Abstract
Estrogen signaling pathways may play a significant role in the pathogenesis of non-small cell lung cancers (NSCLC) as evidenced by the expression of aromatase and estrogen receptors (ERα and ERβ) in many of these tumors. Here we examine whether ERα and ERβ levels in conjunction with aromatase define patient groups with respect to survival outcomes and possible treatment regimens. Immunohistochemistry was performed on a high-density tissue microarray with resulting data and clinical information available for 377 patients. Patients were subdivided by gender, age and tumor histology, and survival data was determined using the Cox proportional hazards model and Kaplan-Meier curves. Neither ERα nor ERβ alone were predictors of survival in NSCLC. However, when coupled with aromatase expression, higher ERβ levels predicted worse survival in patients whose tumors expressed higher levels of aromatase. Although this finding was present in patients of both genders, it was especially pronounced in women ≥ 65 years old, where higher expression of both ERβ and aromatase indicated a markedly worse survival rate than that determined by aromatase alone. Conclusion: Expression of ERβ together with aromatase has predictive value for survival in different gender and age subgroups of NSCLC patients. This predictive value is stronger than each individual marker alone. Our results suggest treatment with aromatase inhibitors alone or combined with estrogen receptor modulators may be of benefit in some subpopulations of these patients.
Keywords: NSCLC, tissue microarray, aromatase, estrogen receptor, immunohistochemistry, prognosis
Introduction
Lung cancer continues to be the leading cause of cancer mortality in both men and women throughout the world. According to the American Cancer Society, in the United States there were an estimated 222,520 new cases of lung cancer and 157,300 deaths from the disease in 2010 [1]. Survival rates for non-small cell lung cancer (NSCLC) continue to be very poor with the 5-year survival rate for all stages at approximately 16% [1]. The search for effective treatment protocols remains elusive although newer agents such as epidermal growth factor receptor (EGFR) inhibitors gefitinib and erlotinib may have beneficial effects in subpopulations of lung cancer patients with certain EGFR mutations [2,3,4,5].
There is increasing evidence to suggest that estrogens and estrogen signaling play a significant role not only in normal lung development but also in lung cancer pathophysiology. In addition to known hormone responsive tissues, estrogen receptors (ERα and ERβ) are expressed in normal lung [6] and in a many non-small cell lung cancer cells [7,8,9,10,11,12,13,14,15]. The most biologically active estrogen, 17β-estradiol, is a mitogen for NSCLC cells and stimulates gene transcription both in vitro and in vivo [9,16,17]. In addition, aromatase, the enzyme that catalyzes formation of estrogens, is expressed in many NSCLCs and correlates with measures of estrogen production in these tumors [11,18]. Thus, localized production of estrogen may contribute to tumor promotion, whether through increased ER signaling or through the formation of oxidative metabolites of estrogen which can lead to formation of unstable DNA adducts and mutagenesis in the lung [19].
Previously we found that, in women 65 years and older with NSCLC, higher aromatase levels in tumor cells conferred a worse prognosis for survival [18]. In the retrospective study described here, we utilized tissue microarray (TMA) technology to examine whether the expression of ERα and ERβ correlated with lung cancer pathology and/or disease outcome. ERβ, but not ERα, showed significantly increased levels of expression with increasing tumor grade. Neither ERα nor ERβ alone were predictors of survival in NSCLC. However, when coupled with levels of aromatase expression, in tumors expressing higher levels of aromatase, ERβ became a strong independent predictor of survival.
Materials and Methods
Patient material
A lung TMA was constructed with archival formalin-fixed, paraffin embedded lung tissue samples as previously described [20,21,22]. All appropriate Institutional Review Board (IRB) and Health Insurance Portability and Accountability Act (HIPPA) regulations were followed. Tissues sampled include primary lung tumor, adjacent non-neoplastic lung parenchyma and metastatic lung carcinoma to lymph nodes and distant sites. Primary lung tumor specimens were derived from patients who underwent segmentectomies or lobectomies with clear surgical margins. All tumors were reviewed by at least two pathologists to confirm the diagnosis. At least three core tissue biopsies, each 0.6 mm in diameter, were taken from select, morphologically representative regions of each paraffin embedded lung tumor and precisely arrayed using a custom built instrument as previously described [20,21,22]. More specific details on the array are included in the Supplemental data section.
For this study, 377 of these patients had sufficient clinical and tissue staining information on primary non-small cell lung tumors. Of these, 192 were women, and 185 were men. Of the cases, 226 were adenocarcinoma (127 women, 99 men), and 93 were squamous cell carcinoma (36 women, 57 men). The remaining histologies included 30 large cell carcinomas and small numbers of adenosquamous carcinoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, carcinoid, and atypical carcinoid. The mean age of patients was 65 years for men and 65.6 years for women. For smoking status, 320 were current or former smokers and 47 were never smokers. No smoking history was available on the remaining patients. Overall, 122 patients had received pre-operative treatment with either chemotherapy, radiation or both.
Immunohistochemistry
The lung TMA was stained using standard two-step indirect immunohistochemistry similar to previous experiments [20,22]. Briefly, TMA sections were cut immediately prior to being stained. After deparaffinization in xylenes, the sections were rehydrated in graded alcohols. Endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol at room temperature (25° C). The sections were placed in a 95° C solution of 0.01M sodium citrate buffer pH 6.0 for antigen retrieval. Blocking nonspecific protein-binding sites was done using background sniper from Biocare Medical (catalog# BS966G/H/L) applied for 5 minutes.
Primary antibody used for detecting ERβ was a mouse anti-ERβ-1 monoclonal antibody (clone PPG5/10, product #MCA1974ST, AbDSerotec, Raleigh, NC) against a synthetic peptide derived from amino acid residues 516 - 530 at the C-terminus of ERβ isoform 1. The primary antibody was applied overnight at 4°C at 1:20 dilution. For ERα, primary rabbit anti-ERα antibody (1D5 obtained from Invitrogen/Zymed, Carlsbad, CA) was applied for 30 minutes at room temperature at 1:800 dilution. For aromatase a goat anti- human CYP19 antibody (C16, Santa Cruz Biotechnology) was used as previously described [18]. Detection was accomplished with the Dako Envision System, followed by chromogen detection with diaminobenzidine (DAB). The sections were counterstained with Harris' haematoxylin, followed by dehydration through graded alcohol solutions and mounting. Positive controls for ERα and ERβ were from breast cancer cases. Positive controls for aromatase were from breast and known positive lung cancer cases. Negative controls were identical array sections stained in the absence of the primary antibody. Semiquantitative scoring of antibody staining on the TMA was performed by one pathologist (VM) and rechecked by a second (MA), without prior knowledge of clinical information. These scores were highly consistent with a correlation coefficient of 0.97. Nuclear and cytoplasmic ERβ staining on array spots was evaluated using staining intensity (0 = not detected, 1 = weak, 2 = moderate, and 3 = strong) and percentage of cells staining at each intensity level (0-100%). A final integrated value of intensity and frequency was derived with the formula: [(3x) + (2y) + (1z)] / 100 where x, y, and z are % staining at intensity 3, 2, and 1, respectively. This value was used for comparing tissue staining.
Statistical analysis
Analyses were performed using the open source R software (http://www.R-project.org) including survival, Design, Hmisc and akima and lattice packages. Pooling criteria are discussed in the Supplemental material section. ERβ expression differences among various subgroups was determined using the Wilcoxon signed rank test or Kruskal-Wallis rank sum test. For dichotomized (high versus low of ERβ and aromatase) expression, the Fisher exact test was used for analysis with categorical variables such as stage, grade and smoking history (Supplement, Table S3). Survival curves were calculated using the Kaplan-Meier method and comparisons were made using the log-rank test. The Cox proportional hazards model (univariate and multivariate) was used to determine the significance of other factors related to survival. The proportional hazards assumption was verified using Schoenfeld and dfbeta residuals. LogRank and Fisher exact P-values were two-sided and a P < 0.05 was considered significant.
Results
Based on our previous work which showed aromatase expression in NSCLC strongly predicting survival in women ≥ 65 years of age, we now examined ERα and ERβ expression in this same setting. These two receptors are the most well characterized effectors of estrogen signaling with possibilities for therapeutic interventions.
Expression profile of ERα and ERβ in NSCLC
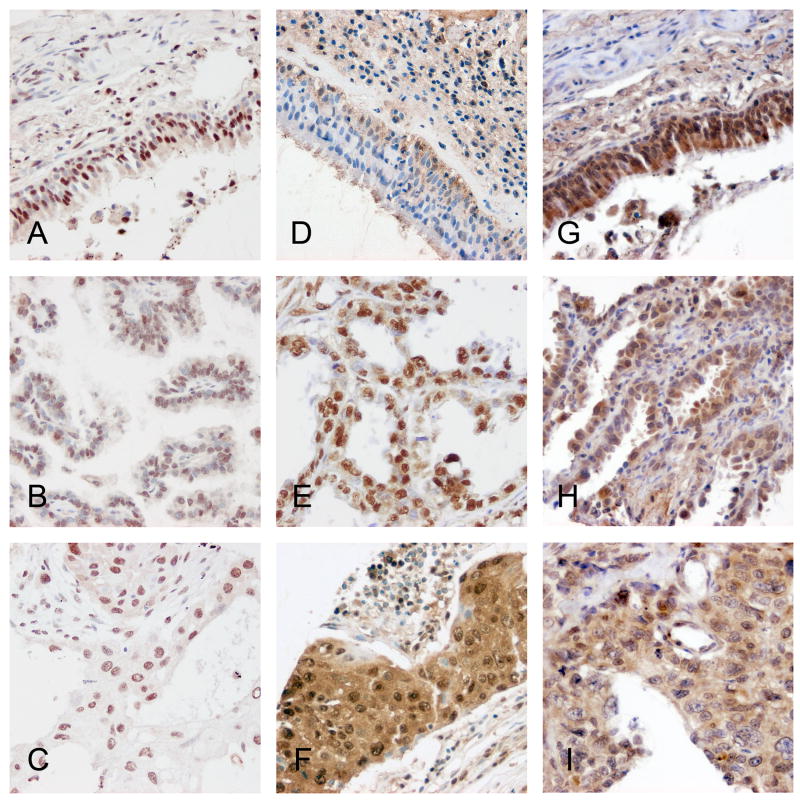
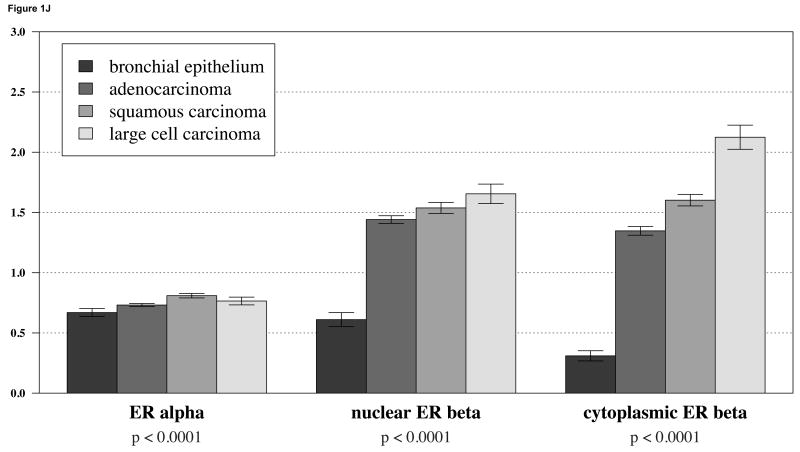
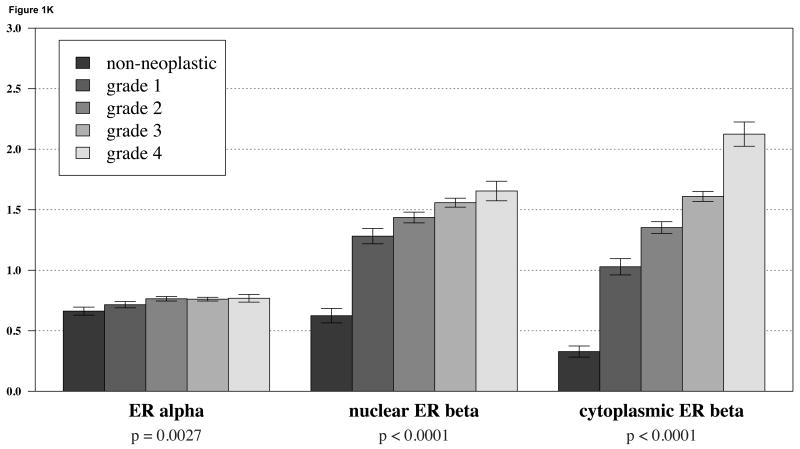
We first considered ERα and ERβ expression separately in lung cancer samples using TMA technology. ERα expression in NSCLC was detected primarily in the nucleus and only very weakly in the cytoplasmic compartment. Representative images are shown in Figures 1A-C. ERβ expression was also observed in both the nuclear and cytoplasmic compartments with relatively strong cytoplasmic staining in some cases (Figure 1D-F) When we considered expression levels based on histologies, we observed that there was only slight difference in ERα expression between non-malignant bronchial epithelial cells and any major subclass of NSCLC (i.e., adenocarcinoma, squamous cell carcinoma, or large cell carcinoma) (Figure 1J). However, both nuclear and cytoplasmic ERβ showed a markedly significant increase in expression in NSCLC compared to bronchial epithelium (Figure 1J). For ERβ higher grade was also associated with notably higher expression, in contrast to ERa where increase in expression was much less prominent (Figure 1K).
Figure 1.
A-C: representative staining of ERα in bronchial epithelium, adenocarcinoma and squamous carcinoma respectively; D-F: representative staining of ERβ in bronchial epithelium, adenocarcinoma and squamous carcinoma; G-I: representative staining of aromatase in bronchial epithelium, adenocarcinoma and squamous carcinoma; J: barplots of ERα and ERβ in different tumor histologies; K: barplots of ERα and ERβ in different tumor grades show a significant increase in cytoplasmic levels of ERβ with increase in grade.
Neither ERα nor ERβ expression alone predicts survival in patients with NSCLC
We next examined whether the expression levels of ERα or ERβ were predictive of disease specific survival. For ERβ we considered both nuclear and cytoplasmic expression. Using both univariate and multivariate Cox models, neither ERα nor ERβ alone was a predictor of survival in NSCLC patients (P=0.57 for nuclear ERα; P=0.38 and 0.99 for cytoplasmic ERβ and nuclear ERβ, respectively). This was the case with expression both as a continuous variable or as a dichotomized variable (i.e., low versus high expression). We further stratified the population to examine whether ERα or ERβ (cytoplasmic or nuclear) was a prognostic marker in a subset of individuals. However, when we considered gender, stage, histopathology subtype, or smoking status, neither protein predicted outcome in any subpopulation examined. We further examined whether expressions of these proteins in combination might predict outcome. The combined expression of ERα and ERβ was not predictive of survival for individuals with NSCLC or any subpopulation that we examined.
ERβ plus aromatase expression predicts survival in NSCLC
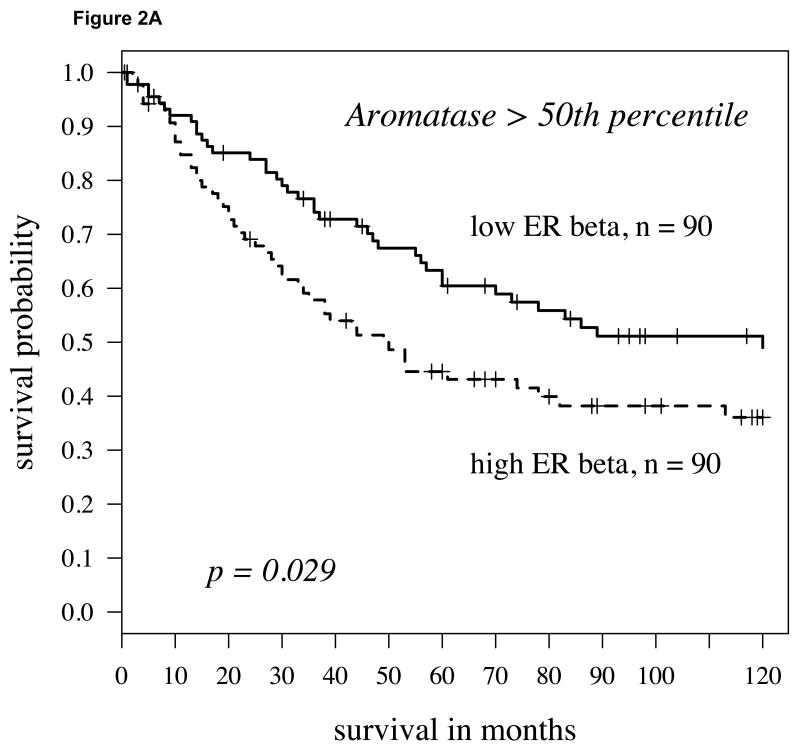
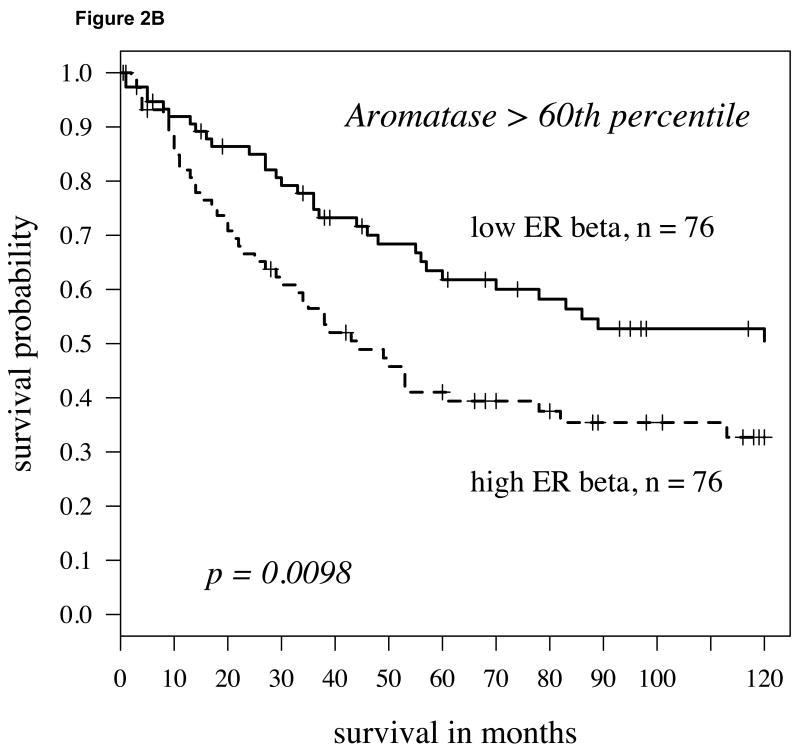
Recently, we and others observed that the enzyme aromatase was expressed in NSCLC cells [11,23,24,25] and that the expression levels were a powerful predictor of disease-specific death in women with NSCLC who were 65 years or older [18]. Aromatase is the final enzyme in the biosynthesis of estrogens. Here we considered whether the combination of aromatase plus ER expression would further segment the NSCLC population and prove to be an even stronger predictor of survival outcome. High versus low expression of all markers was initially defined in a non-biased fashion by dichotomizing at the median value. When such an analysis was conducted, ERα added no predictive value compared to aromatase alone (data not shown). However, when we considered ERβ expression, we found that the combination of high ERβ and high aromatase predicted a significantly poorer outcome in all individuals with NSCLC compared to individuals with higher levels of aromatase but relatively lower levels of ERβ (Figure 2A; P = 0.029). This observation was similar for both cytoplasmic and nuclear expression; however, cytoplasmic ERβ was a considerably stronger predictor as a continuous variable by the univariate Cox model (P= 0.008; hazard ratio = 1.41). Notably, this observation was even stronger if we considered patients with aromatase expression above the 60th percentile (defined as high aromatase expression) and dichotomized based on ERβ levels (Figure 2B; P=0.0098). Under these conditions, ERβ also had a strong predictor of outcome as a continuous variable as well (P = 0.005, hazard ratio = 1.48; see Supplement, Figure S1 and Table S1).
Fig 2.
A: For patients with high aromatase expression (determined by staining intensity above median levels) splitting cytoplasmic ERβ expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with high ERβ (hazard ratio = 1.6, p-value = 0.029); B: Using above the 60th percentile as a cutoff to define high aromatase expression, low cytoplasmic ERβ (again median expression level) conferred a slightly better prognosis (p=0.0098, hazard ratio = 1.81).
We further examined whether in individuals with high aromatase ERβ remained an independent predictor of outcome when compared with additional clinical variables. Indeed, when we considered stage, age, and grade in a multivariate Cox model, of individuals whose tumors had higher aromatase levels, ERβ remained an independent predictor of survival (P=0.007, Table 1).
Table 1. Multivariate Cox proportional hazards model for all patients with high aromatase [defined by higher than median levels], (n=190).
| Variable | Hazard Ratio (95% confidence interval) |
P-value |
|---|---|---|
| Cytoplasmic ERβ mean intensity | 1.48 (1.11 - 1.96) | 0.0073 |
| Stage | 2.32 (1.88 - 2.87) | <0.0001 |
| Grade | 0.93 (0.72 - 1.38) | 0.1200 |
| Age | 1.05 (1.02 - 1.07) | 0.0003 |
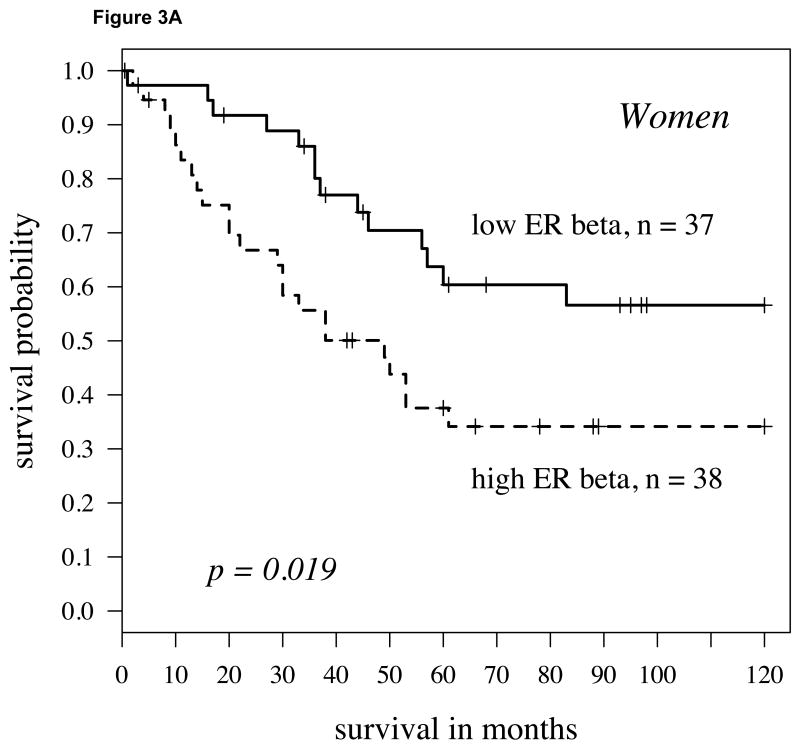
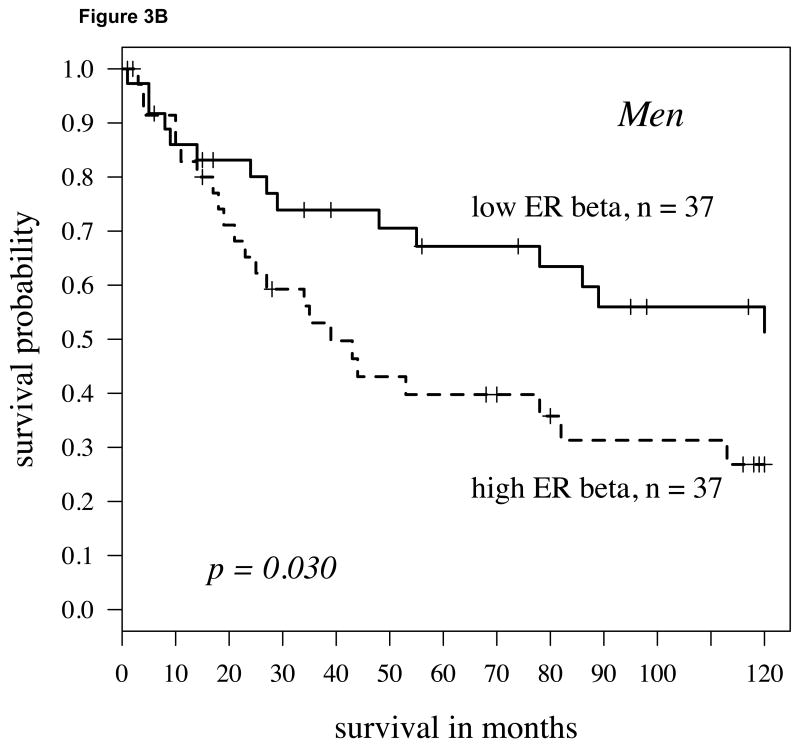
We further stratified the population by gender to test whether the combination of aromatase plus ERβ was a stronger predictor for women or men. For both men and women, the combination of higher aromatase expression with elevated ERβ expression predicted a poor outcome (Figures 3A and 3B; P = 0.019 and 0.030, respectively). However, only for women were the markers significant both as a continuous (P = 0.020) and a dichotomized variable.
Fig 3.
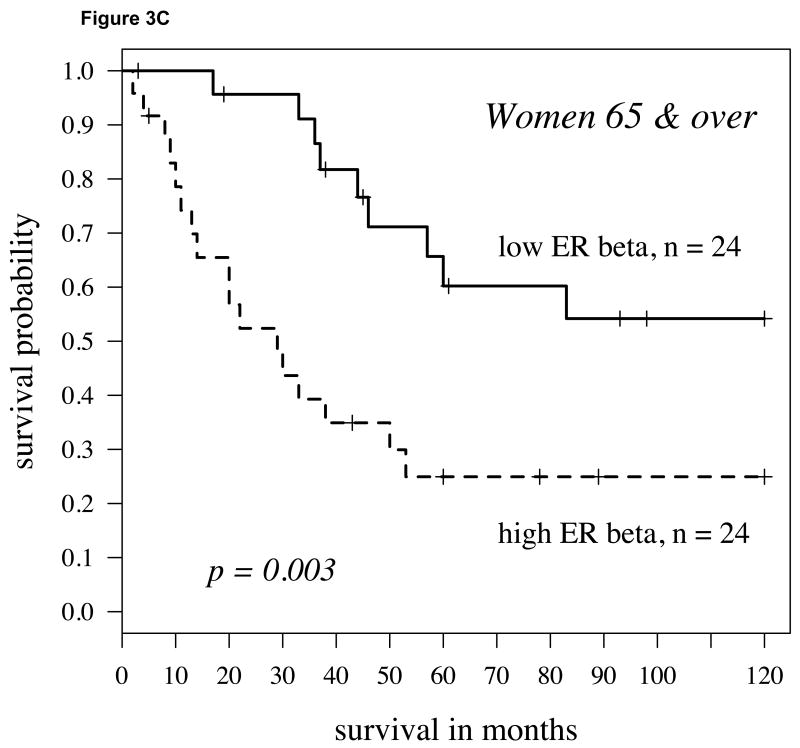
A: For women with high aromatase expression (staining intensity above the 60th percentile splitting cytoplasmic ERβ expression at the midpoint), the Kaplan-Meier survival curve shows worse survival in those patients with high ERβ (hazard ratio = 2.18, p-value = 0.019); B: For men, findings were similar but slightly weaker (hazard ratio = 2.04, P = 0.030). C: In women 65 and over, the findings were stronger than other population subgroups (p=0.003, hazard ratio = 3.25).
As highlighted above, aromatase alone was a strong indicator of survival primarily in women who were 65 years and older [18]. Therefore, we further assessed men and women by separating them into groups of those under or over 65. Stratifying the population by both gender and age groups yielded slightly stronger differences for women 65 years of age or older, with higher aromatase and ERβ predicting a shorter time course to death due to disease (P=0.003, Figure 3C). Although higher levels of ERβ in women under 65 and men 65 and over with higher aromatase were also associated with poorer survival, this difference did not reach significance in either group. In men under 65 with higher aromatase expression, ERβ did not affect survival.
In individuals with higher ERβ expression, aromatase levels predict outcome
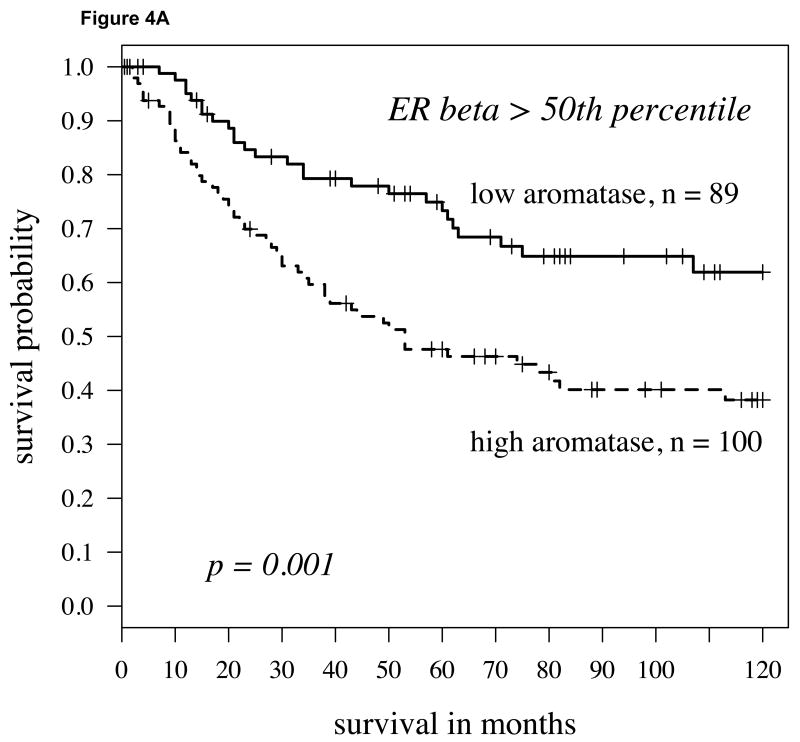
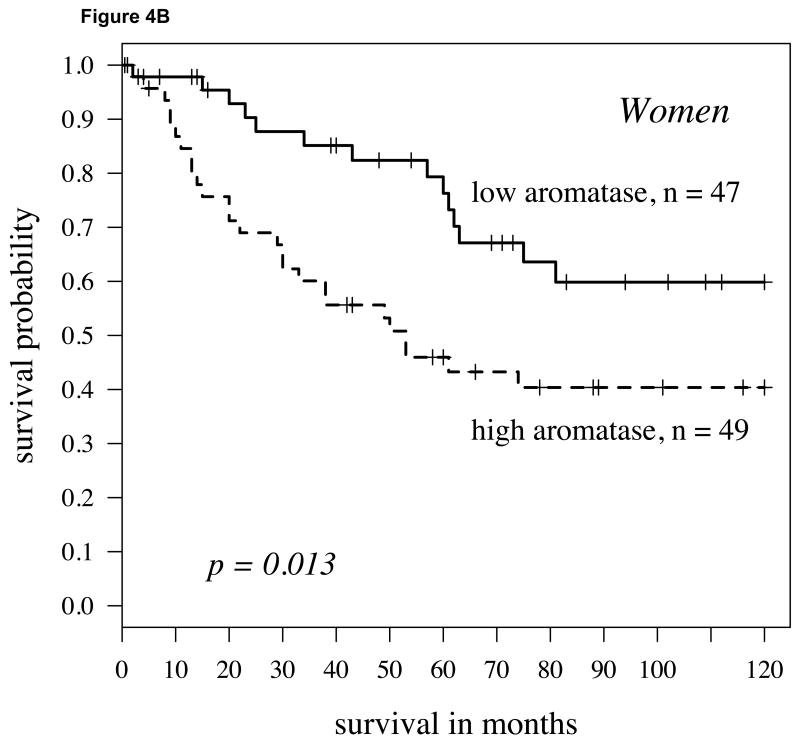
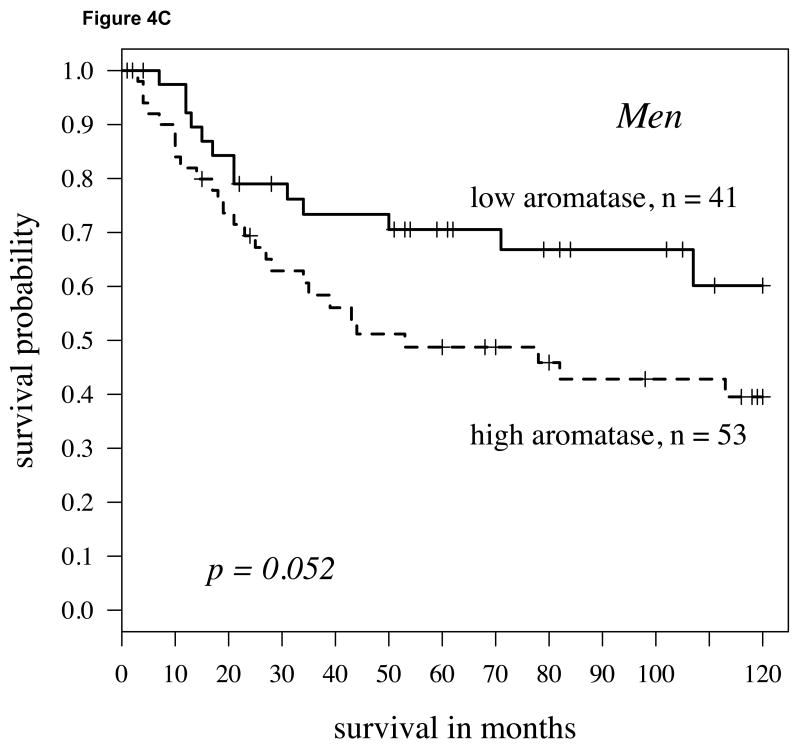
We conducted a comparable analysis to the one described above with first dichotomizing in a non-biased fashion (50% percentile) the population by high versus low ERβ expression. Within the population with higher ERβ expression, individuals with lower aromatase expression had a significantly higher probability of survival than those with higher aromatase levels (Figure 4A; P=0.001). This was similarly the case when aromatase was not dichotomized but considered as continuous variable using the univariate Cox model (P=0.001, hazard ratio = 1.88). Aromatase remained an independent predictor of survival when stage, grade and age were taken into consideration (P=0.0001, Table 2). If the ERβ-high population was stratified by gender, aromatase expression predicted survival differences more strongly in women (Figure 4B; P=0.013) than in men (Figure 4C; P=0.052).
Fig 4.
A: For patients with high cytoplasmic ERβ expression (determined by staining intensity above median levels) splitting aromatase expression at the median level, the Kaplan-Meier survival curve shows significantly worse survival in those patients with aromatase (hazard ratio = 1.47, p-value = 0.001); B: For women with high cytoplasmic ERβ expression, again the Kaplan-Meier survival curve again shows worse survival in those patients with higher aromatase (hazard ratio = 1.49, p-value = 0.013); C: For men, findings were similar but slightly weaker (hazard ratio = 1.38, P = 0.052).
Table 2. Multivariate Cox proportional hazards model for all patients with high cytoplasmic ERβ [defined by higher than median levels], (n=189).
| Variable | Hazard Ratio (95% confidence interval) |
P-value |
|---|---|---|
| Aromatase mean intensity | 2.17 (1.46 - 3.23) | 0.0001 |
| Stage | 2.13 (1.69 - 2.68) | <0.0001 |
| Grade | 1.08 (0.81 - 1.45) | 0.5767 |
| Age | 1.05 (1.02 - 1.08) | 0.0005 |
Discussion
A mounting body of evidence from cell culture and mouse models has shown that estrogen and activation of the estrogen receptor pathways are important not only in lung embryogeneis [26,27] but also in lung cancer pathogenesis [9,28,29] Aromatase mediated conversion of androstenedione to estradiol [30] may also be important for lung cancer progression [29]. Evidence suggests that the majority of intratumoral estradiol is produced locally by aromatase in the lung tumors themselves, possibly from circulating androgens [11]. The exact functions of estrogens in lung cancers however are not clear and gender-based differences also need to be further elucidated. In NSCLC cells, estradiol (E2) was shown to increase cell proliferation in both in vitro and in vivo models [9,28,29,31]. In contrast, agents which blocked estrogen synthesis (such as the aromatase inhibitors anastrazole, exemestane) or interfered with receptor function (such as the pure antiestrogen fulvestrant) inhibited tumor xenograft proliferation [9,28,32]. Effects of estrogen in lung cancers may be mediated directly by receptor binding followed by nuclear localization and activation of transcription or indirectly by extranuclear pathways that engage kinase signaling to modulate transcription and tumor progression. In addition certain oxidative metabolites of estrogen such as catechol estrogen-3,4-quinones can react with DNA to form depurinating adducts, possibly leading to mutations that promote cancer initiation [33].
Gender related differences in lung cancer have been well documented and suggest a role for hormonal influences. Of non-smokers with lung cancer, a higher percentage are women [34]. Women have increased susceptibility to lung cancers from tobacco exposure but have overall better prognoses in some studies but not others [35,36,37,38]. In an analysis of the SEER database, a survival advantage for older (55-59 years) versus younger (40-49 years) women was seen for squamous cell carcinoma and bronchioloalveolar carcinoma suggesting postmenopausal status might be advantageous at least for these histologic subtypes [16]. Also recent studies from the Women's Health Initiative (WHI) and the Vitamins and Lifestyle studies showed a possible increase in incidence and mortality from NSCLC in post-menopausal women treated with combined estrogen and progesterone hormone replacement therapy [39,40,41]. A somewhat analogous finding was reduced lung cancer mortality in breast cancer patients who had been treated with anti-estrogens[42].
We have set out to profile key elements of the estrogen / ER signaling pathway in individuals with lung cancer. Previous proteomics results showed that aromatase expression levels were a strong predictor of survival in women over 65 years of age with NSCLC. Lower levels of aromatase predicted a significantly longer survival. Here, we have continued to assess the estrogen signaling pathway by examining the expression levels and localization of ERα and ERβ. While ERβ in contrast to ERα displayed enhanced expression with increasing grade, neither ERα nor ERβ alone was predictive for survival in individuals with NSCLC nor any patient subgroup examined (gender, histology, stage, or smoking status). However, when we combined ERβ expression with aromatase expression, we found that higher levels of both proteins together predicted a significantly poorer survival outcome. While this observation held true for all individuals with NSCLC examined together, it was somewhat stronger for women of age 65 years and older and weakest in men under 65 years of age.
ER Signaling Pathways
Of note, cytoplasmic levels of ERβ were a stronger predictor of outcome than nuclear expression. Extranuclear estrogen receptor signaling may have considerable importance through interactions with tyrosine kinase receptors (EGFR and IGF1-R), MAP kinase and/or PI3/AKT kinase signaling [16,43,44]. In breast cancer cells, recent data suggest proline-glutamic acid-leucine-rich protein-1 (PELP1) couples ERs to signaling pathways such as Src-MAPK, PI3K-Akt and EGFR-Stat3 [45].
ERs have also been found to cross-communicate with EGFR signaling in NSCLC cells such that combined targeting of ER and EGFR enhances antitumor effects [17,28,32,46,47]. EGFR mutations are well documented in a subset of individuals with lung cancer; such mutations are more prevalent in women in East Asian populations, never-smokers and individuals with adenocarcinoma [48]. Recently, Nose et al. observed that in individuals with EGFR mutations, higher ERβ expression significantly correlated with lung cancer-free survival [49]. While we do not currently have the EGFR mutation status in the cohort we examined, the interplay between ER and EGFR is certainly an aspect that we are actively examining.
The observation that cytoplasmic levels of ERβ were more strongly predictive of survival in the presence of high aromatase than nuclear ERβ levels could suggest that extranuclear signaling, likely in concert with kinase signaling pathways, may have important factors in lung cancer progression. Indeed, it was recently reported that extranuclear ER forms are critical in regulating invasion and metastatic progression in breast cancer [45]. It will be important to determine if similar functions are mediated by extranuclear ERs in lung cancer.
Niikawa et al. recently reported measurements of aromatase and estradiol within lung tumors [11]. They found that estradiol levels correlated with aromatase expression and that median estradiol concentrations were significantly higher in NSCLCs than non-neoplastic lung tissues. In addition, they also observed that when tumors were dichotomized based on the median level of estradiol, the group with both elevated estradiol and ER positivity, tended to have a worse prognosis. Although these earlier results did not quite reach statistical significance, they indicated a trend consistent with our current report.
ER Immunohistochemistry
Published data on both the presence and effects of ERα and ERβ on survival in lung cancer is somewhat varied [11,12,13,14,15,17,28,32,50,51,52]. This might be due to a lack of reproducibility in IHC with different anti-ER antibodies and diverse preparative methods [7,53,54] and/or to the relatively lower levels of ER proteins in lung tissue as compared to breast tissue. Of note, there is currently no standardized IHC assay for the measure of ERs in lung cancer nor for breast cancer [54]. In addition, as is seen for EGFR, ethnic and regional variations in the several patient populations may account for different effects of estrogen signaling. Nevertheless, general trends are starting to emerge with overall higher expression levels of ERβ in lung cancer compared to ERα and a trend towards the former playing a role in lung cancer pathobiology and reflecting disease aggressiveness [8,10,11,13,15,16,50]. As one example, Hershberger et al. reported that ERβ agonists are highly effective in promoting proliferation of lung tumor cells [16]. This contrasts somewhat with what is observed in breast cancers where ERα tends to predominate over ERβ expression [11,55].
Conclusion
The results presented here are consistent with our overall hypothesis that NSCLC cells hijack the estrogen signaling pathway. We predict that the mechanism of progression for most if not all NSCLCs involves dysfunction at one or more nodes in this pathway. It is interesting to note that while aromatase was primarily predictive in women 65 years or older, the combination of aromatase plus ERβ levels was a strong indicator of outcome in both men and women. This observation could reflect hormonal differences as a function of age. Estrogen levels in women decline after menopause while levels remain relatively constant in men throughout life. In postmenopausal women, the ovaries respond to higher gonodotropin levels and therefore contribute significantly to the circulating pool of testosterone and androgens. This appears to be maintained for at least ten years past the onset of menopause [56]. Levels of androgens fall in women during the reproductive years, then may continue to fall [57] or level off after approximately 65 years of age [58]. Effects of other interacting proteins such as sex hormone-binding globulin (SHBG) may also play a role in their bioavailability. Androgens are the substrates used by aromatase for estrogen synthesis. As we continue to map branches of the ER signaling pathway with regard to expression level and activation in men and women with NSCLC, we predict that different nodes will be preferentially enhanced in different substrata of individuals. Such characterization may provide useful clinical tools to determine disease aggressiveness as well as potential targets of therapeutic attack. We continue to explore both of these possibilities.
Supplementary Material
Acknowledgments
Early Detection Research Network NCI CA86366 (L. Goodglick and D. Chia), the National Lung Cancer Partnership (L. Goodglick), the Specialized Programs of Research Excellence in Lung Cancer grant P50-CA90388, NCI (R. Pietras and L. Goodglick), the Stiles Program in Oncology (R. Pietras), the Piansky Family Endowment (M.C. Fishbein).
Abbreviations
- NSCLC
non-small cell lung cancer
- ER
estrogen receptor
- IHC
immunohistochemistry
- TMA
tissue microarray
- DAB
diaminobenzidine
Footnotes
Conflict of Interest Statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka T, Matsuoka M, Sutani A, Gemma A, Maemondo M, et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer. 2009 doi: 10.1002/ijc.24746. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Kosaka T, Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11:190–198. doi: 10.1007/s10147-006-0583-4. [DOI] [PubMed] [Google Scholar]
- 6.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 7.Raso MG, Behrens C, Herynk MH, Liu S, Prudkin L, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;15:5359–5368. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe K, Miki Y, Ono K, Mori M, Kakinuma H, et al. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol. 2009 doi: 10.1016/j.humpath.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 10.Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11:7280–7287. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 11.Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417–4426. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 12.Wu CT, Chang YL, Shih JY, Lee YC. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130:979–986. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Kawai H, Ishii A, Washiya K, Konno T, Kon H, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res. 2005;11:5084–5089. doi: 10.1158/1078-0432.CCR-05-0200. [DOI] [PubMed] [Google Scholar]
- 14.Ali G, Donati V, Loggini B, Servadio A, Dell'Omodarme M, et al. Different estrogen receptor beta expression in distinct histologic subtypes of lung adenocarcinoma. Hum Pathol. 2008;39:1465–1473. doi: 10.1016/j.humpath.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Hershberger PA, Stabile LP, Kanterewicz B, Rothstein ME, Gubish CT, et al. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol. 2009;116:102–109. doi: 10.1016/j.jsbmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 18.Mah V, Seligson DB, Li A, Marquez DC, Wistuba, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 20.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 21.Seligson DB, Horvath S, Shi T, Yu H, Tze S, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 22.Seligson D, Horvath S, Huerta-Yepez S, Hanna S, Garban H, et al. Expression of transcription factor Yin Yang 1 in prostate cancer. Int J Oncol. 2005;27:131–141. [PubMed] [Google Scholar]
- 23.Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 24.Marquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci. 2009;1155:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyama T, Kagawa N, Sugio K, Uramoto H, Hatano O, et al. Expression of aromatase CYP19 and its relationship with parameters in NSCLC. Front Biosci. 2009;14:2285–2292. doi: 10.2741/3379. [DOI] [PubMed] [Google Scholar]
- 26.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23:8542–8552. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morani A, Barros RP, Imamov O, Hultenby K, Arner A, et al. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta-/-) mice. Proc Natl Acad Sci U S A. 2006;103:7165–7169. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, et al. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Hammoud Z, Tan B, Badve S, Bigsby RM. Estrogen promotes tumor progression in a genetically defined mouse model of lung adenocarcinoma. Endocr Relat Cancer. 2008;15:475–483. doi: 10.1677/ERC-08-0002. [DOI] [PubMed] [Google Scholar]
- 30.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 31.Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, et al. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598–1605. doi: 10.1158/0008-5472.CAN-04-2694. [DOI] [PubMed] [Google Scholar]
- 32.Marquez-Garban DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Patel JD. Lung cancer in women. J Clin Oncol. 2005;23:3212–3218. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 35.Henschke CI, Miettinen OS. Women's susceptibility to tobacco carcinogens. Lung Cancer. 2004;43:1–5. doi: 10.1016/j.lungcan.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 37.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 38.Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst. 2004;96:826–834. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 39.Slatore CG, Chien JW, Au DH, Satia JA, White E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol. 2010;28:1540–1546. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. J Natl Cancer Inst. 2010;102:1413–1421. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rapiti E, Verkooijen HM, Fioretta G, Schubert H, Vinh-Hung V, et al. Reduced Lung Cancer Mortality Risk among Breast Cancer Patients Treated with Anti-Estrogens. 2009. [DOI] [PubMed] [Google Scholar]
- 43.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, et al. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids. 2009;74:622–627. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, et al. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res. 2010;70:4092–4101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 47.Liu M, Yang SC, Sharma S, Luo J, Cui X, et al. EGFR signaling is required for TGF-beta 1 mediated COX-2 induction in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007;37:578–588. doi: 10.1165/rcmb.2007-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell DW, Brannigan BW, Matsuo K, Finkelstein DM, Sordella R, et al. Increased prevalence of EGFR-mutant lung cancer in women and in East Asian populations: analysis of estrogen-related polymorphisms. Clin Cancer Res. 2008;14:4079–4084. doi: 10.1158/1078-0432.CCR-07-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27:411–417. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 50.Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of alpha and beta estrogen receptors in human nontumor and tumor lung tissue. Mol Cell Endocrinol. 2002;188:125–140. doi: 10.1016/s0303-7207(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 51.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37:153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 52.Radzikowska E, Langfort R, Giedronowicz D. Estrogen and progesterone receptors in non small cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2002;8:69–73. [PubMed] [Google Scholar]
- 53.Carder PJ, Murphy CE, Dervan P, Kennedy M, McCann A, et al. A multi-centre investigation towards reaching a consensus on the immunohistochemical detection of ERbeta in archival formalin-fixed paraffin embedded human breast tissue. Breast Cancer Res Treat. 2005;92:287–293. doi: 10.1007/s10549-004-4262-8. [DOI] [PubMed] [Google Scholar]
- 54.Speirs V, Green CA, Shaaban AM. Oestrogen receptor beta immunohistochemistry: time to get it right? J Clin Pathol. 2008;61:1150–1151. author reply 1151-1152. [PubMed] [Google Scholar]
- 55.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 56.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 57.Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, et al. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 2007;92:509–516. doi: 10.1210/jc.2006-1399. [DOI] [PubMed] [Google Scholar]
- 58.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.