Abstract
We studied the spatial distribution patterns of mercury (Hg) in lake water, littoral sediments, zooplankton, crayfish, fish, and common loons in 44 lakes of the Adirondacks of New York State, USA, a region that has been characterized as a “biological Hg hotspot”. Our study confirmed this pattern, finding that a substantial fraction of the lakes studied had fish and loon samples exceeding established criteria for human and wildlife health. Factors accounting for the spatial variability of Hg in lake water and biota were lake chemistry (pH, acid neutralizing capacity (ANC), percent carbon in sediments), biology (taxa presence, trophic status) and landscape characteristics (land cover class, lake elevation). Hg concentrations in zooplankton, fish and common loons were negatively associated with the lake water acid-base status (pH, ANC). Bioaccumulation factors (BAF) for methyl Hg (MeHg) increased from crayfish (mean log10 BAF = 5.7), to zooplankton (5.9), to prey fish (6.2), to larger fish (6.3), to common loons (7.2). MeHg BAF values in zooplankton, crayfish, and fish (yellow perch equivalent) all increased with increasing lake elevation. Our findings support the hypothesis that bioaccumulation of MeHg at the base of the food chain is an important controller of Hg concentrations in taxa at higher trophic levels. The characteristics of Adirondack lake-watersheds (sensitivity to acidic deposition; significant forest and wetland land cover; and low nutrient inputs) contribute to elevated Hg concentrations in aquatic biota.
Electronic supplementary material
The online version of this article (doi:10.1007/s10646-011-0717-y) contains supplementary material, which is available to authorized users.
Keywords: Spatial distribution, Methylmercury, Bioaccumulation, Aquatic biota, pH, Acid neutralizing capacity
Introduction
Mercury (Hg) is a toxic metal that can threaten both human and ecological health, due to its strong tendency to bioaccumulate along food webs (Driscoll et al. 2007c; Bookman et al. 2008). Evers et al. (2007) conducted a spatial analysis using indicators of Hg contamination for human health (i.e., Perca flavescens; yellow perch) and wildlife health (i.e., Gavia immer; common loon) across northeastern North America. Five “biological Hg hotspots” were identified based on this analysis. The Adirondack region of New York State, U.S. is considered a biological Hg hotspot (Evers et al. 2007, 2008), largely due to the sensitivity of lake-watersheds in the region to inputs of atmospheric Hg deposition.
Separate indicators have been established to protect humans and wildlife from exposure to Hg. The U.S. Environmental Protection Agency (EPA) has indicated a Hg concentration of 0.3 μg g−1 (wet weight, ww) in fish tissue as a water quality criterion under § 304 of the Clean Water Act to guide human consumption of fish. Due to the detailed quantitative understanding of the physiological and reproductive effects of Hg, the common loon has been widely used, especially in the northeastern U.S., as an indicator of the impacts of Hg contamination on wildlife (Evers et al. 2003, 2007, 2008). Three dietary concentration thresholds of Hg in fish tissues have been established to indicate health impacts of common loons: concentrations above 0.16 μg g−1 Hg significantly decrease loon reproduction (Evers et al. 2008); 0.21 μg g−1 is the Hg concentration associated with a 50% decrease from maximum production of fledged young (Burgess and Meyer 2008); and concentrations above 0.41 μg g−1 result in complete reproductive failure (Burgess and Meyer 2008). In addition, Evers et al. (2007, 2008) indicated that significant adverse physiological, behavioral and reproductive effects occur above blood Hg concentrations of 3.0 μg g−1 in common loons.
There is considerable lake-to-lake variation in Hg concentrations in the Northeast (Chen and Folt 2005). Many studies have been conducted on the factors influencing Hg bioaccumulation in aquatic food webs (Chen and Folt 2005; Kamman et al. 2005; Driscoll et al. 2007c; Bushey et al. 2008; Mason et al. 1996). Lake water chemistry, particularly pH and dissolved organic carbon (DOC), appears to influence the bioavailability of Hg at the base of the aquatic food chain (Adams et al. 2009; Dittman et al. 2009). Chen et al. (2005) proposed that indicators such as pH, acid neutralizing capacity (ANC), lake area, and zooplankton abundance are useful in identifying lakes that likely contain fish with high Hg concentrations. Other studies have shown the importance of wetlands in the transport of Hg and the production and supply of methyl Hg (MeHg; Hurley et al. 1995; Driscoll et al. 1998, 2007a; Selvendiran et al. 2008). Simonin et al. (2008) suggested that outlet dams and the amount of contiguous wetlands affect Hg concentrations in fish. Dittman and Driscoll (2009) indicated that pH and fish condition affect fish Hg concentrations. Indeed, previous studies have focused on three categories of factors affecting Hg bioaccumulation in aquatic biota: lake physico-chemistry (e.g., pH, DOC), biology (e.g., taxa presence, trophic status), and landscape characteristics (e.g., land cover class, amount of connected wetlands, elevation) (George and Batzer 2008). Few studies have been conducted to evaluate spatial factors that influence the bioaccumulation of Hg across the entire aquatic food chain (Kramar et al. 2005; Kamman et al. 2005).
This study was conducted by the Wildlife Conservation Society, BioDiversity Research Institute, and the New York State Department of Environmental Conservation partners in the former Adirondack Cooperative Loon Program to assess the impact of Hg contamination on common loon populations in the Adirondack Park of New York. We analyzed Hg concentrations in lake water, littoral sediments, zooplankton, crayfish, fish, and common loon in 44 lakes of Adirondack Park. We established the following hypotheses for this study: (a) lake-watershed attributes (such as water chemistry, land coverage, elevation) regulate the spatial patterns of Hg and MeHg concentrations in aquatic biota across trophic levels; (b) the sensitivity of lakes to atmospheric Hg deposition is established by physio-chemical characteristics of lake-watersheds and the supply of MeHg to the base of the food chain; and (c) the acid-base status of Adirondack lakes (pH, ANC) is an important controller of bioaccumulation of MeHg.
Study site, field and analytical methods
Study site
The Adirondack Park of New York State, USA (43°59′N, 74°14′W; 2.4 million ha), contains a unique mountainous landscape of wetlands, northern hardwood and boreal forests, alpine tundra, and approximately 2,800 lakes (Driscoll et al. 1991). The variability of lake characteristics, presence of breeding common loons, and the accessibility of the lakes were the main criteria in selecting the study sites. Forty-four lakes were selected for study (Fig. 1), including several listed in the New York State fish consumption advisory (http://www.nyhealth.gov/publications/2779, accessed March 28, 2011).
Fig. 1.
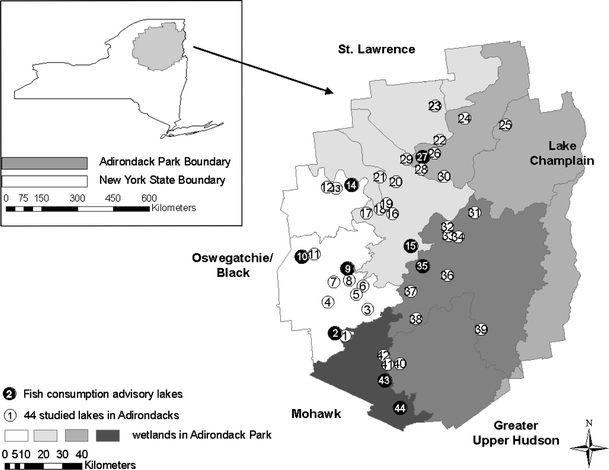
Location of the 44 study lakes in Adirondack Park of New York State. The corresponding lake names for each ID are shown in Table 1
Atmospheric deposition is the dominant source of Hg to the Adirondacks (Driscoll et al. 2007c). Miller et al. (2005) created a spatial distribution model of Hg deposition across northeastern North America. From their model, atmospheric Hg deposition is generally estimated to be higher in the southeastern Adirondacks where elevations are higher, and deposition decreases toward the northwest. Using the Miller et al. (2005) model, the mean total deposited Hg to the study lake-watersheds was estimated to be approximately 20 μg m−2 year−1, with wet deposition of about 8 μg m−2 year−1 and dry deposition about 12 μg m−2 year−1.
Field and laboratory methods
All samples were collected at approximately the same time of year, around August 2003 or 2004. Lake water and sediment samples were collected following “clean hands-dirty hands protocols” (USEPA 2001, 2002). Water samples were collected as grab samples at a depth of half meter near the center of the lakes. Surface sediment samples were collected using a modified 50 mL syringe inserted to a 3–5 cm depth in lake littoral sediments where crayfish were present. Zooplankton samples were collected via tow nets (64 μm pore size). Crayfish were collected by hand from lakes with rocky substrate. Whole body and tail of crayfish samples were measured for Hg concentrations. Four size-classes of fish (5–10 cm, 10–15 cm, 15–20 cm and 20–25 cm) were collected for whole body analyses using seines, rod and reel, and minnow traps. Loons were captured by night-lighting techniques (Evers et al. 2008), and blood samples were non-lethally collected. We have one sample for water, sediment and zooplankton, two or three samples for crayfish, and multiple samples for fish and loons in each of the 44 lakes. We were unable to collect crayfish from 17 of the lakes and also did not collect sediments from those lakes.
The collected samples were sent to Syracuse University (water, sediments, zooplankton) and Texas A&M University (crayfish, fish, loon) for chemical analysis. Total Hg (THg) was analyzed via oxidation, purge and trap, and cold vapor atomic fluorescence spectroscopy (CVAFS, Tekran model 2600) based on USEPA method 1631 (2002, revision E). MeHg was analyzed via distillation, aqueous ethylation, purge and trap, desorption, and CVAFS based on USEPA method 1630 (2001). All samples were analyzed for THg. MeHg was analyzed in water, sediment, and zooplankton. All biota Hg and MeHg concentrations are expressed on a wet weight (ww) basis, except for zooplankton (dry weight, dw). Ancillary water chemistry parameters, including pH, ANC, DOC, dissolved inorganic carbon (DIC), Na, K, Ca, Mg, Si, NH4, NO3, SO4, F, Cl, total phosphorus (P), chlorophyll a, monomeric aluminum (Alm) and non-labile (organic) monomeric aluminum (Alo), were analyzed from the same sample which was analyzed for Hg species according to standard methods (APHA/AWWA/WEF 1998). The determination of THg, MeHg and ancillary chemical properties for all water, sediment and biological samples were subjected to quality assurance (QA) procedures which are summarized in the electronic supplementary material.
Data analysis methods
In this study several different species of fish were collected. We considered golden shiner (Notemigonus crysoleucas), creek chub (Semotilus atromaculatus), and banded killifish (Fundulus diaphanus) to be “prey fish”, while yellow perch, pumpkinseed sunfish (Lepomis gibbosus), brown bullhead (Ictalurus nebulosus), smallmouth bass (Micropterus dolomieu), and largemouth bass (Micropterus salmoides) as “fish”. To facilitate comparison of fish Hg concentrations across lakes, we converted all fish Hg concentrations to yellow perch equivalent values (YPE) based on the New York State Research Development Authority (NYSERDA) fish tissue standardization approach (Kamman et al. 2003). In designing the project dataset, the fish collected were grouped into four size classes (described above). In so doing, we paired yellow perch Hg concentrations with Hg concentrations of other fish species within lake and size categories to calculate predictive linear relationships that took the form of linear regressions or simple adjustment factors. Ultimately, we computed modeled YPE from other fish species for all lakes in this study.
Similarly, to facilitate the comparisons of blood Hg concentrations among samples of loons (including females, males and juveniles), we converted values into equivalent female loon concentrations, or female loon unit (FLU) concentrations. We used a compilation of common loon data from New York state (1998–2008, n = 381), whose subsets contained multiple Hg observations for loons from a single territory and year, to develop the modeled conversion relationships. The equation used to convert male blood Hg concentrations into FLU was: FLU = exp (−0.64939 + 1.354711 * ln(Hgmale)), r 2 = 0.61, 95% confidence limits (CL): 1.073–1.739. While the equations used to convert juvenile blood samples into FLU values were: age <4 weeks, FLU = exp (1.117769 + 0.441887 * ln(Hgjuv)), r 2 = 0.58, 95% CL: 0.324–0.571; age from 4 to 6 weeks, FLU = exp (1.818148 + 0.752218 * ln(Hgjuv)), r 2 = 0.52, 95% CL: 0.568–0.976.
We also used the bioaccumulation factor (BAF) to represent the magnitude of Hg contamination in biota. BAF was expressed as the log10 ratio of MeHg concentration in biota to MeHg in lake water (Dittman and Driscoll 2009). It was assumed that THg and MeHg concentrations are equivalent in crayfish, prey fish, fish and loon samples (crayfish: Pennuto et al. 2005; fish: Watras and Bloom 1992; Lasorsa and Allen-Gil 1995; loon: Driscoll et al. 2007c).
We used the Statistic Analysis System (SAS 9.1.3, SAS Institute Inc., Cary, NC) software to perform data analysis. To investigate the relationships across trophic levels from lake-to-lake, we pooled data sets according to species and lakes using the SAS Analyst and SAS PROC MERGE tools. We explored the inter-correlations of the lake chemistry parameters with SAS VARCLUS. We analyzed the relationships of Hg concentrations in biota and various physicochemical, biological and spatial factors using SAS PROC CORR. We conducted multiple regressions to predict biota Hg concentrations from lake water chemistry and related biology parameters using SAS PROC REG tools with both entry and leaving levels at 0.15. Also, we used analysis of variance (SAS PROC GLM) and Tukey’s multiple comparisons to compare Hg concentrations in biota.
We used Geographic Information System (ESRI ArcGIS 9.3) software to analyze the spatial patterns of Hg concentrations. We obtained both the digital elevation model (DEM) and National Hydrography Dataset (NHD) of the Adirondack Park from the United States Geological Survey (USGS). We delineated the watersheds for the study lakes via Arc Hydro 1.3 tools based on these datasets. We determined the area percentages of land cover classes for each watershed using the National Land Cover Dataset for 2001 (NLCD, Fry et al. 2009). The atmospheric Hg deposition data were obtained from the spatial maps created by Miller et al. (2005).
A key limitation of this synoptic study is the single collection of Hg samples for each lake. An inherent assumption in our approach is that samples collected during a single summer collection are representative of annual conditions in water, littoral sediments and across the aquatic food chain. Investigations have noted marked seasonal and spatial variations in water column Hg concentrations (Selvendiran et al. 2009), and seasonal variations in zooplankton (Slotton et al. 1995). However, multiple collections for each site were beyond the scope of this synoptic survey.
Results and discussion
General lake water chemistry
The watershed and water chemistry characteristics of each study lake are summarized in Table 1. Most of the Adirondack lakes studied were characterized by relatively low-DOC (35 out of 44 lakes <5 mg C L−1). The lakes were largely soft water, with a mean pH of 6.5 (range: 5.3–7.8) and a mean ANC of 105 μeq L−1 (range: 4–331 μeq L−1). Most of the study lakes were oligotrophic, with low concentrations of total P (mean: 2.6 μg L−1, range: 0–6.0 μg L−1) and chlorophyll a (2.8 μg L−1, 0.2–15.7 μg/L).
Table 1.
Lake name, ID, mean major chemistry and lake-watershed characteristics, and location of the 44 study lakes in Adirondacks, the lake IDs correspond to lakes shown in Fig. 1
| Basin | Lake | ID | pH | DOC | ANC | Chl-a | Elev | Area | Latitude | Longitude |
|---|---|---|---|---|---|---|---|---|---|---|
| mg C L−1 | μeq L−1 | μg L−1 | m | ha | ||||||
| Oswegatchie/Black | South Lake | 1 | 5.7 | 2.6 | 12.7 | 1.1 | 563 | 191.5 | 43.50583 | −74.8728 |
| North Lake | 2 | 5.26 | 6.0 | 9.7 | 0.3 | 544 | 263.2 | 43.52033 | −74.9422 | |
| Squaw Lake | 3 | 5.95 | 3.6 | 21.0 | 1.4 | 619 | 22.5 | 43.635 | −74.7367 | |
| Nicks Lake | 4 | 7 | 4.3 | 141.6 | 3.3 | 538 | 55.0 | 43.6695 | −74.9908 | |
| Limekiln Lake | 5 | 6.39 | 2.3 | 38.7 | 0.8 | 511 | 2840.3 | 43.708 | −74.8078 | |
| Seventh Lake | 6 | 7.08 | 4.2 | 188.6 | 0.9 | 563 | 94.0 | 43.742 | −74.759 | |
| Little Safford Lake | 7 | 5.6 | 8.4 | 18.3 | 2.5 | 519 | 18.6 | 43.75667 | −74.9533 | |
| Moss Lake | 8 | 6.6 | 4.4 | 86.0 | 1.9 | 533 | 199.8 | 43.77533 | −74.8518 | |
| Big Moose Lake | 9 | 5.29 | 2.5 | 12.6 | 1.4 | 443 | 0.2 | 43.81733 | −74.8532 | |
| Beaver Lake | 10 | 6.17 | 4.3 | 40.1 | 0.7 | 435 | 261.9 | 43.87617 | −75.157 | |
| Moshier Reservoir | 11 | 6.02 | 4.2 | 21.8 | 0.9 | 531 | 129.6 | 43.88517 | −75.1053 | |
| Newton Falls | 12 | 6.83 | 5.3 | 116.2 | 15.7 | 536 | 556.4 | 44.2085 | −74.9853 | |
| Chaumont Pond | 13 | 6.78 | 4.5 | 119.0 | 2.2 | 469 | 432.2 | 44.205 | −74.942 | |
| Cranberry Lake | 14 | 6.57 | 4.2 | 64.4 | 2.3 | 470 | 154.0 | 44.21917 | −74.8422 | |
| St. Lawrence | South Pond | 15 | 5.99 | 3.7 | 16.1 | 0.8 | 568 | 215.9 | 43.92383 | −74.4528 |
| Round Lake | 16 | 6.74 | 6.7 | 85.6 | 3.1 | 556 | 135.3 | 44.085 | −74.575 | |
| Lows Lake | 17 | 6.55 | 4.5 | 67.2 | 6.6 | 524 | 1344.9 | 44.086 | −74.7415 | |
| Hitchins Pond | 18 | 6.46 | 4.5 | 79.1 | 2.3 | 500 | 164.7 | 44.10817 | −74.6542 | |
| Horseshoe Lake | 19 | 6.56 | 4.6 | 261.9 | 3.1 | 503 | 152.6 | 44.125 | −74.6243 | |
| Piercefield Flow | 20 | 6.77 | 6.2 | 87.1 | 0.9 | 544 | 48.6 | 44.23333 | −74.5597 | |
| Massawepie Lake | 21 | 7.19 | 3.7 | 235.7 | 2.2 | 526 | 179.0 | 44.25 | −74.6575 | |
| Spitfire Lake | 22 | 7.21 | 2.8 | 176.1 | 2.3 | 576 | 36.4 | 44.4205 | −74.2545 | |
| Clear Pond | 23 | 5.67 | 2.1 | 8.3 | 1.2 | 469 | 159.3 | 44.584 | −74.2837 | |
| Lake Champlain | Kushaqua Lake | 24 | 7.39 | 5.8 | 331.5 | 7.2 | 509 | 66.5 | 44.52317 | −74.1022 |
| Taylor Pond | 25 | 7.1 | 3.3 | 162.7 | 1.7 | 641 | 29.5 | 44.48933 | −73.8202 | |
| Little Clear Pond | 26 | 7.26 | 1.6 | 250.1 | 2.5 | 512 | 51.0 | 44.35317 | −74.2848 | |
| Long Pond | 27 | 7.82 | 3.7 | 105.7 | 4.7 | 523 | 1063.5 | 44.34167 | −74.4005 | |
| East Pine Pond | 28 | 6.9 | 3.4 | 163.6 | 5.6 | 487 | 692.4 | 44.33717 | −74.4073 | |
| Dry Channel Pond | 29 | 5.74 | 3.7 | 20.4 | 2 | 476 | 27.3 | 44.34233 | −74.442 | |
| Middle Saranac Lake | 30 | 6.96 | 3.9 | 159.0 | 1.6 | 531 | 40.6 | 44.25883 | −74.2397 | |
| Upper Hudson | Henderson Lake | 31 | 6.26 | 2.7 | 30.8 | 1 | 496 | 0.7 | 44.0885 | −74.0555 |
| Wolf Pond | 32 | 6.93 | 3.0 | 85.9 | 0.3 | 645 | 54.7 | 44.024 | −74.2192 | |
| Arbutus Lake | 33 | 6.72 | 4.1 | 72.5 | 1 | 434 | 44.5 | 43.97633 | −74.2348 | |
| Woodruff Lake | 34 | 7.52 | 6.6 | 283.2 | 12.4 | 653 | 431.7 | 43.95917 | −74.1432 | |
| Lake Durant | 35 | 6.71 | 6.8 | 102.9 | 1.8 | 484 | 104.9 | 43.83733 | −74.3843 | |
| Lake Abanankee | 36 | 6.75 | 3.1 | 106.6 | 2 | 429 | 41.4 | 43.79183 | −74.2245 | |
| Cedar River Flow | 37 | 6.87 | 3.9 | 110.2 | 3.4 | 461 | 358.5 | 43.7225 | −74.47 | |
| Mason Lake | 38 | 6.94 | 3.3 | 172.2 | 2.4 | 525 | 191.0 | 43.58867 | −74.4233 | |
| Garnet Lake | 39 | 7.09 | 3.8 | 203.9 | 4.4 | 494 | 115.2 | 43.53617 | −74.008 | |
| Piseco Lake-Big Bay | 40 | 5.6 | 3.7 | 101.1 | 1.5 | 547 | 199.1 | 43.37533 | −74.5405 | |
| Private Lake #1 | 41 | 6.18 | 6.0 | 55.6 | 3.7 | 555 | 1.2 | 43.372 | −74.6187 | |
| Mohawk | G Lake | 42 | 6.18 | 2.1 | 23.8 | 1 | 491 | 188.2 | 43.4175 | −74.635 |
| Ferris Lake | 43 | 5.94 | 4.3 | 32.0 | 1.6 | 488 | 20.0 | 43.30017 | −74.6335 | |
| Canada Lake | 44 | 6.57 | 2.4 | 58.7 | 0.9 | 450 | 3.7 | 43.15817 | −74.5378 |
Hg concentrations in the Adirondack Park
Water
Concentrations of THg in lake water had a mean of 1.73 ng L−1 and ranged from 0.10 (Clear Pond) to 4.96 ng L−1 (North Lake). Concentrations of MeHg in lake water had a mean of 0.096 ng L−1 and ranged from <0.002 (method detection limit, MDL) to 0.48 ng L−1 (Dry Channel Pond). The fraction of THg occurring as MeHg (% MeHg/THg) had a mean of 6% and ranged from 0 to 48% (Dry Channel Pond). A weak relationship was found between MeHg and THg in water (r 2 = 0.13, p = 0.02). The individual average Hg concentrations in water, sediment and biota of each lake are presented in a table in the supplementary material (electronic version only). Estimated total atmospheric Hg deposition was weakly correlated with Hg concentrations in lake water (r 2 = 0.10, p = 0.03). Although some study lakes are in close proximity, concentrations of THg and MeHg were highly variable. Hg concentrations in lake water were similar to those reported by other studies for the same region (THg: 0.56–5.07 ng L−1, MeHg: 0.03–0.60 ng L−1, Dittman and Driscoll 2009; THg: 1.36–7.01 ng L−1, MeHg: 0.03–0.96 ng L−1, Dennis et al. 2005; MeHg: 0.1–0.4 ng L−1, Driscoll et al. 1998).
We conducted SAS VARCLUS procedure to address the inter-correlations of lake water chemistry measurements. We found 3 clusters of variables which explained 48% of the variability: cluster 1, pH, DIC, ANC, Si, chlorophyll a, Na, Ca, Mg, Cl, NO3; cluster 2, DOC, THg, MeHg, SO4, Alm and Alo; and cluster 3, NH4, total P, K, F. Parameters in cluster 2 were found to be correlated with THg and MeHg concentrations in lake water. DOC was slightly positively correlated with THg (r 2 = 0.18, p = 0.002) but not with MeHg in lake water. Both Alm and Alo were positively related to THg (Alm: r 2 = 0.32, p < 0.0001; Alo: r 2 = 0.34, p < 0.0001). Neither THg nor MeHg in water were correlated with pH.
Sediments
THg concentrations in littoral sediments (dw) had a mean of 17.2 ng g−1 and ranged from 1.7 (Moss Lake) to 88.1 ng g−1 (Mason Lake). MeHg concentrations in littoral sediments had a mean of 0.36 ng g−1 and ranged from <0.002 (MDL, Lake Abanakee) to 3.63 ng g−1 (Mason Lake). Mean % MeHg/THg in littoral sediments was 2% and ranged from 0 (Lake Abanakee) to 16% (Middle Saranac Lake). In contrast to our results for lake water, sediments MeHg concentrations were strongly correlated with THg concentrations (r 2 = 0.57, p < 0.0001, n = 29). We found significant positive correlations between percent organic carbon in littoral sediments with THg (r 2 = 0.92, n = 44), MeHg (r 2 = 0.90, n = 40) and %MeHg/THg (r 2 = 0.59, n = 40, all p values < 0.0001).
Lower food web
THg concentrations in zooplankton (dw) had a mean of 0.31 μg g−1 and ranged from 0.007 (Canada Lake) to 0.89 μg g−1 (North Lake). MeHg concentrations in zooplankton had a mean of 0.07 μg g−1 (dw) and ranged from 0.0007 (Canada Lake) to 0.25 μg g−1 (North Lake). In zooplankton, % MeHg/THg had a mean of 24%, and ranged from 0 (Canada Lake) to 74% (Squaw Lake). THg and MeHg concentrations in zooplankton were positively correlated (r 2 = 0.32, p = 0.0002). There were no significant differences in the mean whole body THg concentrations (mean: 0.05 μg g−1, range: 0.01–0.14 μg g−1, p = 0.44) for the four crayfish species (Orconectes limosus, O. robustus, Procambarus acutus and O. propinquus), nor for whole body THg (mean: 0.05 μg g−1) and tail THg concentrations (mean: 0.06 μg g−1). Crayfish whole body THg concentrations were significantly correlated (r 2 = 0.94, p < 0.0001, n = 39) with tail THg concentrations. Prey fish Hg concentrations (mean, range) were: banded killifish (0.07, 0.04–0.11 μg g−1), golden shiner (0.10, 0.07–0.14 μg g−1), and creek chub (0.11, 0.05–0.15 μg g−1).
Upper food web
Fish total Hg concentrations (mean, range) were: pumpkinseed sunfish (0.10, 0.03–0.19 μg g−1), brown bullhead (0.10, 0.07–0.14 μg g−1), smallmouth bass (0.11, 0.04–0.33 μg g−1), largemouth bass (0.12, 0.04–0.23 μg g−1), and yellow perch (0.16, 0.04–0.46 μg g−1, Fig. 2). Hg concentrations in 7% of all fish and 12% of yellow perch equivalent samples exceeded the EPA tissue criterion for MeHg in fish (0.3 μg g−1). Ten of the 44 lakes (23%) had at least one fish sample with Hg concentration above 0.3 μg g−1 (Fig. 3), and 5 of those 10 lakes were not currently listed on the New York State fish consumption advisory. Note that 28 (64%), 21 (48%) and 4 (9%) of the 44 lakes had at least one fish (YPE) in excess of the 0.16 μg g−1, 0.21 μg g−1, and 0.42 μg g−1 Hg threshold values, respectively, for adverse effects on common loon health (Fig. 3). There was a high density of lakes with elevated Hg concentrations in zooplankton and fish (YPE) in the southwestern part of our study area (Fig. 4). The study conducted by Driscoll et al. (1991) showed a large number of lakes acidified by acidic deposition in the southwestern Adirondacks (Fig. 4).
Fig. 2.
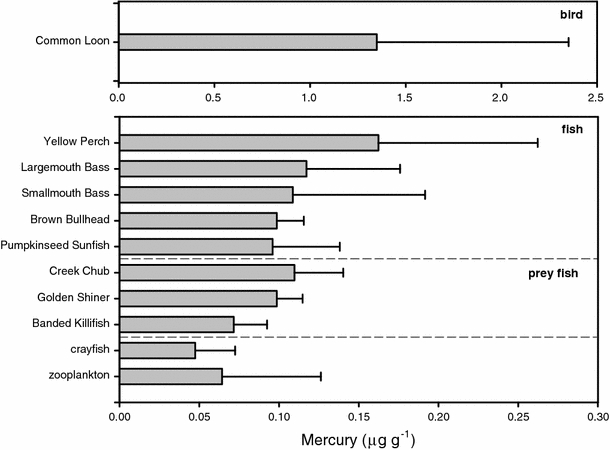
Mercury concentrations (mean ± standard deviation) in biota, whole body, except for zooplankton (dry weight) all samples were based on wet weight
Fig. 3.
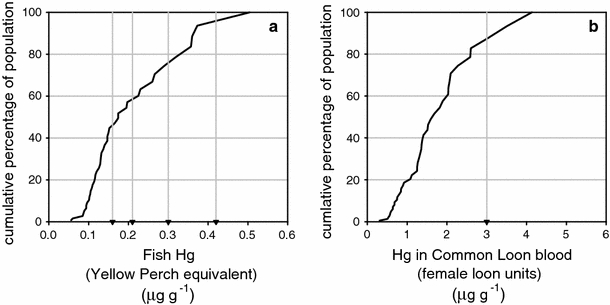
Mean Hg concentrations in fish (whole body, yellow perch equivalent values, μg g−1, ww; a and common loons (blood, female loon unit values, μg g−1; b for the cumulative distribution of lakes sampled. The gray vertical lines in the fish population plot a represent the threshold values of 0.16, 0.21 and 0.42 μg g−1 for adverse effects on common loon and 0.30 μg g−1 for the USEPA fish consumption advisory criterion. The gray vertical line in the loon population plot b represents the threshold concentration of 3.0 μg g−1 for the health concern criterion for the common loon
Fig. 4.
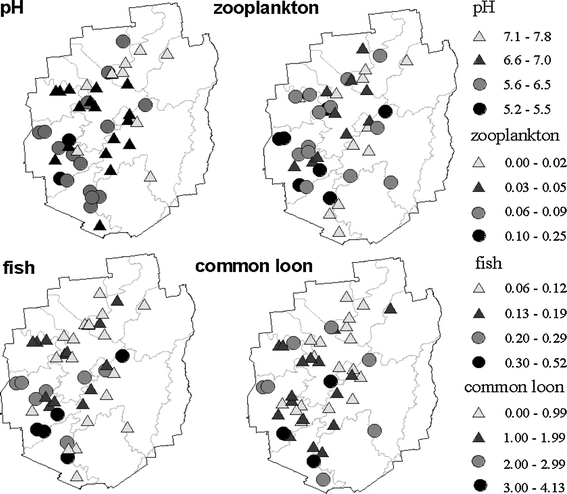
Spatial distributions of pH in lake surface water and Hg concentrations in biota in the 44 Adirondack lakes, μg g−1, i.e. zooplankton (MeHg), crayfish (THg), fish (yellow perch equivalent, YPE), and common loon (blood, female loon unit, FLU)
Total Hg concentrations in common loon blood (FLU) had a mean of 1.34 μg g−1, and ranged from 0.36 (Clear Pond) to 5.87 μg g−1 (Ferris Lake). Hg concentrations in 9% of all the loon blood samples (FLU) were greater than 3.0 μg g−1, while 13 of the study lakes (30%) had at least one loon blood sample with a Hg concentration greater than 3.0 μg g−1. Like zooplankton and fish, the spatial distribution patterns for FLU Hg concentrations in the common loon population showed an abundance of lakes with elevated concentrations in the southwestern Adirondacks (Fig. 4). Overall, Hg concentrations increased across the aquatic food web in the order of crayfish, zooplankton, prey fish, fish and common loon (Fig. 2).
Factors affecting Hg bioaccumulation
Lake chemistry effects
Hg concentrations in aquatic biota were strongly influenced by lake water chemistry. Since collinearity was detected for the lake water chemistry variables in the cluster analysis, linear multiple regressions were used to predict biotic Hg concentrations from lake water chemistry variables. For zooplankton, the models obtained were: MeHgzooplankton = 0.2933 − 0.04068 pH + 0.05084 Alo (p values for each of the estimated parameters were: 0.02, 0.02, and 0.04, respectively; r 2 = 0.51, n = 33), and THgzooplankton = −0.55601 + 0.32578 Alo + 0.00114 elevation (p values: 0.03, <0.0001, and 0.01, respectively; r 2 = 0.47, n = 38). For fish, the model obtained was: HgYPE = 0.28 − 0.019 DOC − 0.00056 ANC + 0.066 Alm − 0.28 MeHg/THg (p values: 0.0045, <0.0001, <0.0001 and 0.0008, respectively; r 2 = 0.41, n = 112). While the regression models did not work well in predicting crayfish and loon blood Hg, we did find a slight correlation between loon blood Hg concentrations with lake water pH (r 2 = 0.17, p = 0.0004, n = 241). We found no significant relationships between Hg concentrations in crayfish and Hg concentrations in littoral sediments. Our analysis suggests that the acid–base status of lake water (pH, ANC and Al) is particularly related to MeHg accumulation in aquatic biota.
Although many studies have reported the important influence of the acid-base status on Hg in aquatic biota, the mechanism contributing to this pattern remains poorly defined. As our and other observations (e.g., Driscoll et al. 1994; Dittman and Driscoll 2009) show, surface water concentrations of THg and MeHg do not vary systematically with pH. Thus, the correlation between lake pH and Hg concentrations in biota may be due to the influence of acidity on the assimilation of MeHg at the bottom of the food web and/or trophic transfer up the food web (Wyn et al. 2009).
The patterns of Hg in biota with ANC may be particularly relevant to air and water quality managers. Although there is variability in our observations, zooplankton, fish and loons all showed exponential increases in Hg concentrations with decreases in ANC (Fig. 5). The highest Hg concentrations in biota occurred in low ANC lakes that are likely to be severely impacted by acidic deposition (Driscoll et al. 2007c) and the biotic Hg concentrations decreased markedly with slight increases in ANC from these low values. This spatial pattern suggests potential interactions of acidic deposition and Hg contamination. Modest increases in ANC, which have been observed in low ANC lakes following atmospheric emission controls of sulfur dioxide and nitrogen oxides (Driscoll et al. 2007b) will likely have the co-benefit of decreasing Hg concentrations in biota.
Fig. 5.
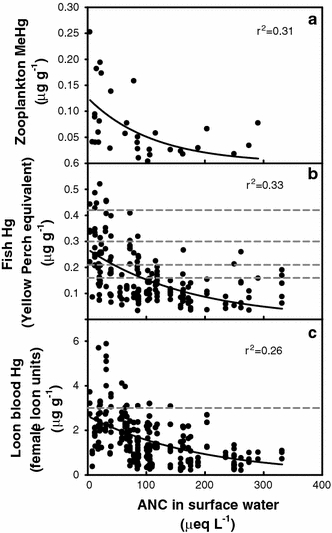
Relationships between acid neutralizing capacity (ANC) and Hg concentrations in zooplankton (a), fish (b), and common loons (c). The exponential decay models fitted were: y = 0.126e−0.009x (a), y = 0.2698e−0.0057x (b), and y = 2.644e−0.0052x (c), respectively. The p values for the three models are 0.002, <0.0001 and <0.0001, respectively. The dashed lines of 0.16, 0.21, 0.3, and 0.42 μg g−1 in plot b, and 3 μg g−1 in plot c represent the same criteria values as in Fig. 3
The strong linkage of Hg with Al is also interesting and has been previously reported (Driscoll et al. 1994), although the operating mechanism is also not clear. Elevated concentrations of Al are generally coincident with low pH values. Driscoll et al. (1994) speculated that Al competed with Hg in binding to the functional sites of DOC, and elevated concentrations of Al would, therefore, increase the bioavailability of Hg. Adams et al. (2009) suggested that elevated Al contributed to nutrient limitations of aquatic productivity of Adirondack lakes and as a result enhanced the bioconcentration of Hg at the base of the food web.
Biologic effects
Generally, MeHg BAFs increased in the order of crayfish (5.7), zooplankton (5.9), prey fish (6.2), fish (6.3) and common loons (7.2). The values of MeHg BAFs found for this study were consistent with those reported in the literature (crayfish, Alpers et al. 2008; zooplankton, Back and Watras 1995; Pickhardt et al. 2005; fish, Driscoll et al. 1994; loons, Evers et al. 2004). Zooplanktons are near the bottom of the aquatic food chain, but their MeHg BAF values are relatively high in view of low trophic position (Driscoll et al. 2007c). These relatively large MeHg BAF values demonstrate generally the importance of the lower food web in setting the magnitude of Hg concentration for higher trophic levels and ultimately controlling exposure to wildlife and human (Driscoll et al. 1994; Kamman et al. 2005). Lake chemistry parameters were more strongly related to Hg concentrations in zooplankton than Hg concentrations in any other biota. Crayfish, one of the largest benthic invertebrates, are an important food source for other organisms, such as predatory fish (yellow perch) and fish-eating birds (common loon) (Pennuto et al. 2005).
The values of fish Hg concentrations were within ranges found in other studies for Adirondack lakes (0.09–1.11 μg g−1, Dittman and Driscoll 2009; 0.001–3.24 μg g−1, Simonin et al. 2008) and water bodies of the northeastern U.S. (Kamman et al. 2005). As observed in many previous studies, fish Hg concentrations increased with fish length. Simonin et al. (2008) found the relationships between Hg concentrations and fish length were more significant (r 2 ranged from 0.76 to 0.87, varied with fish species) for individual lakes than for groups of lakes.
Although we did not find statistically significant differences in blood Hg concentrations in female (mean: 1.48 μg g−1, range: 0.43–5.87 μg g−1) and male loons (1.95, 0.62–3.85 μg g−1), male body burdens exceeded females, and blood Hg concentrations in adult loons (1.71, 0.43–5.87 μg g−1) were significantly higher than chicks (0.26, 0.06–0.82 μg g−1). These patterns follow other studies (Evers et al. 1998; Meyer et al. 1998; Scheuhammer et al. 1998; Rimmer et al. 2010). Hg concentrations in crayfish were positively correlated with Hg concentrations in zooplankton (THg, r 2 = 0.44, p = 0.004, n = 40), fish (YPE, r 2 = 0.14, p < 0.0001, n = 101) and loons (FLU, r 2 = 0.41, p < 0.0001, n = 132). Using linear multiple regression, the equation obtained to predict Hg in loon blood from Hg in yellow perch and zooplankton was: HgFLU = 0.69565 + 3.407 Hgyellow perch + 4.38355 Hgzooplankton (p values: 0.0004, 0.01 and 0.03, respectively; r 2 = 0.19, n = 105). The relationships among Hg concentrations in various taxa of biota reflect the common Hg transfer mechanism(s) along the aquatic food chain and to larger piscivorous animals. Variations of Hg concentrations for similar species (zooplankton, crayfish, fish, and loons) in similar habitats and areas are likely due to food web complexity and dietary patterns.
Spatial landscape effects
The variability of Hg concentrations in biota for nearby lakes indicated that landscape factors affect Hg bioaccumulation in addition to lake chemistry and biology. We hypothesized that landscape characteristics would influence lake physicochemical properties, Hg inputs to lakes and the feeding habitat of biota, and therefore, affect the magnitude of Hg contamination. Most of the land cover area for the watersheds of the study lakes is deciduous forest, as deciduous forest occupies 54%, coniferous forest 14%, mixed forest 6%, woody wetlands 11%, and open water 12%, respectively, of the total area of the watersheds to the study lakes. No significant relationships were found between the percentages of land cover class and lake water chemistry parameters, or Hg concentrations in lake water or aquatic biota. We only found a slight correlation between the percentages of watershed area as mixed forest and Hg concentrations in common loons (FLU, r 2 = 0.10, p < 0.0001). Note the land cover classes of the study lakes did not vary substantially as the dominant land cover is forest. Relationships may have been evident if there was more variation in watershed land cover classes. For example, Kramar et al. (2005) reported a stronger relationship (r 2 = 0.55, p < 0.0001) between Hg concentrations in common loon and land cover classes, (i.e., crop land, shrub land, wetlands) for the major lakes and eleven smaller ponds in northwest Maine.
The elevation in Adirondack Park is generally high along a southwest-northwest transect. The elevation range for the Park is 23–1625 m, while the study lakes range from 429 to 623 m. Both pH (r 2 = 0.11, p = 0.03) and ANC (r 2 = 0.09, p = 0.05) were weakly negatively related with lake elevation. Although we did not find relationships between elevation and Hg concentrations in zooplankton, crayfish, fish and loons, we did find slight positive correlations between elevation and MeHg BAF in zooplankton (r 2 = 0.15, p = 0.01, n = 28), crayfish (r 2 = 0.21, p < 0.0001, n = 24) and fish (r 2 = 0.14, p < 0.0001, n = 62). The mechanism for the elevation effect on Hg bioaccumulation in aquatic ecosystems is not clear. High elevation lakes are likely to receive greater atmospheric Hg deposition (Miller et al. 2005) resulting in newly deposited Hg that is likely more available to biota. Furthermore, high elevation lakes receive greater inputs of acidic deposition and have shallow soils and surficial deposits which make them more sensitive to surface water acidification (Driscoll et al. 1991; Ito et al. 2002). We did not note a relationship between THg or MeHg in lake water and elevation. This lack of association suggests that the spatial patterns of Hg in biota with elevation may be driven more by the influence of lake pH on MeHg bioavailability and/or trophic transfer.
Summary
The spatial distribution patterns of Hg concentrations in lake water, littoral sediments, zooplankton, crayfish, fish and common loon showed considerable variation across the 44 Adirondack lakes. Consistent with previous studies, Hg strongly bioaccumulated in zooplankton, fish and common loon blood. Our spatial analysis indicates that lakes with the highest concentrations of Hg in aquatic biota are generally located in the southwestern portion of the Adirondack Park, where there is a high density of lakes that have been acidified by acidic deposition (Fig. 4; Driscoll et al. 1991). Many of the lakes studied have Hg concentrations in fish and common loon blood which exceed criteria established for the protection of human and wildlife health. We observed a marked increase in the Hg concentration in biota with decreases in lake pH and ANC. Modest increases in ANC in low ANC lakes (associated with atmospheric emission controls and decreased in acidic deposition) would likely have the co-benefit of decreasing Hg contaminations in biota.
The Adirondacks is a region characterized by relatively high concentrations of Hg in aquatic biota (Driscoll et al. 1994; Evers et al. 2007). Several factors undoubtedly contribute to this pattern. Watersheds of the Adirondacks are largely forested. Forests greatly enhance atmospheric deposition of Hg due to the scavenging of gaseous and particulate Hg by the forest canopy. There are an abundance of wetlands in Adirondack watersheds which are important in the transport of Hg to downstream lakes and the production of MeHg (Selvendiran et al. 2008). Adirondack lakes are relatively unproductive, a condition which enhances bioconcentration of Hg (Chen and Folt 2005). Our analyses show that Hg concentrations in fish and common loon are directly related to MeHg concentrations in the lower food web. The most distinct factors influencing biotic Hg concentrations in this study were lake pH and ANC. Many Adirondack lakes are naturally acidic, but have also been acidified by elevated inputs of acidic deposition. It would appear that the status of the Adirondacks as a biological Hg hotspot is due to its landscape characteristics which make the region sensitive to moderate inputs of Hg but also ongoing effects of acidic deposition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1 Mean concentrations of THg and MeHg in water, sediment (dry weight) and zooplankton (dry weight), and mean concentrations of THg in whole body and tail of crayfish, mean and standard deviation of fish (YPE) and loon (FLU) Hg concentrations. Quality control and quality assurance (QA/QC) procedures (PDF 46 kb)
Acknowledgments
The New York State Energy Research and Development Authority, Wildlife Conservation Society, Natural History Museum of the Adirondacks, New York State Department of Environmental Conservation, and the Audubon Society of New York provided support and in-kind assistance for this project. We thank M. Watson and G. Lampman of the New York State Energy Research and Development Authority; K. Roy and S. Capone of the Adirondack Lakes Survey Corporation; E. Osmancevic of the Center for Environmental Systems Engineering Laboratory at Syracuse University; J. Ozard, J. Loukmas, J. Sutherland, D. Adams, B. Bauer, D. Bloomquist, and T. Gudlewski of New York State Department of Environmental Conservation; A. Sauer, G. Lee and the loon monitoring and banding field staff of the former Adirondack Cooperative Loon Program; and D. Pepin formerly of BioDiversity Research Institute.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adams RM, Twiss MR, Driscoll CT. Patterns of mercury accumulation among seston in lakes of the Adirondack Mountains, New York. Environ Sci Technol. 2009;43:4836–4842. doi: 10.1021/es900409b. [DOI] [PubMed] [Google Scholar]
- Alpers CN, Stewart AR, Saiki MK, Marvin-DiPasquale MC, Topping BR, Rider KM, Gallanthine SK, Kester CA, Rye RO, Antweiler RC, De Wild JF (2008) Environmental factors affecting mercury in Camp Far West Reservoir, California, 2001–03. U.S. Geological Survey Scientific Investigations Report 2006-5008
- APHA/AWWA/WEF . Standard methods for the examination of water and wastewater. 20. Washington, DC: American Public Health Association, American Water Works Association, and Water Environment Federation; 1998. [Google Scholar]
- Back RC, Watras CJ. Mercury in zooplankton of Northern Wisconsin Lakes: taxonomic and site-specific trends. Water Air Soil Pollut. 1995;80:931–938. doi: 10.1007/BF01189747. [DOI] [Google Scholar]
- Bookman R, Driscoll CT, Engstrom DR, Effler SW. Local to regional emission sources affecting mercury fluxes to New York lakes. Atmos Environ. 2008;42:6088–6097. doi: 10.1016/j.atmosenv.2008.03.045. [DOI] [Google Scholar]
- Burgess NM, Meyer MW. Methylmercury exposure associated with reduced productivity in common loons. Ecotoxicology. 2008;17:83–91. doi: 10.1007/s10646-007-0167-8. [DOI] [PubMed] [Google Scholar]
- Bushey JT, Driscoll CT, Mitchell MJ, Selvendiran P, Montesdeoca MR. Mercury transport in response to storm events from a northern forest landscape. Hydrol Process. 2008;22:4813–4826. doi: 10.1002/hyp.7091. [DOI] [Google Scholar]
- Chen CY, Folt CL. High plankton densities reduce mercury biomagnification. Environ Sci Technol. 2005;39:115–121. doi: 10.1021/es0403007. [DOI] [PubMed] [Google Scholar]
- Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast US. Ecotoxicology. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- Dennis IF, Clair TA, Driscoll CT, Kamman N, Chalmers A, Shanley J, Norton SA, Kahl S. Distribution patterns of mercury in lakes and rivers of Northeastern North America. Ecotoxicology. 2005;14:113–123. doi: 10.1007/s10646-004-6263-0. [DOI] [PubMed] [Google Scholar]
- Dittman JA, Driscoll CT. Factors influencing changes in mercury concentrations in lake water and yellow perch (Perca flavescens) in Adirondack lakes. Biogeochemistry. 2009;93:179–196. doi: 10.1007/s10533-009-9289-9. [DOI] [Google Scholar]
- Dittman JA, Shanley JB, Driscoll CT, Aiken GR, Chalmers AT, Towse JE. Ultraviolet absorbance as a proxy for total dissolved mercury in streams. Environ Pollut. 2009;157:1953–1956. doi: 10.1016/j.envpol.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Newton RM, Gubala CE, Baker JP, Christensen S. Adirondack mountains. In: Charles DE, editor. Acidic deposition and aquatic ecosystems: regional case studies. New York: Springer; 1991. p. 133. [Google Scholar]
- Driscoll CT, Yan C, Schofield CL, Munson R, Holsapple J. The mercury cycle and fish in the Adirondack lakes. Environ Sci Technol. 1994;28(3):136A–143A. doi: 10.1021/es00052a003. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Holsapple J, Schofield CL, Munson R. The chemistry and transport of mercury in a small wetland in the Adirondack region of New York, USA. Biogeochemistry. 1998;40:137–146. doi: 10.1023/A:1005989229089. [DOI] [Google Scholar]
- Driscoll CT, Abbott ML, Bullock R, Jansen J, Leonard D, Lindberg SE, Munthe J, Pirrone N, Nilles M (2007a) Airsheds and watersheds. In: Harris R, Krabbenhoft DP, Mason R, Murray MW, Reash R, Saltman T (eds) Ecosystem responses to mercury contamination: indicators of change. SETAC, CRC Press, Boca Raton, FL
- Driscoll CT, Driscoll KM, Roy KM, Dukett J. Changes in the chemistry of lakes in the Adirondack region of New York following declines in acidic deposition. Appl Geochem. 2007;22:1181–1188. doi: 10.1016/j.apgeochem.2007.03.009. [DOI] [Google Scholar]
- Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience. 2007;57:17–28. doi: 10.1641/B570106. [DOI] [Google Scholar]
- EPA . Method 1630: methyl mercury in water by distillation, aqueous ethylation, purge and trap, and CVAFS (EPA-821-R-01–020, January 2001). Office of water, office of science and technology, engineering and analysis division 4303. Washington, DC: US Environmental Protection Agency; 2001. [Google Scholar]
- EPA . Method 1631, revision E: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry (EPA-821-R-01-019, August 2002). Office of water, office of science and technology, engineering and analysis division 4303. Washington, DC: US Environmental Protection Agency; 2002. [Google Scholar]
- Evers DC, Kaplan JD, Meyer MW, Reaman PS, Major A, Burgess N, Braselton WE. Bioavailability of environmental mercury measured in Common Loon feathers and blood across North American. Environ Tox Chem. 1998;17:173–183. doi: 10.1002/etc.5620170206. [DOI] [Google Scholar]
- Evers DC, Taylor KM, Major A, Taylor RJ, Poppenga RH, Scheuhammer AM. Common loon eggs as indicators of methylmercury availability in North America. Ecotoxicology. 2003;12:69–81. doi: 10.1023/A:1022593030009. [DOI] [PubMed] [Google Scholar]
- Evers DC, Lane OP, Savoy L, Goodale W (2004) Assessing the impacts of methylmercury on piscivorous wildlife using a wildlife criterion value based on the Common Loon, 1998–2003. Report BRI 2004–05 submitted to the Maine Department of Environmental Protection. BioDiversity Research Institute, Gorham, Maine
- Evers DC, Han YJ, Driscoll CT, Kamman NC, Gooodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T. Biological mercury hotspots in the Northeastern United States and Southeastern Canada. Bioscience. 2007;57:29–43. doi: 10.1641/B570107. [DOI] [Google Scholar]
- Evers DC, Savoy LJ, DeSorbo CR, Yates DE, Hanson W, Taylor KM, Siegel LS, Cooley JH, Jr, Bank MS, Major A, Munney K, Mower BF, Vogel HS, Schoch N, Pokras M, Goodale JF. Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology. 2008;17:69–81. doi: 10.1007/s10646-007-0168-7. [DOI] [PubMed] [Google Scholar]
- Fry JA, Coan MJ, Homer CG, Meyer DK, Wickham JD (2009) Completion of the National Land Cover Database (NLCD) 1992-2001 land cover change retrofit product. U.S. Geological Survey open-file report 2008-1379
- George BM, Batzer D. Spatial and temporal variations of mercury concentrations in Okefenokee invertebrates: Southeast Georgia. Environ Pollut. 2008;152:484–490. doi: 10.1016/j.envpol.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Hurley JP, Benoit JM, Babiarz CL, Shafer MM, Andren AW, Sullivan JR, Hammond R, Webb DA. Influences of watershed characteristics on mercury concentrations in Wisconsin rivers. Environ Sci Technol. 1995;29:1867–1875. doi: 10.1021/es00007a026. [DOI] [PubMed] [Google Scholar]
- Ito M, Mitchell MJ, Driscoll CT. Spatial patterns of precipitation quantity and chemistry and air temperature in the Adirondack region of New York. Atmos Environ. 2002;36:1051–1062. doi: 10.1016/S1352-2310(01)00484-8. [DOI] [Google Scholar]
- Kamman NC, Lorey PM, Driscoll CT, Estabrook R, Major A, Pientka B. Assessment of mercury in waters, sediments, and biota of VT and NH lakes using a geographically randomized design. Environ Tox Chem. 2003;23:5. doi: 10.1897/03-170. [DOI] [PubMed] [Google Scholar]
- Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodale W, Linehan J, Estabrook R, Hutcheson M, Major A, Scheuhammer AM, Scruton DA. Mercury in freshwater fish of Northeast North America—a geographic perspective based on fish tissue monitoring databases. Ecotoxicology. 2005;14:163–180. doi: 10.1007/s10646-004-6267-9. [DOI] [PubMed] [Google Scholar]
- Kramar D, Goodale WM, Kennedy LM, Carstensen LW, Kaur T. Relating land cover characteristics and common loon mercury levels using geographic information systems. Ecotoxicology. 2005;14:253–262. doi: 10.1007/s10646-004-6272-z. [DOI] [PubMed] [Google Scholar]
- Lasorsa B, Allen-Gil S. The methylmercury to total mercury ratio in selected marine, freshwater, and terrestrial organisms. Water Air Soil Pollut. 1995;80:905–913. doi: 10.1007/BF01189743. [DOI] [Google Scholar]
- Mason RP, Reinfelder JR, Morel FMM. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol. 1996;30:1835–1845. doi: 10.1021/es950373d. [DOI] [Google Scholar]
- Meyer MW, Evers DC, Hartigan J. Patterns of Common Loon (Gavia immer) mercury exposure, reproduction, and survival in Wisconsin, USA. Environ Tox Chem. 1998;17:184–190. [Google Scholar]
- Miller EK, Vanarsdale A, Keeler GJ, Chalmers A, Poissant L, Kamman NC, Brulotte R. Estimation and mapping of wet and dry mercury deposition across Northeastern North America. Ecotoxicology. 2005;14:53–70. doi: 10.1007/s10646-004-6259-9. [DOI] [PubMed] [Google Scholar]
- Pennuto CM, Lane OP, Evers DC, Taylor RJ, Loukmas J. Mercury in the Northern Crayfish, Orconectes virilis (Hagen), in New England, USA. Ecotoxicology. 2005;14:149–162. doi: 10.1007/s10646-004-6266-x. [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Impacts of zooplankton composition and algal enrichment on the accumulation of mercury in an experimental freshwater food web. Sci Total Environ. 2005;339:89–101. doi: 10.1016/j.scitotenv.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Rimmer CC, Miller EK, McFarland KP, Taylor RJ, Faccio SD. Mercury bioaccumulation and trophic transfer in the terrestrial food web of a montane forest. Ecotoxicology. 2010;19:697–709. doi: 10.1007/s10646-009-0443-x. [DOI] [PubMed] [Google Scholar]
- Scheuhammer AM, Atchison CM, Wong AHK, Evers DC. Mercury exposure in breeding common loons (Gavia immer) in central Ontario, Canada. Environ Tox Chem. 1998;17(2):191–196. [Google Scholar]
- Selvendiran P, Driscoll CT, Bushey JT, Montesdeoca MR. Wetland influence on mercury fate and transport in a temperate forested watershed. Environ Pollut. 2008;154:46–55. doi: 10.1016/j.envpol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Selvendiran P, Driscoll CT, Montesdeoca MR. Mercury dynamics and transport in two Adirondack lakes. Limnol Oceanogr. 2009;54(2):413–427. doi: 10.4319/lo.2009.54.2.0413. [DOI] [Google Scholar]
- Simonin HA, Jefferey JL, Skinner LC, Roy KM. Lake variability: key factors controlling mercury concentrations in New York State fish. Environ Pollut. 2008;154:107–115. doi: 10.1016/j.envpol.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Slotton DG, Reuter J, Goldman CR. Mercury uptake patterns of biota in a seasonally anoxic northern California Reservoir. Water Air Soil Pollut. 1995;80:841–850. doi: 10.1007/BF01189735. [DOI] [Google Scholar]
- Watras CJ, Bloom NS. Mercury and methylmercury in individual zooplankton: implication for bioaccumulation. Limnol Oceanogr. 1992;37:1313–1318. doi: 10.4319/lo.1992.37.6.1313. [DOI] [Google Scholar]
- Wyn B, Kidd KA, Burgess NM, Curry RA. Mercury biomagnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site, Nova Scotia. Can J Fish Aquat Sci. 2009;66:1532–1545. doi: 10.1139/F09-097. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 Mean concentrations of THg and MeHg in water, sediment (dry weight) and zooplankton (dry weight), and mean concentrations of THg in whole body and tail of crayfish, mean and standard deviation of fish (YPE) and loon (FLU) Hg concentrations. Quality control and quality assurance (QA/QC) procedures (PDF 46 kb)


