Abstract
Purpose
HIV infection has been implicated in dysregulation of the autonomic nervous system.
Method
Cross-sectional study examining the relationship between the presence of persistent detectable HIV viral load with autonomic function, measured by heart rate variability (HRV). Non-virologic suppression (NVS) was defined as having a detectable viral load for at least 3 months prior to autonomic function testing. HRV was measured during the following 4 maneuvers: resting and paced respirations and sustained handgrip and tilt. Inferences on parasympathetic and sympathetic modulations were determined by analyzing time and frequency domains of HRV.
Results
57 participants were enrolled in 3 groups: 22 were HIV-infected participants with HIV virologic suppression (VS; undetectable HIV viral load), 9 were HIV-infected participants who had NVS, and 26 were HIV seronegative controls. There were lower time domain parameters in the HIV-infected group as a whole compared to controls. There were no significant differences in time domain parameters among HIV-infected participants. There were no differences in frequency domain parameters during any of the maneuvers between controls and all HIV-infected participants, nor between the NVS and VS groups.
Conclusion
There were differences in autonomic function between HIV-infected individuals and HIV seronegative controls, but not between the NVS and VS groups.
Keywords: acquired immune deficiency syndrome, autonomic dysfunction, heart rate variability, HIV, viremia
The autonomic nervous system (ANS) is a regulatory structure that helps people adapt to changes in their environment. This system consists of 2 antagonistic subsystems – the parasympathetic and sympathetic nervous systems – which are involved in the homeostatic and physiologic functions. The balance of parasympathetic and sympathetic activity is crucial to the fitness of the cardiovascular system. Increased sympathetic activation is associated with metabolic syndrome, hypertension, ischemic heart disease, arrhythmia, and cardiomyopathy, whereas decreased para-sympathetic activation has been seen with aging and in baroreflex and chemoreflex impairment.1,2 Impaired ANS, particularly among patients with cardiovascular disease (CVD) and diabetes mellitus, is associated with heightened risk of cardiovascular morbidity and cardiovascular and all-cause mortality.3–8 Large epidemiologic studies such as the Atherosclerosis Risk in Communities (ARIC) and Framingham studies have found an association between autonomic dysfunction and the incidence of hypertension, cardiomyopathy, arrhythmia, myocardial infarction, coronary heart disease, and stroke.9–13
The prevalence of any symptoms of clinical autonomic neuropathy in HIV has been variable among different populations and HIV status and treatment, ranging from 0% to 84%.14–16 Compostella et al found that 19% of treated HIV-infected subjects demonstrated severe autonomic neuropathy. Symptoms comprised orthostatic intolerance and secretomotor and gastrointestinal dysfunction. There was no relationship between CD4 cell counts and autonomic symptom scores.17 Subjects reported symptoms of autonomic dysfunction, despite normal or borderline autonomic reflex responses.
The Strategies for Management of Anti-Retroviral Therapy (SMART) trial, which assessed the safety and efficacy of antiretroviral structured treatment interruption (STI) strategies, showed increased morbidity and mortality from CVD in those who interrupted antiretroviral therapy (ART) compared to those who continued to receive ART.18–20 One hypothesis to explain this relatively prompt increase in CVD risk associated with STI is that viremia induced by discontinuation of ART leads to an increase in cardiovascular autonomic dysfunction. This cross-sectional study examined the relationship between cardiovagal autonomic function with the presence of persistent detectable HIV viral load.
METHOD
Subject Characteristics
This cross-sectional study examined the relationship between autonomic function, measured by cardiovagal heart rate variability (HRV), with the presence of detectable HIV viral load. HRV was assessed in 3 groups: HIV-infected participants with HIV virologic suppression (VS), HIV-infected participants who do not have virologic suppression (NVS), and HIV seronegative participants who serve as a control. Subjects were obtained through a convenient sample of participants studied at the Hawaii Center for AIDS. Virologic suppression for the purpose of this study was defined as having an undetectable viral load by Roche Amplicor testing for the preceding 3 months prior to the autonomic function tests. Nonvirologic suppression was defined as having a detectable viral load for at least 3 months. Eligible subjects had to have been on the same highly active antiretroviral therapy (HAART) regimen for at least 3 months. HAART was defined as an antiretroviral regimen that contained a minimum of 3 antiretroviral drugs in any of the following combinations: 2 nucleo-side reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (NNRTI); 3 NRTIs that included abacavir; 2 NRTIs and a protease inhibitor (PI); 2 PIs and an NNRTI; 2 PIs and an NRTI; or an NRTI, NNRTI, and PI. The Human Subjects Review Committee of the University of Hawaii approved the study protocol, and all subjects signed a written informed consent prior to entry. Exclusion criteria were as follows: known cardiovascular disease, arrhythmia, pregnancy, hypertension, and diabetes. Participants currently taking medication known to influence autonomic nerve function (such as antihypertensive medications and tricyclic antidepressants) were excluded. Health characteristics including demographics, clinical exam, past medical history, medication history, fasting lipid panel, fasting glucose and insulin, HIV RNA viral load, CD4 cell count, and plasma measurements of cardiovascular markers were obtained.
Heart Rate Variability Analysis
Autonomic function was measured by HRV. HRV was measured in all subjects at 7:00 to 9:00 a.m., after a minimum of an 8-hour fast. Participants rested for 30 minutes before testing. Electrocardiographic signals were recorded by a bedside electrocardiographic monitor and transmitted to a personal computer for recording (BioPac Systems, Inc, Goleta, California, USA). The collection frequency was set at 1000/s. Continuous EKG interbeat intervals were monitored during the following 4 maneuvers of at least 5 minutes duration each: resting, paced respirations at 0.2 Hz, sustained handgrip, and tilt. Paced respirations amplify the respiratory-related vagal (parasympathetic) modulation of the heart rate. Tilt testing is the most common test of sympathetic vasomotor function. The initial fall in blood pressure during tilt activates baroreceptors with a subsequent reflex increase in sympathetic outflow and parasympathetic inhibition. After lying flat, subjects were tilted to 70° heads up. Static handgrip testing was also conducted to evaluate sympathetic function. Subjects were instructed to maintain static handgrip (30% of maximal voluntary contraction) for 5 minutes. Five-minute rest periods were given between each maneuver.
The recorded electrocardiographic signals were retrieved afterwards to measure the consecutive R-R intervals by using software for the detection of R waves (Acknowledge 3.7.1; Biopac Systems, Inc, Goleta, California, USA). Sinus pause and atrial or ventricular arrhythmia were deleted and the last 512 R-R intervals were obtained for HRV analysis as per recommendations from the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology on autonomic function testing.21 If the percentage of deletion was greater than 5%, the HRV test was excluded from the study.
HRV was analyzed in time and frequency domains. The time domain parameters, which are based on simple statistical measures of variability, address the magnitude of variability and provide information about the vagal (parasympathetic) modulation of the heart, with higher variability generally reflecting greater parasympathetic modulation. Reduced HRV has prognostic implications for future cardiovascular disease events.22 Frequency domain yields information about the amount of the overall variance (or power) in heart period resulting from periodic oscillations of heart rate at various frequencies. Further separation of parasympathetic and sympathetic activities can be inferred from the frequency domain analysis.
The time domain measures were determined as follows. The standard deviation of normal R-R intervals (SDNN) is the statistical measure of variability or spread of the R-R intervals around the average heart rate.23 Other time domain measures such as the percentage of intervals greater than 50 ms different from its predecessor (PNN50) and the root of the mean square of successive differences (RMSSD) were calculated to capture subtle gradual trends in heart rate over time. The PNN50 and RMSSD provide additional measures of variability of the R-R intervals as well as confirmation of the SDNN.
Frequency domain was assessed using power spectral analysis. The power spectrum of the R-R intervals was obtained by means of Fast Fourier transformation (Matlab version 6.5; Mathworks Inc, Natick, Massachusetts, USA) and expressed as the area under the curve of power versus frequency.23 The area under the power spectrum within the range of 0.04–0.15 Hz and 0.15–0.4 Hz was defined as the low-frequency power (mixed sympathetic and parasympathetic origin) and high-frequency power (parasympathetic origin), respectively. The low- to high-frequency power ratio (LF/HF ratio) is thought to provide the best index of sympathetic modulation in HRV. The normalized high-frequency power (100 × high-frequency power/total power) was used as the index of vagal modulation and the low-/high-frequency power ratio was used as the index of sympathetic modulation.24,25
Plasma Cardiovascular Biomarkers
Plasma concentrations of sE-selectin, sVCAM-1, sICAM-1, adiponectin, and tPAI-1 were measured using a fluorescent-labeled microsphere bead multiplex immunoassay (LINCOplex; Linco Research, St. Charles, Missouri, USA) according to the manufacturer's protocols and analyzed using Upstate Beadview (Temecula, California, USA). All cardiovascular markers were measured in duplicate. The sensitivity of the assays were as follows: 79.0 pg/mL for sE-selectin, 16.0 pg/mL for sVCAM-1, 9.0 pg/mL for sICAM-1, 56.0 pg/mL for adiponectin, and 1.0 pg/mL for tPAI-1. Intra-assay coefficients of variation for sE-selectin, sVCAM-1, sICAM-1, adiponectin, and tPAI-1 were 7.5%, 3.8%, 3.8%, 3.4%, and 2.9%, respectively. Inter-assay coefficients of variation for sE-selectin, sVCAM-1, sICAM-1, adiponectin, and tPAI-1 were 10.7%, 6.8%, 10.6%, 8.3%, and 12.6%, respectively.
Statistical Analysis
The primary objective was to assess the relationship of autonomic function measured by HRV between each group. The Shapiro-Wilks normality test was used to assess the homogeneity of variances between and within the study groups. Categorical variables were compared using the chi-square test. Continuous variables were analyzed by Wilcoxon rank test. The correlation between HRV parameters and other continuous variables was assessed using linear regression. A variance stabilizing transformation was applied to the data when needed. A 2-sided probability of P < .05 was used to determine statistical significance. All statistical analyses were performed using the JMP statistical program (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
Baseline characteristics of the 57 participants (26 controls and 31 HIV-infected participants, of whom 22 had virologic suppression and 9 had nonvirologic suppression) are presented (Table 1). Median duration of HIV infection was 7.0 years for the virologic controlled group and 10.5 years for the virologic failure group. Median time on ART was 4.2 years for the virologic controlled group and 6.5 years for the virologic failure group. Neither median duration of HIV infection nor time on ART was statistically different. Baseline characteristics were similar among all groups except for systolic blood pressure, current CD4 count, and CD4 nadir, which were significantly different between the HIV-infected groups. With regard to the cardiovascular biomarkers, sICAM-1 was higher in the HIV-infected group as a whole compared to controls. There were no significant differences in these biomarkers among HIV-infected participants.
Table 1.
Baseline characteristics
| HIV negative |
HIV positive |
|||
|---|---|---|---|---|
| Control | Virologic suppression | Virologic nonsuppression | All HIV-positive participants | |
| n | 26 | 22 | 9 | 31 |
| Males, % | 89 | 91 | 100 | 94 |
| Age, years | 39.0 (32.5, 40.6) | 41.3 (35.7, 49.4) | 37.9 (35.4, 44.8) | 41.2 (35.6, 47.3) |
| Ethnicity, % White | 58 | 50 | 56 | 52 |
| Duration of HIV infection, years | – | 7.0 | 10.5 | |
| Duration of ART use, years | – | 4.2 | 6.5 | |
| Body mass index, kg/m2 | 24.1 (21.7, 28.1) | 24.7 (22.8, 26.0) | 24.8 (22.0, 27.4) | 24.7 (22.8, 26.1) |
| Heart rate, beats/min | 68.0 (60.5, 72.0) | 68.0 (60.0, 73.0) | 68.0 (64.0, 80.0) | 68.0 (64.0, 76.0) |
| Systolic blood pressure, mm Hg | 110.0 (103.5, 115.0) | 106.5 (99.8, 116.8) | 119 (113.0, 123.5)** | 110.0 (103.0, 120.0) |
| Diastolic blood pressure, mm Hg | 73.5 (67.5, 78.3) | 72.5 (69.6, 81.5) | 74.0 (69.5, 79.5) | 73.0 (69.0, 80.0) |
| Nadir CD4, cells/mm3 | – | 313.5 (248.0, 600.8) | 252.0 (37.5, 346.0)** | 305 (183, 531) |
| CD4, cells/mm3 | – | 532.5 (369.5, 707.5) | 252.0 (79.0, 346.0)** | 426 (252, 629) |
| HIV RNA PCR, copies/mL | – | – | 400 (113, 32750)** | 0 (0, 111) |
| Fasting glucose, mg/dL | 86.5 (80.8, 92.0) | 85.0 (77.5, 91.3) | 89.0 (77.0, 91.0) | 86.0 (78.0, 91.0) |
| Insulin, uIU/mL | 4.3 (2.8, 7.7) | 6.0 (4.0, 8.1) | 5.0 (3.5, 11.6) | 6.0 (4.0, 7.5) |
| Homeostasis Model Assessment (HOMA-IR) | 0.9 (0.6, 1.8) | 1.2 (0.9, 1.7) | 1.1 (0.6, 2.6) | 1.2 (0.9, 1.7) |
| sE-Selectin, ng/mL | 23.1 (19.5, 28.8) | 21.6 (16.6, 27.5) | 22.5 (17.3, 31.1) | 22.1 (17.0, 28.7) |
| sVCAM-1, ng/mL | 1115 (956, 1317) | 1240 (1000, 1450) | 1220 (1065, 1385) | 1230 (1023, 1403) |
| sICAM-1, ng/mL | 202.0 (166.0, 246.5) | 212.0 (188.0, 279.0) | 272.0 (207.5, 310.5)* | 229.0 (188.8, 292.0)* |
| adiponectin, ng/mL | 10150 (7463, 14350) | 7800 (4660, 16300) | 9880 (6410, 13050) | 7910 (6033, 13225) |
| tPAI-1, ng/mL | 14.3 (6.7, 20.5) | 12.3 (6.5, 26.9) | 19.5 (8.4, 26.8) | 13.9 (7.6, 25.7) |
Note: Continuous variables are displayed as medians (Q1, Q3).
P < .05 compared to HIV seronegative controls.
P < .05 compared to HIV-infected virologic suppression group.
The unadjusted time domain results are shown in Table 2. There were lower time domain parameters in the HIV-infected group as a whole compared to controls. These differences remained significant during paced breathing and tilt even after adjustment for age and gender. Among HIV-infected participants, there were no significant differences in time domain parameters, although there were trends toward significance for SDNN and RMSSD in the paced breathing. These trends, however, were no longer significant after adjusting for age and gender.
Table 2.
Comparison of time domain analysis parameters by HIV viremia categories
| HIV negative |
HIV positive |
||||
|---|---|---|---|---|---|
| Control | Virologic suppression | Virologic nonsuppression | All HIV-positive participants | ||
| Spontaneous breathing | SDNN, ms | 68.9 (47.6, 90.5) | 53.2 (41.5, 84.2) | 63.0 (40.7, 100.2) | 60.1 (42.1, 87.1) |
| PNN50, % | 26.8 (11.9, 46.1) | 11.5 (2.1, 29.6) | 15.2 (4.4, 33.8 ) | 12.9 (2.7, 29.5)* | |
| RMSSD, ms | 48.9 (34.9, 63.8) | 37.9 (20.7, 54.0) | 42.4 (26.9, 78.6) | 41.4 (25.9, 59.5) | |
| Paced breathing | SDNN, ms | 73.3 (52.6, 82.5) | 63.8 (41.3, 83.5) | 61.9 (39.2, 88.5) | 61.9 (43.2, 85.4) |
| PNN50, % | 38.5 (24.7, 52.4) | 19.7 (2.7, 46.6) | 18.6 (9.5, 40.3) | 18.6 (3.7, 43.4) | |
| RMSSD, ms | 63.7 (40.8, 78.4) | 40.1 (29.1, 66.0) | 41.5 (31.3, 62.7) | 41.5 (30.1, 64.1)* | |
| Hand grip | SDNN, ms | 74.9 (55.3, 104.3) | 76.4 (55.8, 93.7) | 79.7 (45.1, 87.5) | 77.1 (50.9, 92.5) |
| PNN50, % | 20.1 (5.8, 28.9) | 7.6 (1.7, 21.2) | 5.8 (1.8, 34.6) | 6.7 (1.7, 22.8)* | |
| RMSSD, ms | 43.1 (30.5, 58.1) | 36.2 (20.8, 56.6) | 33.3 (23.0, 76.0) | 34.5 (23.2, 60.6) | |
| Tilt | SDNN, ms | 75.9 (58.5, 88.0) | 74.5 (55.4, 88.9) | 67.6 (40.8, 86.8) | 69.8 (51.9, 86.7) |
| PNN50, % | 16.4 (4.2, 29.2) | 5.8 (2.3, 11.0) | 3.2 (1.6, 13.2) | 5.5 (1.7, 11.0)* | |
| RMSSD, ms | 40.5 (25.4, 56.9) | 33.2 (26.6, 43.1) | 39.3 (19.9, 58.6) | 34.2 (26.6, 42.5) | |
Note: Data are presented as median (Q1, Q3), deviation for the standard deviation of normal R-R intervals (SDNN), the percentage of intervals greater than 50 ms different from its predecessor (PNN50) and the root of the mean square of successive differences (RMSSD). A Wilcoxon signed rank test was used to assess the differences between groups.
P < .05 compared with HIV seronegative controls.
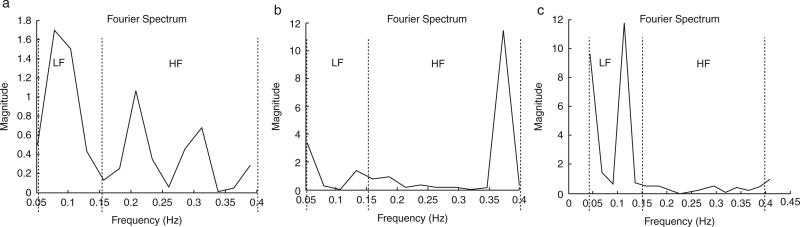
Figure 1 displays representative spectral analysis during (a) rest, (b) deep breathing, and (c) tilt maneuvers. Frequency ranges for LF and HF cutoffs are clearly delineated at each maneuver. Table 3 shows the spectral analysis between groups during the various maneuvers. There were no differences between controls and the HIV-infected group in spectral power parameters during any of the maneuvers. Similarly there were no differences between the NVS and VS groups.
Figure 1.
Representation of spectral analysis during (a) rest, (b) deep breathing, and (c) tilt. HF = high-frequency power; LF = low-frequency power.
Table 3.
Comparison of frequency domain analysis parameters by HIV viremia categories during spontaneous breathing, paced breathing, handgrip, and tilt maneuvers
| HIV negative |
HIV positive |
||||
|---|---|---|---|---|---|
| Control | Virologic suppression | Virologic nonsuppression | All HIV-positive participants | ||
| Spontaneous breathing | Low-frequency power (nu) | 63.3 (51.5, 74.7) | 65.2 (50.6, 81.4) | 56.4 (50.7, 64.1) | 57.0 (51.3, 72.7) |
| High-frequency power (nu) | 36.7 (25.3, 48.5) | 34.8 (18.6, 49.5) | 43.6 (35.9, 49.3) | 43.0 (27.3, 48.7) | |
| Low- to high-frequency power ratio | 1.7 (1.1, 3.0) | 1.9 (1.0, 4.4) | 1.3 (1.0, 1.8) | 1.3 (1.1, 2.7) | |
| Paced breathing | Low-frequency power (nu) | 32.1 (25.0, 38.2) | 42.0 (28.0, 53.7) | 35.7 (24.0, 39.5) | 39.6 (26.4, 52.4) |
| High-frequency power (nu) | 67.9 (61.8, 75.1) | 58.0 (46.3, 72.0) | 64.3 (60.5, 76.0) | 60.4 (47.6, 73.6) | |
| Low- to high-frequency power ratio | 0.5 (0.4, 0.7) | 0.8 (0.4, 1.5) | 0.6 (0.3, 0.7) | 0.7 (0.4, 1.2) | |
| Handgrip | Low-frequency power (nu) | 66.6 (60.2, 73.4) | 64.0 (55.4, 75.9) | 67.5 (52.9, 80.5) | 65.5 (54.9, 78.5) |
| High-frequency power (nu) | 33.4 (26.7, 39.8) | 32.5 (22.6, 39.6) | 32.5 (19.6, 47.1) | 32.5 (21.1, 41.2) | |
| Low- to high-frequency power ratio | 2.0 (1.5, 2.7) | 1.9 (1.4, 3.1) | 2.1 (1.1, 4.2) | 1.9 (1.3, 5.0) | |
| Tilt | Low-frequency power (nu) | 79.9 (67.8, 85.2) | 76.0 (67.2, 82.2) | 68.8 (59.2, 82.2) | 74.5 (66.1, 81.8) |
| High-frequency power (nu) | 20.1 (14.9, 32.3) | 24.0 (17.8, 32.9) | 31.2 (17.8, 40.8) | 25.5 (18.2, 33.9) | |
| Low- to high-frequency power ratio | 4.0 (2.1, 5.9) | 3.2 (2.0, 4.6) | 2.2 (1.5, 4.7) | 2.9 (1.9, 4.5) | |
Note: Data are presented as median (Q1, Q3) deviation for the low-frequency power, high-frequency power, and low- to high-frequency power ratio. A Wilcoxon signed rank test was used to assess the differences between groups.
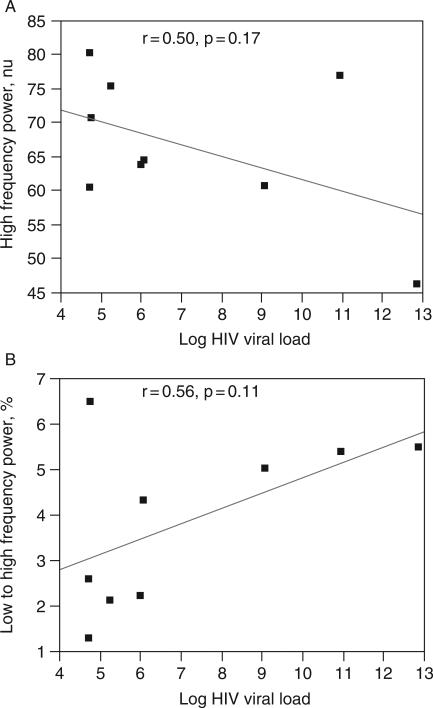
Further analysis was conducted on the NVS group to determine the correlation between HIV viral load and autonomic measures (time and frequency domain measurements). Although none of the measures were statistically significant, there were consistent trends of a negative correlation between HIV viral load and time domain measures (SDNN, PNN50, and RMSSD). Similarly there was a trend toward a negative correlation between HIV viral load and high-frequency power during paced respiration (modulation of parasympathetic activity). A positive trend on HIV viral load and low- to high-frequency power ratio during tilt (modulation of sympathetic activity) was seen (Figure 2).
Figure 2.
Correlation of HIV viral load with frequency domain parameters during (a) paced breathing and (b) tilt.
DISCUSSION
This is the first study to examine the ANS with regard to virologic control in HIV-infected individuals receiving ART. There were differences in HRV between HIV-infected individuals and HIV seronegative controls. However, there were no differences between the NVS and VS groups. There were no significant differences in the plasma cardiovascular biomarkers among the HIV-infected participants. There was a weak correlation between HIV viral load and frequency analysis parameters, but no correlations between the autonomic function tests, CD4 count, and CD4 nadir.
HIV-infected patients have a higher prevalence of autonomic dysfunction compared to the general population.15,26 Symptomatic autonomic dysfunction in HIV-infected individuals is rare.15,27,28 Sympathovagal imbalance has been reported in ART-naïve HIV-infected individuals.29 Autonomic dysfunction was reported to occur in 97% of untreated AIDS patients in Africa.26 Autonomic dysfunction has been associated with HIV infection, correlating with the severity of HIV disease progression.16,30,31
HIV is thought to alter sympathovagal balance, resulting in increased sympathetic tone. The exact mechanism by which HIV modulates autonomic function remains unknown but may involve HIV-induced changes in the brain responsible for ANS function. HIV has a predilection for the central nervous system and localizes in high concentration in the hippocampus, basal ganglia, and other regions involved in hypothalamic regulation.32 The hypothalamus is the basal part of the diencephalon governing the ANS. Intraventricular injection of gp120 HIV envelope protein in rats impaired function of the suprachiasmatic nucleus within the hypothalamus. Injury to the suprachiasmatic nucleus has been associated with increased sympathetic modulation.33 Chimelli et al examined the morphology of sympathetic ganglia in individuals with AIDS and found inflammatory cells in the sympathetic ganglia together with evidence of nerve cell degeneration.34 Immunostaining showed presence of T lymphocytes and an increased number of macrophages. HIV antigens were detected in macrophages. Additionally, a direct effect of HIV on these ganglia is the mechanism postulated to cause dysfunction. Cardiac vagal efferent pathways might also be compromised by peripheral HIV neuropathy leading to sympathovagal imbalance.35
Individuals with AIDS receiving ART have better measures of autonomic function compared to individuals with AIDS and not receiving ART.35 Correia et al found that during tilt, the magnitude of increased sympathetic activity was less dramatic in AIDS patients who were on ART. The investigators speculate that ART may improve autonomic function in individuals with AIDS. Markers of immune activation and disease progression correlate with severity of autonomic dysfunction.36,37
ART may adversely affect the autonomic system. Indinavir, a widely used PI, has been shown to directly inhibit the GLUT-4 transporter.38 The inhibition of GLUT-4 transporters can occur within minutes, without any effects on intracellular signaling of insulin.39 GLUT-4 appears to be expressed in neurons localized to the hypothalamic nuclei. It has been proposed that these transporters might be involved in the hypothalamic glucose-insulin sensing mechanism and thus in the nervous regulation of metabolism.40 Direct influence of these transporters can affect ANS activity, although further investigation is necessary in this area.40 Alternatively, PIs may also directly affect the ANS through a protease mechanism. Proteases play an important role in promoting and mediating neurodegeneration.41 Protease activated receptors (PARs) are expressed throughout the brain. PARs such as PAR-2 and PAR-3 have been found to have the highest densities at the thalamus, hypothalamus, and striatum.42 The localization of PARs to certain brain regions may predispose these brain regions to be affected by PIs. The sample size of the HIV-infected groups was not large enough to compare the various anti-retroviral regimens.
Although limited by small sample size and its observational nature, this study indicated a trend toward a decrease in parasympathetic modulation as assessed by decreased high-frequency power in HIV-infected individuals compared to seronegative controls. The increase in low- to high-frequency power ratio suggests an increase in sympathetic modulation in HIV infection. The weak correlation between HIV viral load and frequency analysis parameters suggests a potential association between autonomic function and HIV viremia. An insufficient power may be the reason for the weak correlation. As the quality of care improves among the HIV-infected population, it is difficult to find HIV-infected individuals in virologic failure for more than 3 months. The majority of the subjects with persistent HIV viremia were on the same antiretroviral regimen for at least 3 months. Further investigations are warranted, especially evaluating the duration and magnitude of HIV viremia on autonomic neuropathy. Larger studies measuring autonomic function and prevalence of autonomic dysfunction symptoms will be needed to quantitate the relationship between autonomic dysfunction and clinical CVD.
HIV results in alterations in autonomic function. There may be an association between increasing autonomic dysfunction with increasing HIV viremia. Similar to findings in the diabetic and coronary heart disease populations, these alterations in cardiovagal autonomic function may be prognostic of increased cardiovascular disease morbidity and mortality.
ACKNOWLEDGMENTS
This study was supported by awards NHLBI K23 HL088981, RCMI NCRR P20RR011091, NCRR 1 R25 RR019321, P50 NS32352, P01 NS4 4344, U54 NS065736, UL1 RR24150, and NCRR 1 UL1 RR024150 from the US National Institutes of Health (NIH). This investigation/manuscript was also supported by a grant from the Hawaii Community Foundation (grant 20051293). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the Hawaii Community Foundation. The JMP statistical software license was supported by NIH grant RR-16467 from the HS-BRIN program of the National Center for Research Resources.
REFERENCES
- 1.Aso Y, Wakabayashi S, Nakano T, Yamamoto R, Takebayashi K, Inukai T. High serum high-sensitivity C-reactive protein concentrations are associated with relative cardiac sympathetic overactivity during the early morning period in type 2 diabetic patients with metabolic syndrome. Metabolism. 2006;55:1014–1021. doi: 10.1016/j.metabol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Dall'ago P, D'Agord Schaan B, da Silva VO, et al. Parasympathetic dysfunction is associated with baroreflex and chemoreflex impairment in streptozotocin-induced diabetes in rats. Auton Neurosci. 2006 doi: 10.1016/j.autneu.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 4.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 5.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49:95–108. [PubMed] [Google Scholar]
- 6.Rathmann W, Enck P, Frieling T, Gries FA. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care. 1991;14:1086–1089. doi: 10.2337/diacare.14.11.1086. [DOI] [PubMed] [Google Scholar]
- 7.Valensi P, Sachs RN, Harfouche B, et al. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339–343. doi: 10.2337/diacare.24.2.339. [DOI] [PubMed] [Google Scholar]
- 8.Toyry JP, Niskanen LK, Lansimies EA, Partanen KP, Uusitupa MI. Autonomic neuropathy predicts the development of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1996;27:1316–1318. doi: 10.1161/01.str.27.8.1316. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 10.Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 11.Singh JP, Larson MG, O'Donnell CJ, et al. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol. 2000;86:309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 12.Liao D, Cai J, Rosamond WD, et al. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 13.Carnethon MR, Anthony MS, Cascio WE, et al. Prospective association between hormone replacement therapy, heart rate, and heart rate variability. The Atherosclerosis risk in communities study. J Clin Epidemiol. 2003;56:565–571. doi: 10.1016/s0895-4356(03)00008-8. [DOI] [PubMed] [Google Scholar]
- 14.Lohmoller G, Matuschke A, Goebel FD. [False-positive test of autonomic neuropathy in HIV infection and AIDS? Case control study of heart rate variability in 62 HIV positive patients]. Med Klin (Munich) 1989;84:242–245. [PubMed] [Google Scholar]
- 15.Brownley KA, Milanovich JR, Motivala SJ, et al. Autonomic and cardiovascular function in HIV spectrum disease: early indications of cardiac pathophysiology. Clin Auton Res. 2001;11:319–326. doi: 10.1007/BF02332978. [DOI] [PubMed] [Google Scholar]
- 16.Rogstad KE, Shah R, Tesfaladet G, Abdullah M, Ahmed-Jushuf I. Cardiovascular autonomic neuropathy in HIV infected patients. Sex Transm Infect. 1999;75:264–267. doi: 10.1136/sti.75.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compostella C, Compostella L, D'Elia R. The symptoms of autonomic dysfunction in HIV-positive Africans. Clin Auton Res. 2008;18:6–12. doi: 10.1007/s10286-007-0451-y. [DOI] [PubMed] [Google Scholar]
- 18.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 19.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 20.El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 21.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 22.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 23.Freeman R. Noninvasive evaluation of heart rate: time and frequency domains. In: Low PA, editor. Clinical Autonomic Disorders. 3rd ed. Wolters Kluwer Lippincort Williams & Wilkins; Philadelphia: 2008. pp. 185–197. [Google Scholar]
- 24.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 25.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 26.Nzuobontane D, Ngu BK, Christopher K. Cardiovascular autonomic dysfunction in Africans infected with human immunodeficiency virus. J R Soc Med. 2002;95:445–447. doi: 10.1258/jrsm.95.9.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen JA, Miller L, Polish L. Orthostatic hypotension in human immunodeficiency virus infection may be the result of generalized autonomic nervous system dysfunction. J Acquir Immune Defic Syndr. 1991;4:31–33. [PubMed] [Google Scholar]
- 28.Lebech AM, Kristoffersen US, Mehlsen J, et al. Autonomic dysfunction in HIV patients on antiretroviral therapy: studies of heart rate variability. Clin Physiol Funct Imaging. 2007;27:363–367. doi: 10.1111/j.1475-097X.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 29.Mittal CM, Wig N, Mishra S, Deepak KK. Heart rate variability in human immunodeficiency virus-positive individuals. Int J Cardiol. 2004;94:1–6. doi: 10.1016/j.ijcard.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 31.Becker K, Gorlach I, Frieling T, Haussinger D. Characterization and natural course of cardiac autonomic nervous dysfunction in HIV-infected patients. AIDS. 1997;11:751–757. doi: 10.1097/00002030-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Yun AJ, Lee PY, Bazar KA. Modulation of host immunity by HIV may be partly achieved through usurping host autonomic functions. Med Hypotheses. 2004;63:362–366. doi: 10.1016/j.mehy.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Raymon LP, Kimes AS, Tabakoff B, London ED. [AIDS and sleep disorders: effect of gp120 on cerebral glucose metabolism]. C R Seances Soc Biol Fil. 1989;183:407–418. [PubMed] [Google Scholar]
- 34.Chimelli L, Martins AR. Degenerative and inflammatory lesions in sympathetic ganglia: further morphological evidence for an autonomic neuropathy in AIDS. J NeuroAIDS. 2002;2:67–82. doi: 10.1300/j128v02n03_05. [DOI] [PubMed] [Google Scholar]
- 35.Correia D, Rodrigues De Resende LA, Molina RJ, et al. Power spectral analysis of heart rate variability in HIV-infected and AIDS patients. Pacing Clin Electrophysiol. 2006;29:53–58. doi: 10.1111/j.1540-8159.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 36.Schifitto G, McDermott MP, Evans T, et al. Autonomic performance and dehydroepiandrosterone sulfate levels in HIV-1-infected individuals: relationship to TH1 and TH2 cytokine profile. Arch Neurol. 2000;57:1027–1032. doi: 10.1001/archneur.57.7.1027. [DOI] [PubMed] [Google Scholar]
- 37.Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. Proc Natl Acad Sci USA. 2001;98:12695–12700. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolte LA, Yarasheski KE, Kawanaka K, Fisher J, Le N, Holloszy JO. The HIV protease inhibitor indinavir decreases insulin- and contraction-stimulated glucose transport in skeletal muscle. Diabetes. 2001;50:1397–1401. doi: 10.2337/diabetes.50.6.1397. [DOI] [PubMed] [Google Scholar]
- 39.Noor MA, Seneviratne T, Aweeka FT, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. AIDS. 2002;16:F1–8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alquier T, Leloup C, Arnaud E, Magnan C, Penicaud L. Altered Glut4 mRNA levels in specific brain areas of hyperglycemic-hyperinsulinemic rats. Neurosci Lett. 2001;308:75–78. doi: 10.1016/s0304-3940(01)01936-x. [DOI] [PubMed] [Google Scholar]
- 41.Siao CJ, Tsirka SE. Extracellular proteases and neuronal cell death. Cell Mol Biol (Noisy-le-grand) 2002;48:151–161. [PubMed] [Google Scholar]
- 42.Striggow F, Riek-Burchardt M, Kiesel A, et al. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]