Abstract
The risk of venous thromboembolism (VTE) is higher after total hip or knee replacement surgery than after almost any other surgical procedure; warfarin sodium is commonly prescribed to reduce this peri-operative risk. Warfarin has a narrow therapeutic window with high inter-individual dose variability and can cause hemorrhage. The Genetics-InFormatics Trial (GIFT) of Warfarin to Prevent Deep Vein Thrombosis (DVT) is a 2×2 factorial-design, randomized controlled trial designed to compare the safety and effectiveness of warfarin-dosing strategies. GIFT will answer two questions: (1) Does pharmacogenetic (PGx) dosing reduce the rate of adverse events in orthopedic patients; and (2) Is a lower target International Normalized Ratio (INR) non-inferior to a higher target INR in orthopedic participants? The composite primary endpoint of the trial is symptomatic and asymptomatic VTE (identified on screening ultrasonography), major hemorrhage, INR ≥ 4, and death.
Keywords: pharmacogenetics, warfarin, randomized controlled trial, dosing algorithm
Introduction
Warfarin sodium and other vitamin K antagonists adjusted to achieve a target INR of 2–3 can be used to prevent and treat venous thromboembolism (VTE, e.g. pulmonary embolism or deep venous thrombosis), but are associated with the doubling of hemorrhagic risk.1 This risk is greatest during the first weeks to months of warfarin therapy 2–6 when the therapeutic dose is generally determined by trial and error. To reduce hemorrhagic risk, experts recommend prescribing a predicted therapeutic dose to patients who are beginning warfarin, rather than use a standard loading dose.7–9 The Genetics-InFormatics Trial (GIFT) of Warfarin to Prevent Deep Vein Thrombosis (DVT) compares two algorithms of predicting the optimal warfarin dose—one using both genetic and clinical information, and the other using clinical factors alone. These algorithms are available through a non-profit web based application (www.WarfarinDosing.org) created by some of the authors. This application tailors warfarin doses to individual participants’ clinical vs. clinical plus pharmacogenetic profiles.
Although there is overwhelming evidence that single nucleotide polymorphisms (SNPs) in the cytochrome P-450 2C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) genes influence warfarin dose requirements, it is unclear whether prospective pharmacogenetic dosing reduces adverse events. 10, 11 CYP2C9 is associated with warfarin inactivation and VKORC1 is associated with the mechanism of action of warfarin. Two multi-centered, randomized trials in progress (EU-PACT 12; COAG NCT00839657) are investigating pharmacogenetic-based warfarin dosing using VKORC1 and CYP2C9. A third gene, cytochrome P-450 4F2 (CYP4F2), has also been associated with warfarin dose requirements.13 but its effect on warfarin safety has yet to be evaluated. Small, single-centered trials 14–18 have not detected any reduction in major adverse events from genotyping, but have been underpowered to detect a putative difference.
The first aim of GIFT is to evaluate how pharmacogenetic management affects incidence of adverse outcomes. GIFT will recruit elderly, hip and knee arthroplasty participants receiving warfarin prophylaxis. This population is at high risk for both thrombotic and hemorrhagic events. The rate of symptomatic venous thromboembolism (VTE) after hip or knee replacement surgery is much higher than after almost any other procedure 19, and these rates are highest in the elderly. 20 Studying this high-risk population will therefore power GIFT to test for a difference in outcomes between pharmacogenetic and clinical warfarin dosing.
The second aim of GIFT is to test the safety and effectiveness of two target INR ranges in arthroplasty participants. The American College of Chest Physicians (ACCP) recommends a target INR of 2.5 while the American Academy of Orthopaedic Surgeons (AAOS) recommends a target INR ≤ 2.0. The AAOS argues that a lower target INR is safer from the standpoint of hemorrhagic risk, and is non-inferior in preventing PE. 21 These incongruous therapeutic targets have not been directly compared in arthroplasty participants. Prior studies that compared different INR goals in participants with a history of deep vein thrombosis (DVT) provided conflicting results. The randomized trial by Ridker et al showed that an INR target of 1.5–2.0 could prevent VTE recurrence without increased risk of major bleeding compared to placebo. 22 In contrast, Kearon et al found that an INR of 2–3 was more effective than an INR of 1.5–1.9 in preventing VTE recurrence and equally safe.23 The Ridker trial, in conjunction with studies showing that target INR values < 2.5 are safe and effective in orthopedic participants 24, 25, has led many orthopedic surgeons to target lower INR values.26 The best way to clarify this question is to do a multi-centered trial, as proposed here.
Materials and Methods
Study population
GIFT plans to enroll 1,600 Medicare beneficiaries undergoing elective total hip or knee replacement surgery at Barnes-Jewish Hospital, Intermountain Healthcare, the Hospital for Special Surgery, and University of Utah Health Care. Research coordinators will recruit and obtain written consent from participants prior to surgery in accordance with the Declaration of Helsinki. Inclusion and exclusion criteria are listed in Table 1. This high-risk population will be screened 4–6 weeks after surgery for asymptomatic DVT by Doppler ultrasound. Assuming an average rate of DVT of 15%, the trial is powered to answer two novel questions: are pharmacogenetic-based dosing and targeting lower INR safer and more effective approaches to the prevention of VTE than clinically-based warfarin dosing and traditional INR target range?
Table 1.
Summary of Inclusion and Exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Elective hip or knee arthroplasty (not hip fracture) | Currently taking warfarin |
| ≥65 years of age | Incarcerated or institutionalized |
| Has Medicare Part B | Thrombocytopenia (platelets < 75K) |
| Warfarin prophylaxis anticipated for at least 4 weeks | History of venous thromboembolism |
| Reliable telephone access | History of thrombophilia |
| Willing to give consent (English only) | History of major bleeding or bleeding disorder (e.g. hemophilia, von Willebrand disease, etc.) |
| Willing/able to have Doppler Ultrasound at 4–6 week follow-up visit | Planned administration of any anticoagulant other than warfarin (except heparin flushes) |
| Baseline INR <1.35 | Warfarin genotypes or prior therapeutic warfarin dose known |
| Life expectancy >6 months | Warfarin allergy |
| Unlikely to be compliant (e.g. history of non-compliance, substance abuse, etc.) | |
| First degree relative with VTE before age 50 | |
| Planned administration of interacting medications, except those taken into account by WarfarinDosing.org* | |
| Alcoholism not in remission for past 6 months |
carbamazepine, rifampin, barbiturates, phenytoin
Blinding and randomization
Using a 2 × 2 factorial design, we will randomize participants to each of the following:
Pharmacogenetic vs. clinical dosing of warfarin
Higher target INR of 2.5 vs. a lower target INR of 1.8
Randomization will be stratified by site, race, and whether participants undergo knee or hip arthroplasty. Lists for block randomization will be prepared in advance by the trial statistician and monitored prospectively. Participants will be randomized after they have been genotyped. Patients and study clinicians will be blinded to patient genotype and study arm, but not to daily warfarin dose. The protocol is to initiate warfarin with similar doses in patients who do or do not have the CYP2C9*2 or *3 variants. After 2 warfarin doses, subsequent doses are reduced to accommodate the decreased metabolism of S-warfarin conferred by these alleles for affected patients. These initial doses are based on pharmacokinetic principles27 that we have prospectively validated. 28, 29 This strategy of prescribing the initial two doses as though all participants were CYP2C9*1*1, in combination with the substantial contributions of clinical factors on warfarin dose requirements, should prevent inadvertent unblinding of genotype.
In contrast to genotype, randomization to standard vs. lower target INR value will not be double-blinded. Technicians performing screening Doppler ultrasounds and physicians adjudicating outcomes will be blinded to trial arm, genotype, and target INR.
Warfarin Dosing
The research team will be responsible for warfarin dosing for all participants from the time of surgery until the completion of treatment, 4–6 weeks postoperatively. Dosing will be guided by www.WarfarinDosing.org for a minimum of the first 11 days of treatment (Figure 1). Patients whose INR values are therapeutic after day 11 will remain on their predicted maintenance dose; others will have their warfarin dose adjusted empirically. Patients with a target INR of 1.8 will have an INR range of 1.5 to 2.1, whereas those assigned to a target INR of 2.5 will have an INR range of 2.0 to 3.0. Beginning with the INR measured after the first 11 days of warfarin therapy, we will dose patients per standard of care, titrated to their target INR.
Figure 1.
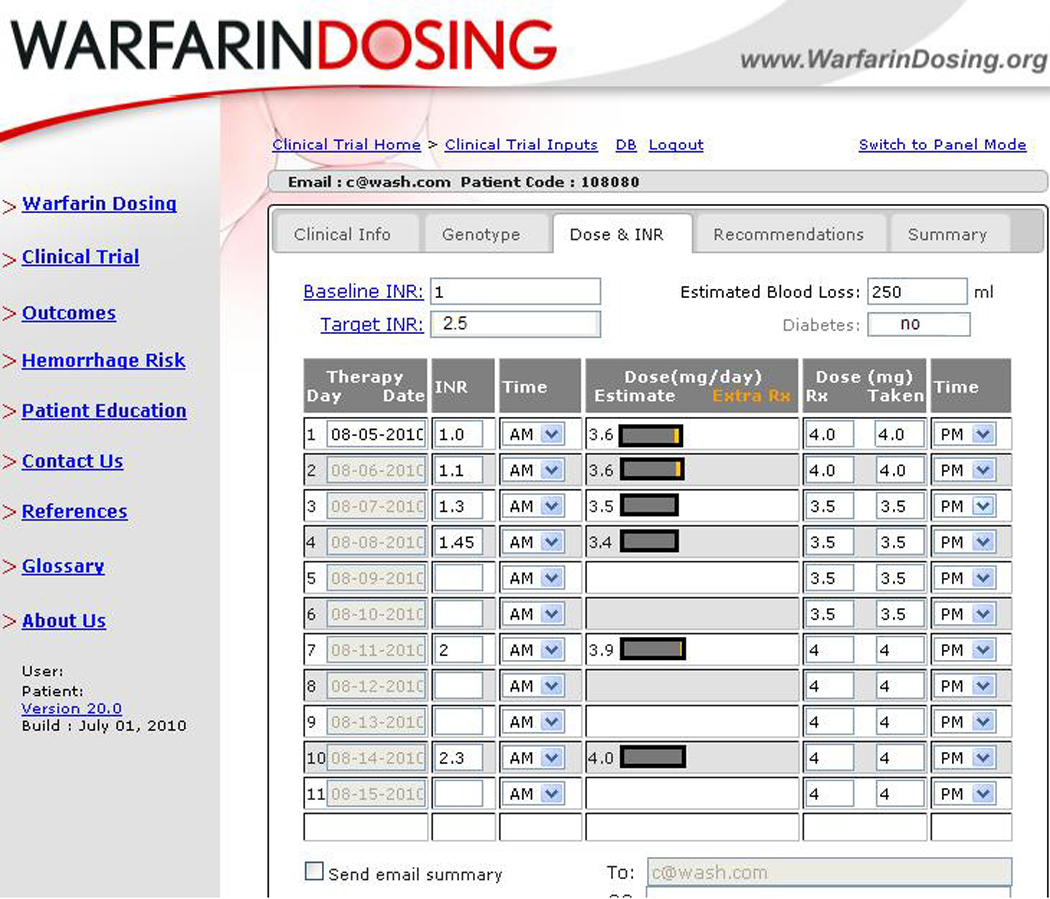
Use of WarfarinDosing.org
While prior studies have used pharmacogenetic or clinical dosing protocols for warfarin initiation, GIFT extends the length of the dosing protocol to the first eleven days of therapy. The GIFT algorithms predict stable warfarin doses over days 1–11 of therapy and incorporate clinical, laboratory, and genetic data gathered from sites across North America (including Missouri, New York and Utah), Europe, and Asia. 28, 30–33 The pharmacogenetic models explained 53%−73% of the variability in warfarin dose with median absolute dosing errors of ≤ 7 mg/week.
To facilitate dosing strategies for GIFT and for the public at large, we have made the non-profit, decision-support web application, www.WarfarinDosing.org, available to the public for investigational use. The website has more than 1000 visitors per week, providing continuous feedback. This extensive testing, feedback, and improvements to the website have produced an accurate, reliable and user-friendly tool critical to support pharmacogenetic study and practice. In March 2011, the US Food and Drug Administration (FDA) issued an Investigational Device Exemption (IDE) for use of WarfarinDosing.org and participant recruitment began on March 24th.
INR testing will be performed per standard practice—daily for inpatients, bi-weekly for non-therapeutic outpatients, and weekly for therapeutic outpatients. Each day that an INR is available, the GIFT research coordinator will enter it into WarfarinDosing.org to obtain a daily estimated dose and an estimated maintenance dose to be used subsequently (until the next INR value). When an INR is not drawn, patients will continue to receive their estimated maintenance dose. The website also indicates whether the dose of warfarin given on the day of INR testing should differ from the estimated therapeutic dose—a feature that allows WarfarinDosing.org to compensate for missed doses, large doses, or other dosing errors.
Data collection
In addition to key variables being entered into the study website for randomization and dosing purposes, all study data will be entered into the GIFT database (GIFT DB). Clinical data including the patient’s age, race, gender, height, weight, medications, and medical and surgical history will be obtained at the time of recruitment. Blood samples for research purposes will be collected to support genotyping and ancillary studies. DNA will be extracted from de-identified blood samples collected in EDTA tubes to determine subjects’ genotypes for CYP2C9 *2 (430C>T),*3 (1075A>C), CYP4F2 V433M (rs2108622 G>A), and VKORC1-1639 G>A. Study laboratory technicians will input the genotype results into www.WarfarinDosing.org. Genotyping for CYP2C9 and VKORC1 will be performed using either the GenMarkDx eSensor genotyping platform or the Simple Probe Warfarin Genotyping Reagents (Idaho Technology Inc), and LightCycler instrumentation (Roche). Genotyping for CYP4F2 will be performed by using either the GenMark eSensor genotyping platform or a melt-curve analysis, with fluorescent resonance energy transfer (FRET) probes, and LightCycler instrumentation (Roche), which was developed by ARUP Laboratories. The Central GIFT Genotyping Laboratory, directed by Dr. Eby, will use Pyrosequencing for verification of all genotypes. The Centers for Medicare & Medicare Services (CMS) will reimburse for the genotyping costs using the Coverage with Evidence Development (CED) mechanism, as implemented on April 5, 2010 (http://www.cms.gov/transmittals/downloads/R111NCD.pdf).
For blinding purposes, genotyping results will not be available to anyone besides the laboratory technicians. Daily warfarin doses, INR values, and other results will be entered into the GIFT DB along with new medications, the results of Doppler ultrasound testing at post-operative week 4–6 and all adverse events. Adverse events will be consistently reported using an electronic case report form
Safety
Following surgery, participants in the hospital will be monitored daily for adverse events, including VTE (DVT or pulmonary embolism), bleeding, stroke, or myocardial infarction, by members of the clinical research team. If symptomatic VTE has not been objectively documented during the period of warfarin therapy, participants will have a Doppler ultrasound of their legs at the time of warfarin completion (4–6 weeks after surgery).
The Biostatistics and Data Management Core will generate reports for the PI and the Data Safety and Monitoring Board (DSMB) regarding rates of adjudicated outcomes. The DSMB will safeguard study participants’ safety, assess effectiveness of study procedures, and monitor the overall conduct of the study. The DSMB is comprised of national experts in clinical trials, cardiology, pharmacogenetics, warfarin research, orthopedics, and statistics. The DSMB meets twice annually.
Primary study outcomes
The primary outcome for Aim 1 is the composite of VTE, major hemorrhage, INR ≥ 4, or death, and for Aim 2 the composite of nonfatal VTE or death (Table 2). Major hemorrhage includes overt bleeding causing a fall in hemoglobin level of 20 g/L or more, overt bleeding in a critical organ, or bleeding causing patient death. We define minor hemorrhage as bleeding that is neither major nor occult.
Table 2.
Summary of Aims, Endpoints, Hypotheses, and Statistical Tests
| Aim | Factor | Endpoints, Primary |
Endpoints, Secondary |
Hypothesis, Primary | Statistical Test |
|---|---|---|---|---|---|
| 1 | Genetic vs. Clinical Dosing | VTE, major hemorrhage, death, or INR ≥ 4 | INR control | Decreased event rate associated with genetic dosing in whole population and in subpopulation whose clinical and genetic predicted doses differ by ≥1 mg/day. | Chi-square test (partitioning alpha) |
| 2 | Target INR 2.5 vs. 1.8 | VTE or death | INR control, bleeding | The event rate is non-inferior in the lower target INR arm | Chi-square test |
VTE = Venous Thromboembolic Event; INR = International Normalized Ratio
Primary analyses for both aims will be on an intent-to-treat basis, but we also will report an on-treatment analysis. The secondary outcomes for this study include the percentage of time in therapeutic range (%TTR) during the first 30 days of warfarin for pharmacogenetic vs. clinical dosing and time to supra-therapeutic INR.
Power and Statistical Analyses
Aim 1. Primary endpoint for clinical vs. pharmacogenetic warfarin dosing
For Aim 1, we will analyze the primary endpoint in the whole population and in the subpopulation whose clinical and genetic predicted doses differ by ≥ 1.0 mg/day (~50% of the population) using a two-sided chi-square test. To preserve the type I error rate of this co-primary endpoint, we will partition our alpha for the tests in the whole group and the subgroup, as described below.
If we recruit 1,600 participants and have an 18% drop-out rate, we will have 1312 participants left for analysis. Using these figures and an overall Type I error rate of 0.05, we have > 95% power to detect a difference in the rate of the co-primary composite endpoint.
We estimate VTE rates (defined as clinically overt VTE or ultrasound detected VTE at 4–6 weeks) of 18% in participants randomized to clinical dosing and 15% in participants randomized to genetic dosing. Historically, DVT rates with warfarin therapy after joint arthroplasty are variable 34, often with rates around 25%. However, because seminal studies (e.g. 35,36) screened for DVT using a more sensitive test, venography, we predict a lower DVT rate in GIFT where participants will be evaluated by Doppler ultrasound.
We suspect that the rate of VTE in the subpopulation whose clinical and genetic predicted doses differ by ≥ 1.0 mg/day will be 1.6 times as high as that in the remaining population. This 1.6-fold increase is an estimate based on a 3-fold increased risk of adverse events in participants who carry at least one copy of CYP2C9*2 and/or CYP2C9*3 but no clear increase in patients homozygous for VKORC1-1639 AA. 37 Major bleeding and death will be uncommon in the trial. In the clinical arm, we anticipate that the rate of major bleeding will be 2.4% and the rate of death will be 1.0%, for a total of 3.4%. In the pharmacogenetic arm, we anticipate the rate of major bleeding or death will be 2.6% (the estimated 32% relative risk reduction in major bleeding is based on a meta-analysis of clinical trials 38 and a similar reduction in a large observational study). 39 Based on prior research, we estimated the rate of INRs ≥ 4.0 in clinical and pharmacogenetic arms to be 12.3% and 7.4% respectively 31. We anticipate that half of the bleeding events will be associated with INRs ≥ 4.0, and account for this correlation in our power calculations.
Alpha partitioning
To preserve a Type 1 error rate of 5% for Aim 1, we partitioned the alpha between the whole group and the subgroup analysis, as recommended. 40 The subgroup consists of participants for whom pharmacogenetic and clinically predicted (per baseline algorithm 28) doses differ by ≥1.0 mg/day. Due to correlation between outcomes in main study and in the subgroup, Bonferroni splitting would be overly conservative.
Partitioning the alpha in this manner maximizes power for Aim 1 while limiting the overall type 1 error rate to 0.05. We elected to partition the alpha a priori, as it maximizes the power for the test in the whole group, without jeopardizing the power in the subgroup. 40 Because the two endpoints are collinear, we used simulation to determine possible pairs of alpha values that preserved the overall 0.05 type 1 error. We selected our alpha value of 0.044 for the tests in the whole group, 0.01 in the subgroup, and 0.05 for the total alpha.
Aim 2. Primary endpoint for low vs. high target INR
We hypothesize that orthopedic participants randomized to a target INR of 1.8 will have a rate of VTE or death that is no higher (non-inferior) than those treated with a target INR of 2.5. Using the average of our estimates above, we expect the rate of VTE or death with standard warfarin therapy and Doppler US screening to be 16.5%. We will have 80% power to reject the null hypothesis of a difference greater than 5% (the non-inferiority margin) in the two arms. Patients with different target INRs will be combined when comparing clinically versus pharmacogenetically-based dosing, but we will also conduct analyses stratified by target INR values and assess for an interaction.
Contingency plan for statistical analyses
If randomization were to result in an unbalanced distribution of any clinical variable associated with VTE [i.e., age, body mass index, hormonal replacement therapy, or male gender 20], we will adjust for the imbalance using logistic regression.
Secondary Study Outcomes
Percentage of Time in Therapeutic Range (%TTR)
We will compare the time spent in therapeutic range (%TTR) during the first 30 days of warfarin for pharmacogenetic versus clinical dosing in a regression model using linear interpolation of INR values between measurements. 41
Time to First Event
We will compare time to first supra-therapeutic INR (the number of days until INR > target INR + 1.5) using the log-rank test or Cox-proportional hazard model, as appropriate. We will censor participants at the time of withdrawal, loss to follow-up, or death. Likewise, we will compare time to the first major or minor bleeding event (safety endpoint).
Secondary Statistical Analysis of Primary Endpoint
As a secondary outcome, we will rank the components of the composite outcomes in ascending order of importance: INR ≥ 4, asymptomatic DVT, symptomatic DVT, major bleed or PE, death, and analyze with ordinal logistic regression.
Discussion
Recent studies emphasize the importance of certain genetic markers in explaining inter-individual variation in warfarin requirements (Table 3).42–44 Of particular importance, common SNPs in the CYP2C9 gene (CYP2C9*2 and CYP2C9*3) are associated with impaired warfarin metabolism, decreased dose requirements and increased time necessary to achieve stable levels of anticoagulation.29, 45–48 Further, SNPs in vitamin K epoxide reductase complex 1 (VKORC1) correlate with warfarin sensitivity.49–52 In recognition of the clinical relevance of these genetic variants, the FDA in 2007, and again in 2010, approved increasingly detailed revisions to the labeling for Coumadin®, a popular formulation of warfarin, to recommend lower initial doses in participants known to carry certain variants in CYP2C9 or VKORC1.53 Although not reflected in drug labeling, recent studies have found that a variant in a third gene (CYP4F2 V433M) is associated with higher warfarin maintenance dose requirements.13
Table 3.
Comparison of completed trials and current multi-centered trials
| Trial | N | % orthopedic |
Gene(s) | Clinical Arm | PGx Dosing (Minimum # Days) |
Blinding | Primary Endpoint |
|---|---|---|---|---|---|---|---|
| Hillman [16] | 38 | 16% | CYP2C9 | Standard 5mg/day | 1 | Arm (pt. only) | Recruitment |
| Caraco [15] | 191 | 0% | CYP2C9 | Computer | 1 | Arm | INR |
| Anderson [14] | 200 | 63% | CYP2C9, VKORC1 | 10 mg warfarin nomogram [49] | 1 | Arm | INR |
| Huang [17] | 121 | 0% | CYP2C9, VKORC1 | Standard 5mg/day | 1 | Arm (pt. only) | INR |
| McMillin [18] | 229 | 100% | CYP2C9, VKORC1 | 3–5 mg/day [17] | 1 | Arm, Outcomes | INR |
| COAG | 1238 | <20% | CYP2C9, VKORC1 | Algorithm mandated | 1–4 | Arm, Dose, Outcomes | INR |
| van Schie [12] | 985* | 0% | CYP2C9, VKORC1 | Algorithm guided | 1–4 | Arm (pt. only) | INR |
| GIFT | 1600 | 100% | CYP2C9, VKORC1, CYP4F2 | Algorithm guided | 1–11 | Arm, Outcomes | AEs |
PGx = pharmacogenetic; pt. = patient; COAG = Clarification of Optimal Anticoagulation through Genetics; GIFT = Genetics-InFormatics Trial of Warfarin to Prevent DVT; AEs = adverse events
2955 patients will be randomized in total for the three trials, one each of warfarin, acenocoumarol, and phenprocoumon
Results from a 2-year randomized trial in Utah suggest that genotype-based dosing did not improve warfarin management as defined by achieving target INR range, though it was associated with fewer thrombotic events. 18 Similarly, the Medco-Mayo Effectiveness Study showed that warfarin genotyping possibly reduces the risk of hospitalization in outpatients initiating warfarin. 39 In contrast to these trials, an Israeli randomized trial 15 found that pharmacogenetic testing significantly reduced the rate of minor hemorrhages. Given these conflicting results, clinicians and researchers are uncertain as to whether they should use genetic testing when initiating warfarin therapy.
GIFT will evaluate novel strategies to improve the safety and effectiveness of warfarin therapy and test the safety and effectiveness of different target INR ranges. Although there is evidence of genotypic influence on warfarin requirements, prior studies of pharmacogenetic vs. clinical warfarin dosing have not been sufficiently powered to determine whether pharmacogenetic testing prevents adverse events. The results from GIFT will illuminate how pharmacogenetic management affects INR control and clinical outcomes while testing the non-inferiority of a target INR of 1.8 versus a target INR of 2.5. GIFT is complementary to other studies (Table 3) and has several advantages: larger sample size, inclusion of high-risk participants, systematic screening of study participants for clinical outcomes, consistent availability of genotype prior to the first warfarin dose, use of CYP4F2, and dosing algorithms that guide warfarin dose for at least the initial 11 days of therapy.
Acknowledgements
This research is funded by the NIH (R01 HL097036). We thank the NIH, FDA and CMS whose constructive criticism improved the trial design. We appreciate the comments of Gerald Moskowitz, PhD, on an earlier draft of the manuscript.
Footnotes
Conflict of interest
The authors declare no conflicts of interest with this study.
References
- 1.White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep-venous thrombosis. Am J Med. 1999;107(5):414–424. doi: 10.1016/s0002-9343(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 2.Landefeld SC, Beyth R. Anticoagulant-related bleeding: Clinical epidemiology, prediction and prevention. Am J Med. 1993;95:315–328. doi: 10.1016/0002-9343(93)90285-w. [DOI] [PubMed] [Google Scholar]
- 3.Fihn SD, McDonell M, Martin D, Henikoff J, Vermes D, Kent D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Annals of internal medicine. 1993;118(7):511–520. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Douketis JD, Foster GA, Crowther MA, Prins MH, Ginsberg JS. Clinical Risk Factors and Timing of Recurrent Venous Thromboembolism During the Initial 3 Months of Anticoagulant Therapy. Arch Intern Med. 2000;160(22):3431–3436. doi: 10.1001/archinte.160.22.3431. [DOI] [PubMed] [Google Scholar]
- 5.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Annals of internal medicine. 2003;139(11):893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 7.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107(12):1692–1711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 8.Ezekowitz MD, James KE, Radford MJ, Rickles FR, Redmond N. Initiating and maintaining patients on warfarin anticoagulation: the importance of monitoring. J Cardiovasc Pharmacol Ther. 1999;4(1):3–8. doi: 10.1177/107424849900400102. [DOI] [PubMed] [Google Scholar]
- 9.Ansell J, Hirsh J, Dalen J, Bussey H, Anderson D, Poller L, et al. Managing oral anticoagulant therapy. Chest. 2001;119(1 Suppl):22S–38S. doi: 10.1378/chest.119.1_suppl.22s. [DOI] [PubMed] [Google Scholar]
- 10.Thacker S, Grice G, Milligan P, Gage B. Dosing Anticoagulant Therapy with Coumarin Drugs: Is Genotyping Clinically Useful? Yes. J Thromb Haemost. 2008 doi: 10.1111/j.1538-7836.2008.03074.x. [DOI] [PubMed] [Google Scholar]
- 11.Bussey HI, Wittkowsky AK, Hylek EM, Walker MB. Genetic testing for warfarin dosing? Not yet ready for prime time. Pharmacotherapy. 2008;28(2):141–143. doi: 10.1592/phco.28.2.141. [DOI] [PubMed] [Google Scholar]
- 12.van Schie RM, Wadelius MI, Kamali F, Daly AK, Manolopoulos VG, de Boer A, et al. Genotype-guided dosing of coumarin derivatives: the European pharmacogenetics of anticoagulant therapy (EU-PACT) trial design. Pharmacogenomics. 2009;10(10):1687–1695. doi: 10.2217/pgs.09.125. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111(8):4106–4112. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116(22):2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 15.Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clinical pharmacology and therapeutics. 2008;83(3):460–470. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 16.Hillman MA, Wilke RA, Yale SH, Vidaillet HJ, Caldwell MD, Glurich I, et al. A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clin Med Res. 2005;3(3):137–145. doi: 10.3121/cmr.3.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang SW, Chen HS, Wang XQ, Huang L, Xu DL, Hu XJ, et al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenetics and genomics. 2009 doi: 10.1097/FPC.0b013e328326e0c7. [DOI] [PubMed] [Google Scholar]
- 18.McMillin GA, Melis R, Wilson A, Strong MB, Wanner NA, Vinik RG, et al. Gene-based warfarin dosing compared with standard of care practices in an orthopedic surgery population: a prospective, parallel cohort study. Therapeutic drug monitoring. 2010;32(3):338–345. doi: 10.1097/FTD.0b013e3181d925bb. [DOI] [PubMed] [Google Scholar]
- 19.White RH, Zhou H, Gage BF. Effect of age on the incidence of venous thromboembolism after major surgery. J Thromb Haemost. 2004;2(8):1327–1333. doi: 10.1046/j.1538-7836.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- 20.Davidson HC, Mazzu D, Gage BF, Jeffrey RB. Screening for deep venous thrombosis in asymptomatic postoperative orthopedic patients using color Doppler sonography: analysis of prevalence and risk factors. AJR Am J Roentgenol. 1996;166(3):659–662. doi: 10.2214/ajr.166.3.8623645. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Orthopaedic Surgeons. Clinical guidelines on prevention of pulmonary embolism in patients undergoing total hip or knee arthroplasty. Rosemont, IL: AAOS; 2007. pp. 1–63. http://www.aaos.org/Research/Guidelines/PE_Guideline.pdf. [Google Scholar]
- 22.Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425–1434. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 23.Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631–639. doi: 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 24.Enyart JJ, Jones RJ. Low-dose warfarin for prevention of symptomatic thromboembolism after orthopedic surgery. Ann Pharmacother. 2005;39(6):1002–1007. doi: 10.1345/aph.1E536. [DOI] [PubMed] [Google Scholar]
- 25.Pendleton RC, Wheeler M, Wanner N, Strong MB, Vinik R, Peters CL. A safe, effective, and easy to use warfarin initiation dosing nomogram for post-joint arthroplasty patients. The Journal of arthroplasty. 2010;25(1):121–127. doi: 10.1016/j.arth.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Anderson FA, Ayers DC, Colwell C, Cushner F, Friedman R, Huo M, Fitzgerald G, Kwong L. Bleeding Concerns Drive Practices Of Orthopedic Surgeons In Prevention Of Venous Thromboembolism In Primary Hip And Knee Arthroplasty. J Thromb Haemost. 2007;Volume 5(Supplement 2) ISTH abstract. [Google Scholar]
- 27.Linder MW, Bon Homme M, Reynolds KK, Gage BF, Eby C, Silvestrov N, et al. Interactive modeling for ongoing utility of pharmacogenetic diagnostic testing: application for warfarin therapy. Clin Chem. 2009;55(10):1861–1868. doi: 10.1373/clinchem.2009.125898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clinical pharmacology and therapeutics. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voora D, Eby C, Linder MW, Milligan PE, Bukaveckas BL, McLeod HL, et al. Prospective dosing of warfarin based on cytochrome P-450 2C9 genotype. Thrombosis and haemostasis. 2005;93(4):700–705. doi: 10.1160/TH04-08-0542. [DOI] [PubMed] [Google Scholar]
- 30.Lenzini P, Grice G, Milligan P, Gatchel S, Deych E, Eby C, et al. Optimal Dose Adjustment in Orthopaedic Patients Beginning Warfarin Therapy. Ann Pharmacother. 2007;41(11):1798–1804. doi: 10.1345/aph.1K197. [DOI] [PubMed] [Google Scholar]
- 31.Lenzini PA, Grice GR, Milligan PE, Dowd MB, Subherwal S, Deych E, et al. Laboratory and clinical outcomes of pharmacogenetic vs. clinical protocols for warfarin initiation in orthopedic patients. J Thromb Haemost. 2008;6(10):1655–1662. doi: 10.1111/j.1538-7836.2008.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King CR, Deych E, Milligan P, Eby C, Lenzini P, Grice G, et al. Gamma-glutamyl carboxylase and its influence on warfarin dose. Thrombosis and haemostasis. 2010;104(4) doi: 10.1160/TH09-11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin D, Horne, Lenzini PA, Wadelius M, Jorgensen AL, Kimmel SE, Eriksson N, Kurnik D, Stein CM, Caldwell MD, Eby CS, Rane A, Lindh JD, Shin JG, Kim HS, Angchaisuksuri P, Chen J, Carlquist JF, Grice GR, Kronquist KE, Gage BF. Pharmacogenetic Dose Refinements Remain Significantly Influenced by Genetic Factors after INR Measurements through One Week of Warfarin Therapy. Am. Coll. Cardiol. 2010;55 A130.E1218 (10) 61219-3. [Google Scholar]
- 34.Geerts W, Bergqvist D, Pineo G, Heit J, Samama C, Lassen M, et al. Prevention of venous thromboembolism. CHEST. 2008;133:381–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 35.Francis CW, Davidson BL, Berkowitz SD, Lotke PA, Ginsberg JS, Lieberman JR, et al. Ximelagatran versus warfarin for the prevention of venous thromboembolism after total knee arthroplasty. A randomized, double-blind trial. Annals of internal medicine. 2002;137(8):648–655. doi: 10.7326/0003-4819-137-8-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Francis CW, Berkowitz SD, Comp PC, Lieberman JR, Ginsberg JS, Paiement G, et al. Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med. 2003;349(18):1703–1712. doi: 10.1056/NEJMoa035162. [DOI] [PubMed] [Google Scholar]
- 37.Limdi NA, Wiener H, Goldstein JA, Acton RT, Beasley TM. Influence of CYP2C9 and VKORC1 on warfarin response during initiation of therapy. Blood Cells Mol Dis. 2009 doi: 10.1016/j.bcmd.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Annals of internal medicine. 2009;150(2):73–83. doi: 10.7326/0003-4819-150-2-200901200-00005. [DOI] [PubMed] [Google Scholar]
- 39.Epstein RS, Moyer TP, Aubert RE, O'Kane DJ, Xia F, Verbrugge RR, et al. Warfarin Genotyping Reduces Hospitalization Rates Results From the MM-WES (Medco-Mayo Warfarin Effectiveness Study) Journal of the American College of Cardiology. 2010 doi: 10.1016/j.jacc.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Joo J, Geller NL, French B, Kimmel SE, Rosenberg Y, Ellenberg JH. Prospective alpha allocation in the Clarification of Optimal Anticoagulation through Genetics (COAG) trial. Clin Trials. 2010;7(5):597–604. doi: 10.1177/1740774510381285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thrombosis and haemostasis. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 42.Millican E, Lenzini P, Milligan P, Grosso L, Eby C, Deych E, et al. Genetic-based dosing in orthopaedic patients beginning warfarin therapy. Blood. 2007;110(5):1511–1515. doi: 10.1182/blood-2007-01-069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schelleman H, Chen Z, Kealey C, Whitehead AS, Christie J, Price M, et al. Warfarin response and vitamin K epoxide reductase complex 1 in African Americans and Caucasians. Clinical pharmacology and therapeutics. 2007;81(5):742–747. doi: 10.1038/sj.clpt.6100144. [DOI] [PubMed] [Google Scholar]
- 44.Limdi NA, McGwin G, Goldstein JA, Beasley TM, Arnett DK, Adler BK, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clinical pharmacology and therapeutics. 2008;83(2):312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, McLeod HL. Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thrombosis and haemostasis. 2004;91(1):87–94. doi: 10.1160/TH03-06-0379. [DOI] [PubMed] [Google Scholar]
- 46.Linder MW, Looney S, Adams JE, 3rd, Johnson N, Antonino-Green D, Lacefield N, et al. Warfarin dose adjustments based on CYP2C9 genetic polymorphisms. Journal of thrombosis and thrombolysis. 2002;14(3):227–232. doi: 10.1023/a:1025052827305. [DOI] [PubMed] [Google Scholar]
- 47.Higashi MK, Veenstra DL, Kondo LM, Wittkowsky AK, Srinouanprachanh SL, Farin FM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287(13):1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 48.Margaglione M, Colaizzo D, D'Andrea G, Brancaccio V, Ciampa A, Grandone E, et al. Genetic modulation of oral anticoagulation with warfarin. Thrombosis and haemostasis. 2000;84(5):775–778. [PubMed] [Google Scholar]
- 49.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352(22):2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 50.Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. The pharmacogenomics journal. 2005;5:262–270. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- 51.D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105(2):645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 52.Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14(13):1745–1751. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 53.Wood S. New Warfarin Labeling Reminds Physicians About Genetic Tests to Help Guide Initial Warfarin Dosing. 2007 http://www.theheart.org/article/807123.do. [Google Scholar]


