Abstract
Glitazones, used for type II diabetes, have been associated with liver damage in humans. A structural feature known as a 2,4-thiazolidinedione (TZD) ring may contribute to this toxicity. TZD rings are of interest since continued human exposure via the glitazones and various prototype drugs is possible. Previously, we found that 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione (DCPT) was hepatotoxic in rats. To evaluate the importance of structure on DCPT toxicity, we therefore studied two series of analogues. The TZD ring was replaced with: a mercaptoacetic acid group ([[[(3,5-dichlorophenyl)amino]carbonyl]thio]acetic acid, DCTA); a methylated TZD ring (3-(3,5-dichlorophenyl)-5-methyl-2,4-thiazolidinedione, DPMT); and isomeric thiazolidinone rings (3-(3,5-dichlorophenyl)-2- and 3-(3,5-dichlorophenyl)-4-thiazolidinone, 2-DCTD and 4-DCTD, respectively). The following phenyl ring-modified analogues were also tested: 3-phenyl-, 3-(4-chlorophenyl)-, 3-(3,5-dimethylphenyl)- and 3-[3,5-bis(trifluoromethyl)phenyl]-2,4-thiazolidinedione (PTZD, CPTD, DMPT and DFMPT, respectively). Toxicity was assessed in male Fischer 344 rats 24 hours after administration of the compounds. In the TZD series only DPMT produced liver damage, as evidenced by elevated serum alanine aminotransferase (ALT) activities at 0.6 and 1.0 mmol/kg (298.6 ± 176.1 and 327.3 ± 102.9 Sigma-Frankel units/ml, respectively) versus corn oil controls (36.0 ± 11.3) and morphological changes in liver sections. Among the phenyl analogues, hepatotoxicity was observed in rats administered PTZD, CPTD and DMPT; with ALT values of 1196.2 ± 133.6, 1622.5 ± 218.5 and 2071.9 ± 217.8, respectively (1.0 mmol/kg doses). Morphological examination revealed severe hepatic necrosis in these animals. Our results suggest that hepatotoxicity of these compounds is critically dependent on the presence of a TZD ring and also the phenyl substituents.
Keywords: 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione; hepatotoxicity; rat; structure-activity relationship; thiazolidinedione
Introduction
As part of an investigation into the potential toxicity of cyclic imide containing compounds, we previously found that 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione (DCPT, Fig. 1) produced liver damage in male rats (Kennedy et al., 2003). We are interested in this compound because it contains a 2,4-thiazolidinedione (TZD) ring. This structural feature is also present in the “glitazone” insulin-sensitizing agents that were originally developed for the treatment of type II diabetes. Troglitazone (Fig. 1), the first member of this class to be marketed, was associated with liver damage in diabetic patients and was removed from the market (Gitlin et al., 1998; Kohlroser et al., 2000; Graham et al., 2003). Rosiglitazone and pioglitazone are still used clinically although there have been several reports of mild hepatic injury with both of these drugs (Gouda et al., 2001; Maeda, 2001; Marcy et al., 2004; El-Naggar et al., 2008). As a class, the glitazones are not recommended for use in patients with existing liver disease (Scheen, 2001). TZD derivatives are also being investigated as potential aldose reductase inhibitors for the treatment of diabetic complications (Bruno et al., 2002; Rakowitz et al., 2006), analgesic and anti-inflammatory agents (Ali et al., 2007) and androgen antagonists (Yang et al., 2008).
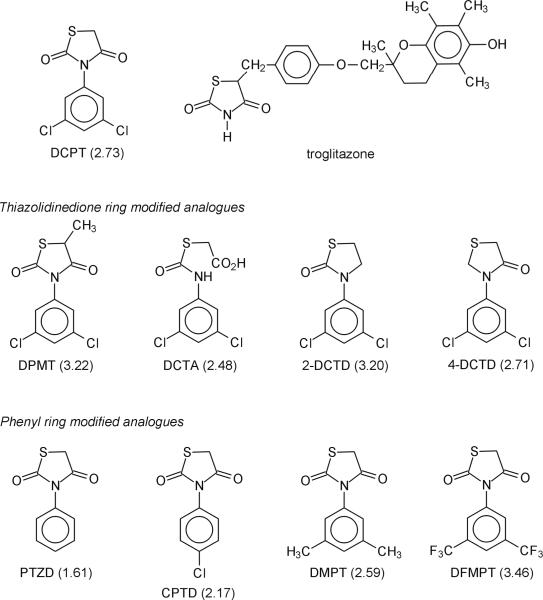
Figure 1.
Structures of thiazolidinedione ring-containing compounds. Calculated log P values for DCPT and its analogues are shown in parenthesis.
Upon further investigation, we found that DCPT-induced hepatotoxicity in rats was dependent on time, dose and gender (Patel et al., 2008). Liver damage, seen as morphological changes and elevations in serum alanine aminotransferase (ALT) levels, was apparent within 3 hr of dosing and was fully established at 24 hr in male rats. In comparion, female rats were less susceptible to hepatotoxicity than males (Patel et al., 2008), which could be due to gender-dependent differences in metabolism (Mugford and Kedderis, 1998; Czerniak, 2001). In separate studies, we evaluated the potential role of cytochromes P450 (CYPs) in DCPT-induced liver damage (Crincoli et al., 2008). Both 1-aminobenzotriazole (non-specific CYP inhibitor) and troleandomycin (CYP3A inhibitor) attenuated DCPT toxicity. In contrast, the CYP3A inducer dexamethasone potentiated hepatic injury. Thus, it seems that a CYP3A-derived DCPT metabolite is responsible for the hepatotoxicity of this compound in male rats (Crincoli et al., 2008).
As noted above, TZD rings are found in rosiglitazone, pioglitazone and several prototype therapeutic agents. Thus, there is potential for continued human exposure to compounds that contain this structural feature. As a consequence, it is important to further explore the potential role of TZD rings in chemically-induced hepatotoxicity. Towards this goal, we decided to conduct a structure-activity relationship (SAR) study. We therefore synthesized, characterized and evaluated the potential toxicity of two different series of DCPT analogues: (1) compounds that contained modifications to the TZD ring, but retained the 3,5-dichlorophenyl ring and (2) compounds that retained the TZD ring, but contained different substituents in the phenyl ring. For the first series of compounds (Fig. 1), the TZD ring was replaced with: its potential hydrolysis product, a mercaptoacetic acid group ([[[(3,5-dichlorophenyl)amino]carbonyl]thio]acetic acid, DCTA); an alkyl substituted TZD ring (3-(3,5-dichlorophenyl)-5-methyl-2,4-thiazolidinedione, DPMT); and isomeric thiazolidinone rings (3-(3,5-dichlorophenyl)-2-thiazolidinone and 3-(3,5-dichlorophenyl)-4-thiazolidinone, 2-DCTD and 4-DCTD, respectively). In the second set of analogues, phenyl substituents were investigated that could exert electron-releasing or electron-withdrawing effects on the TZD ring, which could potentially alter its metabolism and hence toxicity. Therefore, we also evaluated the following phenyl-substituted DCPT analogues (Fig. 1): 3-phenyl-2,4-thiazolidinedione (PTZD), 3-(4-chlorophenyl)-2,4-thiazolidinedione (CPTD), 3-(3,5-dimethylphenyl)-2,4-thiazolidinedione (DMPT) and 3-[3,5-bis(trifluoromethyl)phenyl]-2,4-thiazolidinedione (DFMPT). Based on our results, it can be concluded that an intact TZD ring is an important structural feature for hepatotoxicity and that phenyl ring substituents can alter toxicity of this series of compounds.
Materials and methods
Chemicals/reagents
The starting materials 3,5-dichlorophenyl isocyanate, 3,5-dichlorophenyl thiourea and 3,5-dichloroaniline were obtained from Alfa Aesar (Ward Hill, MA, USA). Phenyl isocyanate, 3,5-dimethylphenyl isocyanate, 4-chlorophenyl isocyanate, 3,5-bis(trifluoromethyl)phenyl isocyanate, ethyl-2-mercaptoacetate, ethyl-2-mercaptopropionate, 1,2-dibromoethane and mercaptoacetic acid were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI, USA). Bio-Rad protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA, USA). 2,4-Dinitrophenylhydrazine, sodium pyruvate, DL-alanine, α-keto-glutaric acid, alanine aminotransferase assay kit (ALT, No. 505-P), blood urea nitrogen assay kit (BUN, No. 640-5) and urine/blood glucose assay kit (No. 510-DA) were all products of Sigma Chemical Co. (St. Louis, MO, USA). These assay kits were later discontinued by the manufacturer. In some experiments, the ALT assay was therefore done using a procedure similar to that described in the Sigma kit. The required solutions were alanine (0.2 M)/α-ketoglutarate (1.8 mM) in 100 mM sodium phosphate buffer, pH 7.4; 2,4-dinitrophenylhydrazine (20 mg/dl) in 1 M HCl; and sodium pyruvate (1.5 mM) in 100 mM sodium phosphate buffer, pH 7.4. A standard curve (0, 23, 50, 83,125 Sigma-Frankel units/ml) was generated by combining the sodium pyruvate (0, 0.5, 0.1, 0.15, 0.2 ml) and alanine/α-ketoglutarate solutions (0.5, 0.45, 0.4, 0.35, 0.3 ml) with water (0.1 ml). Serum samples and standards (0.1 ml) were incubated with the alanine/α-ketoglutarate solution (0.5 ml) for 30 min at 37 °C before addition of 2,4-dinitrophenylhydrazine reagent (0.5 ml). After standing at room temperature for 20 min, 0.4 M NaOH (5 ml) was added and absorbance was measured at 505 nm. Samples outside the range of the standard curve were diluted 1:10 or 1:20 with water before being re-assayed. The BUN assay (No. B7551-120) and urine/blood glucose assay (No. G7518-150) were replaced with the indicated kits obtained from Pointe Scientific (Detroit, MI, USA). Experiments were conducted to ensure that the assay results from the different kits were comparable.
Animals
Male Fischer 344 rats (ca. 200 g) were purchased from Charles River Laboratories (Wilmington, MA, USA). The rats were individually housed in standard stainless steel hanging cages under a 12 h light/dark cycle at ca. 22 °C and 45–50% relative humidity. Food (laboratory rodent diet #5001, PMI Foods, Inc., St. Louis, MO, USA) and water were freely available unless otherwise noted. The animals were held in the Vivarium for a minimum of one week before use in any experiments. All experiments involving the rats were approved by the Institutional Animal Care and Use Committee of the University of the Sciences in Philadelphia.
Syntheses
Melting points were determined with a Thomas-Hoover capillary melting point apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, on a Bruker AVANCE 400 nuclear magnetic resonance (NMR) spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane as the internal standard. The abbreviations used in reporting the splitting patterns are as follows: s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Elemental analyses were performed by Galbraith Laboratories Inc. (Knoxville, TN, USA).
Preparation of TZD ring-modified compounds
The methyl TZD ring compound, 3-(3,5-dichlorophenyl)-5-methyl-2,4-thiazolidinedione (DPMT), was synthesized by a modification of the method of Fujinami et al. (1971) and Kennedy et al. (2003). In step 1, ethyl 2-[[[(3,5-dichlorophenyl)amino]carbonyl]thio]propionate was prepared by refluxing 3,5-dichlorophenyl isocyanate (10.0 g, 0.053 mol) with ethyl 2-mercaptopropionate (6.96 g, 0.052 mol) and pyridine (0.114 ml) in toluene (32 ml) for 22 h. The reaction mixture was cooled in a freezer and the intermediate product was collected and dried in a vacuum oven. For step 2, ethyl 2-[[[(3,5-dichlorophenyl)amino]carbonyl]thio]propionate (5 g, 0.016 mol) and triethylamine (0.25 g, 0.0024 mol) were refluxed in toluene for 24 h. After cooling the reaction mixture in a freezer, the precipitate was filtered and discarded. The filtrate was concentrated on a rotary evaporator to afford the crude product. DPMT was purified by recrystallization using a mixed solvent system of ethanol-water; m.p. 157.5–158.5 °C. 1H NMR (DMSO-d6): δ 7.78 (d, 2H, Ar-H2,6), 7.57 (t, 1H, Ar-H4), 4.63 (q, 1H, CH) and 1.67 (d, 3H, CH3). 13C NMR (DMSO-d6): δ 175.56 (C=O), 171.79 (C=O), 136.77 (Ar-C3,5), 134.80 (Ar-C1), 129.72 (Ar-C4), 128.14 (Ar-C2,6), 45.02 (CH) and 18.82 (CH3). Anal. Calcd. for C10H7Cl2NO2S (276.13): C, 43.50; H, 2.56; N, 5.07; O, 11.59; S, 11.61; Cl, 25.68. Found: C, 43.54; H, 2.51; N, 5.04; S, 11.71; Cl, 26.41.
A modification of the method of Fujinami et al. (1968) was followed for the preparation of [[[(3,5-dichlorophenyl)amino]carbonyl]thio]acetic acid (DCTA). Briefly, 3,5-dichlorophenyl isocyanate (9.4 g, 0.05 mol), pyridine (0.2 ml) and benzoyl peroxide (0.005 g) were dissolved in 25 ml tetrahydrofuran (THF). Mercaptoacetic acid (4.6 g, 0.05 mol) in THF (25 ml) was then added dropwise and the reaction mixture was refluxed at 55 °C for 1 h. After cooling in a freezer, the precipitate was collected and discarded. The filtrate was concentrated on a rotary evaporator to yield the crude product. DCTA was recrystallized using absolute ethanol; m.p. 139–140 °C (lit. mp 122–124.5 °C (Fujinami et al., 1968)). 1H NMR (DMSO-d6): δ 12.08 (s, 1H, COOH), 10.85 (s, 1H, NH), 7.53 (d, 2H, Ar-H2,6), 7.29 (t, 1H, Ar-H4) and 3.73 (s, 1H, CH2). 13C NMR (DMSO-d6): δ 170.82 (C=O), 165.75 (C=O), 143.69 (Ar-C3,5), 134.95 (Ar-C1), 123.58 (Ar-C4), 117.81 (Ar-C2,6) and 32.70 (CH2). Anal. Calcd. for C9H6Cl2NO3S (279.11): C, 38.71; H, 2.17; N, 5.02; O, 17.20; S, 11.49; Cl, 25.40. Found: C, 38.76; H, 2.55; N, 5.00; S, 11.43; Cl, 25.22.
The two-step procedure of Brouwer and Blem (1987) was used for the synthesis of 3-(3,5-dichlorophenyl)-4-thiazolidinone (4-DCTD). First, 3,5-dichloroaniline (20.12 g, 0.125 mol) and mercaptoacetic acid (11.5 g, 0.125 mol) were combined in 95% ethanol with stirring on ice. Formalin solution (10.65 g, 37% by weight) was then added dropwise which led to the formation of a white precipitate. After stirring for 24 h, water (15 ml) was added to the reaction mixture and stirring was continued for several hours. The precipitated compound, 2-[[(3,5-dichlorophenyl)amino]methyl]thioacetic acid, was filtered and air dried. This intermediate (30 g, 0.112 mol) was then refluxed in xylene (217 ml) for 6 h with azeotropic removal of water (Dean-Stark trap) to yield 4-DCTD. The crude 4-DCTD was recrystallized using absolute ethanol; m.p. 103.5–105.5 °C (lit. mp 87–92 °C (Brouwer and Blem, 1987)). 1H NMR (DMSO-d6): δ 7.68 (d, 2H, Ar-H2,6), 7.48 (t, 1H, Ar-H4), 4.93 (s, 1H, CH2) and 3.73 (s, 1H, CH2). 13C NMR (DMSO-d6): δ 172.22 (C=O), 142.20 (Ar-C3,5), 134.90 (Ar-C1), 125.57 (Ar-C4), 120.93 (Ar-C2,6), 49.73 (CH2) and 33.19 (CH2). Anal. Calcd. for C9H7Cl2NOS (248.12): C, 43.57; H, 2.84; N, 5.64; O, 6.45; S, 12.92; Cl, 28.58. Found: C, 43.76; H, 3.06; N, 5.68; S, 12.87; Cl, 29.60.
Synthesis of 3-(3,5-dichlorophenyl)-2-thiazolidinone (2-DCTD) proceeded in two steps. The intermediate 2-methylimino-3-(3,5-dichlorophenyl)thiazolidine was first synthesized using a variation on the method of Toldy et al. (1972). Briefly, 3,5-dichlorophenyl thiourea (10 g, 0.045 mol) and 1,2-dibromoethane (19.27 g, 0.013 mol) were refluxed in isoamyl alcohol (25 ml) for 4 h. The resulting intermediate, 2-methylimino-3-(3,5-dichlorophenyl)thiazolidine was hydrolyzed to 2-DCTD via the diazonium salt, using a modification of the method of Tashiro and Nakamura (1985). Thus, 2-methylimino-3-(3,5-dichlorophenyl)thiazolidine (4.5 g, 0.018 mol) was dissolved in water (20 ml), the solution was acidified (pH 2.5–3.5) with HCl, and xylene (20 ml) was added. The reaction mixture was heated to 60 °C for 30 min and then aqueous sodium nitrite (5 ml, 5% solution) was added. The temperature was raised to 90 °C and heating was continued until the color of the reaction mixture became pale yellow. The crude product (2-DCTD) was extracted with xylene and the solvent was removed on a rotary evaporator. 2-DCTD was purified by column chromatography on silica gel (60–200 mesh) using benzene and then benzene-hexane (9:1) as eluents; m.p. 105–106 °C. 1H NMR (DMSO-d6): δ 7.56 (d, 2H, Ar-H2,6), 7.42 (t, 1H, Ar-H4), 4.21 (t, 2H, CH2) and 3.46 (t, 1H, CH2). 13C NMR (DMSO-d6): δ 172.17 (C=O), 141.89 (Ar-C3,5), 134.92 (Ar-C1), 124.91 (Ar-C4), 120.54 (Ar-C2,6), 50.88 (CH2) and 25.85 (CH2). Anal. Calcd. for C9H7Cl2NOS (248.12): C, 43.57; H, 2.84; N, 5.64; O, 6.45; S, 12.92; Cl, 28.58. Found: C, 44.00; H, 3.13; N, 5.68; S, 13.18; Cl, 29.31.
Preparation of phenyl ring-modified compounds
All analogues with modifications in the phenyl ring were synthesized similarly to DPMT. In brief, phenyl isocyanate or appropriately substituted derivatives were condensed with ethyl 2-mercaptoacetate, followed by base-catalyzed cyclization to the TZD ring-containing compounds.
Recrystallization of the unsubstituted analogue, 3-phenyl-2,4-thiazolidinedione (PTZD) using 95% ethanol yielded pure product; m.p. 142.5–143.5 °C. 1H NMR (DMSO-d6): δ 7.52–7.30 (m, 5H, Ar-H), 4.31 (s, 2H, CH2). 13C NMR (DMSO-d6): δ 172.84 (C=O), 172.28 (C=O), 134.18 (Ar-C3,5), 129.98 (Ar-C1), 129.78 (Ar-C4), 128.75 (Ar-C2,6) and 34.28 (CH2). Anal. Calcd. for C9H7NO2S (193.22): C, 55.95; H, 3.65; N, 7.25; O, 16.56; S, 16.59. Found: C, 55.96; H, 3.55; N, 7.22; S, 17.01.
Purification of 3-(3,5-dimethylphenyl)-2,4-thiazolidinedione (DMPT) was accomplished via recrystallization using 95% ethanol; m.p. 183–184 °C. 1H NMR (DMSO-d6): δ 7.10 (s, 1H, Ar-H4), 6.90 (s, 2H, Ar-H2,6), 4.29 (s, 2H, CH2) and 2.30 (s, 6H, Ar-CH3). 13C NMR (DMSO-d6): δ 172.73 (C=O), 172.26 (C=O), 139.30 (Ar-C3,5), 134.12 (Ar-C1), 131.06 (Ar-C4), 126.80 (Ar-C2,6), 34.28 (CH2) and 24.03 (Ar-CH3). Anal. Calcd. for C11H11NO2S (221.27): C, 59.71; H, 5.01; N, 6.33; O, 14.46; S, 14.40. Found: C, 59.78; H, 5.08; N, 6.22; S, 14.38.
The para-subsituted compound, 3-(4-chlorophenyl)-2,4-thiazolidinedione (CPTD), was recrystallized using a mixed solvent system of absolute ethanol-water; m.p. 143–144 °C. 1H NMR (DMSO-d6): δ 7.59 (m, 2H, Ar-H3,5), 7.37 (m, 2H, Ar-H2,6) and 4.29 (s, 2H, CH2). 13C NMR (DMSO-d6): δ 172.65 (C=O), 172.03 (C=O), 134.39 (Ar-C3,5), 132.08 (Ar-C1), 130.54 (Ar-C4), 130.00 (Ar-C2,6) and 35.22 (CH2). Anal. Calcd. for C9H6ClNO2S (227.66): C, 47.48; H, 2.66; N, 6.15; O, 14.06; S, 14.08; Cl, 15.57. Found: C, 47.31; H, 2.70; N, 5.91; S, 14.16; Cl, 15.66.
Purification of 3-[3,5-bis(trifluoromethyl)phenyl]-2,4-thiazolidinedione (DFMPT) was accomplished via recrystallization from hexane; m.p. 115–116 °C. 1H NMR (DMSO-d6): δ 8.28 (s, 1H, Ar-H4), 8.18 (s, 2H, Ar-H2,6) and 4.32 (s, 2H, CH2). C NMR (DMSO-d6): δ 172.56, 171.80, 136.09, 132.11 (q), 130.21, 127.75, 125.04, 123.73 (d), 122.33, 119.62 and 35.45. Anal. Calcd. for C11H5F6NO2S (329.21): C, 40.13; H, 1.53; N, 4.25; O, 9.72; S, 9.74; F, 34.62. Found: C, 40.37; H, 1.57; N, 4.27; S, 9.72; F, 34.43.
In vivo toxicity
The experimental method is similar to our previously published 24 h procedure (Patel et al., 2008). Rats were randomly divided into treatment groups (N= 3–4 rats/group) and transferred to individual stainless steel metabolism cages (Allentown Caging Equipment Co., Allentown, NJ, USA) to allow for the collection of urine. The animals were kept in the metabolism cages for a minimum of two days before dosing.
On the first day of the experiment (control day, before any treatments), urine was collected for six hours during which food and water were removed from the cages to avoid contamination or dilution of the urine sample. All six hour urine samples were stored at −78 °C for quantitative determination of protein levels. After the 6 h period, food (ca. 40 g) and water (150 ml) were returned into the cages. Food and water intake, and the amount of urine excreted over the next 18 h were also measured.
On day two, each animal received a single dose of a DCPT analogue (0.2, 0.4, 0.6 or 1.0 mmol/kg, i.p in corn oil) or corn oil only (4 ml/kg). Dosages >1.0 mmol/kg are generally impractical due to solubility problems. Rats treated with DCTA at 0.6 mmol/kg appeared to be moribund (sluggish movements and low body temperatures) 24 h after dosing; therefore, this compound was only evaluated at three doses (0.2, 0.4 and 0.6 mmol/kg, i.p. in corn oil). All animals were returned to the metabolism cages immediately after treatment and urine was collected as described above. Food and water intake, and the amount of urine excreted over the next 18 h were also measured. Twenty-four hour after dosing, the rats were anesthetized using isoflurane. While under anesthesia, a blood sample was obtained by cardiac puncture and then the animals were euthanized by cervical dislocation. The blood samples were allowed to clot for 15 min at room temperature and centrifuged at 12,000 rpm for 20 min. Analysis for alanine aminotransferase (ALT) levels was conducted using fresh serum and the serum samples were then stored at −78 °C for subsequent analysis of blood urea nitrogen (BUN) levels. The kidneys and livers were removed, weighed, and the right kidney and a section of liver were fixed in formalin for histological analysis. Tissue sections were prepared and stained with hematoxylin and eosin (H&E) by American Histolabs, Inc. (Gaithersburg, MD, USA).
Computational analyses
As an estimate of relative lipophilicities, log P values were calculated using the ChemDraw Ultra 8.0 software package (CambridgeSoft, Cambridge, MA, USA).
Statistics
Results are expressed as means ± SE (N = 3 or 4). The data were analyzed by a one way ANOVA followed by a Student-Newman-Keuls post hoc test. When the normality or equal variance tests failed, the data were analyzed by a one way ANOVA on ranks. Differences in the means were considered significant when p < 0.05.
Results
TZD ring-modified compounds
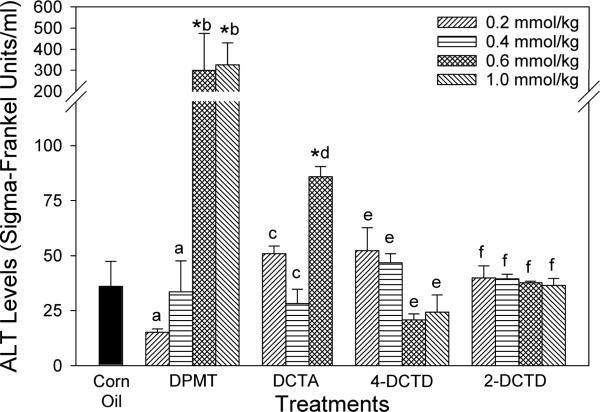
Serum ALT levels (Fig. 2) were measured in all rats as an index of liver damage. Compared to the two lower doses and corn oil controls, rats that received DPMT at 0.6 and 1.0 mmol/kg exhibited a significant elevation in ALT concentrations. Administration of DCTA at 0.6 mmol/kg, which resulted in reduced body temperatures and sluggish movements in the animals, produced a modest increase (about 2-fold) in ALT levels; but at 0.2 and 0.4 mmol/kg no changes were noted. 4-DCTD- and 2-DCTD-treated animals did not exhibit any significant changes in serum ALT levels at any of the doses evaluated. No significant changes were observed in liver weights with any of the treatments when compared across doses for a compound or to the corn oil controls (Table 1).
Figure 2.
Effect of thiazolidinedione ring modifications on serum alanine aminotransferase (ALT) levels in rats. Compounds were administered i.p. in corn oil. Controls received corn oil only (4 ml/kg). Values are means ± SE (N = 3–4). An asterisk (*) indicates that the value is significantly different (p < 0.05) from the corn oil control. Different letters within a treatment group indicate that the doses are significantly different (p < 0.05).
Table 1.
Effect of thiazolidinedione ring modifications on blood urea nitrogen, urine volume and protein content, or liver and kidney weights in rats. Compounds were administered i.p. in corn oil. Controls received corn oil only (4 ml/kg). Values are means ± SE (N = 3–4). An asterisk (*) indicates that the value is significantly different (p < 0.05) from the corn oil control. Different letters within a treatment group indicate that the doses are significantly different (p < 0.05).
| Treatment | Dose (mg/kg) | Blood urea nitrogen (mg/dl) | Twenty-four-hour urine volume (ml) | Six-hour urine protein content (mg) | Liver weight (g/100% BW) | Kidney weight (g/ 100% BW) |
|---|---|---|---|---|---|---|
| Corn Oil | - | 20.2 ± 2.0 | 3.7 ± 0.5 | 1.5 ± 0.4 | 3.49 ± 0.12 | 0.37 ± 0.01 |
| DPMT | 0.2 | 19.6 ± 0.8a | 6.5 ± 2.1a | 2.1 ± 0.7a | 3.67 ± 0.31a | 0.37 ± 0.01a |
| 0.4 | 24.6 ± 1.3a | 8.1 ± 1.7a | 1.6 ± 0.1a | 3.81 ± 0.22a | 0.38 ± 0.01a | |
| 0.6 | 19.8 ± 2.8a | 11.6 ± 1.0*,a | 2.6 ± 0.8a | 4.03 ± 0.27a | 0.39 ± 0.01a | |
| 1.0 | 20.0 ± 2.2a | 10.8 ± 1.3*,a | 2.5 ± 0.3a | 4.10 ± 0.07a | 0.41 ± 0.01a | |
| DCTA | 0.2 | 21.0 ± 1.0b | 12.5 ± 3.8b | 2.9 ± 0.9b | 3.58 ± 0.21b | 0.40 ± 0.01b |
| 0.4 | 62.2 ± 11.2*,c | 13.1 ± 2.4b | 2.5 ± 0.4b | 3.62 ± 0.05b | 0.43 ± 0.01 *,b,c | |
| 0.6 | 67.8 ± 17.9*,c | 9.5 ± 5.0b | 2.5 ± 1.1b | 3.46 ± 0.17b | 0.45 ± 0.01 *,c | |
| 4-DCTD | 0.2 | 26.8 ± 1.5d | 12.3 ± 1.7*,c | 1.8 ± 0.3c | 3.74 ± 0.23c | 0.40 ± 0.01d |
| 0.4 | 25.6 ± 1.9d | 11.3 ± 2.2*,c | 1.6 ± 0.2c | 3.96 ± 0.18c | 0.37 ± 0.01d | |
| 0.6 | 33.8 ± 2.4*,e | 9.1 ± 1.6*,c | 1.9 ± 0.4c | 3.46 ± 0.09c | 0.41 ± 0.01d | |
| 1.0 | 42.5 ± 1.1*,f | 12.3 ± 1.0*,c | 2.8 ± 0.3c | 3.81 ± 0.15c | 0.42 ± 0.01d | |
| 2-DCTD | 0.2 | 23.0 ± 1.2g | 6.0 ± 0.8d | 1.6 ± 0.4d | 3.96 ± 0.10d | 0.39 ± 0.01e |
| 0.4 | 22.3 ± 1.1g | 4.3 ± 0.3d | 1.8 ± 0.1d | 3.71 ± 0.11d | 0.38 ± 0.01e | |
| 0.6 | 23.9 ± 0.9g | 5.0 ± 0.7d | 2.2 ± 0.1d | 3.74 ± 0.11d | 0.40 ± 0.01e | |
| 1.0 | 24.4 ± 0.7g | 4.4 ± 1.3d | 2.0 ± 0.1d | 3.85 ± 0.15d | 0.37 ± 0.01e |
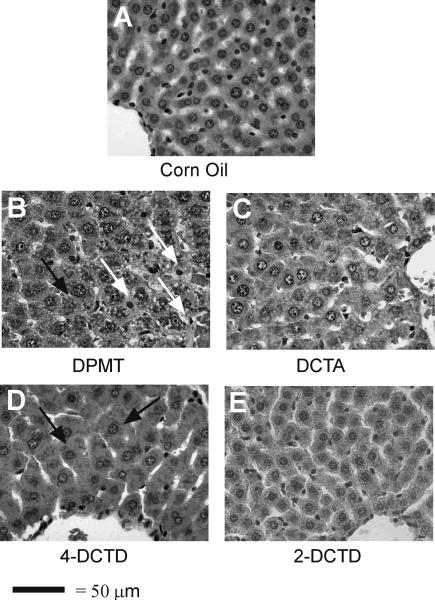
Representative photomicrographs of liver sections from the animals treated with corn oil or a TZD-ring modified DCPT analogue (highest dose for each compound) are shown in Fig 3. The liver from a corn oil-treated animal appeared normal (Fig. 3A). Hepatocytes were healthy in appearance and were arranged in a cord-like fashion radiating out of the central veins. Periportal hepatocytes also appeared normal with rounded nuclei and distinct cytoplasm. DPMT-treated animals at 0.2 and 0.4 mmol/kg (data not shown) did not show any noticeable changes in liver morphology compared to corn oil control animals. At the 0.6 mmol/kg DPMT dose, sections showed some damage near the centrilobular vein (data not shown). Cytoplasm in the cells was condensed and irregularly stained. Sinusoids showed more blood than the controls and some leakage of inflammatory cells. Liver sections from rats that received 1.0 mmol/kg DPMT exhibited more damaged hepatocytes (Fig. 3B). Vacuolization was noted in the cytoplasm giving it a lacey appearance. Swollen hepatocytes were evident. Nuclei were also not as round and distinct as in corn oil controls, although no necrotic lesions were visible. DCTA treatment did not produce any significant changes at the two lower doses investigated (0.2 and 0.4 mmol/kg, data not shown). Sections appeared normal with healthy looking hepatocytes radiating out of the central vein. Also, no changes were observed in periportal hepatocytes and the bile ducts were normal. At the 0.6 mmol/kg DCTA dosage nuclear condensation was apparent in some hepatocytes (Fig. 3C). Apart from some swollen hepatocytes and narrowed sinusoidal spaces, liver sections from 4-DCTD-treated animals showed no changes in hepatic morphology at any dose (1.0 mmol/kg shown in Fig. 3D) compared to corn oil-treated controls. Liver sections from the 2-DCTD treatment groups (0.2, 0.4 and 0.6 mmol/kg, data not shown; 1.0 mmol/kg, Fig. 3E) were generally normal, although some nuclear condensation was evident.
Figure 3.
Effect of thiazolidinedione ring modifications on rat hepatic morphology. Representative photomicrographs are shown for the highest dose administered for each compound (1.0 mmol/kg for DPMT, 2-DCTD and 4-DCTD; 0.6 mmol/kg for DCTA; i.p. in corn oil). Controls received corn oil only (4 ml/kg). Vacuolated cells and swollen hepatocytes are indicated by white arrows and black arrows, respectively. Magnification is 400×.
BUN levels, urine volume, urine protein and kidney weights were determined to assess the potential nephrotoxicity of the compounds (Table 1). BUN concentrations were slightly elevated with 4-DCTD treatment at 0.6 and 1.0 mmol/kg only. Compared to corn oil and the 0.2 mmol/kg dose, DCTA treatment at 0.4 and 0.6 mmol/kg also produced a significant increase (about 3-fold) in serum BUN concentrations. In contrast, treatment with DPMT and 2-DCTD did not produce any significant changes in BUN levels. Urine protein levels were not significantly elevated by any treatment at any of the doses that were administered. Diuresis (approximately three and a half-fold increase) was noted with 4-DCTD treatment at all doses compared to controls, but was not dose-dependent. An increase in urine volume (about three-fold increase) was also noted with DPMT treatment at 0.6 and 1.0 mmol/kg dosages. The increases in urine volumes seen with DCTA and 2-DCTD were not statistically significant. A slight increase in kidney weight was noted with DCTA treatment at 0.6 and 1.0 mmol/kg treatment; however none of other treatments product any significant changes in this parameter (Table 1).
When compared to the corn oil controls, DPMT- and DCTA-treated animals did not exhibit obvious changes in kidney morphology at any doses (data not shown). Minor swelling in the proximal tubular cells and slight dilation of collecting tubules was observed with 4-DCTD, which could have contributed to the increased urinary flow (data not shown). In contrast, 2-DCTD treatment did not produce any noticeable changes in the kidney morphology at any of the doses that were evaluated (data not shown).
Log P values were calculated as an estimate of the lipophilicity of the compounds. For the TZD ring modified analogues of DCPT, the relative ranking (most to least lipophilic, Fig. 1) was: DPMT > 2-DCTD > 4-DCTD > DCTA.
Phenyl ring-modified compounds
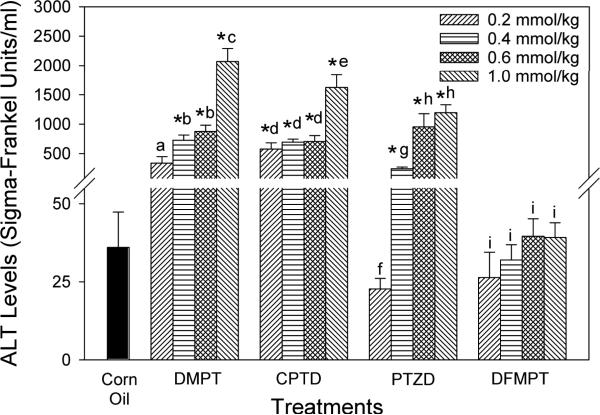
Serum ALT results for this series of compounds are summarized in Fig. 4. Except for the lowest dose, treatment with the dimethyl analogue, DMPT, resulted in marked increases in serum ALT levels when compared to the corn oil controls. ALT values were significantly elevated with CPTD treatment at all four doses. At 1.0 mmol/kg, both DMPT and CPTD produced significant elevations in ALT values when compared to the three lower doses of each compound. The lowest dose of PTZD (0.2 mmol/kg) did not produce a significant change compared to the corn oil controls, but ALT levels were elevated approximately 7-, 26- and 33-fold at the 0.4, 0.6, and 1.0 mmol/kg PTZD doses, respectively. With this compound, ALTs were significantly elevated at 0.6 and 1.0 mmol/kg compared to the two lower doses. By contrast, serum ALT levels were normal in rats that received DFMPT at all four doses. Compared to controls, a slight, but statistically significant increase in liver weights was observed in animals treated with DMPT (0.4, 0.6 and 1.0 mmol/kg), CPTD (0.6 and 1.0 mmol/kg), and PTZD and DFMPT (1.0 mmol/kg dose, Table 2). Liver weights in rats that received DMPT at the 0.2 mmol/kg dose were significantly lower than those seen with the higher doses.
Figure 4.
Effect of phenyl ring modifications on serum alanine aminotransferase (ALT) levels in rats. Compounds were administered i.p. in corn oil. Controls received corn oil only (4 ml/kg). Values are means ± SE (N = 3–4). An asterisk (*) indicates that the value is significantly different (p < 0.05) from the corn oil control. Different letters within a treatment group indicate that the doses are significantly different (p < 0.05).
Table 2.
Effect of phenyl ring ring modifications on blood urea nitrogen, urine volume and protein content, or liver and kidney weights in rats. Compounds were administered i.p. in corn oil. Controls received corn oil only (4 ml/kg). Values are means ± SE (N = 3–4). An asterisk (*) indicates that the value is significantly different (p < 0.05) from the corn oil control. Different letters within a treatment group indicate that the doses are significantly different (p < 0.05).
| Treatment | Dose (mg/kg) | Blood urea nitrogen (mg/dl) | Twenty-four-hour urine volume (ml) | Six-hour urine protein content (mg) | Liver weight (g/100% BW) | Kidney weight (g/ 100% BW) |
|---|---|---|---|---|---|---|
| Corn Oil | - | 20.2 ± 2.0 | 3.7 ± 0.5 | 1.5 ± 0.4 | 3.49 ± 0.12 | 0.37 ± 0.01 |
| DMPT | 0.2 | 18.1 ± 1.3a | 5.8 ± 1.4*,a | 0.8 ± 0.3a | 3.47 ± 0.20a | 0.38 ± 0.01a |
| 0.4 | 22.2 ± 0.4a | 9.9 ± 2.2*,a | 1.4 ± 0.1a | 4.10 ± 0.20*,b | 0.40 ± 0.02a | |
| 0.6 | 27.5 ± 1.4*,b | 8.9 ± 1.8*,a | 1.9 ± 0.2a | 4.20 ± 0.05*,b | 0.39 ± 0.00a | |
| 1.0 | 32.8 ± 3.1*,b | 11.3 ± 2.5*,a | 2.8 ± 0.4*,b | 3.97 ± 0.04*,b | 0.38 ± 0.01a | |
| CPTD | 0.2 | 37.9 ± 0.6*,c | 5.7 ± 1.5b | 2.0 ± 0.1c | 3.62 ± 0.16c | 0.39 ± 0.02b |
| 0.4 | 38.1 ± 1.7*,c | 12.4 ± 4.6*,c | 1.9 ± 0.1c | 3.84 ± 0.07c | 0.41 ± 0.01b | |
| 0.6 | 40.4 ± 2.7*,c | 16.6 ± 0.8*,c | 2.0 ± 0.1c | 4.36 ± 0.06*,d | 0.46 ± 0.02*,c | |
| 1.0 | 34.9 ± 1.8*,c | 15.3 ± 2.7*,c | 1.9 ± 0.1c | 3.98 ± 0.14*,c | 0.51 ± 0.04*,c | |
| PTZD | 0.2 | 24.8 ± 1.9d | 6.1 ± 1.7d | 2.1 ± 0.5d | 3.69 ± 0.25e | 0.36 ± 0.01d |
| 0.4 | 24.1 ± 0.9d | 11.5 ± 3.2d | 2.1 ± 0.2d | 3.68 ± 0.18e | 0.40 ± 0.00*,e | |
| 0.6 | 28.6 ± 2.0*,d | 12.5 ± 2.4d | 3.4 ± 0.9d | 4.08 ± 0.08e | 0.41 ± 0.01*,e | |
| 1.0 | 31.3 ± 2.4*,d | 22.6 ± 0.4*,e | 2.3 ± 0.6d | 4.24 ± 0.06*,e | 0.47 ± 0.01 *,f | |
| DFMPT | 0.2 | 19.6 ± 0.5e | 10.1 ± 1.3*,f | 1.1 ± 0.3e | 3.82 ± 0.12f | 0.40 ± 0.01g |
| 0.4 | 21.7 ± 0.4e | 14.1 ± 2.6*,f,g | 1.6 ± 0.2e | 3.59 ± 0.10f | 0.42 ± 0.03g | |
| 0.6 | 22.5 ± 0.5e | 20.0 ± 3.1*,g,h | 1.5 ± 0.2e | 3.78 ± 0.07f | 0.30 ± 0.09g | |
| 1.0 | 32.7 ± 1.4*,f | 23.5 ± 1.8*,h | 1.0 ± 0.2e | 4.05 ± 0.15*,f | 0.48 ± 0.01*,h |
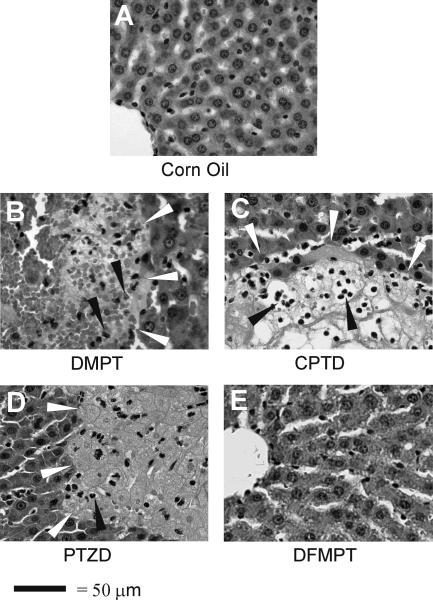
Representative photomicrographs of liver sections from the animals treated with DMPT, CPTD, PTZD and DFMPT (1.0 mmol/kg doses) are shown in Fig. 5. Corn oil (4 ml/kg) is included again for comparison (Fig. 5A). Liver sections from DMPT-treated animals at 0.2 and 0.4 mmol/kg doses (data not shown) exhibited necrotic lesions with infiltration of inflammatory cells, neutrophils and lymphocytes. The damage was more scattered and not limited to the centrilobular region. At higher doses, 0.6 mmol/kg (data not shown) and 1.0 mmol/kg (Fig. 5B), necrotic lesions were widespread with many inflammatory cells seen around the lesions. Typical signs of liquefactive necrosis were evident, such as cell lysis and digestion of cellular material by proteolytic enzymes that produced holes. Sections from CPTD-treated animals exhibited necrotic lesions with acute inflammatory response at all doses. The origin of these lesions was not very clear and normal organization of cells was lost. Signs of coagulative necrosis, such as denaturation of proteins and loss of cytoplasmic material with preservation of cell outlines were evident in animals at lower doses (data not shown). At 1.0 mmol/kg CPTD (Fig. 5C), liver sections showed signs of liquefactive necrosis. The necrotic lesions were widespread with infiltration of neutrophils and lymphocytes. Blood was also present at the site of lesions with a complete loss of normal cellular arrangement. PTZD-treated animals did not show any signs of hepatic damage at the 0.2 mmol/kg dose (data not shown). At the 0.4 mmol/kg dose (data not shown) cells had begun to show some damage with granular and irregularly stained cytoplasm and indistinct nuclei. With increasing dose, more noticeable damage was seen. At the highest dose (1.0 mmol/kg, Fig. 5D), larger necrotic lesions were present and an acute inflammatory response occurred. Liver sections from DFMPT-treated animals did not show any noticeable changes in morphology at all doses (lower doses, data not shown; 1.0 mmol/kg dose, Fig. 5E). Sections appeared normal and healthy with hepatocytes arranged in row-like fashion radiating out of central vein. The bile ducts were intact and also the periportal hepatocytes exhibited no significant changes.
Figure 5.
Effect of phenyl ring modifications on rat hepatic morphology. Representative photomicrographs are shown for the highest dose administered for each compound (1.0 mmol/kg; i.p. in corn oil). Controls received corn oil only (4 ml/kg). White arrowheads define regions of necrosis. Inflammatory cells are indicated by black arrowheads. Magnification is 400×.
Compared to corn oil, DMPT and PTZD treatments produced a statistically significant, but small elevation in BUN levels at the two highest doses (Table 2). Animals treated with CPTD exhibited a statistically significant, but modest (1.8-fold) increase in BUN levels at all dosages compared to the corn oil controls. DFPMT treatment also produced a significant elevation in BUN concentration at the highest dosage (1.0 mmol/kg) compared to controls and the three lower doses. A significant increase in urine protein was only observed with the highest dose of DMPT (Table 2). Diuresis was seen after administration of DMPT and DFMPT at all doses compared to controls and was especially pronounced at the highest doses of the latter compound (Table 2). An increase in urine volume was also noted with PTZD (1.0 mmol/kg) and CPTD (0.4, 0.6 and 1.0 mmol/kg) treatments. Kidney weights were not significantly altered by DMPT treatment at any doses compared to the corn oil-treated animals or to each other (Table 2). However, a small increase was noted with CPTD (0.6 and 1.0 mmol/kg), PTZD (0.4, 0.6 and 1.0 mmol/kg) and DFMPT (1.0 mmol/kg) treatments.
Renal morphology was normal in the DMPT treatment groups (data not shown). Kidney sections from rats that were administered CPTD and DFMPT exhibited minor swelling in some proximal tubular cells (data not shown). Collecting tubules were slightly dilated which could have contributed to the increased urinary flow. PTZD-treated rats exhibited dilation of distal tubules and collecting tubules (data not shown). Overall, only relatively mild kidney damage was seen with all phenyl ring analogues.
Based on log P values (Fig. 1), the relative order of lipophilicity for the phenyl ring-modified DCPT analogues was (most to least lipophilic): DFMPT > DMPT > CPTD > PTZD.
Discussion
The glitazones do not cause liver damage in common laboratory animal species (Chojkier, 2005) and are not suitable for evaluating mechanism of toxicity for TZD ring-containing compounds in vivo. More recently, 5-(4-methoxybenzylidene)thiazolidine-2,4-dione, which has a similar TZD ring substitution pattern as the glitazones, was found to be non-toxic in mice (Luo et al., 2010). Thus, DCPT, which reproducibly produces liver damage in rats, may be a useful compound to explore the potential hepatotoxicity of TZD ring derivatives (Patel et al., 2008). For example, we obtained ALT values of 26.7 ± 6.6, 231.5 ± 50.0, 281.7 ± 103.0 and 506.9 ± 99.4 SF units/ml in male rats at DCPT doses of 0.2, 0.4, 0.6 and 1.0 mmol/kg, respectively. Morphological changes, which included necrosis and inflammation, also worsened with increasing dosage (Patel et al., 2008). In addition to the TZD ring, DCPT contains a meta-substituted dichlorobenzene (DCB) ring (Fig. 1). Although DCBs have been shown to produce hepatic injury in rats (Stine et al., 1991; Valentovic et al., 1993), we do not believe that this structural feature is a critical factor in the liver damage associated with DCPT (Crincoli et al., 2008; Patel et al., 2008). The experiments described here were designed to further explore the effect of structure on DCPT-induced hepatotoxicity in rats.
In contrast to our previous structure-activity relationship study (Kennedy et al., 2003), all of the analogues described herein contain a sulfur atom, which is a potential site for oxidative metabolism. In fact, CYP3A-mediated TZD ring sulfoxidation was proposed as an initial step in the generation of reactive intermediates from the glitazones (Kassahun et al., 2001; Tettey et al., 2001; He et al., 2004; Baughman et al., 2005). Furthermore, we found that DCPT-induced hepatotoxicity was CYP3A-dependent in rats (Crincoli et al., 2008). Generation of a sulfoxide may therefore also be necessary for the conversion of DCPT into a putative toxic species. We observed here that attachment of a methyl group to the TZD ring (i.e. DPMT, Fig. 1) resulted in a compound that was hepatotoxic. Since this substituent should sterically hinder biotransformation at the methylene carbon atom in DPMT, we believe that this position is not a primary site for metabolic activation in this compound. However, oxidation on the sulfur atom of DPMT is still a possibility, which could account for its toxicity.
A modest elevation in ALT levels was observed with the ring-opened compound DCTA (Fig. 1, a thioglycolic acid derivative), but no evidence of significant morphological damage was seen in the rat liver sections. DCTA is the only analogue that contains an ionizable functional group. As an aliphatic carboxylic acid it should be extensively ionized in plasma. Hence, reduced distribution into the liver may be a limiting factor for DCTA hepatotoxicity, compared to the other non-ionizable, neutral compounds. One possible explanation for the systemic effects that we observed with DCTA could be degradation to 3,5-dichlorophenyl isocyanate. If this potentially reactive compound was generated in vivo, protein carbamoylation and toxicity could result (Brown et al., 1987). Although we have no direct evidence that this occurred in the rats with DCTA, the structurally analogous compound S-(methylaminocarbonyl)thioglycolic acid was reported to undergo base-catalyzed decomposition to methyl isocyanate (Machacek et al., 1981).
4-DCTD and 2-DCTD (Fig. 1) were synthesized and tested to address the impact of replacing the unsymmetrical TZD ring of DCPT with regioisomeric thiazolidinone rings. These compounds did not produce any significant liver damage at any dose, which suggests that DCPT metabolic activation is dependent on the presence of both carbonyl groups in the TZD ring. The lack of hepatic injury from these two compounds further suggests that the DCB ring is not contributing to hepatotoxicity in our rat model. Also, 4-DCTD and 2-DCTD probably do not undergo extensive biotransformation to DCPT in vivo (i.e., the thiazolidinone ring in each compound is not metabolized to a TZD ring), otherwise liver damage should have been observed.
Lipophilicity can be an important factor in pharmacokinetics (van de Waterbeemd et al., 2001; Giaginis and Tsantili-Kakoulidou, 2008). Log P values were therefore calculated to determine the octanol/water partition coefficients (relative lipophilicities) of the DCPT analogues. Based on this analysis, the non-toxic analogue 2-DCTD and the toxic compound DPMT have similar estimated lipophilicities (log P values = 3.20 and 3.22, respectively). Furthermore, the hepatotoxic “parent” compound DCPT (Patel et al., 2008) has a similar log P value to the non-toxic analogue 4-DCTD (2.73 and 2.71, respectively). These results suggest that there is no correlation between the lipophilicities of the compounds and their ability to produce liver damage in rats.
Among the phenyl substituted compounds (Fig. 1), DMPT, CPTD and PTZD produced severe liver damage including large areas of necrosis with infiltration of neutrophils. Hepatotoxicity, as evidenced by ALT measurements, tended to increase with dose. DFMPT treatment, on the other hand, did not produce any changes in ALT levels and the histological examination showed no signs of toxicity compared to corn oil controls. These results indicate that liver damage is highly dependent on the substituents in the phenyl ring and dose. As we observed with the TZD ring analogues, hepatotoxicity did not correlate with lipophilicity. In fact, PTZD (log P = 1.61), the least lipophilic compound in this series, produced significant liver damage; whereas the most lipophilic analogue DFMPT (log P = 3.48) was not hepatotoxic. However, analogues which could release electrons into the TZD ring via inductive or resonance effects (DMPT and CPTD) were more toxic than the compound that would withdraw electrons (DFMPT). Conceivably, the phenyl ring substituents could alter electron density in the TZD ring. This, in turn, could modify biotransformation in the TZD ring, which we believe is an important factor in hepatotoxicity (Crincoli et al., 2008).
We cannot exclude the possibility that other factors, such as alternative biotransformation pathways, could also contribute to toxicity in the phenyl series of compounds. For example, the phenyl ring in DMPT is structurally analogous to 1,3-dimethylbenzene (m-xylene). Metabolism of xylenes is believed to occur by an initial benzylic oxidation (Low et al., 1989). Administration of m-xylene to rats (4.0 mmol/kg, i.p.) resulted in 45% loss of hepatic glutathione levels within 3 hours (van Doorn et al., 1980). The same investigators found that 1.3% of a total m-xylene dose (3.0 mmol/kg, i.p.) was excreted in urine as thioether conjugates. These findings suggest that reactive intermediates can be formed from m-xylene, although the doses were 3–4 times higher than we used with DMPT. CPTD contains a chlorophenyl moiety and, in analogy to chlorobenzene, phenolic metabolites could be formed via epoxide intermediates or direct oxidation (Selander et al., 1975). In fact, chlorobenzene was shown to cause an elevation in serum ALT levels in rats (Dalich and Larson, 1985); however, the dose used was nearly 10-fold greater than we used with CPTD.
Consistent with our previous findings for DCPT (Kennedy et al., 2003; Patel et al., 2008), both series of analogues produced relatively mild effects on rat kidneys. This is in spite of the fact that DCPT has close structural similarity to the nephrotoxicant N-(3,5-dichlorophenyl)succinimide (NDPS) (Rankin, 1982). This disparity may be due to differences in metabolism and distribution of the compounds. The NDPS succinimide ring undergoes biotransformation in rat liver (Rankin, 2004; Cui et al, 2005). These hepatic metabolites are then believed to undergo transport through the blood to the kidneys, where they may accumulate; thereby causing nephrotoxicity. Although biotransformation of the toxic TZD derivatives described herein has not been studied yet, it is conceivable that putative reactive metabolite(s) may exert damage directly in the hepatocytes where they are produced. As noted above, TZD ring sulfoxidation has been proposed as a possible bioactivation mechanism for the glitazones (Kassahun et al., 2001; Tettey et al., 2001; He et al., 2004; Baughman et al., 2005).
In conclusion, our findings with the DCPT analogues (TZD ring-modified series) strongly suggest that an intact TZD ring is crucial for these compounds to exert liver damage in rats. We believe that biotransformation involving the TZD ring sulfur atom may be a factor in hepatotoxicity for the following reasons: (1) among the TZD ring-modified compounds, only DPMT produced significant liver injury (above); (2) DCPT was the only hepatotoxic analogue among a series of compounds containing different cyclic imides (i.e. succinimide, oxazolidinedione, etc.) attached directly to a 1,3-DCB ring (Kennedy et al., 2003); and (3) CYP inhibitors attenuated DCPT toxicity, whereas a CYP inducer potentiated liver damage in male rats (Crincoli et al., 2008). However, hepatotoxicity is also dependent on the nature of the substituents in the phenyl ring and may be due to differences in biotransformation among these compounds.
Short Abstract.
We conducted a structure-activity relationship study into 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione (DCPT)-induced hepatotoxicity in rats. Attachment of a methyl group in the thiazolidinedione (TZD) ring was still associated with liver damage. By contrast, hepatotoxicity was abolished with other TZD ring modifications such as ring opening or removal of either carbonyl group. The toxicity of DCPT analogues was also highly dependent on the nature of the substituents in the phenyl ring. Our results suggest that an intact TZD ring is required for hepatotoxicity.
Acknowledgements
The authors would like to thank Dr. Joan B. Tarloff, USP Department of Pharmaceutical Sciences, for her assistance with the cardiac punctures. This publication was made possible by grant number ES012499 (P.J.H.) from the National Institute of Environmental Health Sciences (NIEHS), NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
References
- Ali AM, Saber GE, Mahfouz NM, El-Gendy MA, Radwan AA, Hamid MA. Synthesis and three-dimensional qualitative structure selectivity relationships of 3,5-disubstituted-2,4-thiazolidinedione derivatives as COX2 inhibitors. Arch. Pharm. Res. 2007;30:1186–1204. doi: 10.1007/BF02980259. [DOI] [PubMed] [Google Scholar]
- Baughman TM, Graham RA, Wells-Knecht K, Silver IS, Tyler LO, Wells-Knecht M, Zhao K. Metabolic activation of pioglitazone identified from rat and human liver microsomes and freshly isolated hepatocytes. Drug Metab. Dispos. 2005;33:733–738. doi: 10.1124/dmd.104.002683. [DOI] [PubMed] [Google Scholar]
- Brouwer WG, Blem AR. Substituted thiazolidinones useful as plant growth regulators. #4,664,694 US patent. 1987
- Brown WE, Green AH, Cedel TE, Cairns J. Biochemistry of protein-isocyanate interactions: A comparison of the effects of aryl vs. alkyl isocyanates. Environ. Health Perspect. 1987;72:5–11. doi: 10.1289/ehp.87725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita MG. Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg. Med. Chem. 2002;10:1077–1084. doi: 10.1016/s0968-0896(01)00366-2. [DOI] [PubMed] [Google Scholar]
- Chojkier M. Troglitazone and liver injury: In search of answers. Hepatology. 2005;41:237–246. doi: 10.1002/hep.20567. [DOI] [PubMed] [Google Scholar]
- Crincoli CM, Patel NN, Tchao R, Harvison PJ. Role of biotransformation in 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione-induced hepatotoxicity in Fischer 344 rats. Toxicology. 2008;250:100–108. doi: 10.1016/j.tox.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Rankin GO, Harvison PJ. Metabolism of the nephrotoxicant N-(3,5-dichlorophenyl)succinimide in rats: evidence for bioactivation through alcohol-O-glucuronidation and O-sulfation. Chem. Res. Toxicol. 2005;18:991–1003. doi: 10.1021/tx0496587. [DOI] [PubMed] [Google Scholar]
- Czerniak R. Gender-based differences in pharmacokinetics in laboratory animal models. Int. J. Toxicol. 2001;20:161–163. doi: 10.1080/109158101317097746. [DOI] [PubMed] [Google Scholar]
- Dalich GM, Larson RE. Temporal and dose-response of monochlorobenzene hepatotoxicity in rats. Fund. Appl. Toxicol. 1985;5:105–116. doi: 10.1016/0272-0590(85)90054-5. [DOI] [PubMed] [Google Scholar]
- El-Naggar MHM, Helmy A, Moawad M, Al-Omary M, Al-Kadhi Y, Habib B. Late-onset rosiglitazone-associated acute liver failure in a patient with Hodgkin's lymphoma. Ann. Pharmacother. 2008;42:713–718. doi: 10.1345/aph.1K543. [DOI] [PubMed] [Google Scholar]
- Fujinami AA, Nodera KN, Tanaka KT, Hyogo OT, Yamamoto ST, Akiba KI, Ooishi TM. # 1,953,431 German patent. 1968
- Fujinami A, Ozaki T, Yamamoto S. Studies on biological activity of cyclic imide compounds. Part I. Antimicrobial activity of 3-phenyloxazolidine-2,4-diones and related compounds. Agric. Biol. Chem. 1971;35:1707–1719. [Google Scholar]
- Giaginis C, Tsantili-Kakoulidou A. Alternative measures of lipophilicity: from octanol-water partitioning to IAM retention. J. Pharm. Sci. 2008;97:2984–3004. doi: 10.1002/jps.21244. [DOI] [PubMed] [Google Scholar]
- Gitlin N, Julie NL, Spurr CL, Lim KN, Juarbe HM. Two cases of severe clinical and histologic hepatotoxicity associated with troglitazone. Ann. Intern. Med. 1998;129:36–38. doi: 10.7326/0003-4819-129-1-199807010-00008. [DOI] [PubMed] [Google Scholar]
- Gouda HE, Khan A, Schwartz J, Cohen RI. Liver failure in a patient treated with long-term rosiglitazone therapy. Am. J. Med. 2001;111:584–585. doi: 10.1016/s0002-9343(01)00926-3. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Green L, Senior JR, Nourjah P. Troglitazone-induced liver failure: A case study. Am. J. Med. 2003;114:299–306. doi: 10.1016/s0002-9343(02)01529-2. [DOI] [PubMed] [Google Scholar]
- He K, Talaat RE, Pool WF, Reily MD, Reed JE, Bridges AJ, Woolf TF. Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab. Dispos. 2004;32:639–646. doi: 10.1124/dmd.32.6.639. [DOI] [PubMed] [Google Scholar]
- Kassahun K, Pearson PG, Tang W, McIntosh I, Leung K, Elmore C, Dean O, Wang R, Doss G, Baillie TA. Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem. Res. Toxicol. 2001;14:62–70. doi: 10.1021/tx000180q. [DOI] [PubMed] [Google Scholar]
- Kennedy EL, Tchao R, Harvison PJ. Nephrotoxic and hepatotoxic potential of imidazolidinedione-, oxazolidinedione-, and thiazolidinedione-containing analogues of N-(3,5-dichlorophenyl)succinimide (NDPS) in Fischer 344 rats. Toxicology. 2003;186:79–91. doi: 10.1016/s0300-483x(02)00692-3. [DOI] [PubMed] [Google Scholar]
- Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: Report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am. J. Gastroenterol. 2000;95:272–276. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- Low LK, Meeks JR, Mackerer CR. Health effects of the alkylbenzenes. II. Xylenes. Toxicol. Ind. Health. 1989;5:85–105. doi: 10.1177/074823378900500108. [DOI] [PubMed] [Google Scholar]
- Luo Y, Ma L, Zheng H, Chen L, Li R, He C, Yang S, Ye X, Chen Z, Li Z, Gao Y, Han J, He G, Yang L, Wei Y. Discovery of (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione, a readily available and orally active glitazone for the treatment of concanavalin A-induced acute liver injury of BALB/c mice. J. Med. Chem. 2010;53:273–281. doi: 10.1021/jm901183d. [DOI] [PubMed] [Google Scholar]
- Machacek V, Sterba V, Zahradnickova H. Solvolysis kinetics and mechanism of 3-methyl-1,3-thiazolidine-2,4-dione. Coll. Czech. Chem. Commun. 1981;46:3097–3103. [Google Scholar]
- Maeda K. Hepatocellular injury in a patient receiving pioglitazone. Ann. Intern. Med. 2001;135:306. doi: 10.7326/0003-4819-135-4-200108210-00029. [DOI] [PubMed] [Google Scholar]
- Marcy TR, Britton ML, Blevins SM. Second-generation thiazolidinediones and hepatotoxicity. Ann. Pharmacother. 2004;38:1419–1423. doi: 10.1345/aph.1E072. [DOI] [PubMed] [Google Scholar]
- Mugford C, Kedderis G. Sex-dependent metabolism of xenobiotics. Drug. Metab. Rev. 1998;30:441–498. doi: 10.3109/03602539808996322. [DOI] [PubMed] [Google Scholar]
- Patel NN, Crincoli CM, Kennedy EL, Frederick DM, Tchao R, Harvison PJ. Effect of gender, dose and time on 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione (DCPT)-induced hepatotoxicity in Fischer 344 rats. Xenobiotica. 2008;38:435–449. doi: 10.1080/00498250701830267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowitz D, Maccari R, Ottana R, Vigorita MG. In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones. Bioorg. Med. Chem. 2006;14:567–574. doi: 10.1016/j.bmc.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Rankin GO. Nephrotoxicity following acute administration of N-(3,5-dichlorophenyl)succinimide in rats. Toxicology. 1982;23:21–31. doi: 10.1016/0300-483x(82)90038-5. [DOI] [PubMed] [Google Scholar]
- Rankin GO. Nephrotoxicity induced by C- and N-arylsuccinimides. J. Toxicol. Environ. Health, Pt. B. 2004;7:399–416. doi: 10.1080/10937400490486113. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. Hepatotoxicity with thiazolidinediones: Is it a class effect? Drug Safety. 2001;24:873–888. doi: 10.2165/00002018-200124120-00002. [DOI] [PubMed] [Google Scholar]
- Selander HG, Jerina DM, Daly JW. Metabolism of chlorobenzene with hepatic microsomes and solubilized cytochrome P450 systems. Arch. Biochem. BIophys. 1975;168:309–321. doi: 10.1016/0003-9861(75)90255-6. [DOI] [PubMed] [Google Scholar]
- Stine ER, Gunawardhana L, Sipes IG. The acute hepatotoxicity of the isomers of dichlorobenzene in Fischer-344 and Sprague-Dawley rats: isomer-specific and strain-specific differential toxicity. Toxicol. Appl. Pharmacol. 1991;109:472–481. doi: 10.1016/0041-008x(91)90010-c. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Nakamura M. 3-Substituted 2(3H)-benzothiazolones. # 60-166673. JP patent. 1985 [Procedure from Patent Abstracts of Japan (English translation): http://www19.ipdl.inpit.go.jp/PA1/result/detail/main/wAAAuPayW3DA360166673P1.htm.
- Tettey JN, Maggs JI, Rapeport WG, Piromohamed M, Park BK. Enzyme-induction dependent bioactivation of troglitazone and troglitazone quinone in vivo. Chem. Res. Toxicol. 2001;14:965–974. doi: 10.1021/tx0001981. [DOI] [PubMed] [Google Scholar]
- Toldy L, Borsi J, Elek S, Elekes I, Andrasi Certain 3-(2,6-dichlorophenyl)-2-iminothiazolidines. # 3,671,537 US patent. 1972
- Valentovic MA, Ball JG, Anestis D, Madan E. Acute hepatic and renal toxicity of dichlorobenzene isomers in Fischer 344 rats. J. Appl. Toxicol. 1993;13:1–7. doi: 10.1002/jat.2550130103. [DOI] [PubMed] [Google Scholar]
- van Doorn R, Bos RP, Brouns RME, Leijdekkers Ch-M, Henderson PTh. Effect of toluene and xylenes on liver glutathione and their urinary excretion as mercapturic acids in the rat. Arch. Toxicol. 1980;43:293–304. doi: 10.1007/BF00366185. [DOI] [PubMed] [Google Scholar]
- van de Waterbeemd H, Smith DA, Jones BC. Lipophilicity in PK design: methyl, ethyl, futile. J. Comput. Aided Mol. Des. 2001;15:273–286. doi: 10.1023/a:1008192010023. [DOI] [PubMed] [Google Scholar]
- Yang J, Wei S, Wang D-S, Wang Y-C, Kulp SK, Chen C-S. Pharmacological exploitation of the peroxisome proliferator-activated receptor γ agonist ciglitazone to develop a novel class of androgen-receptor-ablative agents. J. Med. Chem. 2008;51:2100–2107. doi: 10.1021/jm701212m. [DOI] [PMC free article] [PubMed] [Google Scholar]