Abstract
This study was performed in the aim to identify potential targets for the development of novel therapy to treat cancer with poor outcome or treatment efficacy. We show that the negatively charged phospholipid phosphatidylserine (PS) is exposed in the outer leaflet of their plasma membrane not only in tumor cell lines, but also in metastases and primary cultures thereof, which contrasts with a lack of PS exposure by differentiated non-tumorigenic counterparts. Studied tumor cell lines were derived from non-tumorigenic and malignant melanomas, prostate- and renal cancer, glioblastoma and a rhabdomyosarcoma. Importantly, also metastases of melanoma expose PS and there is a correlation between malignancy of melanoma cell lines from different stages of tumor progression and PS exposure. The PS exposure we found was neither of apoptotic nor of experimental artificial origin. Finally potentially malignant and non-malignant cells could be differentiated by sorting of a primary cell culture derived from a glioblastoma based on PS exposure, which has so far not been possible within one culture due to lack of a specific marker. Our data provide clear evidence that PS could serve as uniform marker of tumor cells and metastases as well as a target for novel therapeutic approaches based on e.g. PS-specific host defense derived peptides.
Keywords: Cancer plasma membrane, Phosphatidylserine exposure, Tumor marker, Peptide target
Highlights
► Novel marker of glioblastoma, renal cancer, rhabdomyosarcoma ► Novel marker of metastasis ► Marker also present in primary cultures ► Increase of exposure of marker with malignancy ► Demonstration of universality of a novel target for antitumor therapy
1. Introduction
Cancer is the leading cause of death worldwide (http://www.who.int/mediacentre/factsheets/fs297/en/). In the last decades significant progress has been made in respect of diagnostics and therapeutic approaches. However, despite favorable advancements, state of the art chemotherapy is still not successful in many cancers. Potential toxicity and many side effects of chemotherapeutics can mainly be explained by a lack of adequate specificity for tumor cells. Chemotherapy, due to resistance and other reasons, is essentially inefficient for prostate cancer, metastatic melanoma, and bladder, kidney and pancreatic cancer. Further, the benefit of chemotherapy in general is modest, since the increase of survival rate after 5 years for all adult cancers is only by 2% [1]. Consistently, the search for more specific targets generally expressed within a certain tumor is a major issue in anti-cancer research. Cell membrane, growth factor receptors and proteins involved in cell–cell-signaling are of broad interest, but relatively little is known about changes in lipid composition of the cell membrane in carcinogenesis. Nevertheless, in recent years lipidomics in cancer research has gained more attention.
The outer leaflet of eukaryotic plasma membranes normally comprises neutral phospholipids, like phosphatidylcholine (PC) and sphingomyelin (SM), whereas the negatively charged phospholipid phosphatidylserine (PS) as well as phosphatidylethanolamine (PE) are located in the inner leaflet [2]. It has been reported earlier that this asymmetry may be lost due to reduced activity of the ATP dependent phospholipid translocase, which specifically transports PS and PE between bilayer leaflets and is susceptible to oxidative injury, or due to the activation of a scramblase induced by increased intracellular Ca2+ levels which leads to non-specific movement of phospholipids [3]. This loss of asymmetry results in exposure of the negatively charged PS on the surface of cancerous and other pathological cells [4,5], as well as apoptotic cells [6,7] and thus PS represents a promising target for cationic host defense peptides. The fact that platelets and erythrocytes also expose PS upon activation [8] seems to cause minimal side effects compared to much higher side effects observed with other applied therapies since these peptides can act specifically within the organism.
Surface exposure of PS by malignant cells was shown in tumorigenic Friend erythroleukemic cells [9], other leukemia and neuroblastoma cells [10], malignant melanoma [11] and human gastric carcinoma cells [12]. In vivo exposure of anionic phospholipids could be shown on the surface of endothelial cells in tumor blood vessels [13]. In an attempt to correlate changes in the phospholipid bilayer of malignant melanoma (MM) cell membranes with progression of these tumors Cichorek et al. showed slightly higher exposure of PS in the more progressive amelanotic transplantable hamster cell lines than in melanotic ones [14]. Lack of surface exposure of PS was demonstrated for non-cancer cells as normal human epidermal keratinocytes [11], 3T3-fibroblasts [15] and lymphocytes [10].
In general, apoptotic cells exposing PS in the outer leaflet of the plasma membrane are specifically recognized by macrophages [6,7] and dendritic cells [16]. However, tumor cells, although exposing PS, circumvent apoptosis and prevent the recognition by macrophages [17,18]. Therefore, membrane-active peptides have been proposed to constitute a promising approach in the development of anticancer drugs [10,19–21]. The positively charged small peptides are produced by the innate immune system and are able to discriminate between different cell membranes. These host defense peptides specifically interact with negatively charged lipids on their target cell membrane [22–25], which would minimize side effects upon treatment and could reach all cancer types exposing PS, even those resistant to conventional chemotherapy.
The discovery of a specific target generally expressed within all tumor types as well as by metastases would be a major success in cancer research. Therefore, the present study focuses on PS exposure of malignancies with poor outcome or treatment efficacy, which require new pharmaceutical therapies and would enormously benefit from cancer specific peptide drugs. This study has to be considered as an extension of the pioneering work by Utsugi and coworkers [9,11] who proved PS expression in the outer leaflet of three different tumor cell lines. We extended the study by increasing the number of cancer types and including metastases and primary cell cultures. We have therefore clearly demonstrated that PS-exposure is a general phenomenon not only in primary lesions of cancer but also in metastases, cancer cell lines and primary cultures and a by far underestimated Achilles’ heel of cancer.
2. Materials and methods
2.1. Cells and culture
2.1.1. Cell lines
Glioblastoma (U87-mg) purchased from CLS (Cell Line Service Heidelberg, Germany) and rhabdomyosarcoma cell lines (TE671) purchased from ECAAC (Health Protection Agency Culture Collections Salisbury, UK) were cultured in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen, UK) with addition of 2 mM glutamine, 10% FBS (fetal bovine serum; Lot A10109-0523, PAA Laboratory, Pasching, Austria) and 1% Pen/Strep (Penicillin/Streptomycin, PAA Laboratory, Austria). Kidney carcinoma (769-P) and prostate adenocarcinoma cell lines (LNCaP) purchased from CLS were cultured in RPMI1640 medium (Invitrogen, UK) with addition of 10% FBS, 2 mM l-glutamine, 1% PenStrep and 1 mM sodium pyruvate (Invitrogen, UK).
The melanocytic cell lines were kindly provided by Dr. Meenhard Herlyn (The Wistar Institute, Philadelphia, PA). The primary human melanoma cell lines SBcl2, WM35 and metastatic melanoma cell lines WM9, WM164 were maintained in RPMI (Sigma, St. Louis, MO) supplemented with 2% FBS, 2% l-glutamine and 1% Pen/Strep.
All cell lines were purchased frozen and thawed freshly before the experiments. During culturing they were kept in a 5% CO2 atmosphere at 37 °C. At 90% confluence cell cultures were passaged with accutase (PAA, Pasching, Austria). All cell cultures were periodically checked for mycoplasma and passaged in the laboratory no longer than 1 month after first thawing.
2.1.2. Primary cell cultures
A surgical specimen of primary glioblastoma (GBM), histologically independently verified by two pathologists (GFAP positive, Ki67 index above 50%), was blended mechanically and transferred to culture flask containing growth medium (DMEM, 10% FBS, 1% l-glutamine, 1% Pen/Strep, 1% Amphotericin B; PAA Laboratory, Austria) [26]. Tumorigenicity has been shown in nude mice. A melanoma metastasis to the brain (MMB), was blended mechanically and transferred to a culture flask containing growth medium (RPMI, 5% FBS, 1% l-glutamine, 1%Pen/Strep, 1% Amphotericin). Human melanocytes (FOM101), derived from human foreskin were cultured in human melanocytes growth medium (PromoCell GmbH, Heidelberg, Germany). Normal human dermal fibroblasts (NHDF) purchased from PromoCell GmbH (Heidelberg, Germany) were cultured in fibroblast growth medium 2 (PromoCell GmbH, Heidelberg, Germany).
Synoviocytes were isolated from synovium of Osteoarthritis (OA) patients undergoing joint replacement surgery, who fulfilled the criteria of the 1986 American College of Rheumatology. Random biopsies of synovial membrane were obtained aseptically from joints of OA patients. Synovial membrane tissue was separated macroscopically from ligaments, fat and other non-synovial tissue, minced in 1 mm slices and washed with PBS (phosphate buffered saline; GIBCO Invitrogen) supplemented with Pen/Strep and 0.25 μg Amphotericin B (PAA Laboratory, Austria). Synovial membrane specimens were rinsed several times with 1× PBS, finely dissected and digested with 0.2% Collegenase B (Roche Diagnostics, Germany) in high glucose Dulbecco's-modified Eagle's medium (DMEM-HG; GIBCO Invitrogen), containing 10% FBS, 1% l-glutamine, Pen/Strep and Amphotericin B (PAA Laboratory, Austria). Following overnight incubation at 37 °C, the cell suspension was filtered on a nylon membrane (Cell Strainer 40 μm; BD Bioscience). Cells were collected by centrifugation, washed twice, re-suspended in DMEM-HG growth medium, plated in culture flasks and allowed to attach for 3 days. OA cells were authenticated by flow cytometry.
Primary culture T22 was obtained after surgical excision of a Rhabdomyosarcoma, histologically specified by a pathologist. The tissue was minced mechanically, washed twice and transferred to Collagenase B and transferred to culture flask containing growth medium (DMEM-HG, 10% FBS, 1% l-glutamine, 1% Pen/Strep).
Primary cell cultures were used between passages 2 and 6 (except for FOM101 and GBM, which were passaged up to 12). All cell cultures were periodically checked for mycoplasma. All patients consented and ethics approval for the use of patient-derived material was given by the local research ethics committee.
2.2. Flow cytometry analysis
2.2.1. PS exposure
For Annexin V binding cultured cells with ~ 90% confluence were treated with accutase for 2–3 min at 37 °C. Cells were removed gently from the plate, dispensed into fresh media and washed once with media. 106 cells were re-suspended in 1 ml 1× binding buffer (Abcam, Cambridge, USA). 100 μl (105 cells) cell suspension was stained with 5 μl Annexin V-FITC and 5 μl propidium iodide (PI) (Abcam, Cambridge, GB), i.e. incubated at room temperature for 5 min in the dark. Flow cytometry analysis of PS-surface content of the cells was performed with an LSRII Flow cytometer (Becton Dickinson, USA). 10.000 events were collected. Cells were identified in the side scatter and forward scatter with linear scale. Fluorescence signal was analyzed with logarithmic scale. Analysis was done with the FACS Diva software (Becton Dickinson, USA). The graphical analysis was performed in FCS-express (De Novo Software, Los Angeles, USA).
2.2.2. Cell sorting
1.5 × 107 primary glioblastoma cells were stained with 50 μl Annexin V-FITC for 15 min at RT in the dark. Dead cells were discriminated by PI staining. Annexin negative cells and Annexin positive cells were analyzed and sorted by a live sterile cell sorting system (BD FACSAria Cell-Sorting System BD, Biosciences, San Jose, CA, USA).
2.2.3. Apoptosis
Cleavage of caspase-3: 2 × 106 cells/ml were fixed with formaldehyde for 10 min at 37 °C and permeabilized with methanol. The pellet was re-suspended in incubation-buffer and then stained with FITC-conjugated monoclonal active caspase-3 antibody (Cell Signaling, USA). Cells were then analyzed by flow cytometry (FACS Calibur, BD, Biosciences, USA). Untreated cells were used as negative control. As positive control apoptosis was induced by treatment with 10 μg/ml of petrol ether extract of roots of the plant Onosma paniculatum [27] up to 72 h. Analysis was performed with CellQuest (BD, Biosciences, USA)
2.3. Immunocytochemistry
After cell sorting Annexin negative and Annexin positive cells were suspended in DMEM culture medium and seeded onto chamber slides (BD Falcon). After overnight incubation the cells were washed with PBS and fixed with 4% formaldehyde/PBS. Staining with Ki67 (Mib-1) antibody purchased from Ventana was performed using standardized automated procedures with CC1 predilute antigen retrieval and Ventana iView detection system (Ventana Medical Systems, Tucson, AZ, USA and Dako Cytomation, Glostrup, Denmark). Counterstaining was performed with haematoxylin.
2.4. Electron microscopy
After cell sorting Annexin negative and Annexin positive cells were separately suspended in complete DMEM culture medium, washed with PBS, and replaced with fixatives (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M cacodylate buffer/pH 7.4) for 30 min at RT. Fixatives were removed and replaced with cacodylate buffer/pH 7.4 and the tissue was post fixed in 2% osmium tetraoxide solution, dehydrated in a series of graded ethanol and embedded in TAAB epoxy resin. Sections were cut at app. 70 nm thickness, counter stained with uranyl acetate and lead citrate, and visualized using a Zeiss EM 902 transmission electron microscope.
2.5. Fluorescence microscopy
Cells (2 × 104) were seeded onto 8 wells chamber slides (ibidy GmbH, Martinsried, Germany) and grown in 300 μl medium for 2–3 days in a confluent layer. Medium was removed and cells were washed twice with 1x Annexin binding buffer (ABB) of Vybrant Apoptosis Assay Kit #2 (Molecular Probes™, Invitrogen). 5 μl Annexin V-Alexa Fluor 488 (staining PS exposing cells) and 5 μl PI (staining DNA of necrotic cells) in 300 μl ABB were added and incubated at room temperature in the dark. After incubation cells were washed twice with ABB to remove unbound Annexin V-Alexa Fluor 488 and covered with ABB. A Leica DMI6000 B with IMC in connection with a Leica DFC360 FX camera and AF 6000 software was used for imaging.
Brightfield transmission, green (Annexin V-Alexa Fluor 488, λex = 488 nm and λem = 530 nm) and red fluorescence (PI, λex = 536 nm and λem = 617 nm) were measured in the respective channels. Exposure time, intensity and gain were fixed for measurement of all cell lines and primary cell cultures studied.
2.6. Fluorescence spectroscopy
Cells were cultured to ~ 90% confluence and then detached by accutase/EDTA. Accutase activity was stopped with media containing 10% FBS. Cells were washed with 1× Annexin binding buffer (ABB) of an Annexin V-FITC Apoptosis Detection Kit (Abcam, Cambridge, UK). 105 cells per experiment were re-suspended in 500 μl ABB and incubated with 5 μl Annexin V-FITC and 5 μl PI for 5 min at RT in the dark. Cells were washed with ABB and re-suspended in 100 μl ABB and measured with a fluorescence spectrometer (Fluoromax 3) with a λex = 488 nm and λem = 530 nm. Five independent experiments were performed.
3. Results
In the present study, we evaluated anionic phospholipids exposed by cancer cells as targets for cationic host defense peptides and thereof derived antitumor peptides. In particular, the specificity and significance of the presence of negatively charged PS in the outer leaflet of the plasma membrane of tumor cells and metastases of cell lines and primary cultures were investigated.
3.1. Cancer cells and metastases specifically and significantly expose PS
A standard Annexin V affinity assay, frequently used for detection of apoptosis, has been applied to specifically elucidate the exposure of PS by malignant melanoma, soft tissue, prostate, kidney and brain cancer cell lines. However, to avoid membrane stress, adherent cells were detached before Annexin V-FITC binding with accutase/low EDTA instead of the standard trypsin/EDTA detachment, since trypsinization or scrapping of the cells was shown to lead to increase of Annexin V binding (PS exposure) (PhD thesis Maximilian Martin Gemeinhardt, Munich, Faculty of Medicine, 2008). This is probably due to non-repairable membrane damage.
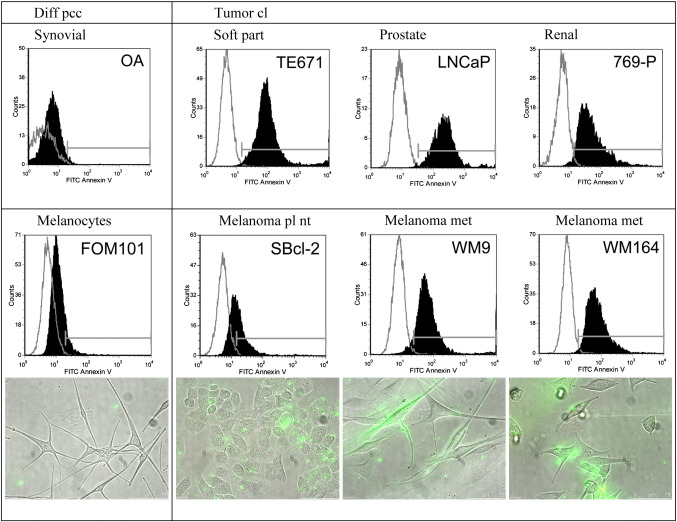
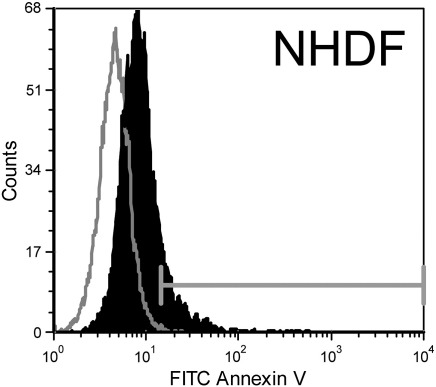
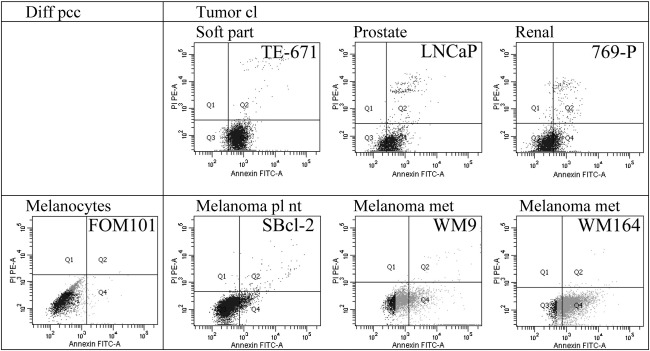
As demonstrated by flow cytometry the different cancer cell lines of rhabdomyosarcoma, a malignant soft tissue tumor of mesenchymal origin (TE671), prostate cancer (LNCaP), renal cell cancer (769-P) (Fig. 1), melanoma of primary (SBcl-2, WM35) and metastatic lesions (WM9 and WM164) and glioblastoma (U-87 mg) (Figs. 1 and 2) exposed significant amounts of PS compared to non-tumorigenic cells (Fig. 1 and supplementary Fig. 1S). This is indicated by selective binding of Annexin V-FITC to PS on the surface of tumor cells, revealed by a strong shift in fluorescence intensity of Annexin V-FITC labeled tumor cells in contrast to unlabeled tumor cells compared to a negligible shift of the labeled corresponding “non-tumor” control cells like differentiated synoviocyte cells of OA patients (non-tumor counterpart to TE671), melanocytes derived of foreskin (FOM101) (Fig. 1) and normal human dermal fibroblasts (NHDF) (supplementary Fig. 1S).
Fig. 1.
Top: Flow cytometry plots of Annexin V-FITC/PI labeled (black filled) and unlabeled cells (grey line) comprising differentiated control cells of primary cell cultures (diff pcc) of synoviocytes, isolated from synovium of Osteoarthritis (OA) patients and tumor cell lines (cl) of different cancer types, as rhabdomyosarcoma, a malignant soft tissue tumor of mesenchymal origin (TE671), prostate cancer (LNCaP) and renal cell cancer (769-P). Plots indicate a strong binding of Annexin V to PS on tumor cell lines. Second panel: Flow cytometry plots of Annexin V-FITC/PI labeled (black filled) and unlabeled cells (grey line) comprising differentiated control cells of primary cultures (diff pcc) of melanocytes (FOM101) and melanoma cell lines (cl) of primary lesions (pl) (nt non-tumorigenic) (SBcl-2) and more highly malignant melanoma metastases (met) (WM9 and WM164). Annexin V binds significantly to melanoma cell lines of primary and metastatic lesions. Fluorescence microscopy pictures (overlay of bright field and fluorescence channels) of melanocytes and melanoma cell lines indicate specific binding of Annexin V-Alexa Fluor 488 (green) to PS on the outside of melanoma cells. No PI (red) is integrated assuring integrity of cells studied.
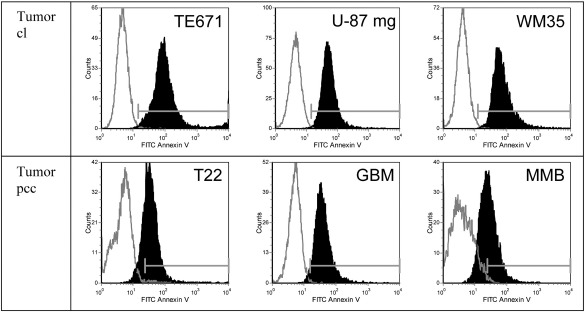
Fig. 2.
Comparison of Annexin V binding by tumor cell lines and correlating primary tumor cell cultures of a rhabdomyosarcoma (T22) (correlating to rhabdomyosarcoma cell line TE671), a glioblastoma (GBM) (correlating to glioblastoma cell line U-87 mg) and primary cell cultures of a malignant melanoma metastasis to the brain (MMB) (correlating to melanoma cell line WM35). Flow cytometry plots of Annexin V-FITC/PI labeled cells (black filled) and unlabeled cells (grey line) comprising tumor cell lines (top) and primary cell cultures of tumor tissue (bottom) (cl cell line; pcc primary cell culture). Tumor primary cell culture, as well significantly expose PS indicated by Annexin V binding.
To exclude false positive results by Annexin V labeled necrotic cells, the assays were always performed in the presence of propidium iodide (PI) staining necrotic cells that exhibit membrane damage and therefore also internalize Annexin V labels. Thus, PS levels referred to are derived from Annexin positive, but PI negative cells. In all cell types studied, PI positive necrotic cells (Q2, supplements, Fig. 2S) constituted only a minor population of less than 5%, whereas cells in Annexin positive cells (Q4, supplements, Fig. 2S) have to be ascribed to cells exposing PS. Thus, cells studied were not undergoing necrosis. In summary, results confirmed a cancer cell specific high exposure of PS by all the cancer cell lines investigated.
3.2. Correlation of level of PS exposure with malignancy and cancer type
To determine a possible correlation between PS exposure and tumor progression, four human malignant melanoma (MM) cell lines isolated from different stages were compared to melanocytes (Table 1).
Table 1.
PS exposure (Annexin V binding) of different tumor cell lines and tumor primary cell cultures related to PS exposure of melanocytes (fluorescence spectroscopy; 4 independent experiments of each cell line).
| Cells | Annexin V binding | Absolute intensity/105 cells |
|---|---|---|
| Melanocytes pcca | 1.0 ± 0.4 | 0.31∙105 |
| SBcl-2clb | 4.1 ± 0.6 | 1.28∙105 |
| WM35 cl | 7.0 ± 1.1 | 2.20∙105 |
| WM9 cl | 7.6 ± 2.1 | 2.38∙105 |
| WM164 cl | 11.0 ± 3.9 | 3.44∙105 |
| 769-P cl | 6.7 ± 0.3 | 2.10∙105 |
| TE671 cl | 14.9 ± 4.1 | 4.65∙105 |
| GBM pcc | 4.7 ± 0.5 | 1.46∙105 |
apcc primary cell culture; bcl cell line.
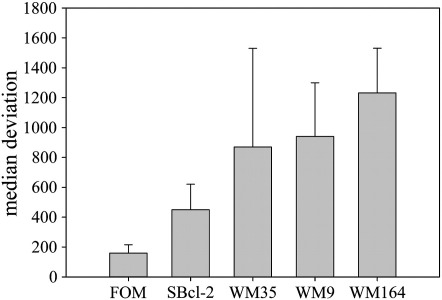
Fluorescence spectroscopy experiments enabled a comparison of the PS levels (Annexin V-FITC bound) exposed by different MM cell lines (Table 1). Results revealed a correlation between tumor malignancy and PS levels exposed. For instance, a nearly doubling in PS exposure was observed by WM35 [28] compared to SBcl-2, a non-tumorigenic cell line in SCID mice [29]. In SBcl-2 cells PS exposure was increased 4-fold compared to melanocytes whereas in cancer cells of higher malignancy, WM9 and WM164 of metastatic lesions, exposure was increased by 8–11 fold. Thus, a clear correlation between malignant potential within a tumor entity and the level of PS exposed was found. These results are in agreement with data obtained by flow cytometry (Fig. 1) (Section 3.1.) (median deviation, supplements, Fig. 3S).
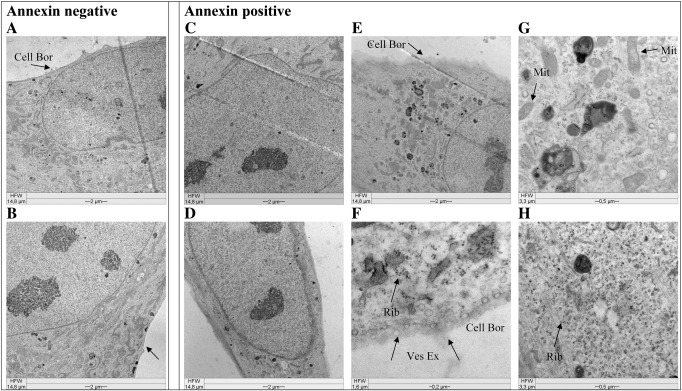
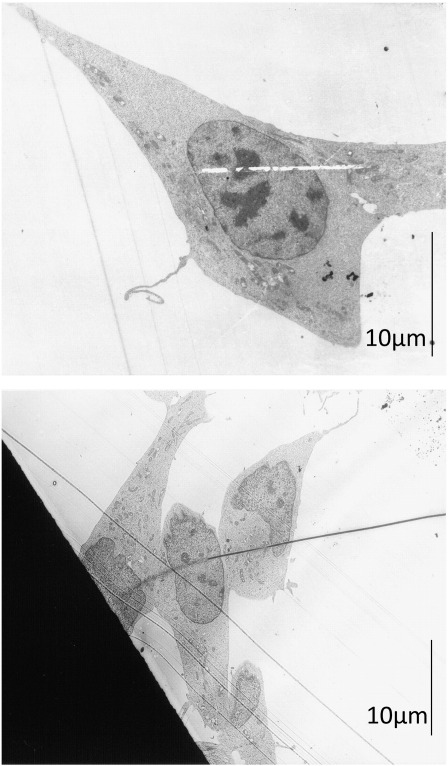
Fig. 3.
Comparison of morphology of Annexin V negative and positive cells. Pictures are taken by a Zeiss EM 902 transmission electron microscope after sorting of an Annexin V labeled primary culture of glioblastoma cells (GBM) by a BD FACSAria Cell-Sorting System. Annexin negative (A, B) and Annexin positive (C–H) cells are non-PS exposing and PS exposing cells, respectively. Arrows indicate diffuse cellular border (Cell Bor), Ribosomes (Rib), (exocytosed) vesicles (Ves Ex), normal mitochondria (Mit). Annexin positive cells show characteristics of glioblastoma (tumor) cells, but not of apoptosis.
Some striking differences were observed in terms of levels of PS exposed by other cancer types. In the case of the rhabdomyosarcoma cell line TE671, the amount of PS exposed was significantly higher in comparison to malignant melanoma or 769-P cells (Table 1), which suggests that this cancer type may be an even better candidate for efficient treatment by PS-specific peptides.
The PS level exposed by the primary cell culture of a glioblastoma (GBM) (see also Section 3.3.) is lower than that of melanoma cell lines WM9 or WM164, which is quite feasible, since it is not yet a homogenous tumor cell line. Nevertheless, the PS level is still increased compared to melanocytes.
Fluorescence labeling of vital cells (Fig. 1) grown on slides proved that PS (Annexin V-Alexa Fluor 488 bound, green fluorescence) is exposed by MM cells, even when not being detached before labeling (like for flow cytometry) and therefore “not stressed”, whereas non neoplastic melanocytes showed no Annexin V binding, thus no PS exposure. Furthermore, the quite interesting observation could be made that PS on the surface of MM cells appeared to be clustered and not distributed homogeneously over the plasma membrane, as would be the case if Annexin V stained PS in the inner leaflet of necrotic cells. Thus, to exclude false positive results by necrotic cells, cells were always co-labeled with PI, but only a negligible number of PI-stained necrotic cells were detected.
3.3. PS exposure — not an artifact of cell lines!
Cell culturing, which comprises several passages of the cells by repeated detachment and seeding of cells, is a standard procedure for cancer cells. The studied non-tumor cells however were primary cultures that can only be passaged a few times. Therefore tumor cell lines could suffer membrane damage through cell culturing procedures. In order to exclude this experimentally artificial reason for PS exposure, in addition to studies on cancer cell lines we also investigated primary cancer cell cultures of a glioblastoma (GBM) (correlating to glioblastoma cell line U-87 mg), primary cells of a rhabdomyosarcoma (T22) (correlating to rhabdomyosarcoma cell line TE671) and a malignant melanoma metastasis to the brain (MMB) (correlating to melanoma cell line WM35) (Fig. 2). The studied primary tumor cells, like the cell lines showed significant binding of Annexin V-FITC. These results clearly indicate that not only cancer cell lines but also primary cell cultures of malignant tumors and even metastases expose significant levels of PS. This further demonstrates that PS exposure to the outer lipid leaflet of cancer cells is not caused by cell culturing procedures but is an original and permanent characteristic of cancer cells. As mentioned, it has to be taken into account that primary cell cultures of tumors also include non neoplastic cells with a certain extent of stromal cells and therefore the PS level exposed can be lower compared to homogenous cancer cell lines. This is for instance nicely demonstrated by the primary cell culture of the melanoma metastases (MMB), which shows less shift of the Annexin V labeled cells (Fig. 2) meaning slightly reduced PS exposure compared to melanoma cell line WM35, but still significantly increased levels compared to differentiated melanocytes (Fig. 1).
3.4. PS — a potential common marker of cancer cells, but “no reliable eat me” signal
There is no universal tumor marker known so far. To prove that PS could really be an accurate marker for all cancer cells, we tried to distinguish between tumor and non-tumor cells just by the characteristic of exposure or non-exposure of PS, respectively.
Therefore, a primary cell culture of a histologically verified glioblastoma (WHO grade IV) was evaluated. Even when the specimen for the culture is obtained from the center of the tumor, it is very likely to contain non neoplastic cells as well as glial tumor cells, like microglia and stromal cells, macrophages and maybe reactive glial cells. A fact being of advantage in this case, since we should be able to observe malignant and to some extent benign cell types in one culture. Microscopic inspection of the cell culture was insufficient to distinguish between the different cell types.
After Annexin V labeling and exclusion of necrotic factors by PI, strongly increased PS levels on the cell surface of a part of the cells were detected. In a second step the cells were sorted using a FACS ARIA II TM cell sorter according to their Annexin V binding events. It was possible to obtain Annexin positive (A+) and Annexin negative (A−) cells. These different cell types were returned to culture to let the cells recover. The two different cultures were then investigated by electron microscopy (Fig. 3) and immunocytochemistry using the proliferation marker Mib-1 (Fig. 4).
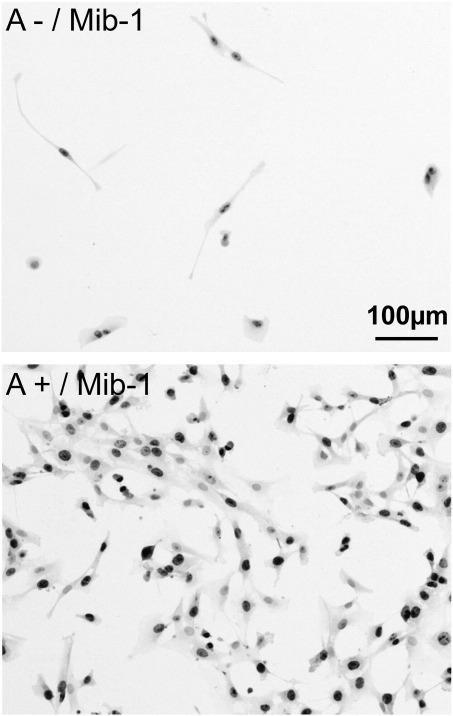
Fig. 4.
Morphology and Mib-1 immunocytochemistry of a FACS ARIA II TM cell sorted Annexin V labeled primary cell culture of glioblastoma. Top: Annexin negative cells (100×); bottom: Annexin positive cells (100×). Annexin positive cells express higher levels of Mib-1.
The electron microscopy pictures revealed that the Annexin positive cells (Fig. 3 C–H) (potential glial tumor cells carrying the PS marker) showed some homogenous morphological characteristics indicating that these cells were tumor cells. Size and shape of nuclei of Annexin positive cells (Fig. 3 C and D) were partially changed compared to those of Annexin negative cells (Fig. 3 A and B), indicated by an elongated or polymorphic shape and sizes up to 25 μm, which is twice the normal size. Increased nucleus/plasma ratios, as well as polymorphic nuclei are characteristics of tumor cells. Nevertheless, also the few Annexin negative cells exhibited quite large nuclei (10–15 μm), potentially representing reactive astrocytes. Furthermore, a structural change of the plasma membrane of the Annexin positive cells could be seen, characterized by a quite diffuse cellular border (D, E, F, arrows Cell Bor) compared to a sharp border exhibited by Annexin negative cells (A and B). This membrane feature is probably reflecting a difference between tumor and stromal cells. Another characteristic of the Annexin positive cells was a strong increase in formation of ribosomes and vesicles (F and H, arrow Rib) and an increase in exocytosis of vesicles (F, arrow Ves Ex), a feature of GBM cells described in detail recently by Skog et al. [30]. For better representation of intracellular changes only parts of cells are shown, whole cell images before sorting are presented in the supplements (Fig. 4S).
The aforementioned findings were supported by slowly growing Annexin negative cells in contrast to well proliferating Annexin positive cells and the fact that proliferation marker Mib-1 (Fig. 4) was slightly higher expressed in A+ than in A− cells, a feature of glioblastoma cells. However, it has to be noted that the amount of Annexin negative cells within the primary cell culture was quite low.
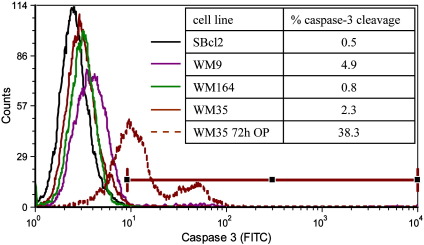
Further the Annexin positive cells of glioblastoma were checked for morphological changes due to apoptosis, like swelling of mitochondria, typical apoptotic blebbing or condensation of chromatin (Fig. 3 G, D, E, F, arrow Mit (normal mitochondria)). The Annexin positive cells however were lacking all these signs of apoptosis. This was also supported by caspase-3 cleavage assays with the melanoma cancer cell lines used within this study (Fig. 5) revealing no significant caspase-3 cleavage (below 5%), thus excluding PS exposure of studied cancer cells to be a sign of cells undergoing apoptosis. This is further confirmed by a positive control, where apoptosis was induced in the melanoma cell line WM35 by 72 h treatment with a plant extract of Onosma paniculatum resulting in significant increase of caspase-3 cleavage from 2.3% by untreated up to 38% by treated cells, indicated by a strong shift in fluorescence intensity. Inducibility of cell death in a caspase dependent manner by treatment with the plant extract was also reported for the studied melanoma cell lines SBcl-2, WM35, WM9 and WM164 recently by Rinner et al. [27].
Fig. 5.
Minor caspase cleavage of melanoma cell lines. Flow cytometry plots of melanoma cell lines were stained with FITC-conjugated monoclonal active caspase-3 antibody. Untreated cells were used as negative control. Analysis was performed with CellQuest (BD, Biosciences, USA). No significant cleavage of caspase-3 was detected for untreated melanoma cell lines represented by solid lines, indicating no apoptosis. As a positive control, WM35was treated with 10 μg/ml petrol ether extract of roots of the plant Onosma paniculatum (OP) for 72 h (brown dashed line) [27]. Treatment caused clear apoptosis indicated by 38% of caspase-3 cleavage.
4. Discussion
After several decades of cancer research no common marker for malignant cells has been identified so far, which would offer many possibilities, not only for therapy, but also for fast and accurate diagnosis. Already in 1991 Utsugi et al. [11] reported about elevated expression of PS in the outer leaflet of human tumor cells. In 2002 Ran et al. [13] showed that the monoclonal antibody 9D2 and Annexin V, the natural ligand to anionic phospholipids, specifically localized to tumor vessels and tumor cells in and around necrotic regions after i.v. injection into tumor-bearing mice, whereas none of the blood vessels in normal tissues had detectable externalized anionic phospholipids. Our study strongly supports these findings and more importantly clearly demonstrates that exposure of PS to the outer leaflet of membranes is not restricted to blood vessels but is a general phenomenon for cancer plasma membranes independently on cancer type and is also valid for metastases and many cancer types including those with poor outcome or treatability, like glioblastoma or malignant melanoma. Thus, the anionic phospholipid PS could serve as a common cancer marker as well as a therapeutic target. Since the standard labeling technique with Annexin V requires an intact plasma membrane and apoptotic or necrobiotic cells also show increased levels of PS in their outer leaflet, there may be some limitations. Therefore in our study “false positive” results by increased Annexin V binding to PS by cells undergoing apoptosis had to be excluded. In fact, the cancer cells tested showed elevated PS levels, but without any sign of apoptosis. This is an important aspect, since it is mainly assumed that cancer cells expose higher levels of PS due to their higher rate of cell division and apoptosis, as e.g. described by Boersma et al. [31]. On the other hand PS exposed could mean that cancer cells should be fated to die but are clever enough to avoid the last step of being killed. As mentioned earlier there are several ways for cancer cells to prevent apoptosis like melanoma cells inhibiting the expression of the gene encoding Apaf-1, the apoptotic protease activating factor-1 [17].
It is not fully understood how and why cancer cells expose PS to the outer plasma membrane leaflet. When separating phospholipids of a total cell lipid extract of human melanocytes (FOM101) and two melanoma cell lines (SBcl-2 and WM164) by two-dimensional thin-layer chromatography and quantification by the method of Broekhuyse [32], no increase in the total amount of PS was found in the cancer cells (data not shown). Therefore, not the overall PS content but only the asymmetric PS distribution in the plasma membrane seems to get lost. It has been reported that the combined action of an amino phospholipid translocase, responsible for the specific movement of PS and PE to the inner leaflet of plasma membranes, and a less specific floppase, responsible for the movement of PS and PC to the outside, both dependent on ATP, seems to equip the cell with a mechanism that corrects for alterations in lipid distribution to avoid potential pathological consequences [5]. Nevertheless, as reported, inactivation of these activities in vitro did not result in loss of asymmetry. However, activation of a third protein, the lipid scramblase, by influx of Ca2+ into the cytoplasm can cause a rapid transbilayer phospholipid mixing leading to a nearly symmetric distribution across the membrane bilayer [5]. Thus, cellular changes like depletion of ATP and/or influx of Ca2+ into the cytoplasm seem to be important factors accounting for exposure of PS. Furthermore, it has been proposed by Ran et al. [13] that injury and activation of tumor endothelium by cytokines and reactive oxygen species might induce PS exposure of tumor vessels. As mentioned in Section 3.2., we were able to observe by fluorescence microscopy that PS, visualized by Annexin V-Alexa Fluor 488, was localized in clusters on the plasma membrane of cancer cells. Interestingly some of these PS exposing clusters budded off the cells were quite mobile and even moved from one cell to the other in the form of small PS exposing blebs (unpublished observation). In this context it is of interest to note that loss of membrane asymmetry is often accompanied by blebbing and subsequent shedding of lipid-symmetric microvesicles from the cell surface [33,34]. It is known that eukaryotic cells are able to perform intercellular communication by e.g., exosomes [35]. If these vesicles still carry the PS marker, one could then assume that host defense peptides, which can target PS would also be able to prevent possible intercellular communication between cancer cells.
Definitely, PS constitutes an Achilles' heel of cancer cells, because the anionic lipid represents a cancer type independent target for host defense peptides that rapidly kill cells exposing negatively charged lipids. A number of studies revealed that cationic amphipathic peptides specifically interact with PS containing membranes [10,36–38]. So far highly active antimicrobial peptides derived from host defense could be designed to selectively target bacterial membranes [39–43], whereas antitumor peptides to target cancer cell membranes are in development [20,24,37,44,45]. The high levels of exposed PS found in our study for cancer cell lines, derived from metastatic lesions, suggest that these peptides can even be beneficial as a therapeutic intervention in the metastatic cascade, which is difficult so far. Furthermore, all cancer types including cancer with poor prognosis and treatability, like glioblastoma and melanoma, amongst others, would highly benefit using the PS target for novel approaches in diagnosis and therapy. Successful sorting of a primary culture of a glioblastoma within this study gives hope for possible differentiation between tumor and non-tumor cells and selective targeted therapy.
Conclusions
Our study constitutes an extension of previous work [11] regarding cancer types with a focus on cancer with poor outcome and treatment efficacy, including metastases and primary cell cultures using another assay. We show that PS exposure is also a characteristic of metastases, a very important aspect regarding therapy. Furthermore, we compared several primary cancer cell cultures with correlating cell lines to rule out cell culturing effects on the membrane and to prove originality of PS exposure. Cancer specific PS exposure was not a sign of apoptosis. By using PS as a marker it was possible to distinguish within a primary culture of glioblastoma between potential malignant and benign cells.
The study aimed at supplying evidence for a uniform and novel target for cancer therapy in the form of the negatively charged lipid phosphatidylserine. We prove that PS is specifically exposed by cancer cells and metastases, which delivers the important basis and target for the further development of cationic antitumor peptide drugs, to one day support or even partially substitute chemotherapy.
The following are the supplementary materials related to this article.
Fig. 1S.
Flow cytometry plots of Annexin V-FITC/PI labeled (black filled) and unlabeled cells (grey line) comprising differentiated control cells of primary cultures of normal human dermal fibroblasts (NHDF). Plot indicates a lack of binding of Annexin V to PS on non-tumor cells meaning lack of PS exposure by these cells (supplement to Fig. 1).
Fig. 2S.
2D-flow cytometry plots of AnnexinV-FITC/PI labeled differentiated primary cell cultures (pcc) of melanocytes (FOM101) and tumor cell lines (cl) of melanoma (SBcl-2, WM9, WM164), prostate cancer (LNCaP), renal cancer (769-P) and a rhabdomyosarcoma (TE671). Events in section Q4 indicate binding of Annexin V-FITC, events in section Q2 indicate PI-positive cells. Plots demonstrate that amount of PI-positive cells is minor than 5%, thus Annexin V binding of tumor cells is due to PS exposure (supplement to Fig. 1).
Figure 3S.
Median deviation of flow cytometry experiments (mean value of 4 independent experiments) with Annexin V-FITC/PI labeled melanocytes (FOM101) and melanoma cells (SBcl-2, WM35, WM9, WM164) (supplement to Fig. 1 and Table 1). Annexin V-FITC binding is increased in tumor cell lines and is higher in higher malign cell lines of metastases like WM9 and WM164.
Figure 4S.
Pictures of whole cells of a primary culture of glioblastoma cells (GBM) before sorting taken by a Zeiss EM 902 transmission electron microscope (supplement to Fig. 3).
Acknowledgement
We want to thank Dr. R. Hofmann-Wellenhof from the Department of Dermatology at the Medical University of Graz and Dr. Gerd Leitinger from the Core Facility Ultrastructure Analysis at the Center of Medical Research in Graz for their help regarding melanocytes and melanoma cell cultures and performance of electron microscopy experiments, respectively. We thank S. Tumer for technical support. Further we thank Dr. Sylvie Blondelle from Sanford-Burnham Medical Research Institute in La Jolla, USA, for carefully reading the manuscript. Financial support from the Austrian Science Foundation FWF (grant no. P20760-B11 to D.Z) is acknowledged.
References
- 1.Morgan G., Ward R., Barton M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin. Oncol. (R. Coll. Radiol.) 2004;16:549–560. doi: 10.1016/j.clon.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Bevers E.M., Comfurius P., Zwaal R.F. Regulatory mechanisms in maintenance and modulation of transmembrane lipid asymmetry: pathophysiological implications. Lupus. 1996;5:480–487. doi: 10.1177/096120339600500531. [DOI] [PubMed] [Google Scholar]
- 3.Seigneuret M., Devaux P.F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. U.S.A. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwaal R.F., Comfurius P., Bevers E.M. Surface exposure of phosphatidylserine in pathological cells. Cell Mol. Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PubMed] [Google Scholar]
- 5.Zwaal R.F., Schroit A.J. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 6.Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 7.Martin S., Reutelingsperger C.P., McGahon A., Rader J., van Schie R., La Face D., Green D.R. Early redistribution of plasma membrane phophatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Ab1. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson P., Bevers E.M., Smeets E.F., Comfurius P., Schlegel R.A., Zwaal R.F. Continuous analysis of the mechanism of activated transbilayer lipid movement in platelets. Biochemistry. 1995;34:10448–10455. doi: 10.1021/bi00033a017. [DOI] [PubMed] [Google Scholar]
- 9.Connor J., Bucana C., Fidler I.J., Schroit A.J. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3184–3188. doi: 10.1073/pnas.86.9.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroder-Borm H., Bakalova R., Andrä J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579:6128–6134. doi: 10.1016/j.febslet.2005.09.084. [DOI] [PubMed] [Google Scholar]
- 11.Utsugi T., Schroit A.J., Connor J., Bucana C.D., Fidler I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- 12.Woehlecke H., Pohl A., Alder-Baerens N., Lage H., Herrmann A. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2) Biochem. J. 2003;376:489–495. doi: 10.1042/BJ20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ran S., Downes A., Thorpe P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 14.Cichorek M., Kozlowska K., Witkowski J.M., Zarzeczna M. Flow cytometric estimation of the plasma membrane diversity of transplantable melanomas, using annexin V. Folia Histochem. Cytobiol. 2000;38:41–43. [PubMed] [Google Scholar]
- 15.Papo N., Seger D., Makovitzki A., Kalchenko V., Eshhar Z., Degani H., Shai Y. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res. 2006;66:5371–5378. doi: 10.1158/0008-5472.CAN-05-4569. [DOI] [PubMed] [Google Scholar]
- 16.Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 17.Soengas M.S., Capodieci P., Polsky D., Mora J., Esteller M., Opitz-Araya X., McCombie R., Herman J.G., Gerald W.L., Lazebnik Y.A., Cordon-Cardo C., Lowe S.W. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita T., Reed J.C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- 19.Papo N., Braunstein A., Eshhar Z., Shai Y. Suppression of human prostate tumor growth in mice by a cytolytic D-, L-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 2004;64:5779–5786. doi: 10.1158/0008-5472.CAN-04-1438. [DOI] [PubMed] [Google Scholar]
- 20.Yang N., Stensen W., Svendsen J.S., Rekdal O. Enhanced antitumor activity and selectivity of lactoferrin-derived peptides. J. Pept. Res. 2002;60:187–197. doi: 10.1034/j.1399-3011.2002.21008.x. [DOI] [PubMed] [Google Scholar]
- 21.Gifford J.L., Hunter H.N., Vogel H.J. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol. Life Sci. 2005;62:2588–2598. doi: 10.1007/s00018-005-5373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohner K., Latal A., Lehrer R.I., Ganz T. Differential scanning microcalorimetry indicates that human defensin, HNP-2, interacts specifically with biomembrane mimetic systems. Biochemistry. 1997;36:1525–1531. doi: 10.1021/bi961300p. [DOI] [PubMed] [Google Scholar]
- 23.Papo N., Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol. Life Sci. 2005;62:784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PubMed] [Google Scholar]
- 24.Hoskin D.W., Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel H.J., Schibli D.J., Jing W., Lohmeier-Vogel E.M., Epand R.F., Epand R.M. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell Biol. 2002;80:49–63. doi: 10.1139/o01-213. [DOI] [PubMed] [Google Scholar]
- 26.Spiegl-Kreinecker S., Buchroithner J., Elbling L., Steiner E., Wurm G., Bodenteich A., Fischer J., Micksche M., Berger W. Expression and functional activity of the ABC-transporter proteins P-glycoprotein and multidrug-resistance protein 1 in human brain tumor cells and astrocytes. J. Neurooncol. 2002;57:27–36. doi: 10.1023/a:1015735815111. [DOI] [PubMed] [Google Scholar]
- 27.Rinner B., Kretschmer N., Knausz H., Mayer A., Boechzelt H., Hao X.J., Heubl G., Efferth T., Schaider H., Bauer R. A petrol ether extract of the roots of Onosma paniculatum induces cell death in a caspase dependent manner. J. Ethnopharmacol. 2010;129:182–188. doi: 10.1016/j.jep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Satyamoorthy K., DeJesus E., Linnenbach A.J., Kraj B., Kornreich D.L., Rendle S., Elder D.E., Herlyn M. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7(Suppl 2):S35–S42. [PubMed] [Google Scholar]
- 29.Nesbit M., Schaider H., Miller T.H., Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J. Immunol. 2001;166:6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 30.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boersma H.H., Kietselaer B.L., Stolk L.M., Bennaghmouch A., Hofstra L., Narula J., Heidendal G.A., Reutelingsperger C.P. Past, present, and future of annexin A5: from protein discovery to clinical applications. J. Nucl. Med. 2005;46:2035–2050. [PubMed] [Google Scholar]
- 32.Broekhuyse R.M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys Acta. 1968;152:307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- 33.Comfurius P., Senden J.M., Tilly R.H., Schroit A.J., Bevers E.M., Zwaal R.F. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim. Biophys. Acta. 2005;1026:153–160. doi: 10.1016/0005-2736(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 34.Wiedmer T., Shattil S.J., Cunningham M., Sims P.J. Role of calcium and calpain in complement-induced vesiculation of the platelet plasma membrane and in the exposure of the platelet factor Va receptor. Biochemistry. 1990;29:623–632. doi: 10.1021/bi00455a005. [DOI] [PubMed] [Google Scholar]
- 35.Belting M., Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J. Cell Biol. 2008;183:1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manavbasi Y., Zweytick D., Willumeit R., Lohner K. Phosphatidylserine selective peptides as novel anti-cancer agents. Biophys. J. 2009;96:157. [Google Scholar]
- 37.Riedl S., Rinner B., Tumer S., Schaider H., Lohner K., Zweytick D. Targeting the cancer cell membrane specifically with human lactoferricin derivatives. Ann. Oncol. 2011;22(Suppl. 3):33. [Google Scholar]
- 38.Papo N., Seger D., Makovitzki A., Kalchenko V., Eshhar Z., Degani H., Shai Y. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res. 2006;66:5371–5378. doi: 10.1158/0008-5472.CAN-05-4569. [DOI] [PubMed] [Google Scholar]
- 39.Lohner K., Blondelle S.E. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 2005;8:241–256. doi: 10.2174/1386207053764576. [DOI] [PubMed] [Google Scholar]
- 40.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 41.Shai Y., Oren Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22:1629–1641. doi: 10.1016/s0196-9781(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 42.Blondelle S.E., Lohner K. Optimization and high-throughput screening of antimicrobial peptides. Curr. Pharm. Des. 2010;16:3204–3211. doi: 10.2174/138161210793292438. [DOI] [PubMed] [Google Scholar]
- 43.Hallock K.J., Lee D.K., Ramamoorthy A. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid bilayer structure via positive curvature strain. Biophys. J. 2005;84:3052–3060. doi: 10.1016/S0006-3495(03)70031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papo N., Shai Y. New lytic peptides based on the D,L-amphipathic helix motif preferentially kill tumor cells compared to normal cells. Biochemistry. 2003;42:9346–9354. doi: 10.1021/bi027212o. [DOI] [PubMed] [Google Scholar]
- 45.Eliassen L.T., Berge G., Sveinbjornsson B., Svendsen J.S., Vorland L.H., Rekdal O. Evidence for a direct antitumor mechanism of action of bovine lactoferricin. Anticancer. Res. 2002;22:2703–2710. [PubMed] [Google Scholar]