test the predictive value of DTI‐derived parameters, we conducted partial correlation analyses of tract‐specific FA, λ∥ and λ⟂ with proportional change in WMFT as well as baseline WMFT scores. We accounted for age, time post‐stroke and lesion size as covariates in all correlation analyses because these variables have been shown to have an effect on outcome in stroke patients [Cramer, 2008a].
Additional multiple regression analyses were conducted to test which tract's diffusivity values would be a superior predictor. Because of the considerable overlap of the two corticospinal tracts (PT and aMF) and the resulting collinearity problem, we abstained from entering both tracts separately into this analysis. Instead, we merged the two tracts and extracted tract‐specific diffusivity parameters for a combined corticospinal tract. This allowed comparisons of the predictive value of corticospinal versus transcallosal tracts.
To further evaluate differences between the patient and control group, we applied an FDA [Ramsay and Dalzell, 1991], which was used in a recent DTI study [Schaechter et al., 2009]. FA values taken from voxels belonging to the descending corticospinal tracts of each hemisphere were fit to continuous curves in a function space for every single subject. We generated 100 data points for each individual tract and applied t‐tests to localize significant differences between corticospinal tracts of the patient and control groups (see Fig. 3). To obtain a measure of the degree to which FA curves of individual patients differed from those in healthy subjects, we subtracted individual areas under the curve (AUC) of patients from the mean AUC of the control group. This yielded a single value for each tract for every patient. The lower these values, the more similar the respective FA curve was to those of healthy subjects. To test for the predictive value of this novel AUC variable, we conducted partial correlation analyses with baseline WMFT scores and change scores in WMFT scores. This analysis could only be performed for the corticospinal tracts because the U‐shaped course of the transcallosal fibers did not allow for an adequate slice‐by‐slice extraction of FA values.
Figure 3.
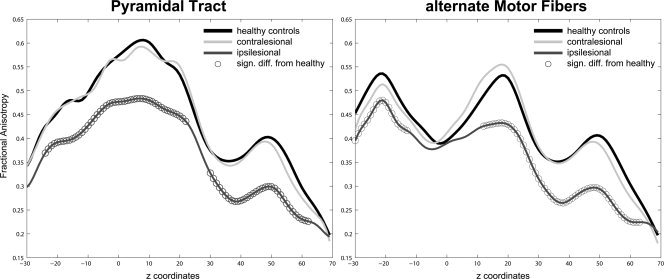
FA curves of pyramidal tract and alternate motor fibers. Slice‐by‐slice comparison of ipsilesional (dark grey) and contralesional tracts (light grey) with the mean tracts of the control group (black): FDA‐derived continuous FA values of PT (A) and aMF (B). Significant differences (P< 0.05) between patients' curves and the mean curve of healthy subjects are marked with circles. Note the characteristic differential shape of PT and aMF curves.
RESULTS
Behavioral Measures
WMFT scores changed significantly from 0.83 ± 0.48 at baseline to 0.70 ± 0.45 sec[log] after the intervention (P < 0.001), corresponding to a mean improvement of −0.14 ± 0.09 sec[log] (range −0.36 to −0.02 sec[log]) or −19.6% ± 9.9% proportional change (range −33.0% to −1.8%). A partial correlation analysis (controlling for age, time post‐stroke and lesion size) revealed an association of baseline WMFT and proportional change in WMFT (r = 0.60; P = 0.041). Therefore, to rule out the possibility that our main results could be explained by simple correlations between baseline impairment level and diffusivity measures, we also ran all further analyses using baseline WMFT as an additional covariate (see Supporting Information).
Tract Volumes
Ipsilesional corticospinal tracts had lower volumes as compared to contralesional tracts (P < 0.005) and tracts of healthy subjects (all P < 0.015). Furthermore, the patients' transcallosal motor tracts were reduced in their number of voxels as compared to the controls (P = 0.001). Contralesional tracts did not differ from the tracts of healthy subjects (all P > 0.20).
Diffusivity of Ipsilesional Corticospinal Tracts
The patients' FA values of ipsilesional PT and aMF were lower and directional diffusivity values were higher compared to the control group (see Table II). Within the patient group, corticospinal tracts differed between the hemispheres with lower ipsilesional FA values (all P < 0.001) and higher ipsilesional λ∥ (all P < 0.025) as well as λ⟂ values (all P < 0.015).
Table II.
Tract‐specific diffusivity parameters
| Patient group | Control group | Patients vs. controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tracts | FA | λ∥ | λ⟂ | Tracts | FA | λ∥ | λ⟂ | FA | λ∥ | λ⟂ |
| PTi | 0.32 ± 0.06 | 1.48 ± 0.29 | 0.97 ± 0.27 | PTL | 0.42 ± 0.03 | 1.21 ± 0.11 | 0.65 ± 0.10 | ↓* | ↑* | ↑ * |
| PTc | 0.40 ± 0.03 | 1.28 ± 0.08 | 0.72 ± 0.09 | PTR | 0.40 ± 0.04 | 1.24 ± 0.12 | 0.71 ± 0.14 | — | ↑ | — |
| aMFi | 0.32 ± 0.06 | 1.42 ± 0.27 | 0.93 ± 0.27 | aMFL | 0.41 ± 0.03 | 1.18 ± 0.08 | 0.64 ± 0.07 | ↓* | ↑* | ↑* |
| aMFc | 0.39 ± 0.03 | 1.24 ± 0.07 | 0.72 ± 0.09 | aMFR | 0.40 ± 0.04 | 1.19 ± 0.10 | 0.68 ± 0.12 | — | ↑ | ↑ |
| M1‐M1 | 0.27 ± 0.05 | 1.36 ± 0.15 | 0.85 ± 0.19 | M1‐M1 | 0.33 ± 0.03 | 1.28 ± 0.10 | 0.79 ± 0.09 | ↓* | ↑ | ↑* |
Directional diffusivity values are given as mean μm2/ms ± standard deviation. Arrows in the three columns on the right show the direction of differences between the patient and control groups (asterisk [*] indicates P < 0.05; post‐hoc Bonferroni‐corrected for multiple comparisons [K = 5]).
Abbreviations: aMF, alternate motor fibers; c, contralesional hemisphere; FA, fractional anisotropy; i, ipsilesional hemisphere; L, left; λ∥, axial diffusivity; λ⟂, radial diffusivity; M1‐M1, transcallosal fibers connecting primary motor cortices; PT, pyramidal tract; R, right.
Partial correlation analyses of tract‐specific FA with proportional WMFT change yielded significant results. The correlations of λ⟂ values and change in motor scores showed a strong trend to be significant, whereas λ∥ values were only weakly related to WMFT change (see Fig. 4). No significant results were obtained when DTI‐derived measures were correlated with baseline WMFT scores (see Table III for Pearson's coefficients of all correlation analyses).
Figure 4.
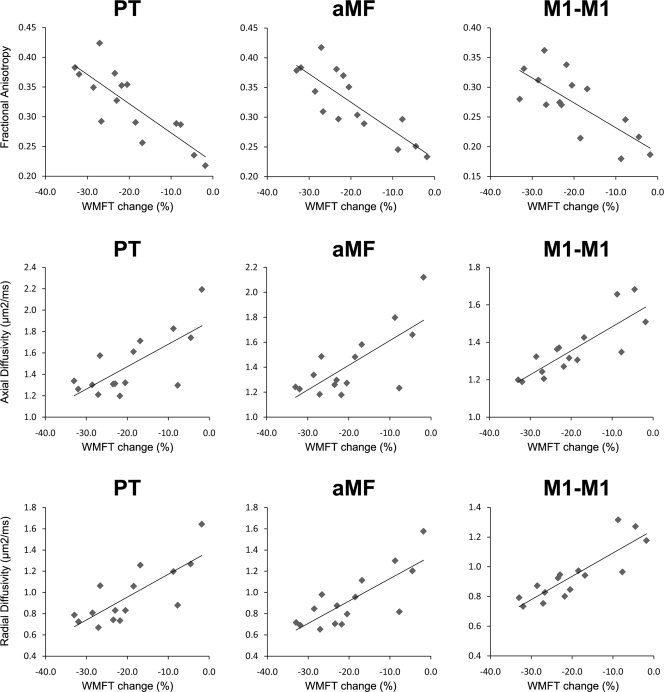
Scatter plot of correlation analyses. Correlation analyses of tract‐specific diffusivity and proportional change in Wolf Motor Function Test (WMFT) scores for ipsilesional PT, ipsilesional aMF, and M1‐M1. Pearson's coefficients of all correlations can be found in Table III; the regression lines in the graph are derived from the raw data (i.e., without accounting for covariates).
Table III.
Partial correlation analyses
| FA | λ∥ | λ⟂ | ||||
|---|---|---|---|---|---|---|
| bWMFT | ΔWMFT | bWMFT | ΔWMFT | bWMFT | ΔWMFT | |
| PTi | −0.37 | −0.80* | 0.27 | 0.58 | 0.23 | 0.67 |
| PTc | −0.30 | −0.40 | −0.19 | −0.09 | −0.08 | 0.05 |
| aMFi | −0.46 | −0.87* | 0.24 | 0.58 | 0.27 | 0.72 |
| aMFc | −0.38 | −0.48 | −0.05 | −0.07 | 0.06 | 0.27 |
| M1‐M1 | −0.77* | −0.70 | 0.39 | 0.80* | 0.58 | 0.82* |
Table III shows results of partial correlations, controlling for age, time post‐stroke, and lesion size as covariates; values in the table are Pearson's coefficients (r); results in bold indicate 0.10> P > 0.05, an asterisk (*) indicates P < 0.05 (two‐tailed; post‐hoc Bonferroni‐corrected for multiple comparisons [K = 5]).
Abbreviations: bWMFT, baseline WMFT scores; ΔWMFT, proportional change in WMFT scores from baseline to post‐intervention.
The analysis of tract‐specific FA profiles revealed that differences between the patients' individual AUC and the control group's mean AUC correlated with WMFT change and baseline WMFT scores for both PT and aMF (see Supporting Information). FDA‐derived region‐specific differences between FA curves of patients and controls are depicted in Figure 3.
Diffusivity of Contralesional Corticospinal Tracts
FA values of contralesional PT and aMF did not differ from the values in our group of healthy age‐matched control subjects. Similarly, directional diffusivities of the PT did not differ between the two groups, but trends were found for higher directional diffusivity values of the aMF in the patient group compared to the control group (Table II). Partial correlation analyses revealed no correlations between tract‐specific DTI‐derived parameters and WMFT change or baseline WMFT scores (Table III). The analysis of FA profiles demonstrated that AUC difference values of contralesional tracts did not significantly predict changes in WMFT, but correlated with baseline WMFT scores (see Table III and Supporting Information).
Diffusivity of Transcallosal Motor Fibers
FA values of transcallosal motor fibers in the patient group were significantly lower than in the control group. λ⟂ values were significantly higher, but there was no significant difference in λ∥ values (Table II). Partial correlations with change in WMFT yielded significant results for all directional diffusivities and a strong trend for FA (see Fig. 4). Partial correlations with baseline WMFT scores revealed significant results only for FA, but not for λ∥ or λ⟂ values (Table III).
Diffusivity of the Control Tract (Uncinate Fasciculus)
None of the ipsi‐ or contralesional tract‐specific diffusivity parameters correlated with baseline WMFT (all P > 0.08) or change in WMFT (all P > 0.30).
Multiple Regression Analyses
A first regression analysis was conducted using tract‐specific FA of corticospinal tracts and transcallosal M1‐M1 fibers as predictors of WMFT change (adjusted r = 0.78, P = 0.011). A strong trend was found for the corticospinal tracts (combined PT and aMF) to predict WMFT change (partial r = −0.53, P = 0.052) while M1‐M1 did not predict WMFT change (partial r = −0.09, P = 0.750). A second regression analysis of tract‐specific λ∥ values (adjusted r = 0.81, P = 0.001) demonstrated that transcallosal M1‐M1 fibers were a significant predictor of WMFT change (partial r = 0.64, P = 0.013) whereas ipsilesional corticospinal tracts did not predict motor improvement (partial r = 0.31, P = 0.282) in this model. Similarly, the regression analysis of tract‐specific λ⟂ values (adjusted r = 0.83, P < 0.001) showed transcallosal M1‐M1 fibers to be a significant predictor of WMFT change (partial r = 0.59, P = 0.027) while the ipsilesional corticospinal tracts did not predict change in motor scores (partial r = 0.23, P = 0.427).
DISCUSSION
We found a distinct pattern of diffusivity alterations in transcallosal and ipsilesional corticospinal tracts of chronic stroke patients with decreased FA values and increased directional diffusivities as compared to the control group. This pattern is characteristic of chronic white matter degeneration [Concha et al., 2006; Sidaros et al., 2008; Yu et al., 2009].
Prediction of Functional Motor Improvement
DTI‐derived measures of the PT, aMF and M1‐M1 predicted functional gains in our experimental rehabilitation trial [Lindenberg et al., 2010b]. This finding was complemented by the results of the functional data analysis, which revealed that the closer the FA profiles of corticospinal tracts resembled those of healthy age‐matched controls, the greater the improvement in WMFT scores. Our rationale for investigating two different corticospinal tracts originating from primary motor cortex was based on previous work suggesting that the integrity of all descending fibers is related to the level of motor impairment after stroke [Fries et al., 1991; Lang and Schieber, 2003; Lindenberg et al., 2010a]. Our results complement these studies and provide evidence that the integrity of both the PT and aMF is directly related to the functional potential for further recovery in the chronic stage after stroke.
In addition to corticospinal tracts, we tracked transcallosal fibers connecting the primary motor cortices. Dense callosal connections between homologous motor areas have been demonstrated in animal studies [Jenny, 1979; Pandya et al., 1969], and electrophysiological investigations added evidence of their functional significance within inhibitory and facilitatory circuits in humans [Ferbert et al., 1992; Hanajima et al., 2001]. Using a combination of DTI and transcranial magnetic stimulation, a recent study demonstrated the close relationship of inter‐hemispheric inhibition between primary motor hand regions and diffusivity of transcallosal fibers connecting these regions [Wahl et al., 2007]. This allowed us to use transcallosal M1‐M1 fibers as a structural substrate of inter‐hemispheric interactions in our present study. The observed correlations between transcallosal diffusivity parameters and change in WMFT scores provide strong evidence for the importance of inter‐hemispheric interactions in addition to uni‐hemispheric circuits for motor function after stroke. Although the exact role of contralesional motor regions for recovery remains elusive [Johansen‐Berg et al., 2002; Werhahn et al., 2003], our present study suggests that its connection with ipsilesional motor cortex is critical for functional improvement in the chronic stroke phase [Cramer, 2008b; Perez and Cohen, 2009]. Since DTI does not provide information about fiber direction or the functional role of fibers (e.g., excitatory or inhibitory), we cannot determine whether the integrity of connections from contralesional to ipsilesional motor cortex or vice versa accounted for the therapeutic response observed in our patient sample.
The superior predictive power of M1‐M1 diffusivity parameters as compared to unihemispheric corticospinal tracts needs to be interpreted within the context of our experimental brain stimulation trial. The simultaneous modulation of excitability of ipsilesional and contralesional motor cortices influences both sides of a hypothetical model of imbalance in inter‐hemispheric inhibition [Schlaug et al., 2008; Vines et al., 2008], possibly increasing the importance of intact M1‐M1 fibers. Future studies would need to test the value of motor tract diffusivity measures as predictors of recovery potential comparing different rehabilitative approaches. Furthermore, we cannot exclude the possibility that inter‐individual pre‐morbid differences in transcallosal fibers accounted for the observed relationship with WMFT changes [Johansen‐Berg et al., 2007].
Alteration of Fractional Anisotropy
The use of regional FA values as surrogate markers of motor impairment after stroke has been well established in several cross‐sectional studies of the corticospinal system: lower ipsilesional values correlated with poorer motor function [Lindenberg et al., 2010a; ; Schaechter et al., 2009; Thomalla et al., 2004]. In addition to their role as structural correlates of motor function, regional FA values of the internal capsule predicted changes in motor impairment after 30 days of practice [Stinear et al., 2007]. Using a comparable number of chronic stroke patients, our present study suggests that tract‐specific FA may be a valuable alternative to FA of predetermined ROIs in predicting functional potential, especially when DTI is used without additional electrophysiological measures.
Contralesional tract‐specific FA values correlated with motor impairment at baseline in our chronic stroke patients, replicating a previous finding [Schaechter et al., 2009]. Interestingly, although FA profiles of contralesional PT and aMF (derived using the FDA) correlated with baseline WMFT score, it did not predict functional potential for recovery in our present analysis. This suggests that if remodeling of a contralesional motor tract occurs after stroke [Benowitz and Carmichael, 2009], it might not necessarily provide the structural basis for further functional gains in the chronic phase.
Alteration of Directional Diffusivity
The use of directional diffusivity as an indicator of fiber integrity is based on animal studies, which have provided evidence for distinct pathological processes underlying changes in λ∥ and λ⟂ [Mac Donald et al., 2007; Song et al., 2003; Wu et al., 2007]. A decrease in λ∥ is thought to be indicative of axonal damage and associated with retraction in the acute phase after injury, whereas an elevation due to degenerative processes has been reported in the chronic phase. In contrast, λ⟂ was shown to increase shortly after injury and to stabilize on an elevated level in the chronic phase compared to normal values [Sidaros et al., 2008; Yu et al., 2009]. This increase in λ⟂ was suggested to primarily reflect demyelination [Budde et al., 2007; Song et al., 2003]. However, the model of separable axial and radial diffusivities and their specific connection to discrete pathological processes appears to be controversial, especially in regions of complex fiber architecture [Wheeler‐Kingshott and Cercignani, 2009]. Since our tracts of interest comprised major pathways with bundles of coherently aligned fibers, the issue of complex fiber orientations might not be as problematic so that the general interpretation of diffusivity changes outlined above may still apply for the selected tracts.
Higher λ∥ values of corticospinal and transcallosal tracts were associated with less improvement through the intervention. Higher axial diffusivity values (as compared to the contralesional hemisphere or control subjects) might reflect a composite of degeneration and subsequent structural compensation [Sidaros et al., 2008]. The latter does not necessarily yield functionally meaningful connections [Benowitz and Carmichael, 2009; Dancause et al., 2005], hence the magnitude of axial diffusivity might be driven by factors which actually interfere with recovery and cause the inverse correlation with WMFT change scores.
Similarly, higher λ⟂ values of transcallosal and ipsilesional corticospinal tracts were associated with lower functional gains. This corresponds to results of a study of children with motor dysfunction where higher λ⟂ of corticospinal motor fibers predicted less improvement in motor function at a follow‐up assessment [Ludeman et al., 2008]. Similarly, in an investigation of diffusivity changes due to optic neuritis, λ⟂ of the optic nerve in the subacute phase correlated with clinical scores in the chronic phase [Naismith et al., 2009]. Interestingly, we found substantial alterations not only in corticospinal tracts, which showed overlap with the lesion in every single patient in our group, but also M1‐M1, which showed overlap with the lesion only in 8 of the 15 patients (see Table I). Thus, the alterations cannot solely be caused by retro‐ or anterograde changes directly related to the stroke lesions. In fact, remote changes within the broader “motor network” have been consistently described in previous studies (e.g., [Schaechter et al., 2009; Thomas et al., 2005]) and could be explained by diaschisis (e.g., [Seitz et al., 1999]).
Limitations
The novel results found in this study open avenues for further research. In particular, we chose to use a short DTI sequence in order to minimize movement artifacts in the sample of moderately to severely impaired patients and to test the predictive value of DTI parameters acquired with a sequence that could be easily implemented in standard clinical settings (4:20 min scanning time). However, the resulting voxels are non‐isotropic so that partial volume effects and angular resolution vary in different axes, limiting the degree to which valid comparisons can be made between tracts that are oriented along these different axes. Furthermore, the limited number of diffusion directions may hinder an appropriate modeling of multiple fibers per voxels. While DTI parameters used in the current study may be appropriate for clinical applications, studies with higher resolution and isotropic voxels as well as greater number of diffusion directions would be needed to more accurately test the predictive value of tract‐specific diffusivity measures.
CONCLUSION
DTI‐derived parameters predicted functional improvement in chronic stroke patients undergoing an experimental rehabilitation trial. The more the diffusivity profiles of their affected tracts resembled those observed in healthy subjects, the greater their potential for functional recovery. Furthermore, measures of transcallosal fibers were superior predictors as compared to corticospinal tracts in the context of our bihemispheric stimulation approach. In conclusion, diffusivity parameters of individual tracts and tract combinations may help in assessing potential benefits of brain stimulation applied to ipsilesional and contralesional motor cortices and in determining optimal neurorehabilitative interventions.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank Catherine Wan and Psyche Loui for comments on an earlier draft of the manuscript.
Contributor Information
Robert Lindenberg, Email: rlindenb@bidmc.harvard.edu.
Gottfried Schlaug, Email: gschlaug@bidmc.harvard.edu.
REFERENCES
- Acosta‐Cabronero J, Williams GB, Pengas G, Nestor PJ ( 2010): Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain 133: 529–539. [DOI] [PubMed] [Google Scholar]
- Basser PJ ( 1995): Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed 8: 333–344. [DOI] [PubMed] [Google Scholar]
- Beaulieu C ( 2009): The biological basis of diffusion anisotropy In: Johansen‐Berg H, Behrens TE, editors. Diffusion MRI: From Quantitative Measurement to In Vivo Neuroanatomy. London: Academic Press; pp 105–126. [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM ( 2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW ( 2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Carmichael ST ( 2009): Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis 37: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK ( 2007): Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57: 688–695. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman G, Corbetta M ( 2010): Resting inter‐hemispheric fMRI connectivity predicts performance after stroke. Ann Neurol 67: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C ( 2006): Diffusion tensor imaging of time‐dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage 32: 1090–1099. [DOI] [PubMed] [Google Scholar]
- Cramer SC ( 2008a): Repairing the human brain after stroke. I. Mechanisms of spontaneous recovery. Ann Neurol 63: 272–287. [DOI] [PubMed] [Google Scholar]
- Cramer SC ( 2008b): Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol 63: 549–560. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI ( 1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ ( 2005): Extensive cortical rewiring after brain injury. J Neurosci 25: 10167–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD ( 1992): Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM ( 1999): Cortical surface‐based analysis. II. Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fries W, Danek A, Witt TN ( 1991): Motor responses after transcranial electrical stimulation of cerebral hemispheres with a degenerated pyramidal tract. Ann Neurol 29: 646–650. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR ( 2008): Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 63: 236–246. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I ( 2001): Interhemispheric facilitation of the hand motor area in humans. J Physiol 531: 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiervang E, Behrens TE, Mackay CE, Robson MD, Johansen‐Berg H ( 2006): Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage 33: 867–877. [DOI] [PubMed] [Google Scholar]
- Jang SH, Bai D, Son SM, Lee J, Kim DS, Sakong J, Kim DG, Yang DS ( 2008): Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol 64: 460–465. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S ( 2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jenny AB ( 1979): Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol 188: 137–145. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM ( 2002): The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA 99: 14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Della‐Maggiore V, Behrens TE, Smith SM, Paus T ( 2007): Integrity of white matter in the corpus callosum correlates with bimanual co‐ordination skills. Neuroimage 36( Suppl 2): T16–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA ( 2004): MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol 25: 677–691. [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Schieber MH ( 2003): Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol 90: 1160–1170. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G ( 2010a): Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 74: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G ( 2010b): Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 74: 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludeman NA, Berman JI, Wu YW, Jeremy RJ, Kornak J, Bartha AI, Barkovich AJ, Ferriero DM, Henry RG, Glenn OA ( 2008): Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology 71: 1676–1682. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D ( 2007): Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 27: 11869–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW ( 2009): Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol 65: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DM, Uswatte G, Crago JE, Cook EW III, Taub E ( 2001): The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil 82: 750–755. [DOI] [PubMed] [Google Scholar]
- Naismith RT, Xu J, Tutlam NT, Snyder A, Benzinger T, Shimony J, Shepherd J, Trinkaus K, Cross AH, Song SK ( 2009): Disability in optic neuritis correlates with diffusion tensor‐derived directional diffusivities. Neurology 72: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Huijzen CV ( 2008): The Human Central Nervous System. Berlin: Springer. [Google Scholar]
- Pandya DN, Gold D, Berger T ( 1969): Interhemispheric connections of the precentral motor cortex in the rhesus monkey. Brain Res 15: 594–596. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG ( 2009): Interhemispheric inhibition between primary motor cortices: What have we learned? J Physiol 587: 725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, Marshall RS, Krakauer JW ( 2008): Inter‐individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair 22: 64–71. [DOI] [PubMed] [Google Scholar]
- Ramsay JO, Dalzell CJ ( 1991): Some tools for functional data analysis. J R Stat Soc B 53: 539–572. [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N ( 2009): Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 30: 3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D ( 2008): Transcranial direct current stimulation in stroke recovery. Arch Neurol 65: 1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Azari NP, Knorr U, Binkofski F, Herzog H, Freund HJ ( 1999): The role of diaschisis in stroke recovery. Stroke 30: 1844–1850. [DOI] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E ( 2008): Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain 131: 559–572. [DOI] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH ( 2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD ( 2007): Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 130: 170–180. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK ( 2008): Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage 40: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ ( 2008): Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex 18: 1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC ( 2006): Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol 117: 1641–1659. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J ( 2004): Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 22: 1767–1774. [DOI] [PubMed] [Google Scholar]
- Thomas B, Eyssen M, Peeters R, Molenaers G, Van Hecke P, De Cock P, Sunaert S ( 2005): Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain 128: 2562–2577. [DOI] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G ( 2008): Dual‐hemisphere tDCS facilitates greater improvements for healthy subjects' non‐dominant hand compared to uni‐hemisphere stimulation. BMC Neurosci 9: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach‐Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U ( 2007): Human motor corpus callosum: Topography, somatotopy, and link between microstructure and function. J Neurosci 27: 12132–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG ( 2003): Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol 54: 464–472. [DOI] [PubMed] [Google Scholar]
- Wheeler‐Kingshott CA, Cercignani M ( 2009): About “axial” and “radial” diffusivities. Magn Reson Med 61: 1255–1260. [DOI] [PubMed] [Google Scholar]
- Whitall J, Savin DN Jr, Harris‐Love M, Waller SM ( 2006): Psychometric properties of a modified Wolf Motor Function test for people with mild and moderate upper‐extremity hemiparesis. Arch Phys Med Rehabil 87: 656–660. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols‐Larsen D ( 2006): Effect of constraint‐induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA 296: 2095–2104. [DOI] [PubMed] [Google Scholar]
- Wu Q, Butzkueven H, Gresle M, Kirchhoff F, Friedhuber A, Yang Q, Wang H, Fang K, Lei H, Egan GF, Kilpatrick TJ. ( 2007): MR diffusion changes correlate with ultra‐structurally defined axonal degeneration in murine optic nerve. Neuroimage 37: 1138–1147. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, Li K ( 2009): A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage 47: 451–458. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G ( 2010): Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 41: 910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


