Abstract
Our aim was to examine the association between serum dehydroepiandrosterone sulfate (DHEAS) at baseline and BMD change at the femoral neck (FN) and lumbar spine (LS) in postmenopausal women during a 15-year follow-up. All participants were from the Chingford Study. BMD at the FN and LS were measured eight times during the 15-year follow-up by dual-energy X-ray absorptiometry. DHEAS at baseline was measured using radioimmunoassay. Data on height, weight, and hormone-replacement therapy (HRT) status were obtained at each visit. Multilevel linear regression modeling was used to examine the association between longitudinal BMD change at the FN and LS and DHEAS at baseline. Postmenopausal women (n = 1,003) aged 45–68 years (mean 54.7) at baseline were included in the study. After adjustment for baseline age, estradiol, HRT, and BMI, BMD at the FN decreased on average 0.49% (95% CI 0.31–0.71%) per year; and the decline was slowed down by 0.028% per squared year. Increase of DHEAS (each micromole per liter) was associated with 0.49% less bone loss at the FN (95% CI 0.21–0.71%, P = 0.001). However, this strong association became slightly weaker over time. Similar but weaker results were obtained for LS BMD. Our data suggest that high serum DHEAS at baseline is associated with less bone loss at both FN and LS and this association diminishes over time. The nature of the association is unclear, but such an association implies that, in managing BMD loss, women might benefit from maintaining a high level of DHEAS.
Keywords: BMD, DHEAS, Osteoporosis, Longitudinal study, Postmenopausal
Osteoporosis is defined as a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with consequent increases in bone fragility and susceptibility to fractures [1, 2]. It affects 4–6 million postmenopausal white women in the United States [3], and costs US $17 billion per year in direct expenditure [4]. In the United Kingdom, osteoporosis costs the National Health Service £940 million annually [2].
Osteoporosis is defined clinically by the measurement of bone mineral density (BMD), which remains the single best predictor of primary osteoporotic fracture [5]. BMD declines with increasing age, and the rate of decline is more pronounced after menopause [6]. This decline can be attributed to a number of factors: age, genetics, estrogen deficiency, adverse lifestyle factors, or the prolonged use of certain medication [7].
Dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) are the most abundant circulating steroids. They are secreted by the adrenal glands, decline with increasing age, and have been implicated in a variety of age-related pathophysiologic conditions including osteoporosis [8]. DHEA may have a role in protecting against BMD loss. Postulated mechanisms include its conversion to androgen and estrogen, alteration in hormone–receptor sites, and regulation of enzymatic activity, all plausibly related to bone metabolism [9]. Several studies have examined the relationship between DHEA or DHEAS and osteoporosis but with conflicting results [10–15]. This discrepancy could be due to small sample size, heterogeneity of the study populations, or cross-sectional design.
We utilized a longitudinal study design to examine the association between serum DHEAS level at baseline and BMD change over a 15-year follow-up in a large population-based sample.
Subjects and Methods
Study participants were from the Chingford Study, a well-described prospective population-based longitudinal study of osteoarthritis and osteoporosis, comprising 1,003 women aged 43 years or above at entry, derived from the age/sex register of a large general practice in Chingford, north London. All the women lived within 5 miles of the general practice, 98% of the women were white, and they were predominantly middle class. Participants were similar to women in the UK general population in terms of weight, height, and smoking characteristics [16]. The study was approved by the local ethics committee, and written consent was obtained from each woman and has been described in detail previously [16, 17].
DXA Scan
BMD was measured at the FN and LS at L1–L4 by dual-energy X-ray absorptiometry (DXA; Hologic QDR 1000 for year 1 to year 3; Hologic QDR 2000 for year 4 to year 6; Hologic Delphi W for year 8 to year 10, and Hologic Discovery for year 15; Hologic, Waltham, MA). A cross-calibration was performed each time the machine was upgraded. The cross-calibration involved scanning 30 patients on the old scanner and then again on the new one on the same day. The coefficient of variation for interscanner variation was 0.05% for BMD. Intrascanner reproducibility, expressed as a coefficient of variation from duplicate measurements in healthy volunteers 1 week apart, was 1.6% at the FN and 0.8% at the LS. Quality control was performed regularly using a phantom to ensure the reliability of the densitometer. All BMD measurements were performed by a standardized protocol.
DHEAS Measurement
Serum samples were collected without any special patient preparation requirements at the first visit and stored at −45°C in a freezer until assayed. Serum DHEAS levels were measured by radioimmunoassay (RIA) from the same batch—a nonextraction, nonchromatographic method using 125I ligand as described previously [18]. The within- and between-assay precisions were 4–10% and 6–14%, respectively.
Other Measurements
At each biannual visit, height and weight were measured and body mass index (BMI, weight [kg]/height [m2]) was calculated. In addition, information on smoking (never-smokers, ex-smokers, and current smokers), physical activity (inactive, moderately inactive, moderately active, and active), diet (milk pints, ounce of cheese, yoghurt pots) per week, estradiol, polypharmacy (current medication for blood pressure, diabetes, diuretics, and thyroid), family history (arthritis at hand and knee of child, mother, father, brother, sister, aunt, and grandmother), and rheumatoid arthritis (RA) was collected at the first visit. All women completed a standardized, nurse-administered questionnaire on medical history for a number of known risk factors for osteoporosis. Medication information was detailed, particularly for those drugs that influence bone loss such as bisphosphonates and steroids. Use of hormone-replacement therapy (HRT) was assessed, and women were classified as “current” and “no HRT” use for each visit year except year 5.
Statistical Analysis
Since a series of BMD measurements during the 15-year follow-up period were available and the number of measurements and their time interval differed across individuals, a multilevel regression model for longitudinal data was chosen for the analysis. Repeated measures of BMD at the FN and LS, sufficiently normally distributed, taken on a maximum of eight occasions per participant between baseline and the most recent clinical visit were used as outcomes in the analyses. A time variable, measured in years since baseline, indicated when each measurement was taken and represented follow-up time. The model allowed the investigation of changes in the influence of DHEAS on BMD over time by including DHEAS-by-time interaction terms. The coefficient for the DHEAS variable then indicated its influence on BMD at time zero (thus, baseline BMD), while the interaction term indicated the influence of DHEAS on BMD change per year from baseline (slope). These models account for within-subject correlation between repeated measures and allow for incomplete outcome data as long as a missing-at-random process can be assumed. Therefore, all participants with at least two BMD measurements make a contribution to the estimates of association. The repeated measures of BMD were modeled to represent a linear change of BMD over time (slope). Nonlinear (time squared) changes of BMD were also considered; this was significant and, therefore, included in the model. The basic model was therefore reduced to include baseline DHEAS, time, time squared, and the DHEAS-by-time interaction. Wald tests were performed to assess levels of significance for the fixed-effect parameters. The model fit was evaluated by a likelihood ratio test (LRT). Based on the LRT, a random intercept model fitted the data well compared to other possible models. This allowed an estimate of the variability across individual intercept and intraclass correlation. Separate models were produced for the FN and LS BMD. To investigate whether the associations between baseline DHEAS and BMD at both sites were confounded, age, estradiol at baseline, and time-varying HRT and BMI as covariates were added to the model. Since DHEAS was not associated with smoking, physical activity, diet, polypharmacy, family history, and RA, these variables were not included in the final model. From the final adjusted model, predicted average BMD trajectories over the follow-up time were also graphically presented by baseline DHEAS interquartile ranges.
The model fit was as follows:
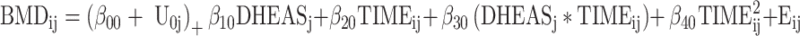 |
where BMDij denotes BMD at the FN or LS on measurement occasion i (i = 1–8) of subject j (j = 1 − n), DHEASj was DHEAS at the baseline, and TIMEij and TIME2ij were defined as the linear and quadratic forms of the time in years after baseline at which each measurement was taken, respectively. The terms U0j and Eij, denoting the random intercept and error components, were assumed to be normally, independently, and identically distributed with mean zero and variance σ2u0, σ2e, respectively.
The intraclass coefficient of the BMD that attributed to subject effect (level 2) was estimated using the formula 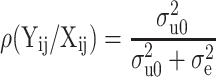 .
.
A coefficient estimate was considered statistically significant if its P value (two-sided) was <0.05. All statistical analyses were performed using STATA/SE version 10 for Windows (StataCorp, College Station, TX).
Results
A total of 1,003 postmenopausal women with a mean age of 54.7 years (range 45–68) at baseline participated in the study. Thirty-three women were lost to follow-up (1 died, 14 moved, 5 withdrew, and 13 unknown) after the year 2 visit. By the year 15 visit, 111 women had died, 21 were unable to attend for review, and 217 were lost to follow-up. However, the majority of them (n = 301) were still included in the analysis as they had baseline serum DHEAS measurements and multiple DXA measures.
Only four women were on calcium and/or vitamin D supplementation at baseline, but 178 women were at year 15. At years 10 and 15, there were 52 and 145 women reported to be on either bisphosphonates or steroids, respectively; and they were excluded from the analysis after this point. The average duration of HRT use was 27 months for current HRT users.
Women were invited to come back for examination every year, and a DXA scan was performed at baseline and years 2, 3, 4, 5, 6, 8, and 10. On average, women had their BMD measured at the FN and LS six times. There were 953 participants who had at least two FN BMD measurements and 947 for LS BMD. Seventy-five subjects had no baseline DHEAS measurements. The mean baseline BMD was 0.79 and 0.98 g/cm2 at the FN and LS, respectively, and the mean baseline DHEAS was 3.99 (μmol/L) (Table 1). Baseline DHEAS decrease with increasing baseline age (ρ = −0.23, P < 0.0001), positively correlated with baseline estradiol (ρ = 0.1, P = 0.007) and was significantly lower in current HRT users than nonusers (P = 0.002). Means and ranges at different baseline age categories were calculated and reported (Table 1). Descriptive statistics at each visit of the study population are presented in Table 2.
Table 1.
Descriptive summary of baseline DHEAS and BMD at both sites at different baseline age categories
| Variable | Baseline age (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| Age < 50 | 50 ≤ Age < 55 | 55 ≤ Age < 60 | Age ≥ 60 | |||||
| Mean (n) | Range | Mean (n) | Range | Mean (n) | Range | Mean (n) | Range | |
| LS (g/cm2) | 1.05 (262) | 0.71–1.50 | 1.00 (191) | 0.66–1.49 | 0.93 (127) | 0.60–1.40 | 0.89 (151) | 0.57–1.31 |
| FN (g/cm2) | 0.81 (99) | 0.57–1.07 | 0.79 (107) | 0.57–1.19 | 0.75 (20) | 0.58–0.90 | 0.71 (22) | 0.51–1.29 |
| DHEAS (μmol/L) | 4.91 (277) | 0.20–20.0 | 4.04 (220) | 0.30–11.60 | 3.60 (196) | 0.40–20.0 | 3.19 (235) | 0.20–13.4 |
| Estradiol (pmol/L) | 174.04 (197) | 20.0–398.0 | 79.6 (189) | 20.0–389.0 | 46.6 (194) | 20.0–348.0 | 31.38 (238) | 10.0–321.0 |
n, number of observations at each age group; BMD, bone mineral density; DHEAS, dehydroepiandrosterone sulfate; FN, femoral neck BMD; LS, lumbar spine BMD
Table 2.
Demographic characteristics and FN and LS BMD at every visit
| Variable | Baseline | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| Age (years) | 1,003 | 54.68 ± 6.02 | 944 | 55.11 ± 5.99 | 634 | 54.55 ± 5.69 | 548 | 54.35 ± 4.77 | 724 | 57.98 ± 5.92 | 843 | 59.28 ± 5.88 | 843 | 61.19 ± 5.9 | 1,002 | 64.46 ± 6.16 |
| Weight (kg) | 1,003 | 66.93 ± 11.84 | 814 | 67.41 ± 11.90 | 663 | 67.74 ± 12.03 | 856 | 68.04 ± 12.20 | 836 | 68.30 ± 12.16 | 851 | 68.73 ± 12.29 | 842 | 69.10 ± 12.84 | 810 | 69.23 ± 12.75 |
| Height (cm) | 1,003 | 161.62 ± 5.92 | 814 | 161.57 ± 5.82 | 663 | 161.22 ± 8.68 | 856 | 161.33 ± 5.94 | 836 | 161.08 ± 5.94 | 851 | 160.40 ± 8.18 | 842 | 160.71 ± 5.90 | 810 | 160.75 ± 6.03 |
| BMI (kg/m2) | 1,003 | 25.61 ± 4.3 | 814 | 25.81 ± 4.35 | 663 | 25.96 ± 4.32 | 856 | 26.12 ± 4.35 | 836 | 26.31 ± 4.4 | 851 | 26.64 ± 4.52 | 842 | 26.74 ± 4.72 | 810 | 26.78 ± 4.73 |
| HRTa | 238 | 24% | 152 | 16% | 194 | 21% | 254 | 28% | – | – | 250 | 28% | 246 | 29% | 195 | 27% |
| FN (g/cm2) | 248 | 0.785 ± 0.13 | 653 | 0.772 ± 0.12 | 630 | 0.774 ± 0.13 | 540 | 0.777 ± 0.13 | 586 | 0.758 ± 0.12 | 845 | 0.747 ± 0.12 | 830 | 0.751 ± 0.12 | 805 | 0.748 ± 0.12 |
| LS (g/cm2) | 731 | 0.983 ± 0.16 | 696 | 0.987 ± 0.16 | 497 | 0.991 ± 0.16 | 511 | 0.983 ± 0.16 | 723 | 0.954 ± 0.15 | 817 | 0.955 ± 0.15 | 834 | 0.951 ± 0.15 | 810 | 0.956 ± 0.16 |
aPercentage of HRT users instead of mean ± SD, and no measurement of HRT was taken at visit 5
DHEAS dehydroepiandrosterone sulfate, FN femoral neck BMD, LS lumbar spine BMD, BMI body mass index, n number of observations at each visit, HRT hormone-replacement therapy
The final analyses were restricted to people who had baseline DHEAS measurements and at least two DXA scans (n = 887 for FN and n = 879 for LS). There was no significant difference in baseline BMD at the FN and LS, age, BMI, and HRT use between people with and without DHEAS measurements (all P > 0.05). However, those who had only one DXA scan, < 6% of the total study subjects, were excluded from the analysis and were significantly older and heavier and had higher DHEAS levels than those who had at least two DXA scans and were included in the analysis (all P < 0.02).
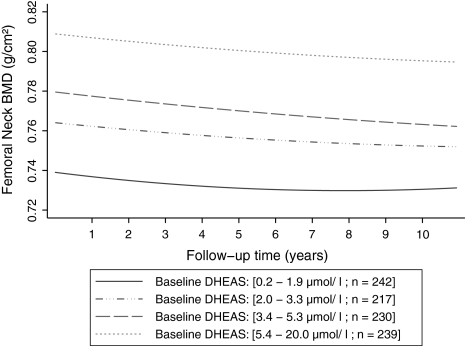
The results of the unadjusted and adjusted associations between DHEAS and BMD change at FN and LS are presented in Tables 3 and 4, respectively. BMD at the FN decreased 0.45% (95% CI 0.30–0.60%) per year, and the rate of decline was slowed down by 0.025% per squared year (Table 3, Fig. 1). The serum level of DHEAS at baseline was strongly and positively associated with the FN BMD at baseline. Increase of DHEAS (each micromole per liter) was associated with 0.92% less bone loss at the FN (95% CI 0.56–1.27%, P < 0.0001). However, this strong association became slightly weaker over time by 0.017% (95% CI 0.0036–0.031%) per year as indicated by a significant DHEAS-by-time interaction (P = 0.013) (Table 3). The negative and significant interaction between DHEAS and time reflects a small change in the degree to which baseline DHEAS predicts bone loss at the FN during the follow-up. The significance remained after adjustment for age and estradiol at baseline and time-varying BMI and HRT (fully adjusted beta = 0.49%, 95% CI 0.21–0.71%, P = 0.001), and all covariates were significant in the model, as expected (Table 3). The results remained the same when the analyses were restricted to those women who were never on HRT during the 15-year follow-up. Figure 1 illustrates the average FN BMD trajectories over time by baseline DHEAS interquartile ranges, which were predicted from the final adjusted multilevel model.
Table 3.
Multilevel linear regression of BMD at the FN with regard to the study factors
| Unadjusted analysis | Multiple adjusted analysisa | Fully adjusted analysisb | ||||
|---|---|---|---|---|---|---|
| FN | Coeff. (95% CI) | P | Coeff. (95% CI) | P | Coeff. (95% CI) | P |
| DHEAS (μmol/L) | 0.92 (0.56–1.27) | <0.0001 | 0.35 (0.11–0.57) | 0.007 | 0.49 (0.21–0.71) | 0.001 |
| Time (year) | −0.45 (−0.60 to −0.30) | <0.0001 | −0.48 (−0.65 to −0.34) | <0.0001 | −0.49 (−0.71 to −0.31) | <0.0001 |
| Time2 (year2) | 0.025 (0.013–0.036) | <0.0001 | 0.027 (0.018–0.035) | <0.0001 | 0.028 (0.018–0.036) | <0.0001 |
| DHEAS*time | −0.017 (–0.031 to −0.0036) | 0.013 | −0.017 (−0.030 to −0.0057) | 0.002 | −0.020 (−0.037 to −0.0063) | 0.003 |
| Baseline age (year) | −0.68 (−0.87 to −0.53) | <0.0001 | −0.61(−0.86 to −0.41) | < 0.0001 | ||
| BMI (kg/m2) | 0.53 (0.48–0.56) | <0.0001 | 0.61 (0.57–0.63) | <0.0001 | ||
| HRT | 0.81 (0.50–1.07) | <0.0001 | 0.92 (0.556–1.21) | <0.0001 | ||
| Estradiol (pmol/L) | 0.018 (0.01–0.025) | <0.0001 | ||||
The βs are interpreted as percentage change in BMD at femoral neck per unit increase in the study factors listed in the table. For example, −0.45 for the time variable represents a 0.45% decrease in BMD at the femoral neck per year during the follow-up period
aAdjusted for baseline age, time-varying (BMI and HRT)
bFurther adjusted for estradiol
DHEAS, dehydroepiandrosterone sulfate; FN, femoral neck BMD, BMI, body mass index; HRT, hormone-replacement therapy; DHEAS * Time, baseline DHEAS and time interaction; Time2, quadratic form of time; BMD, bone mineral density
Table 4.
Multilevel linear regression of BMD at LS with regard to the study factors
| Unadjusted analysis | Multiple adjusted analysisa | Fully adjusted analysisb | ||||
|---|---|---|---|---|---|---|
| LS | Coeff. (95% CI) | P | Coeff. (95% CI) | P | Coeff. (95% CI) | P |
| DHEAS (μmol/L) | 0.72 (0.35–1.06) | <0.0001 | 0.25 (−0.015 to 0.48) | 0.063 | 0.29 (−0.038 to 0.56) | 0.078 |
| Time (year) | −0.46 (−0.60 to −0.31) | <0.0001 | −0.45 (−0.62 to −0.31) | <0.0001 | −0.47 (−0.69 to −0.29) | <0.0001 |
| Time2 (year2) | 0.038 (0.026–0.049) | <0.0001 | 0.035 (0.026–0.042) | <0.0001 | 0.039 (0.028–0.047) | <0.0001 |
| DHEAS * time | −0.037 (−0.052 to −0.023) | <0.0001 | −0.035 (−0.050 to −0.022) | <0.0001 | −0.033 (−0.053 to −0.017) | <0.0001 |
| Baseline age (year) | −0.63 (−0.82 to −0.47) | <0.0001 | −0.51 (−0.77 to −0.30) | <0.0001 | ||
| BMI (kg/m2) | 0.44 (0.38−0.49) | <0.0001 | 0.55 (0.50–0.60) | <0.0001 | ||
| HRT | 1.52 (1.25–1.76) | <0.0001 | 1.69 (1.36–1.95) | <0.0001 | ||
| Estradiol (pmol/L) | 0.022 (0.013–0.029) | <0.0001 | ||||
The βs are interpreted as percentage change in BMD at lumbar spine per unit increase in the study factors listed in the table. For example: −0.46 for time variable represents a 0.46% decrease in BMD at the lumbar spine per year during the follow-up period
aAdjusted for baseline age, time-varying (BMI and HRT)
bFurther adjusted for estradiol
DHEAS dehydroepiandrosterone sulfate, LS lumbar spine BMD, BMI body mass index, HRT hormone-replacement therapy, DHEAS * time baseline DHEAS and time interaction, Time 2 quadratic form of time, BMD bone mineral density
Fig. 1.
Average predicted FN BMD trajectories over follow-up time by baseline DHEAS interquartile ranges, from the fully adjusted model, for the postmenopausal women
The variance component corresponding to the random intercept is 0.014. Since this estimate is substantially larger than its standard error (0.00068), there appears to be significant variation in the between-subject means. The two-variance components can be used to partition the variance across levels. The intraclass correlation coefficient or the proportion variance at the person level is estimated as ρ = 0.94, meaning that roughly 94% of the variance of the FN BMD measures were attributable to the subject level; about 6% is variance within individuals across time. This suggested that the variance within an individual across time does not account for much of the additional variance of the FN BMD.
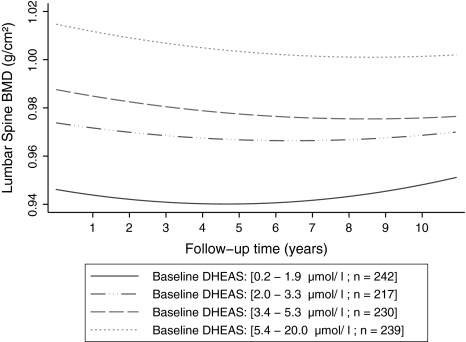
Similarly, BMD at the LS decreased 0.46% (95% CI 0.31–0.60%) per year, and the rate of decline was slowed down by 0.038% per squared year (Table 4, Fig. 2). The serum level of DHEAS at baseline was strongly and positively associated with the LS BMD at baseline. Increase of DHEAS (each micromole per liter) was associated with 0.72% less bone loss at the LS (95% CI 0.35–1.06%, P < 0.0001). However, this strong association became slightly weaker over time by 0.037% (95% CI 0.023–0.052%) per year as indicated by a significant DHEAS-by-time interaction (P < 0.0001) (Table 4). The association became weaker after adjustment for other covariates including age and estradiol at baseline and time-varying BMI and HRT (fully adjusted beta = 0.29%, 95% CI –0.038% to 0.56%, P = 0.078) (Table 4). However, the association became stronger (P = 0.02) when the analyses were restricted to those women who were never on HRT during the 15-year follow-up. Figure 2 shows the average LS BMD trajectories over time by baseline DHEAS interquartile ranges, which were predicted from the final adjusted multilevel model. The graphs, at different quartiles, start to converge slowly over the follow-up time, implying that the effect of baseline DHEAS on LS BMD loss diminishes over time.
Fig. 2.
Average predicted LS BMD trajectories over follow-up time by baseline DHEAS interquartile ranges, from the fully adjusted model, for the postmenopausal women
The fixed-effect model estimates the repeated-measures variance as 0.0018 (within-subject variability) and the person level (between-subject variability) as 0.023. This estimates the total LS BMD variance as 0.025. The intraclass correlation at the person level is estimated as ρ = 0.93. This implies that about 93% of the variance in the LS BMD measures was variance between individuals (subjects) and that about 7% was variance within individuals across time.
Discussion
This large prospective population-based study examined the association between DHEAS level at baseline and BMD change at the FN and LS in postmenopausal women over a 15-year follow-up period. The data suggest that serum level of DHEAS at baseline is strongly associated with BMD but the association became slightly weaker over time. Although the relationship between DHEAS and BMD at the FN and LS was reduced after adjusting for age and estradiol at baseline and time-varying BMI and HRT, an independent effect on BMD was still apparent at both sites. Further, women who had higher DHEAS levels at baseline also had higher BMD at both FN and LS at the end of the 15-year follow-up.
The results from previous cross-sectional studies on the effect of DHEAS on BMD in postmenopausal women with DHEAS in the normal range are inconsistent. Our findings concur with studies that report a positive association between serum DHEAS and BMD [19–21]. Szathmari et al. [21] found a positive relationship between DHEAS and BMD at both FN and LS sites in 105 women aged 45–69 years, 76 postmenopausal and 29 premenopausal. In contrast, other studies found no association of circulating DHEAS either with absolute value of BMD [22, 23] or with rate of bone loss [24]. Zofkova et al. [22] found no association between DHEAS and BMD at both FN and LS in 147 healthy or osteoporotic but otherwise normal premenopausal (n = 26 and n = 13, respectively) or postmenopausal (n = 40 and n = 68, respectively) women aged 40.1 ± 9.9 and 61.9 ± 8.9 years, respectively. Likewise, Murphy et al. [23] found no significant association in a cross-sectional study of 90 community-based women, all at least 1 year since their last menstrual period, who were on average 9.6 years postmenopausal (mean 9.6 ± 4.9 years, range 1–22). In a prospective study of 256 men (aged 50–74 years) and 162 women (aged 55–74 years), Barrett-Connor et al. [10] found no association between DHEAS levels with BMD at any site in either sex, both before and after adjusting for age, obesity, cigarette smoking, and use of antihypertensive medications. A similar study [24] found an insignificant association between DHEAS and BMD at the FN and LS in a population-based cohort of 159, non-HRT user, Australian-born women who at baseline had a mean age of 50.0 years (SD = 2.4) with reported menstruation within the previous 3 months. The reason for the discrepancy is unclear; possible explanations include small sample size, methodological differences [22, 23], and relatively short duration [10, 24].
Studies have suggested the complexity of the potential mechanisms of the effects of DHEA on BMD loss. Estrogen may play a role in mediating the effect of DHEA on bone loss. DHEA can be converted to estrogen, which is a potential antiresorptive agent [25]. There was evidence for a transient increase in serum estrogen for a few hours after oral DHEA administration [26], and the correlations linking serum DHEAS levels to bone turnover markers, BMD, and osteoporotic fractures are strongest in elderly women, in whom peripheral DHEA conversion is the only source of estrogens [27]. However, the effect size of DHEAS on bone loss was even increased after further adjustment for estradiol in the current study, suggesting that DHEA may have a direct effect on bone loss that is independent of estradiol. There is also a possibility that the effect of DHEA on bone was liaised by the increase in serum testosterone concentration that occurs in response to DHEA [25]. Testosterone replacement has been shown to inhibit bone resorption and stimulate formation in hypogonadal men [28]. DHEA replacement resulted in a marked increase in circulating IGF-I levels, which may exert anabolic effects on bone [25]. Furthermore, treatment of oophorectomized rats with DHEA increased BMD above that of intact animals in parallel with the increase in alkaline phosphatase, which may indicate a direct positive effect of DHEA on osteoblasts [29]. DHEA administration reduced the loss of cancellous bone volume in rats in a dose-dependent manner by reducing bone remodeling [22]. However, findings in rodents are of limited significance for humans as DHEA physiology in humans is distinctly different from that in nonprimate mammals [30]. Further studies are needed to elucidate the potential mechanisms for the observed association.
There are some limitations in the current study. The study population was all female; thus, the results may not be generalizable. However, a similar significant association in males has been reported [31]. Multilevel analysis was used to take account of the correlation between repeated measures of FN and LS BMD, BMI, and HRT for the same individual. Although missing outcome data can be comprised in this method of analysis, the “missing-at-random assumption” might be violated if missing occurs systematically with regard to DHEAS levels. However, the majority of loss to follow-up in the study was due to censoring, and there was no difference in BMD, age, BMI, and HRT use between women with and without DHEAS measurements, suggesting that this is not an issue. Although the models fit the data properly, there was inconsiderable variability of BMD between and within subjects that remained unexplained; this could be due to unknown genetic and environmental factors which were not taken into account. In addition, since DHEAS was measured only at baseline in this study, we could not exclude the possibility that changes of DHEAS accompanying BMD changes may be significant for the regulation of BMD.
In conclusion, our data suggest that a high serum DHEAS level at baseline is associated with reduced bone loss at both FN and LS and this association diminishes over time. The nature of the association is unclear, but such an association implies that women might benefit from maintaining a high level of DHEAS for their BMD.
Acknowledgements
We thank all the participants and staff in the Chingford 1,003 woman study for their time and dedication, including Dr. David Doyle, who helped set up the original study, and Dr. Les Perry, for measuring serum DHEAS. The Arthritis Research Campaign, the Wellcome Trust, and Guy’s & St. Thomas’ NHS Foundation Trust and KCL Biomedical Centre supported this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The authors have stated that they have no conflict of interest.
References
- 1.(1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650 [DOI] [PubMed]
- 2.Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M. Treatment of established osteoporosis: a systematic review and cost–utility analysis. Health Technol Assess. 2002;6:1–146. doi: 10.1007/978-94-010-0481-7_1. [DOI] [PubMed] [Google Scholar]
- 3.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt) 2006;15:1028–1034. doi: 10.1089/jwh.2006.15.1028. [DOI] [PubMed] [Google Scholar]
- 4.Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, Burger HG, Wark JD. A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int. 1998;8:282–290. doi: 10.1007/s001980050066. [DOI] [PubMed] [Google Scholar]
- 8.Leowattana W. DHEA(S): the fountain of youth. J Med Assoc Thai. 2001;84(Suppl 2):S605–S612. [PubMed] [Google Scholar]
- 9.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/S0039-128X(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Kritz-Silverstein D, Edelstein SL. A prospective study of dehydroepiandrosterone sulfate (DHEAS) and bone mineral density in older men and women. Am J Epidemiol. 1993;137:201–206. doi: 10.1093/oxfordjournals.aje.a116660. [DOI] [PubMed] [Google Scholar]
- 11.Lambrinoudaki I, Christodoulakos G, Aravantinos L, Antoniou A, Rizos D, Chondros C, Kountouris A, Chrysofakis G, Creatsas G. Endogenous sex steroids and bone mineral density in healthy Greek postmenopausal women. J Bone Miner Metab. 2006;24:65–71. doi: 10.1007/s00774-005-0648-x. [DOI] [PubMed] [Google Scholar]
- 12.Osmanagaoglu MA, Okumus B, Osmanagaoglu T, Bozkaya H. The relationship between serum dehydroepiandrosterone sulfate concentration and bone mineral density, lipids, and hormone replacement therapy in premenopausal and postmenopausal women. J Womens Health (Larchmt) 2004;13:993–999. doi: 10.1089/jwh.2004.13.993. [DOI] [PubMed] [Google Scholar]
- 13.Spector TD, Thompson PW, Perry LA, McGarrigle HH, Edwards AC. The relationship between sex steroids and bone mineral content in women soon after the menopause. Clin Endocrinol (Oxf) 1991;34:37–41. doi: 10.1111/j.1365-2265.1991.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 14.Sun AJ, Jing T, Heymsfield SB, Phillips GB. Relationship of leptin and sex hormones to bone mineral density in men. Acta Diabetol. 2003;40(Suppl 1):S101–S105. doi: 10.1007/s00592-003-0039-5. [DOI] [PubMed] [Google Scholar]
- 15.Bacsi K, Kosa JP, Borgulya G, Balla B, Lazary A, Nagy Z, Horvath C, Speer G, Lakatos P. CYP3A7*1C polymorphism, serum dehydroepiandrosterone sulfate level, and bone mineral density in postmenopausal women. Calcif Tissue Int. 2007;80:154–159. doi: 10.1007/s00223-006-0227-8. [DOI] [PubMed] [Google Scholar]
- 16.Hart DJ, Spector TD. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J Rheumatol. 1993;20:331–335. [PubMed] [Google Scholar]
- 17.Zhai G, Hart DJ, Valdes AM, Kato BS, Richards JB, Hakim A, Spector TD. Natural history and risk factors for bone loss in postmenopausal Caucasian women: a 15-year follow-up population-based study. Osteoporos Int. 2008;19:1211–1217. doi: 10.1007/s00198-008-0562-x. [DOI] [PubMed] [Google Scholar]
- 18.Wathen NC, Perry LA, Rubenstein E, Chard T. A relationship between sex hormone binding globulin and dehydroepiandrosterone sulfate in normally menstruating females. Gynecol Endocrinol. 1987;1:47–50. doi: 10.3109/09513598709082695. [DOI] [PubMed] [Google Scholar]
- 19.Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S. The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas. 2004;48:235–242. doi: 10.1016/j.maturitas.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jc.83.7.2266. [DOI] [PubMed] [Google Scholar]
- 21.Szathmari M, Szucs J, Feher T, Hollo I. Dehydroepiandrosterone sulphate and bone mineral density. Osteoporos Int. 1994;4:84–88. doi: 10.1007/BF01623229. [DOI] [PubMed] [Google Scholar]
- 22.Zofkova I, Bahbouh R, Hill M. The pathophysiological implications of circulating androgens on bone mineral density in a normal female population. Steroids. 2000;65:857–861. doi: 10.1016/S0039-128X(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 23.Murphy S, Khaw KT, Sneyd MJ, Compston JE. Endogenous sex hormones and bone mineral density among community-based postmenopausal women. Postgrad Med J. 1992;68:908–913. doi: 10.1136/pgmj.68.805.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthrie JR, Lehert P, Dennerstein L, Burger HG, Ebeling PR, Wark JD. The relative effect of endogenous estradiol and androgens on menopausal bone loss: a longitudinal study. Osteoporos Int. 2004;15:881–886. doi: 10.1007/s00198-004-1624-3. [DOI] [PubMed] [Google Scholar]
- 25.Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf) 2000;53:561–568. doi: 10.1046/j.1365-2265.2000.01131.x. [DOI] [PubMed] [Google Scholar]
- 26.Arlt W, Haas J, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte HM, Allolio B. Biotransformation of oral dehydroepiandrosterone in elderly men: significant increase in circulating estrogens. J Clin Endocrinol Metab. 1999;84:2170–2176. doi: 10.1210/jc.84.6.2170. [DOI] [PubMed] [Google Scholar]
- 27.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Eyre DR, Clark R, et al. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3654–3662. doi: 10.1210/jc.81.10.3654. [DOI] [PubMed] [Google Scholar]
- 29.Martel C, Sourla A, Pelletier G, Labrie C, Fournier M, Picard S, Li S, Stojanovic M, Labrie F. Predominant androgenic component in the stimulatory effect of dehydroepiandrosterone on bone mineral density in the rat. J Endocrinol. 1998;157:433–442. doi: 10.1677/joe.0.1570433. [DOI] [PubMed] [Google Scholar]
- 30.Cutler GB, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche—survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 31.Haren MT, Malmstrom TK, Banks WA, Patrick P, Miller DK, Morley JE. Lower serum DHEAS levels are associated with a higher degree of physical disability and depressive symptoms in middle-aged to older African American women. Maturitas. 2007;57:347–360. doi: 10.1016/j.maturitas.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]