Abstract
Background. Wilms' tumor suppressor gene (WT1) is essential for normal podocyte function, and transforming growth factor (TGF)-beta contributes to focal segmental glomerulosclerosis (FSGS). We aimed to address whether TGF-beta affects WT1 expression in podocytes.
Methods. A human podocyte cell line treated with TGF-beta1 and kidneys in Alb/TGF-beta1-transgenic mice were analyzed for WT1 expression.
Results. In cultured podocytes, TGF-beta1 reduced WT1 protein expression determined by western blotting beginning at 8 h and decreased WT1 messenger RNA (mRNA) expression measured by quantitative reverse transcription–polymerase chain reaction beginning at 3 h. Knockdown of Smad4 by small hairpin (sh) RNA partially rescued the TGF-beta1-induced reduction of both WT1 protein and mRNA expressions in the cultured podocytes. TGF-beta1 did not alter luciferase activity of the reporter construct for a human WT1 promoter but reduced that for a human WT1 5′ enhancer construct, suggesting that TGF-beta1 may regulate WT1 expression by altering the 5′ enhancer activity. In the transgenic mice, WT1 protein expression in podocytes was decreased at 1 and 3 weeks of age, while glomeruloclerosis developed after 3 weeks.
Conclusion. TGF-beta1 reduces WT1 expression in cultured human podocytes and podocytes in mice before overt glomerulosclerosis begins. The effects are at least partially Smad4 dependent. Our findings identify a novel pathway linking TGF-beta1 to podocyte injury and FSGS. The WT1 reduction may be a useful marker for early podocyte injury.
Keywords: FSGS, podocytes, TGF-beta1, Wilms' tumor suppressor gene
Introduction
Focal segmental glomerulosclerosis (FSGS) and collapsing glomerulopathy are important causes of proteinuria and chronic kidney disease. Podocyte injury has been implicated in the initiation and progression of FSGS [1, 2].
Wilms' tumor suppressor gene (WT1) encodes a zinc finger transcription factor that regulates expression of target genes by binding to both DNA and messenger RNA (mRNA). WT1 was identified in 11p13 by positional cloning in an effort to determine a responsible gene for Wilms tumor, a malignant tumor of the kidney [3, 4]. WT1 is expressed in all stages of kidney development, while the expression becomes restricted to podocytes in the mature kidney.
Mutations in various regions of WT1 have distinct phenotypes. Mutations in exon 8 or 9, which encode the zinc finger domain of WT1, can lead to Denys–Drash syndrome; this syndrome manifests an early-onset nephrotic syndrome with diffuse mesangial sclerosis and, less frequently, FSGS [5]. Mutations in the donor splice site in intron 9 of WT1 result in a reduction of KTS+ isoform and are responsible for the Frasier syndrome, characterized by pseudohermaphroditim and FSGS [5, 6]. Deletion of several neighboring genes in 11p13 including WT1 and PAX6 can cause WAGR syndrome (Wilms' tumor, aniridia, genitourinary anomalies and mental retardation), which is also associated with FSGS [5, 7]. In addition, podocyte WT1 expression is decreased in idiopathic FSGS and HIV-associated collapsing glomerulopathy [2, 8]. WT1-null mice fail to develop kidneys and urogenital tract [9], and reduction of WT1 expression in mice results in crescentic glomerulonephritis and mesangial sclerosis [10]. Substantial evidence suggests an essential role for WT1 in mature podocytes as well as developing kidney.
Transforming growth factor (TGF)-beta expression is increased in podocytes in idiopathic FSGS and HIV-associated collapsing glomerulopathy [8, 11, 12]. TGF-beta induces podocyte injury through various mechanisms, including apoptosis [13], proliferation [14], epithelial–mesenchymal transition (EMT) [15] and increased production of extracellular matrix proteins including collagen [16].
In the present study, we have addressed the hypothesis that TGF-beta1 reduces WT1 expression in podocytes. We have used a conditionally immortalized human podocyte cell line and Alb/TGF-beta1 transgenic mice, in which elevated circulating levels of active TGF-beta1 are associated with progressive glomerulosclerosis [17]. We found that WT1 expression was significantly decreased in both TGF-beta1-treated cultured podocytes and podocytes in transgenic mice kidneys. We also demonstrated that Smad4 mediated the suppression of WT1 by TGF-beta1 and that TGF-beta1 reduced a recently identified 5′ enhancer activity, which is conserved among vertebrates [18].
Methods
Cell culture
A conditionally immortalized human podocyte cell line AB8/13 (provided by Dr Moin Saleem) [19] was grown on type-I collagen-coated culture ware (BD Biosciences, San Jose, CA) in RPMI supplemented with 10% fetal bovine serum, 100 U/mL of penicillin G sodium, 100 μg/mL of streptomycin sulfate and insulin–transferrin–selenium G supplement (Invitrogen, Carlsbad, CA). For each experiment, cells were differentiated by culture for 5 days under growth-restricted conditions at 37°C unless specially noted. Recombinant human TGF-beta1 was purchased from PeproTech (Rocky Hill, NJ).
Smad4 knockdown with small hairpin RNA
pLKO.1-puro lentiviral plasmids encoding small hairpin (sh) RNA for human Smad4 or nontarget control (NTC), together with a puromycin-resistant gene, were purchased from Sigma–Aldrich (St Louis, MO). HIV-1-based lentiviral particles were generated in HEK293FT cells (American Type Culture Collection, Manassas, VA) by cotransfection of shRNA or NTC plasmid with pCMV R8.91 and pCMV-VSV-G using FuGENE6 reagent (Roche, Indianapolis, IN). Cultured podocytes were infected with the lentiviral particles in the presence of 8 μg/mL polybrene for 5 h at 33°C. Effective viral titer was confirmed by observation of cellular survival in the presence of 1 μg/mL puromycin in a preliminary experiment. Four shRNA clones were tested for the ability to knockdown Smad4 mRNA, and the best-performing clone was selected.
Ectopic expression of SNAIL
pBabe-puro-hSNAIL.ER.NoTag, a retroviral expression vector for human SNAIL tagged with mouse estrogen receptor (ER-SNAIL) [20], and pBabe-puro, a control empty vector, was provided from each Dr Eric R. Fearon and Dr Bob Weinberg through Addgene (Cambridge, MA). Retroviral particles were generated in Phoenix-Ampho cells by transfecting each vector using FuGENE6. Cultured podocytes were infected with retroviral particles in the presence of 8 μg/mL polybrene at 33°C overnight. At 72 h after infection, the cells were incubated with 1 μg/mL puromycin, a selection drug for each vector, for 5 days to eliminate uninfected cells. The cells stably expressing ER-SNAIL or control cells were treated with or without 4-hydroxytamoxifen (4-OHT) (Sigma–Aldrich) at indicated concentration for 8 h to induce nuclear translocation of ER-SNAIL [20].
Luciferase reporter assay
p3TP-lux, a TGF-beta-responsive luciferase reporter construct containing a part of human PAI-1 promoter and three TPA responsive elements was provided from Dr Jeff Wrana and Dr Joan Massague through Addgene [21]. p3TP-lux harbors Smad3/Smad4-binding sites and is widely used to monitor a DNA-binding activity of the Smad complex. Reporter constructs for a human WT1 full-length promoter, hW10/luc (−443 to +182), were gifts from Dr Youqi Han and Dr Mark Minden [22]. A reporter construct for human WT1 5′ enhancer, WTA, was supplied by Dr Frank Bollig and Dr Christoph Englert [18]. Empty control vectors for these constructs (pGL2 basic for p3TP-lux, pGL2 enhancer for hW10/luc and pGL3 promoter for WTA) were purchased from Promega (Madison, WI). The reporter vectors or empty vectors were cotransfected into the cultured podocytes with a control reporter construct, pCMV-beta-gal, using FuGENE6 at 33°C. After 24 h, the culture medium including plasmids and Fugene6 was replaced with fresh medium with or without 5 ng/mL TGF-beta1. After another 24-h incubation, the cells were lysed in luciferase lysis buffer (Promega) and luciferase activity was determined with a luciferase reporter assay system (Promega). The data were normalized to beta-galactosidase activity derived from pCMV-beta-gal measured with Galactosto-Star System (Applied Biosystems, Foster City, CA).
TGF-beta1 transgenic mice
Albumin/TGF-beta1 transgenic mice, in which the albumin promoter drives expression of an active TGF-beta1 molecule, have elevated circulating levels of TGF-beta1 at the earliest time point studied (2 weeks of age) and develop progressive glomerulosclerosis [17]. Male transgenic and wild-type C57B6 × CBA mice were sacrificed at 1, 3 and 5 weeks of age (N = 5 or 6 of each genotype at each time point), and kidneys were collected. The experiments were approved in advance by the NIDDK Animal Care and Use Committee and performed under the NIH Guide for the Care and Use of Laboratory Animals.
Glomerular isolation
Isolation of glomeruli from transgenic or wild-type mice kidneys at 3 weeks of age was carried out as previously reported [23]. Briefly, the mice were anesthetized and perfused through the heart with Dynabeads M-450 tosylactivated (Invitrogen) prepared in Hank’s buffered salt solution with calcium and magnesium. Kidneys were minced with a razor blade, digested with collagenase and DNAse for 30 min and passed through 100-μm cell strainer. Gomeruli were collected with a magnet and washed with phosphate-buffered saline (PBS), and RNA was extracted. Equal amounts of glomerular RNA were obtained from mice in each group (wild-type, 2.05 ± 0.45 μg per a mouse; transgenic, 1.98 ± 0.51 μg).
Western blotting
Cultured podocytes were lysed or mouse kidney tissues were homogenized in RIPA lysis buffer (50 mM Tris–HCl, pH 7.4; 150 mM NaCl; 2.5% deoxycholic acid; 1% NP-40; 1 mM ethylenediaminetetraacetic acid) with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Total protein lysates equal to 20 μg of protein were separated on NuPAGE 4–12% bis–tris gels (Invitrogen) under reducing condition and transferred to nitrocellulose membranes (Invitrogen). Membrane were probed with the following antibodies: mouse monoclonal anti-WT1 antibody, Clone 6F-H2 (Millipore, Billerica, MA) at a dilution of 1:500; mouse monoclonal anti-Smad4 antibody, clone SMD46 (Abcam, Cambridge, MA) at 1:250; mouse monoclonal anti-beta-actin antibody (Sigma–Aldrich) at 1:5000; rabbit polyclonal anti-Snail antibody (Abcam) at 1:500; rabbit polyclonal anti-phospho-Smad2 (Ser245/250/255) (Cell Signaling Technology, Danvers, MA) at 1:1000; rabbit monoclonal anti-phospho-Smad 3 antibody, clone EP823Y (Epitomics, Burlingame, CA) at 1:1000 and goat polyclonal anti-Smad2/3 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:200 as a primary antibody. After incubation with horseradish peroxidase-conjugated secondary antibodies, signals were detected by chemiluminescence. The intensity of bands was quantified by densitometory.
Immunofluorescent staining
Cells cultured on type I collagen-coated cover slips (BD Biosciences) or mouse-frozen kidney sections were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature, permeabilized with 0.2 % Triton X-100 for 10 min and incubated with mouse monoclonal anti-WT1 antibody (Millipore) at a dilution of 1:100 or rabbit anti-podocin antibody at 1:200 as a primary antibody (kind gift from Dr Peter Mundel). The first antibody was detected with Alexa 588-conjugated anti-mouse antibody or Alexa 594-conjugated anti-rabbit antibody (Invitrogen).
Quantitative reverse transcription–polymerase chain reaction
Total RNAs were purified from cultured cells or mouse kidney tissues with miRNAeasy mini-kit (Qiagen, Valencia, CA). Sufficient purity of RNA was confirmed by observing 260/280 absorbance ratio of 1.8–2.2, measured by a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). Complementary DNA was synthesized with High Capacity RNA-to-cDNA Kit (Applied Biosystems) according to the manufacturers instruction. Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) was carried out in ABI 7900 sequence detection system (Applied Biosystems) with Power SYBR green PCR master mix (Applied Biosystems). Corresponding primers were as follows: human WT1 forward (F) primer ATAACCACACAACGCCCATC, reverse (R) primer TCAGATGCCGACCGTACAAG; human Snail (F) GCACATCCGAAGCCACAC, (R) GGGAGAAGGTCCGAGCACAC; human Slug (F) GCCCGCCGCGATGCTGTAGG, (R) CTTGCCAGCGGGTCTGGCGG; human Zeb1 (F) GCTCTAACCCGCCTTCATCC, (R) TGTCCCAGTGTTTCAGTTTCTCTG; human Zeb2 (F) AGGCGCAAACAAGCCAATC, (R) GCTGGACTCGTCTCCTGGTC; human beta-actin (F) AGCACAGAGCCTCGCCTTTG, (R) AGCGCGGCGATATCATCATC; mouse WT1 (F) CCAGCCTACCATCCGCAAC, (R) GGGTCCTCGTGTTTGAAGG and mouse beta-actin (F) GTCCACACCCGCCACCAG, (R) TGACCCATTCCCACCATCAC.
Statistics
Data are presented as mean ± SD. Statistical significance was evaluated by one-way analysis of variance, using Bonferroni testing for intergroup comparisons. A P value of <0.05 was taken as significant.
Results
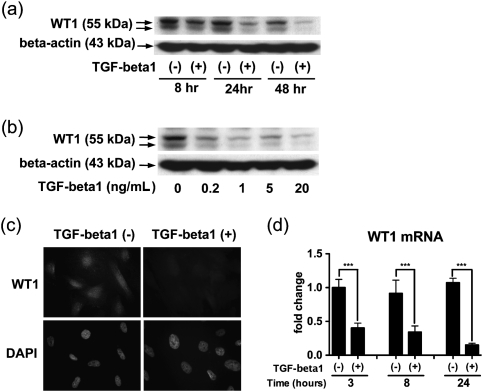
We addressed whether TGF-beta1 affected WT1 expression in a human podocyte cell line AB8/13 (Figure 1). TGF-beta1 (5 ng/mL) reduced WT1 protein expression in the podocytes compared with the control at 8, 24 and 48 h, although the reduction is modest at 8 h (Figure 1a). In a dose–response study, TGF-beta1 at every concentration spanning 0.2–20 ng/mL decreased WT1 protein expression (Figure 1b). In immunolocalization studies, WT1 was localized to the nuclei, and the staining was substantially diminished by exposure to TGF-beta1 (5 ng/mL) for 24 h (Figure 1c). TGF-beta1 (5 ng/mL) exposure for 3, 8 and 24 h reduced WT1 mRNA expression, assessed by qRT–PCR (Figure 1d).
Fig. 1.
TGF-beta1 reduces WT1 expression in cultured human podocytes. (a) A human podocyte cell line AB8/13 was treated with 5 ng/mL TGF-beta1 or vehicle control, and western blotting was performed at 8, 24 and 48 h for WT1 and the loading control, beta-actin. WT1 protein expression was decreased in the podocytes with TGF-beta1 compared with vehicle control at 8, 24 and 48 h. The double bands suggest the presence of splicing variants characteristic of WT1. (b) Podocytes were incubated with TGF-beta1 for 24 h in a dose-response study. WT1 protein expression was decreased in the cells treated with TGF-beta1 at each concentration tested. (c) Podocytes were cultured in the presence or absence of 5 ng/mL TGF-beta1 for 24 h, followed by immunofluorescent staining for WT1 and nuclear staining with 4′,6-diamidino-2-phenylindole. WT1 was expressed predominantly in the nuclei in the control podocytes, and this staining largely disappeared following exposure to TGF-beta1. Original magnification, x400. (d) TGF-beta1 (5 ng/mL) or vehicle control was added to podocyte cultures, and WT1 mRNA expression was measured by qRT-PCR at 3, 8 and 24 h. At all three time points, WT1 mRNA expression was significantly lower in the cells exposed to TGF-beta1. Data were normalized to beta-actin mRNA and shown as mean ± SD for three samples relative to those of the control cells at 3 h. ***P < 0.001.
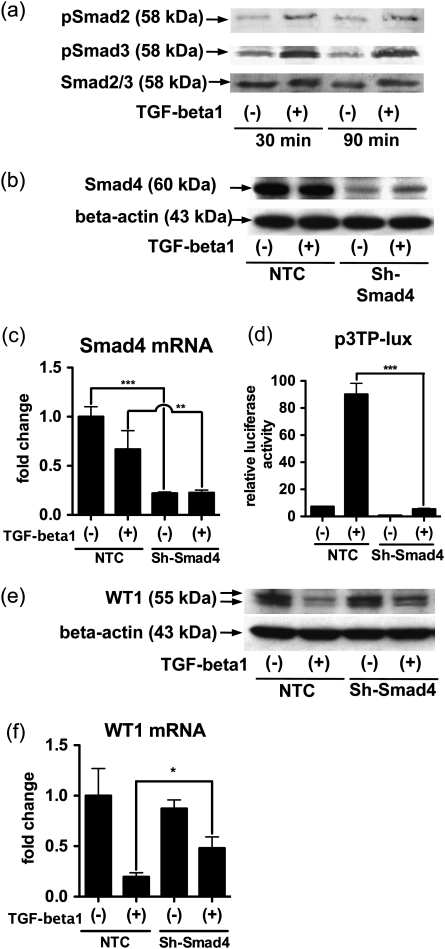
TGF-beta signaling often involves the Smad family, although other pathways also contribute to signal transduction [24, 25]. The TGF-beta type I receptor phosphorylates receptor-regulated (R)-Smads (Smad2 and Smad3), and each phosphorylated R-Smad binds the common mediator Smad (Smad4). The R-Smad/Smad4 complex translocates to the nucleus and regulates gene transcription. We confirmed by western blotting that incubations with TGF-beta1 (5 ng/mL) for 30 and 90 min induced phosphorylation of Smad2 and Smad3 in the cultured podocytes (Figure 2a). To examine whether the Smad signaling mediates WT1 gene regulation by TGF-beta1, we carried out a knockdown assay for Smad4 with shRNA. Comparing podocytes infected with the lentiviral ShRNA vectors targeting Smad4 (Sh-Smad4) with the cells with the vectors encoding NTC, we confirmed an effective knockdown of Smad4 by observing significant reduction of both Smad4 mRNA measured by qRT–PCR (Figure 2b) and Smad4 protein assessed by western blotting (Figure 2c). We performed luciferase reporter assay using p3TP-lux, which is activated by TGF-beta via Smad signaling [21]. Following treatment with TGF-beta1, the luciferase activity was abrogated in podocytes expressing Sh-Smad4 compared with the negative control (NTC), suggesting that Sh-Smad4 effectively inhibits the Smad signal transduction (Figure 2d). We addressed the effect of Sh-Smad4 on WT1 protein and mRNA expressions in the podocytes. Smad4 knockdown substantially rescued the TGF-beta1-induced suppression of WT1 protein after 24 h (Figure 2e). Furthermore, Sh-Smad4 exposure for 8 h partially blocked TGF-beta1 suppression of WT1 mRNA (Figure 2f). Taken together, these data suggest that TGF-beta1 suppression of WT1 mRNA and protein are mediated at least in part by the Smad pathway.
Fig. 2.
Smad4 partly mediates the reduction of WT1 induced by TGF-beta1 in human podocytes. (a) Human cultured podocytes were treated with 5 ng/mL TGF-beta1 for 30 or 90 min, and expression of phosphorylated Smad2 (pSmad2), phosphorylated Smad3 (pSmad3) and total Smad2/3 (Smad2/3) as a loading control were determined by western blotting. pSmad2 and pSmad3 expressions were increased by TGF-beta1 at both 30 and 90 min. (b-f) Podocytes were infected with lentiviral particle encoding NTC shRNA or shRNA targeting Smad4 (sh-Smad4). (b and c) Podocytes infected by each virus vector were treated with 5 ng/mL TGF-beta1. Smad4 protein expression was determined by western blotting at 24 h (b) and the mRNA expression was measured by qRT-PCR at 8 h (c). Smad4 protein and mRNA were decreased by induction of Sh-Smad4. (d) The luciferase activity of p3TP-lux, a reporter construct for Smad-binding element, was determined after incubation with 5 ng/mL TGF-beta1 for 24 h. TGF-beta1 increased the luciferase activity by ∼12-fold in the presence of the NTC shRNA, while this increase was largely prevented by Sh-Smad4. The luciferase activity of p3TP-lux was normalized to beta-galactosidase activity of pCMV-beta-gal and shown as relative values to that of pGL2 basic. (e and f) Podocytes exposed to NTC or Sh-Smad4 were cultured in the presence or absence of 5 ng/mL TGF-beta1. WT1 protein was analyzed by western blotting after 24-h incubation (e) and WT1 mRNA was determined after 8 h. (f) In the cells with NTC, both protein and mRNA expressions of WT1 were suppressed by TGF-beta1. In the cells with Sh-Smad4, the suppression of WT1 was partially recovered both protein and mRNA levels. Blotting for beta-actin served as a loading control in panel (b) and (e). The mRNA expression of Smad4 or WT1 was normalized to that of beta-actin in panel (c) and (f). Mean ± SD for three samples are shown in panel (c) and (f), and those for four samples are presented in panel (d). The data are presented as relative values to those with NTC without TGF-beta1 in panel (c) and (f). *P < 0.05; **P < 0.01; ***P < 0.001.
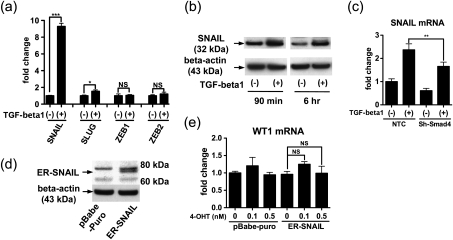
TGF-beta control of EMT typically involves induction of one or more zinc finger transcription factors, such as Snail, Slug, Zeb1 and Zeb2 [26], and therefore, we examined a possible role for these proteins in the observed effects on WT1 expression. We cultured the podocytes in the presence or absence of TGF-beta1 (5 ng/mL) for 30 min and measured mRNA expression of the four genes by qRT–PCR. Among these, TGF-beta1 increased Snail mRNA by nearly 10-fold, increased Slug mRNA to a minor degree and unaffected Zeb1 and Zeb2 mRNA expression (Figure 3a). At 90 min, only Snail mRNA was upregulated (data not shown). After podocytes were exposed to TGF-beta1 (5 ng/mL), Snail protein expression was upregulated after 90 min and 6 h (Figure 3b). The addition of Smad4 shRNA was able to partially decrease Snail mRNA induction by TGF-beta1 at 8 h (Figure 3c), suggesting that Smad signaling contributed to the Snail upregulation. We next sought to elucidate a functional role for Snail in WT1 expression; we used human Snail tagged with mouse estrogen receptor (ER-Snail), a chimeric molecule which makes Snail nuclear translocation dependent on 4-hydroxytamoxifen (4-OHT) [20]. Expression of ER-Snail was confirmed by western blotting (Figure 3d) and by qRT–PCR (data not shown). Unexpectedly, administration of 4-OHT did not alter WT1 mRNA expression (Figure 3e), suggesting that Snail may not be involved in WT1 regulation.
Fig. 3.
Snail is induced by TGF-beta1 but does not alter WT1 expression in cultured podocytes. (a) A human podocyte cell line AB8/13 was incubated with or without 5 ng/mL TGF-beta1 for 30 min, and mRNA expression for Snail, Slug, ZEB1 and ZEB2 was determined by qRT-PCR. TGF-beta1 increased expression of Snail mRNA and to modest extent Slug. Data were normalized to beta-actin mRNA expression and mean ± SD for three samples are shown as relative values to control. (b) Podocytes were treated with 5 ng/mL TGF-beta1 or vehicle for 90 min and 6 h, and Snail protein expression was examined by western blotting. TGF-beta1 increased Snail protein at each time point. (c) Podocytes were treated with lentivirus vector encoding NTC or shRNA targeting Smad4 (sh-Smad4). The cells were incubated with or without 5 ng/mL TGF-beta1 for 8 h, and expression of SNAIL mRNA was determined by qRT-PCR. At the same concentration of TGF-beta1, Snail mRNA expression was significantly lower in the cells with sh-Smad4 than NTC, suggesting that TGF-beta1 induction of SNAIL was at least partly mediated by Smad4. Data were normalized to beta-actin mRNA expression and are shown as relative values (mean ± SD for three samples) to NTC without TGF-beta1. (d) Human Snail protein, tagged with mouse estrogen receptor (ER-Snail) was stably expressed in the podocytes by a retrovirus vector as confirmed by western blotting. (e) Podocytes were subjected to retrovirul infection with virus lacking (pBabe-puro) or containing ER-SNAIL and treated with or without 0.1 or 0.5 nM 4-hydroxytamoxifene (4-OHT) for 8 h to facilitate nuclear translocation. WT1 mRNA expression was analyzed by qRT-PCR. Expression of ER-Snail did not alter WT1 mRNA expression, suggesting that Snail does not regulate WT1 expression in podocytes. WT1 mRNA levels are normalized to beta-actin mRNA and mean ± SD for three samples are shown relative to the cells with pBabe-puro and without 4-OHT. NS, not significant. *P < 0.05; **P < 0.01; ***P < 0.001.
We analyzed the effect of TGF-beta1 on human WT1 promoter activity using a reporter construct hW10/luc [22]. TGF-beta1 (5 ng/mL) did not alter luciferase activity of the construct (Figure 4a). We tested the effect of TGF-beta1 on a human WT1 enhancer located ∼4.3 kb upstream of the transcription start site using a reporter vector WTA [18]. As shown in Figure 4b, TGF-beta1 reduced luciferase activity of WTA by ∼40%. While this effect was modest, it would be consistent with a direct suppressive effect of TGF-beta1 on WT1 transcription.
Fig. 4.
TGF-beta1 reduces WT1 5' enhancer activity but does not alter promoter activity. (a) Podocytes were treated with 5 ng/mL TGF-beta1 or vehicle control for 24 h, and luciferase activity of a WT1 promoter reporter construct hW10/luc (−443 to +182) was measured. The promoter activity was not altered by TGF-beta1. Data are normalized to galactosidase activity of transfection control vector pCMV-beta-gal and shown as relative values (mean ± SD for four samples) to luciferase activity of pGL2 enhancer. NS, not significant. (b) Podocytes were cultured in the presence of 5 ng/mL TGF-beta1 or vehicle, and luciferase activity of a WT1 5' enhancer construct WTA was determined. TGF-beta1 reduced the enhancer activity by ∼40%. Data are normalized to beta-galactosidase activity of the pCMV-beta-gal and shown as relative values (mean ± SD for four samples) to luciferase activity of pGL3-enhancer. **P < 0.01.
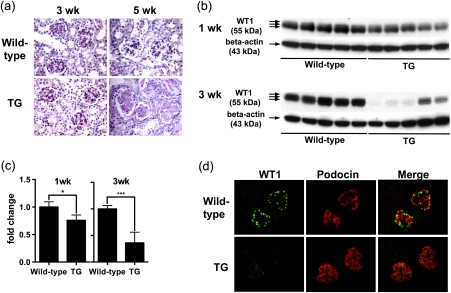
To address whether these observations in cultured podocytes might have relevance to in vivo, we studied Alb/TGF-beta1 transgenic mice, which develop severe progressive global glomerulosclerosis [17]. We carried out histological evaluation for glomerulosclerosis using Periodic acid-Schiff-stained kidney sections of the transgenic and wild-type mice at 1, 3 and 5 weeks of age (Figure 5a, 1-week-old mice kidneys are not shown). Glomeruli in 1 and 3-week-old transgenic mice were histologically intact, while glomerulosclerosis was evident in the transgenic mice at 5 weeks of age, with diffuse mesangial matrix expansion and glomerular capillary obliteration. In the previous study, we reported that the transgenic mice manifested glomerulosclerosis at 3 weeks of age, earlier than the present study [17]. This discrepancy represents phenotypic drift, as the selection of breeder mice at 6–8 weeks of age likely selects for a milder renal phenotype. To evaluate whether WT1 expression might be an early marker of podocyte injury, and because podocyte numbers were likely reduced at 5 weeks of age, we used 1- and 3-week-old transgenic and wild-type mice kidneys. Using whole kidney lysates for western blotting, WT1 protein expression, normalized to beta-actin expression, was significantly lower in transgenic mice kidneys at both 1 week of age (75.8 ± 0.10%) and 3 weeks of age (36.1 ± 20.2%) when compared with wild-type mice (Figure 5b and c). We carried out double-immunofluorescent staining for WT1 (green) and podocin (red) (Figure 5d). In wild-type mice kidneys, WT1 localized to the nuclei of podocytes, as identified by cytoplasmic podocin expression. In transgenic mice kidneys, WT1 expression was greatly diminished, while podocin was unaffected. We measured WT1 mRNA expression by qRT–PCR in isolated glomeruli from 3-week-old mice and found no significant difference between transgenic and wild-type mice (data not shown).
Fig. 5.
WT1 protein expression is reduced in podocytes in Alb/TGF-beta1 transgenic mice. (a) Representative Periodic acid-Schiff stained kidney sections were obtained from wild-type or Alb/TGF-beta1 transgenic (TG) mice at 3 and 5 weeks of age. At 3 weeks of age, WT and transgenic mice glomeruli showed no pathologic changes. At 5 weeks of age, transgenic mice showed moderate-to-severe diffuse global glomerulosclerosis characterized by mesangial matrix expansion and occlusion of the glomerular capillary tuft. Original magnification, x400. (b) WT1 protein expression in whole kidney lysates of wild-type or TG mice at 1 or 3 weeks of age was analyzed by western blotting. The triple bands indicate the presence of WT1 splice variants. (c) The intensity of bands in panel (b) was quantitated by densitometry and normalized to beta-actin protein. WT1 protein expression was significantly reduced in transgenic mice compared with wild-type mice at both 1 and 3 weeks of age, although the difference was modest at 1 week. For each age, relative mean ± SD values are shown, normalized to values for wild-type mice. *P < 0.05; ***P < 0.001. (d) Kidney sections of 3-week-old wild-type or transgenic mice were subjected to double immunostaining for WT1 and podocin. WT1 (green) is located in the nuclei of glomerular cells that also expressed podocin in the cytoplasm (red) in wild-type mice kidneys. WT1 expression was attenuated in transgenic mice kidneys, while podocin expression was retained. Original magnification, x400.
Discussion
In the present study, we have shown that TGF-beta1 reduced WT1 mRNA and protein expression in a human podocyte cell line and in podocytes in mouse kidneys, and this suppressive effect is mediated at least in part via Smad signal transduction. It may be of relevance that TGF-beta1 partially suppressed activity of a WT1 enhancer recently identified ∼4.3 kb upstream of the transcriptional start site, although the effect was modest and the functional importance of this effect remains to be shown.
WT1 is recognized as an important transcriptional regulator of podocyte phenotype [10, 27], but the regulation of WT1 in podocytes is not well understood. TGF-beta1 increases both WT1 protein and mRNA expressions in cultured hepatocytes, which is divergent from our findings and suggests that tissue-specific regulatory networks may be operating [28]. Kashikar et al. [29] showed that serine threonine receptor-associated protein (STRAP) reduced WT1 and E-cadherin expression in mouse embryonic fibroblasts and that STRAP is involved in TGF-beta signaling as a negative regulator [30], although the relationship between TGF-beta and WT1 was not defined. To our knowledge, the current study is the first to show that TGF-beta1 can suppress WT1 expression at the protein and mRNA levels.
TGF-beta is a well-recognized cause of podocyte injury and glomerulosclerosis [17], and reduced expression of WT1 also causes glomerulosclerosis [10]. The data presented here are compatible with a model in which TGF-beta1 acts via multiple pathways to induce podocyte injury, one of which is to reduce WT1 expression and thereby reduce expression of certain critical WT1-dependent genes. We note that the reduction in podocyte WT1 expression was observed in transgenic mice prior to the onset of histologic injury, suggesting that it may be an early injury marker.
One discrepancy in the data presented here is that TGF-beta1 reduced expression of both WT1 mRNA and WT1 protein in a human podocyte cell line, while WT protein but not WT1 mRNA was reduced in Alb/TGF-beta1 transgenic mouse glomeruli. These results suggest that additional factors are operative in vivo.
The transcriptional regulation of WT1 in podocytes is not well understood. In human embryonic kidney (HEK) cells, a yeast artificial chromosome (YAC) containing ∼620 kb including 5′ and 3′ sequence of mouse WT1 gene can induce WT1 expression to a considerable level, and deletion of 15 kb upstream site from transcription start site in the HEK cells with the YAC causes complete loss of the gene expression [31]. To date, retinoic acid [18], PAX2 [32], PAX8 [32], Sp-1 [33], NF-κB [34], GATA-1 [35], GATA-2 [35] and WT1 itself [22, 36] are known to interact with a promoter or an enhancer region of the WT1 gene and modulate WT1 gene expression. In this study, we showed, using a reporter assay, that TGF-beta1 reduced activity of a 5′ enhancer of the WT1 gene, while it did not alter the promoter activity. The 5′ enhancer is located in conserved region at ∼4.3 kb upstream of the human WT1 transcription start site, responds to retinoic acid and contributes to WT1a expression in embryonic zebra fish in the intermediate mesoderm at an early developmental stage and in glomeruli at a later stage [18]. The present study lacks data addressing whether Smads or other regulatory proteins, stimulated by TGF-beta1, interact with the WT1 5′ enhancer sequence. We acknowledge this as an important limitation of our work that will require further effort to clarify.
In conclusion, we have demonstrated that TGF-beta1 reduced WT1 expression in a human podocyte cell line and in podocytes in Alb/TGF-beta1 transgenic mice, prior to the onset of overt glomerular injury and that the effects on WT1 gene expression are at least partially Smad4 dependent. Our findings suggest a novel mechanism by which TGF-beta1 may contribute to podocyte injury and glomerulosclerosis and further suggest that reduced WT1 expression might prove to be a useful marker of early podocyte injury.
Acknowledgments
We are grateful to Hideko Takahashi and Huiyan Lu for technical assistance, Youqi Han, Mark Minden, Frank Bolig, Christoph Englert, Jeff Wrana, Joan Massague, Eric Fearon and Bob Weinberg for providing plasmids, Moin Saleem for providing a human podocyte cell line, Peter Mundel for providing anti-podocin antibody and Jurgen Schnermann for a critical review.
Funding. This study was supported by Intramural Research Program, NIDDK, NIH.
Conflict of interest statement. None declared.
References
- 1.Kriz W. The pathogenesis of ‘classic' focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant. 2003;18(Suppl. 6):vi39–vi44. doi: 10.1093/ndt/gfg1064. [DOI] [PubMed] [Google Scholar]
- 2.Barisoni L, Kriz W, Mundel P, et al. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 3.Call KM, Glaser T, Ito CY, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- 4.Gessler M, Poustka A, Cavenee W, et al. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990;343:774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- 5.Niaudet P, Gubler MC. WT1 and glomerular diseases. Pediatr Nephrol. 2006;21:1653–1660. doi: 10.1007/s00467-006-0208-1. [DOI] [PubMed] [Google Scholar]
- 6.Little M, Wells C. A clinical overview of WT1 gene mutations. Hum Mutat. 1997;9:209–225. doi: 10.1002/(SICI)1098-1004(1997)9:3<209::AID-HUMU2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Fischbach BV, Trout KL, Lewis J, et al. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005;116:984–988. doi: 10.1542/peds.2004-0467. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim BK, Moon KC, et al. Activation of the TGF-beta/Smad signaling pathway in focal segmental glomerulosclerosis. Kidney Int. 2003;64:1715–1721. doi: 10.1046/j.1523-1755.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- 9.Moore AW, McInnes L, Kreidberg J, et al. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 10.Guo JK, Menke AL, Gubler MC, et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet. 2002;11:651–659. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 11.Bodi I, Kimmel PL, Abraham AA, et al. Renal TGF-beta in HIV-associated kidney diseases. Kidney Int. 1997;51:1568–1577. doi: 10.1038/ki.1997.215. [DOI] [PubMed] [Google Scholar]
- 12.Bennett MR, Czech KA, Arend LJ, et al. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp Nephrol. 2007;107:e30–e40. doi: 10.1159/000106775. [DOI] [PubMed] [Google Scholar]
- 13.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–16. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HS, Song CY. Effects of TGF-beta on podocyte growth and disease progression in proliferative podocytopathies. Kidney Blood Press Res. 2010;33:24–29. doi: 10.1159/000285844. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Kang YS, Dai C, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozes MM, Bottinger EP, Jacot TA, et al. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol. 1999;10:271–80. doi: 10.1681/ASN.V102271. [DOI] [PubMed] [Google Scholar]
- 17.Kopp JB, Factor VM, Mozes M, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- 18.Bollig F, Perner B, Besenbeck B, et al. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development. 2009;136:2883–2892. doi: 10.1242/dev.031773. [DOI] [PubMed] [Google Scholar]
- 19.Saleem MA, O'Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 20.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 21.Wrana JL, Attisano L, Carcamo J, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 22.Han Y, San-Marina S, Yang L, et al. The zinc finger domain of Wilms' tumor 1 suppressor gene (WT1) behaves as a dominant negative, leading to abrogation of WT1 oncogenic potential in breast cancer cells. Breast Cancer Res. 2007;9:R43. doi: 10.1186/bcr1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakairi T, Abe Y, Jat PS, et al. Cell-cell contact regulates gene expression in CDK4-transformed mouse podocytes. Am J Physiol Renal Physiol. 2010;299:F802–F809. doi: 10.1152/ajprenal.00205.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 25.Mehra A, Wrana JL. TGF-beta and the Smad signal transduction pathway. Biochem Cell Biol. 2002;80:605–622. doi: 10.1139/o02-161. [DOI] [PubMed] [Google Scholar]
- 26.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 27.Guo G, Morrison DJ, Licht JD, et al. WT1 activates a glomerular-specific enhancer identified from the human nephrin gene. J Am Soc Nephrol. 2004;15:2851–2856. doi: 10.1097/01.ASN.0000143474.91362.C4. [DOI] [PubMed] [Google Scholar]
- 28.Berasain C, Herrero JI, Garcia-Trevijano ER, et al. Expression of Wilms' tumor suppressor in the liver with cirrhosis: relation to hepatocyte nuclear factor 4 and hepatocellular function. Hepatology. 2003;38:148–157. doi: 10.1053/jhep.2003.50269. [DOI] [PubMed] [Google Scholar]
- 29.Kashikar ND, Reiner J, Datta A, et al. Serine threonine receptor-associated protein (STRAP) plays a role in the maintenance of mesenchymal morphology. Cell Signal. 2009;22:138–149. doi: 10.1016/j.cellsig.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta PK, Moses HL. STRAP and Smad7 synergize in the inhibition of transforming growth factor beta signaling. Mol Cell Biol. 2000;20:3157–3167. doi: 10.1128/mcb.20.9.3157-3167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholz H, Bossone SA, Cohen HT, et al. A far upstream cis-element is required for Wilms' tumor-1 (WT1) gene expression in renal cell culture. J Biol Chem. 1997;272:32836–32846. doi: 10.1074/jbc.272.52.32836. [DOI] [PubMed] [Google Scholar]
- 32.Siehl JM, Thiel E, Heufelder K, et al. Possible regulation of Wilms' tumour gene 1 (WT1) expression by the paired box genes PAX2 and PAX8 and by the haematopoietic transcription factor GATA-1 in human acute myeloid leukaemias. Br J Haematol. 2003;123:235–242. doi: 10.1046/j.1365-2141.2003.04622.x. [DOI] [PubMed] [Google Scholar]
- 33.Cohen HT, Bossone SA, Zhu G, et al. Sp1 is a critical regulator of the Wilms' tumor-1 gene. J Biol Chem. 1997;272:2901–2913. doi: 10.1074/jbc.272.5.2901. [DOI] [PubMed] [Google Scholar]
- 34.Dehbi M, Hiscott J, Pelletier J. Activation of the wt1 Wilms' tumor suppressor gene by NF-kappaB. Oncogene. 1998;16:2033–2039. doi: 10.1038/sj.onc.1201747. [DOI] [PubMed] [Google Scholar]
- 35.Furuhata A, Murakami M, Ito H, et al. GATA-1 and GATA-2 binding to 3' enhancer of WT1 gene is essential for its transcription in acute leukemia and solid tumor cell lines. Leukemia. 2009;23:1270–1277. doi: 10.1038/leu.2009.13. [DOI] [PubMed] [Google Scholar]
- 36.Rupprecht HD, Drummond IA, Madden SL, et al. The Wilms' tumor suppressor gene WT1 is negatively autoregulated. J Biol Chem. 1994;269:6198–6206. [PubMed] [Google Scholar]