Abstract
Opiate/narcotic analgesics are the most effective treatments for chronic severe pain, but their clinical utility is often hampered by the development of analgesic tolerance. Recent evidence suggests chronic morphine may activate glial cells to release proinflammatory cytokines. In this study, we used herpes simplex virus (HSV) vectors-based gene transfer to dorsal root ganglion to produce a local release of p55 TNF soluble receptor in the spinal cord in rats with morphine tolerance. Subcutaneous inoculation of HSV vectors expressing p55 TNF soluble receptor into the plantar surface of the hindpaws, enhanced the antinociceptive effect of acute morphine in rats. Subcutaneous inoculation of those vectors into hindpaws also delayed the development of chronic morphine tolerance in rats. TNF soluble receptor expressed by HSV vector reduced gene transcription of mRNA of spinal TNFα and IL-1β induced by repeated morphine. Furthermore, we found that TNF soluble receptor mediated by HSV, reversed the upregulation of TNFα, IL-1β and phosphorylation of p38 mitogen-activated protein kinase (MAPK) induced by repeated morphine. These results support the concept that proinflammatory cytokines may play an important role in the pathogenesis induced by morphine. This study provides a novel approach to treating morphine tolerance.
Keywords: morphine tolerance, cytokines, gene therapy
INTRODUCTION
Chronic pain resulting from tissue injury or damage to structures in the nervous system, is a multidimensional phenomenon involving physical states and psychosocial variables, and is a significant clinical problem. Opiate/narcotic analgesics are the most effective treatments for severe pain, but their clinical utility is often hampered by the development of analgesic tolerance, as well as by de novo painful hypersensitivity to innocuous and noxious stimuli, phenomena observed in both animal and human studies1–4. Classical concepts (the prevailing neuron-centered view) have evolved in recent years with the realization that alterations in neuronal functions fail to explain all of the crucial mechanisms involved5, 6. Since early 1990's, the studies have progressively challenged the classical concept that glia only serve a passive support function in the CNS. Glial cells are considered to be crucial sources of nitric oxide (NO), cytokines (e.g. TNFα, IL-1β), and cyclooxygenase products that influence synaptic transmission in the CNS7. The chronic morphine-induced activation of glial proinflammatory immune responses could activate the MAPK and protein kinase C (PKC) pathways, which are key players in the intracellular signaling cascade leading to the development of morphine tolerance8. Administration of the glial metabolic inhibitor fluorocitrate has been found to attenuate the development of morphine tolerance9. Pentoxifylline (a glia inhibitor) significantly blocked the development of morphine tolerance in naive mice, as well as in a model of neuropathic pain10. Evidence that intrathecal pretreatment with minocycline (microglia inhibitor) attenuates not only the development of morphine antinociceptive tolerance, but also the activities of spinal glia induced by chronic morphine treatment11. Chronic morphine increased glia activation releasing TNFα and/or IL-1β in the spinal cord3, 9, 12, 13, and inhibition of these factors may delay morphine tolerance3, 12. The mechanism underlying the involvement of glial cell products in morphine tolerance is unclear in detail.
Gene transfer offers the possibility to produce and release bioactive macromolecules in a local site in vivo. The local production of neurotransmitters achieved by therapeutic gene transfer, may be used to achieve desired outcomes, while avoiding unwanted adverse side effects that would result from activation of the same receptors in other pathways by a systemically administered drug14. Among the several gene transfer vectors that are available, herpes simplex virus (HSV) is particularly well suited for delivery of a gene in the peripheral nervous system15. Recombinant vectors that are entirely replication defective, retain the ability to establish a persistent quiescent state in neurons, but are unable to replicate (or reactivate) in the nervous system15. We have constructed highly replication-defective HSV genomic vectors that establish a persistent state that can be used to deliver and express transgenes in DRG neurons. Transduction of sensory neurons of the DRG by footpad inoculation with HSV-based vectors coding for human proenkephalin, produces an antihyperanalgesic effect in models of acute thermal pain16, is antinociceptive in subacute inflammatory pain17, and reduces both nocifensive behavior and joint destruction in a rodent model of arthritis18. DRG neurons transduced with an HSV vector transport transgenecoded enkephalin both centrally and peripherally in the bipolar axon of the primary sensory afferent19. Recently, we have demonstrated that highly defective HSV-based vectors can be used to transduce neurons of the DRG to release bioactive peptides from nerve terminals in the spinal cord, to produce antinociceptive effects in models of inflammatory and neuropathic pain20–22. HSV vectors encoding preproenkephalin demonstrated physiological improvement in visceral pain induced by bladder irritation either23. In the current study we investigated the effect of HSV-mediated expression of p55 TNF soluble receptor (TNFSR) on morphine tolerance in rats. We found that HSV vector-mediated TNFSR expression enhanced the antinociceptive effects of acute morphine and attenuated tolerance of chronic morphine in rats.
RESULTS
Expression of TNF soluble receptor mediated by T0TNFSR vector in vivo
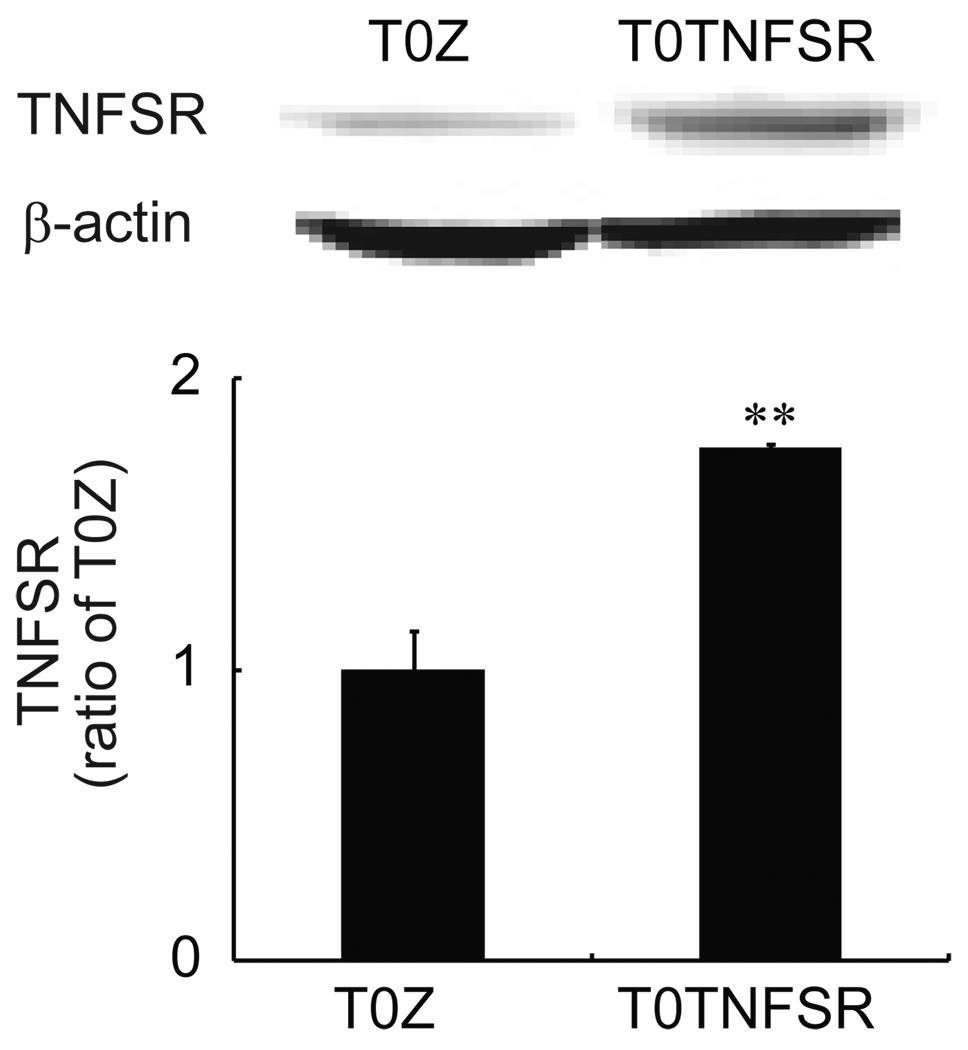
We have previously reported that the non-replicating herpes HSV vector T0TNFSR produces p55 TNFSR in primary DRG neurons in culture in vitro24, and in the lumbar DRG and spinal cord in vivo following subcutaneous inoculation with the vector in the hindpaws of rats24. In the current study, the expression of spinal TNFSR mediated by HSV in morphine tolerance rats was tested. We injected vectors (T0TNFSR or control vector T0Z) into the rat hindpaws. One week after vector injection, rats received chronic morphine for 7 days. Then the spinal cords were harvested, and Western blots were carried out for the expression of TNFSR. We found that inoculation of T0TNFSR significantly upregulated the expression of TNFSR in the spinal dorsal horn (Fig.1).
Figure 1.
The expression of p55 TNFSR mediated by HSV vectors. Seven days after the vectors were inoculated into the hindpaws, rats received chronic morphine for 7 days. After the last dose of morphine, the spinal cord was harvested and a Western blot was conducted for testing signals of TNFSR. T0TNFSR injection significantly induced the expression of TNFSR. * p < 0.05 vs T0Z, n=3–4, ANOVA.
The effect of HSV vector expressing TNFSR on acute morphine antinociception
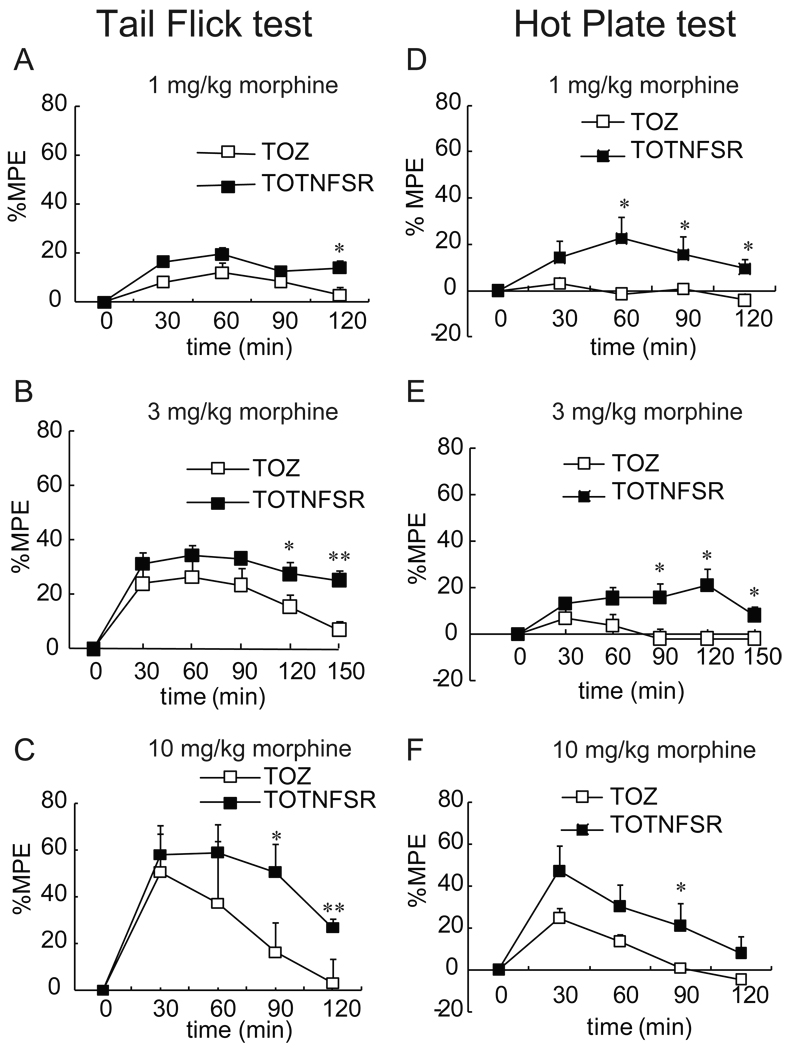
Previous evidence shows that intrathecal injection of acute morphine produces analgesia that dissipates by ~100 min, following which intrathecal interleukin 1 receptor antagonist (IL-1ra), soluble TNF receptor or interleukin 6 (IL-6) neutralizing antibody, unmasked significant continuing analgesia (135 min and 155 min)25, 26. Morphine analgesia is extended in mice with transgenic over-expression of IL-1ra within the brain and spinal cord or mice with targeted deletion of IL-1 receptor26. In the current study, rats were inoculated into two hindpaws with T0TNFSR or T0Z. One week after vector inoculation, animals received acute morphine (1, 3, or 10 mg/kg, IP). Thermal tail flick test and hot plate test were conducted to get thermal latency. Antinociceptive response induced by acute morphine progressively declined at 90 min, being almost completely abolished at 120–150 min in rats inoculated with T0Z. However, rats inoculated with T0TNFSR still showed the antinociceptive effect at 120–150 min. Thus, we think that HSV vector T0TNFSR expressing TNFSR enhanced antinociceptive effect of acute morphine (Fig.2).
Figure 2.
The time courses of acute morphine antinociception with the effect of T0TNFSR or T0Z were described. The non-replicating herpes simplex virus (HSV) vector T0TNFSR producing p55 TNFSR, and control vector T0Z were used. Seven days after vector inoculation, rats received a single dose of morphine (1, 3, and 10 mg/kg, IP). Thermal latency was tested in the tail flick test (A, B and C) and hotplate test (D, E and F). The MPE of morphine in rats with T0TNFSR was significantly higher than that with T0Z at 90–150 min. *p < 0.05, ** p < 0.01 vs T0Z, n=5–6, t test.
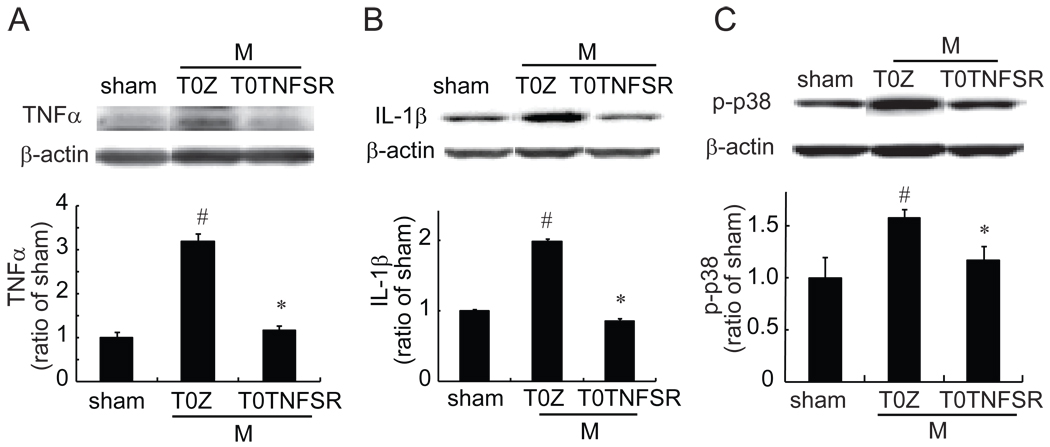
The changes of the spinal TNFα, IL-1β and p-p38 in morphine tolerance rats
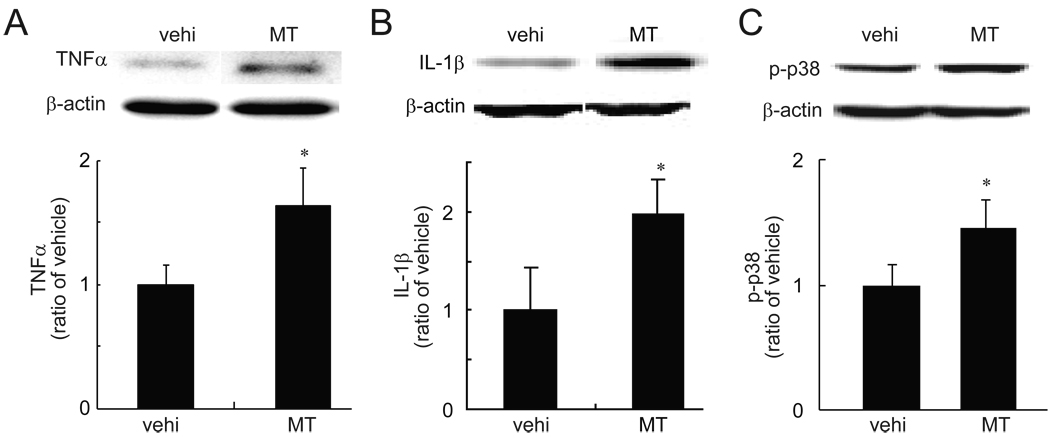
Evidence demonstrates that intrathecally or systemically repeated morphine induced upregulation of proinflammatory cytokines (e.g. TNFα, IL-1β) and phosphorylation of p383, 12, 27, 28. In our model of morphine tolerance, saline or morphine (17.5 mg/kg, IP) were administered once daily for 7 days. Thermal tail flick test and hot plate test were conducted at 60 min after morphine to get thermal latency. After a 7-day treatment with chronic morphine, rats showed a significant loss of the antinociceptive effect compared to that on day 1 (data not shown). After a 7-day treatment with chronic morphine or saline, spinal TNFα and IL-1β were examined with a Western blot. Chronic morphine significantly upregulated spinal TNFα and IL-1β, and enhanced phosphorylation of p38 (Fig.3) compared to vehicle.
Figure 3.
The expression of TNFα, IL-1β and p-p38 in morphine tolerance (MT). Rats received a repeated morphine dose for 7 days. At day 7, 1 hour after the last dose of morphine, L4/5 spinal dorsal horns were harvested, and the expression of TNFα (A), IL-1β (B) and p-p38 (B) was tested using western blots. Repeated morphine significantly induced upregulation of spinal TNFα, IL-1β and p-p38, * p < 0.05 vs vehicle (vehi), n=3–4, t test.
The effect of HSV vector expressing TNFSR on morphine tolerance behavioral response
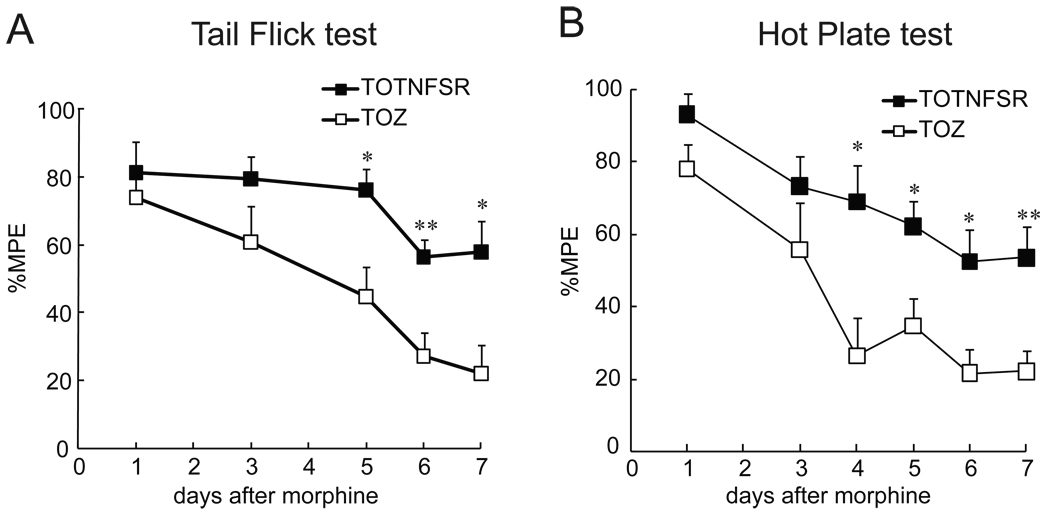
Recent studies demonstrate that repeated morphine induced upregulation of proinflammatory cytokines (e.g. TNFα, IL-1β, and IL-6), and that morphine tolerance is markedly attenuated by genetic or pharmacological blockade of IL-1 signaling3, 12, 26. A soluble TNF receptor may prevent TNFα from binding TNF receptor on the surface of cells. In this study, we examined whether overexpression of TNFSR mediated by HSV vectors changed chronic morphine tolerance. One week after vectors, rats received chronic morphine for 7 days. A thermal tail-flick test and hotplate test were conducted to get thermal latency. Rats with T0TNFSR showed a higher MPE at day 5–7 after morphine compared to rats with T0Z. Thus, HSV vectors expressing TNFSR delayed the development of morphine tolerance (Fig. 4).
Figure 4.
The effect of T0TNFSR or T0Z on chronic morphine tolerance. The non-replicating herpes simplex virus (HSV) vector T0TNFSR producing p55 TNFSR, and control vector T0Z were used. Seven days after the vector inoculation, rats received a repeated dose of morphine for 7 days. Thermal latency was tested in the tail flick test (A) and hotplate test (B) at 60 min after each dose of morphine. The MPE of morphine in rats with T0TNFSR was significantly higher than that with T0Z at day 5–7. *p < 0.05, ** p < 0.01 vs T0Z, n=5–6, t test.
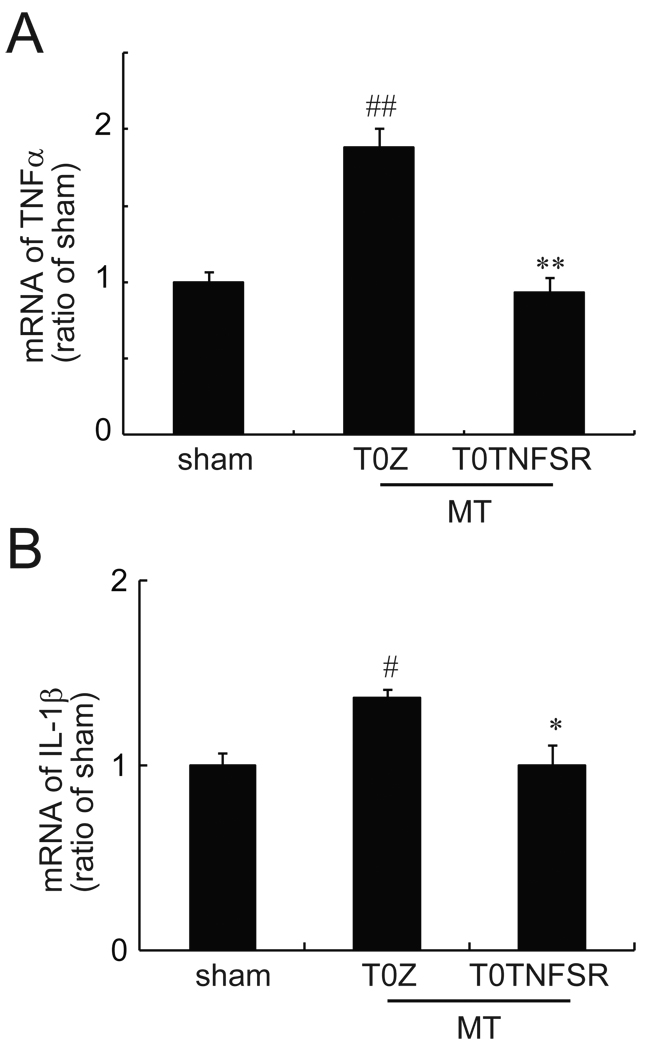
The effects of HSV vector expressing TNFSR on mRNA of spinal TNFα and IL-1β in morphine tolerance rats
Glia in the central nervous system are activated by chronic morphine and increase gene transcription of proinflammatory cytokines12. In this study, the expression of mRNA for spinal TNFα and IL-1β was measured by qRT-PCR. In the sham group, rats received T0Z and chronic saline for 7 days. Chronic morphine significantly increased mRNA levels of TNFα and IL-1β in rats with T0Z compared to the sham group. The expression of TNFα and IL-1β mRNA in morphine tolerance rats with T0TNFSR was significantly lower than that in morphine tolerance rats with T0Z, suggesting that HSV vector expressing TNFSR significantly suppressed the overexpression of mRNA of TNFα and IL-1β (Fig. 5).
Figure 5.
The effect of T0TNFSR or T0Z on the expression of mRNA of spinal TNFα and IL-1β. The non-replicating herpes simplex virus (HSV) vector T0TNFSR producing p55 TNFSR, and control vector T0Z were used. Rats received a repeated morphine dose at 1 week after vectors. In the sham group, rats receive T0Z and a repeated dose of saline. At day 7, 1 hour after the last dose of morphine or saline, L4/5 spinal dorsal horns were harvested, and the expression of TNFα and IL-1β was tested using quantitative real time PCR. Repeated morphine significantly induced upregulation of spinal TNFα (A) and IL-1β (B). The expression of TNFα and IL-1β mRNA in morphine tolerance rats with T0TNFSR was significantly lower than that in morphine tolerance rats with T0Z. # p<0.05, ## p<0.01 vs sham; * p < 0.05, **p<0.01vs T0Z, n=4–6, ANOVA, post hoc comparisons using Fisher's PLSD test (StatViewJ 5.2).
The effects of HSV vector expressing TNFSR on TNFα, IL-1β and p-p38 in the DRG in morphine tolerance rats
Evidence indicates that the DRG is essential for morphine antinociceptive tolerance29. Although there are reports that repeated morphine induces proinflammatory cytokines release from the spinal glia cells7, 12, few studies investigate the change of proinflammatory cytokines in the DRG level. Previous studies demonstrate that chronic morphine exposure increased phosphorylation of mitogen-activated protein kinases (MAPKs), including p38 in DRG neurons30, 31. In the current study, we examined whether overexpression of TNFSR mediated by HSV vector reduced neurochemical changes in morphine tolerance. In the sham group, animals received T0Z and chronic saline for 7 days. Animals inoculated with T0Z showed a statistically significant increase in TNFα, IL-1β and p-p38 in the DRG in the chronic morphine state compared to sham. TNFα, IL-1β, and p-p38 in morphine tolerance rats with T0TNFSR in the DRG, was significantly lower than that in morphine tolerance rats with T0Z (Fig.6).
Figure 6.
The effect of T0TNFSR or T0Z on the expression of TNFα, IL-1β, and p-p38 in the DRG. The non-replicating herpes simplex virus (HSV) vector T0TNFSR producing p55 TNFSR, and control vector T0Z were used. Rats received a repeated morphine dose 1 week after vectors. In the sham group, rats receive T0Z and a repeated dose of saline. At day 7, 1 hour after the last dose of morphine or saline, L4/5 DRGs were harvested, and the expression of TNFα, IL-1β and p-p38 was tested using Western blots. Repeated morphine admimistrations significantly induced upregulation of TNFα, IL-1β and p-p38. The expression of TNFα (A), IL-1β (B) and p-p38 (C) in morphine tolerance rats with T0TNFSR, was significantly lower than that in morphine tolerance rats with T0Z. # p<0.05 vs sham; * p < 0.05, n=4–6, ANOVA, post hoc comparisons using Fisher's PLSD test (StatViewJ 5.2).
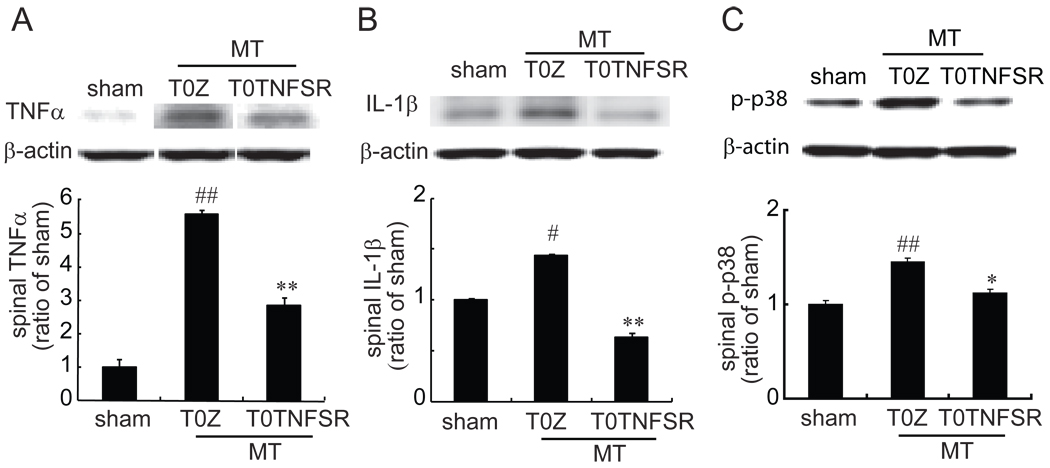
The effects of HSV vector expressing TNFSR on the spinal TNFα, IL-1β and p-p38 in morphine tolerance rats
In this experiment, we challenged whether overexpression of TNFSR mediated by HSV vector reduced neurochemical changes in morphine tolerance. In the sham group, animals received T0Z and chronic saline for 7 days. Animals inoculated with T0Z showed a statistically significant increase in the spinal TNFα, IL-1β and p-p38 in the chronic morphine state compared to sham. The expression of spinal TNFα, IL-1β and p-p38 in morphine tolerance rats with T0TNFSR, was significantly lower than that in morphine tolerance rats with T0Z. Thus, the results showed that HSV vector expressing TNFSR significantly suppressed the upregulation of TNFα, IL-1β and p-p38 induced by chronic morphine in the spinal cord (Fig.7).
Figure 7.
The effect of T0TNFSR or T0Z on the expression of spinal TNFα, IL-1β and p-p38. The non-replicating herpes simplex virus (HSV) vector T0TNFSR producing p55 TNFSR, and control vector T0Z were used. Rats were inoculated with T0TNFSR or T0Z. Rats received a repeated morphine dose at 1 week after vectors. In the sham group, rats receive T0Z and a repeated dose of saline. At day 7, 1 hour after the last dose of morphine or saline, L4/5 spinal dorsal horns were harvested, and the expression of TNFα, IL-1β and p-p38 was tested using western blots. Repeated morphine admimistrations significantly induced upregulation of spinal TNFα, IL-1β and p-p38. The expression of spinal TNFα (A), IL-1β (B) and p-p38 (C) in morphine tolerance rats with T0TNFSR, was significantly lower than that in morphine tolerance rats with T0Z. # p<0.05, ## p<0.01 vs sham; * p < 0.05, **p<0.01vs T0Z, n=4–6, ANOVA, post hoc comparisons using Fisher's PLSD test (StatViewJ 5.2).
DISCUSSION
Evidence suggests that proinflammatory cytokines play an important role in the phenomena of morphine tolerance9, 12, 25. The results of the current study demonstrated that 1) the expression of TNFSR mediated by HSV vectors enhanced acute morphine antinociception; 2) spinal TNFα, IL-1β and p-p38 were increased in morphine tolerance rats; 3) HSV vector expressing TNFSR suppressed mRNA of spinal TNFα and IL-1β in morphine tolerance; and 4) HSV vector expressing TNFSR reversed upregulation of spinal TNFα, IL-1β and p-p38 induced by repeated morphine.
Morphine has profound immunomodulatory effects, influencing immune cells directly via opioid receptors and indirectly by modulating neuroendocrine systems that regulate immune function32, 33. Systemic morphine induced upregulation of spinal proinflammatory cytokines12. Moreover, brain-to-spinal cord projections contribute markedly to systemic opioid tolerance34. Chronic morphine given intrathecally induced morphine tolerance and activation of the spinal proinflammatory systems3. Thus, activation of spinal proinflammatory systems after systemic morphine may be, at least in part, involved in morphine tolerance.
It is notable that the expression of glial activation markers increases in the spinal cord and brain only after chronic exposure to morphine9, 12, 35, 36, and that activated glia release proinflammatory cytokines7. Recent evidence shows that proinflammatory cytokines (e.g. TNFα, IL-1β) play an important role in the antagonism of morphine analgesia and the development of morphine tolerance and withdrawal3, 9, 12, 35. Glial inhibitor potentiated the antinociceptive effect of acute morphine37. In the acute morphine experiment, intrathecal injection of morphine produces antinociceptive effect that dissipates by about 100 min, following which intrathecal TNFα soluble receptor unmasks significant continuing analgesia (135 min to 155 min)25. An important finding from the present study is that overexpression of soluble TNF receptor by HSV vector enhanced the acute antinociceptive effect of morphine, which is consistent with the previous results3. Spinal IL-1 protein also opposes systemic opioid analgesia25. The systemic IL-1 receptor antagonist potentiates acute systemic morphine antinociception26. Acute morphine and methadone administration causes a proinflammatory cytokines-mediated opposition of acute intrathecal and/or systemic opioid antinociception25. These data above demonstrate that opioid-induced proinflammatory mediators contribute significantly to the opposition of morphine analgesia even upon the first exposure to opioids25.
Evidence demonstrates that either intrathecallly or systemically repeated morphine induced upregulation of spinal proinflammatory cytokines (e.g. TNFα, IL-1β) and phosphorylation of p383, 12, 25, 27, 28. TNF soluble receptor may prevent TNFα from binding the TNF receptor on the surface of cells. Propentofylline (inhibitor of the activation of glia) reduces chronic morphine-induced upregulation of mRNA for IL-1β, IL-6 and TNFα at the L5 lumbar spinal cord13. Chronic administration of a combination of IL-1ra, TNFSR and anti-IL-6 antibody restores acute morphine antinociception in nerve-injured rats and also significantly reversed the development of morphine tolerance12. Antinociceptive effects of morphine is extended in strains of mice genetically impaired in IL-1 signaling; acute or chronic blockade of IL-1 signaling by various IL-1ra, or IL-1 tri-peptide antagonist significantly prolongs and potentiates antinociceptive effect of morphine26. Proinflammatory mediators often synergize and interact in the morphine tolerance. P38 MAPK activation is pivotal to the release of several inflammatory cytokines38, 39, and has been implicated in glia mediated morphine anti-analgesia40. Etanercept is a recombinant TNF soluble receptor; it binds to the released TNFα, and prevents its interaction with membrane receptors. Recent study demonstrates that etanercept restores the antinoceptive effect of morphine in morphine-tolerant rats by inhibition of proinflammatory cytokine TNF-α, IL-1β, and IL-6 expression and spinal neuroinflammation41. Application of a mitogen-activated protein kinases (MAPKs) inhibitor reduces morphine tolerance42. Chronic morphine has been shown to activate several MAPKs including p38, known to regulate the production of proinflammatory mediators27, 28, 43–46.
There are reports that chronic morphine induces proinflammatory cytokines release from the spinal glia cells7, 12, but few studies investigate the change of proinflammatory cytokines in the DRG level. Previous studies demonstrate that chronic morphine exposure induced increases in phosphorylation of MAPKs, including p38 in DRG neurons30, 31. In the current study, to our knowledge, we are the first to report that cytokines (TNFα and IL-1β) in the DRGs were upregulated in morphine tolerance.
The intracellular transduction mechanisms by which chronic morphine modulates cytokine formation are not known with certainty, but a few possibilities are proposed based on existing literature in diverse fields. Recent studies demonstrate that toll like receptor 4 (TLR4), ceramide and ROS, likely play important roles in the modulation of cytokine release in the face of morphine tolerance. A growing body of evidence has emerged recently that implicates activation of TLR4 on glial cells in the development of opiate-induced hyperalgesia and antinociceptive tolerance as well as neuropathic pain45, 47. Recent evidence shows that morphine and its metabolite (morphine-3-glucoronide) activate glial TLR4 receptor in a non-stereoselective pattern48, 49. The activation of the TLR4-derived signaling pathway by morphine45 is one potential pathway that links chronic morphine administration to activation of the proinflammatory cytokines-p38 MAPK pathway, hence, the development of hyperalgesia and antinociceptive tolerance to morphine. Also, evidence demonstrates that the development of antinociceptive tolerance to repeated doses of morphine is consistently associated with the appearance of several tyrosine-nitrated proteins in the dorsal horn of the spinal cord; blocking protein nitration attenuates proinflammatory cytokines (e.g. TNFα, IL-1β), and prevents the development of tolerance to morphine44 in mice. Ceramide is a potent proinflammatory and proapoptotic sphingolipid50. Chronic administration of morphine activates the ceramide metabolic pathway in spinal glial cells, resulting in increased production in spinal sphingosine-1-phosphate (S1P, the end-product of ceramide metabolism) by sphingosine kinases. After its extracellular release, S1P would then bind to its receptors on glial cells, initiating a series of signaling pathways, culminating in enhanced production of IL-1β and TNFα3, 7, 43, 44, 51.
In summary, our results demonstrated the release of spinal proinflammatory molecules as necessary for chronic morphine tolerance. Blocking the proinflammatory cytokines was able to delay the development of morphine tolerance. These studies will prove a novel approach to morphine tolerance.
METHOD
Construction of herpes simplex virus vector expressing the p55 TNFSR
The vector T0TNFSR contains the coding sequence for amino acids 1 to 211 of the human p55 TNFSR, which is under the regulatory control of the HSV ICP0 immediate early promoter. This is in the UL41 locus of an HSV recombinant defective for HSV genes ICP4, ICP22, and ICP27. T0TNFSR was generated as described previously20, 24. Control vector T0Z is identical to T0TNFSR except that it contains the E. coli lacZ gene in place of p55 TNFSR. The schematic representation for the HSV vector construction has been shown20.
Animal and evaluation of morphine tolerance
Male Sprague-Dawley rats (body weight 225–250 g) were housed one to two per cage approximately 1 week prior to the beginning of the study, with free access to food and water and maintained on a 12:12, light: dark schedule at 21°C and 60% humidity. All housing conditions and experimental procedures were approved by the University IACUC. Animals were inoculated subcutaneously into the plantar surface of the hindpaws with 30 µl of T0TNFSR or T0Z. To induce antinociceptive tolerance to morphine, 7 days after vector inoculation rats received a repeated dose of morphine (17.5 mg/kg, once a day, IP) for 1 week. Morphine injection and behavior counting were carried out by a blinded operator.
Behavioral testing
Tail-flick test
The hot-water tail-flick test was performed by placing the distal third of the tail in a water bath maintained at 55±0.3°C. Rats were restrained in a hard plastic box. The latency until tail withdrawal from the bath was determined. The intensity was adjusted to produce a baseline latency of approximately 3 seconds. If rats did not show tail withdrawal response in 10 seconds, we ceased the experiment, and 10 seconds were used as cutoff to avoid tissue damage.
Hot plate test
Hot-plate thermal latency was performed with a Harvard Apparatus Ltd. hot plate apparatus (Edenbridge, Kent, United Kingdom). In the test, the rats were kept inside a circular transparent plastic cage on the hot plate (52±0.3°C). Licking or shaking the hind paw or jumping was considered as a sign of thermal nociceptive response. Time to the first reaction was measured. To avoid tissue damage, the cutoff time was set to 40s.
Western blot analysis
One hour after the last dose of morphine administration, the L4/5 spinal dorsal horn was harvested. Samples were rapidly homogenized in lysis buffer (50mM Tris HCl, 150mM NaCl, 1mM EDTA, 0.1% SDS, 1% NP-40, 1% sodium deoxycholate). After homogenization, samples were put on ice for 10 min, sonicated, then centrifuged at a 13000×g for 10 min. Supernatants were collected and stored at −80°C. Protein concentration for each sample was tested using Bio-Rad DC Protein Assay (Bio-Rad, USA). Protein samples with equal amounts (40µg/lane) were loaded and separated on SDS-PAGE gels and transferred to PVDF membranes (Millipore, USA). Membranes were blocked using 5% nonfat milk for 1 h, and incubated overnight at 4°C with a primary antibody of anti-TNFα IgG (1:500, Millipore), rabbit anti-IL-1β IgG (1:2000, Chemicon), rabbit anti-p-p38 IgG (1:200, Santa Cruz), or mouse anti-β-actin antibody (1:5000, Sigma). Membranes were then incubated in HRP-conjugated secondary antibody for 1 hour at room temperature, and followed by enhanced chemiluminescence detection (Amersham Biosciences, Arlington Heights, IL). Quantification of Western blots was done from the obtained chemiluminescence values (BioRad ChemiDoc). A ratio of the intensity of the band of interest to β-actin as an internal control was determined using Bio-Rad Imaging systems.
Quantitative Real-time PCR
Total RNA was isolated from the spinal cord using the TRIzol reagent (Invitrogen), treated with RNase-free DNase-I (Roche) and re-purified, and then quantified spectrophotometrically. Total RNA (1µg) was reverse transcribed (Omniscript RT kit, Qiagen) using random hexamers PCR primer. cDNA prepared from mRNA was amplified using the following primer sets: GAPDH- forward 5’-GTTTGTGATGGGTGTGAACC-3’ and -reverse 5’-TCTTCTGAGTGGCAGTGATG-3’; TNFα-forward 5’-CTTCAAGGGACAAGGCTG-3’ and -reverse 5’-GAGGCTGACTTTCTCCTG-3’; IL-1β-forward 5’-CATTGTGGCTGTGGAGAAG-3’ and -reverse 5’-ATCATCCCACGAGTCACAG-3’. PCR was performed with equal amounts of cDNA in the GeneAmp 7700 sequence detection system (Applied Biosystems), using SYBR® Green PCR Master Mix (Applied Biosystems). Reactions (total volume, 25 μl) were incubated at 50°C for 2 min, at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Each sample was measured, and data points were examined for integrity by analysis of the amplification plot. The comparative cycle threshold (Ct) method was used for relative quantification of gene expression. The amount of mRNA, normalized to the endogenous control (GAPDH) and relative to a calibrator, is given by 2−ΛΛCt, with Ct indicating the cycle number at which the fluorescence signal of the PCR product crosses an arbitrary threshold set within the exponential phase of the PCR, and Ct = [(Cttarget (unknown sample) − Ctend.control (unknown sample))] − [(Cttarget (calibrator sample) − Ctend. control (calibrator sample))] as previously described52.
Drugs and Data Analysis
Morphine hydrochloride was obtained from Elkins–Sinn (Cherry Hill, NJ). Antinociceptive effect was assessed 30, 60, 90 and 120 min after administration of acute morphine. Drugs were dissolved in physiological (0.9%) saline. In the tail flick and hotplate tests, the antinociceptive effects of morphine were represented as a percentage of maximum possible effect (%MPE) using the formula %MPE = [(Test-Baseline)/(Cutoff-Baseline)] × 100%. Mean±SEM value of %MPE was calculated for each group. The statistical significances of the differences were determined by ANOVA (StatViewJ 5.2) followed by post hoc comparisons using Fisher's PLSD test or by t test. P-values of less than 0.05 were considered to be statistically significant.
Acknowledgements
This work was supported by grants from the NIH DA020078 (S.H.), DA026734 (S.H.), DA025527 (S.H.) and NS066792 (S.H.), Department of Veterans Affairs and the NINDS NS038850 and NIDDK DK044935 (D.J.F and M.M). We acknowledge the excellent technical assistance of Vikram Thakur.
Footnotes
Conflict of Interest
David Fink receives compensation for professional services from the University of Michigan and from the Department of Veterans Affairs. He also receives payments from the University of Pittsburgh for patents owned by the University on which he is a co-inventor. None of other authors have received compensation for professional services or anticipate receiving such compensation in the near future.
References
- 1.Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17(5):498–510. doi: 10.1097/MJT.0b013e3181ed83a0. [DOI] [PubMed] [Google Scholar]
- 2.Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25(2):409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24(33):7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22(18):8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28(2):101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28(12):661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60(3):297–307. [PubMed] [Google Scholar]
- 9.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39(3):281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 10.Mika J, Korostynski M, Kaminska D, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, et al. Interleukin-1 alpha has antiallodynic and antihyperalgesic activities in a rat neuropathic pain model. Pain. 2008;138(3):587–597. doi: 10.1016/j.pain.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, et al. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22(1):114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22(22):9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29(2):327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- 14.Glorioso JC, Mata M, Fink DJ. Therapeutic gene transfer to the nervous system using viral vectors. J Neurovirol. 2003;9(2):165–172. doi: 10.1080/13550280390193984. [DOI] [PubMed] [Google Scholar]
- 15.Fink DJ, DeLuca NA, Goins WF, Glorioso JC. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96(6):3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8(7):551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 18.Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21(20):7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antunes bras J, Becker C, Bourgoin S, Lombard M, Cesselin F, Hamon M, et al. Met-enkephalin is preferentially transported into the peripheral processes of primary afferent fibres in both control and HSV1-driven proenkephalin A overexpressing rats. Neuroscience. 2001;103(4):1073–1083. doi: 10.1016/s0306-4522(01)00034-3. [DOI] [PubMed] [Google Scholar]
- 20.Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14(13):1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- 21.Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15(3):183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama H, Sasaki K, Franks ME, Goins WF, Goss JR, de Groat WC, et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2009;20(1):63–71. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59(5):843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22(8):1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115(1–2):50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069(1):235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23(8):2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- 29.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107(29):13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci. 2001;14(7):1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci. 2008;28(22):5836–5845. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83(1–2):19–28. doi: 10.1016/s0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62(2):111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 34.Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92(1–2):5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 35.Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, et al. The Role of TNFalpha in the Periaqueductal Gray During Naloxone-Precipitated Morphine Withdrawal in Rats. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61(5):1766–1773. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast) Brain Behav Immun. 2009;23(2):240–250. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86(6):1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu HE, Sun HS, Cheng CW, Tseng LF. p38 mitogen-activated protein kinase inhibitor SB203580 reverses the antianalgesia induced by dextro-morphine or morphine in the mouse spinal cord. Eur J Pharmacol. 2006;550(1–3):91–94. doi: 10.1016/j.ejphar.2006.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu HE, Thompson J, Sun HS, Terashvili M, Tseng LF. Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J Pharmacol Exp Ther. 2005;314(3):1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]
- 41.Shen CH, Tsai RY, Shih MS, Lin SL, Tai YH, Chien CC, et al. Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesth Analg. 2011;112(2):454–459. doi: 10.1213/ANE.0b013e3182025b15. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40(2):101–107. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- 43.Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, et al. Counter-Regulation of Opioid Analgesia by Glial-Derived Bioactive Sphingolipids. J Neurosci. 2010;30(46):15400–15408. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, et al. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117(11):3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30(11):581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169(2):843–854. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102(16):5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Scientific World Journal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165(2):569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 51.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 52.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]