Less than 1% of HIV patients control HIV replication to an almost undetectable level without the aid of antiretroviral therapy (ARV), and are termed natural viral suppressors (NVSs)1. A recent study demonstrated that NVSs demonstrate immunological control toward HIV and not HCV or other chronic viral infections similar to HCV 2. The impact of effective immunological control of HIV replication in NVSs on response to Interferon-based immune therapy in HCV has not been studied.
Studies have demonstrated that induction of host immunity is a key phenomenon associated with virologic response to IFN and ribavirin (RBV) treatment 3. In this regard, HIV/HCV co-infected patients have significantly lower host immune response and rates of sustained virologic response (SVR) to IFN/RBV compared to HIV negative, HCV infected patients4. Our study compares the effect of superior immunological control of HIV replication in NVSs to that in patients with chronic HIV (CHIV) on the clearance of HCV after treatment with IFN/RBV.
Methods
Forty-eight patients (NVSs: n=7, 14.5%; CHIV: n=41, 85.4%) previously treated for Hepatitis C Genotype 1 in three previous studies were retrospectively analyzed. Treatments for Hepatitis C included: Pegylated IFN-α 2a (180ug weekly), Pegylated IFN-α 2b (1.5ug/kg weekly), or albIFN-α 2b (900ug every other week) all in combination with ribavirin (1-1.2g/day) for 48 weeks. Patients with CHIV were treated with ARVs per standard of care. NVSs had stable CD4 counts and HIV-1 viral loads <1000 for at least three years. Patients were seen at the National Institute of Allergy and Infectious Diseases (NIAID) clinics at the National Institutes of Health. Close follow-up was facilitated with the aid of a case manager and had patients signed informed consent for participation approved by NIAID.
HCV viral load was measured using Abbott Assays (Abbott Laboratories, Abbott Park, Illinois, USA). Pearson’s correlations, Fisher Exact Tests, and Student’s t-tests were used to calculate p-values between the two groups. Repeated measures ANOVA was used to determine differences in HCV viral load during treatment. Analyses were conducted using SAS version 9.1 and GraphPad Prism version 5.03.
Results
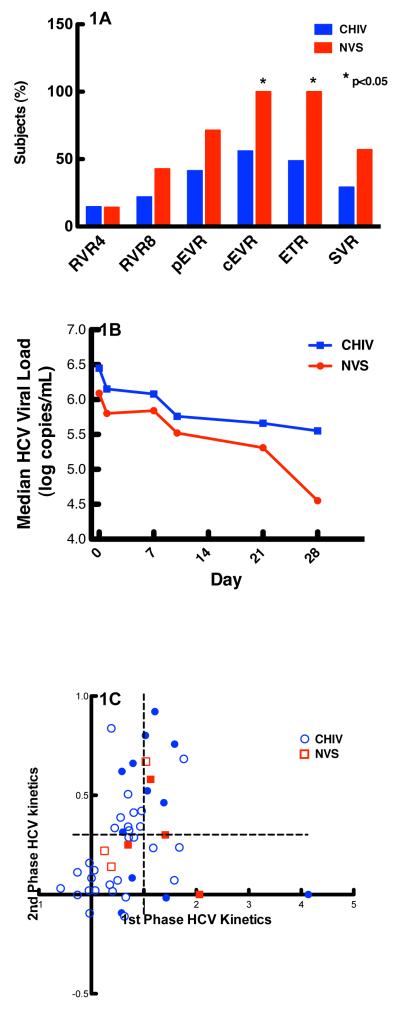
Baseline characteristics in NVSs and CHIV patients including age (45.6 ± 4.2 vs. 47.80 ± 8.08 yrs respectively), gender (100% vs. 80.5% male respectively), race (57.1% vs. 51.2% black), risk factors for acquisition (28.6% vs. 48.8% IVDU and 57.1% vs. 34.2% MSM respectively), pre-HCV therapy CD4 (772 ± 274 vs. 559 ± 268 cells/mm3 respectively), IL-28 haplotype (42.9% vs. 29% AA and 14.3% vs. 34.1% GG respectively), baseline ISHAK fibrosis score (1.7 ± 0.4 vs. 2.7 ± 0.2 respectively) and HCV treatment duration (47.9 ± 0.1 vs. 35.7 ± 16.4 weeks respectively) were not statistically different (p>0.05), except for CD4 nadir which was significantly lower in CHIV patients (615 ± 273 vs. 207 ± 158 cells/ mm3 NVSs and CHIV respectively; p<0.0001) and number of patients on ARV’s (100% of CHIV patients). When we measured early clinical endpoints in NVSs and CHIV patients (Figure 1A), NVSs had higher percentages of response rates; differences in RVR 4 (less then detectable HCV RNA at week four; 14.3% NVS vs. 14.6% CHIV), RVR 8 (42.9% NVS vs. 21.9% CHIV), and cEVR (less than detectable HCV RNA at week 12; 71.4% NVS vs. 41.5% CHIV) were not statistically significant. Of note, there were significantly more NVSs who achieved pEVR (≥ two log reduction in HCV RNA from baseline at week 12; 100% NVS, 56.1% CHIV; p<0.04) and ETR (undetectable HCV RNA at end of treatment; 100% NVS vs. 48.8% CHIV; p<0.01). Fifty-seven percent of NVSs achieved SVR as compared to 29% of CHIV patients (p=0.20).
Figure 1. Comparisons of clinical and HCV viral Kinetic parameters between Chronic HIV patients and NVSs.
Figure 1A. Comparison of clinically relevant end points between CHIV and NVSs. Percentage of patients with a RVR4, RVR8, cEVR (HCV viral load below the level of detection at weeks 4, 8, and 12 respectively), pEVR (≥ 2 log drop from baseline HCV VL at week 12), ETR (HCV viral load below the level of detection at week 48 or when treatment stopped), and SVR (patients with HCV viral load below the level of detection at week 72 or 24 weeks after stopping treatment). NVS patients had significantly higher rates of pEVR and ETR.
Fig 1B. Comparison of early HCV viral load (Days 0-28) between CHIV and NVSs. NVS patients (red) have a lower median HCV viral load from Day 0 to Day 28 of treatment than patients with CHIV (blue) (p=0.003).
Figure 1C. Comparison of 1st and 2nd phase HCV kinetics between CHIV and NVS patients. NVSs (red) and CHIV (blue) patients have similar first and second phase HCV kinetics suggesting similar effect of Interferon-based anti-HCV therapy. Data points of patients who achieved SVR are in solid colors. NVS and CHIV patients with a first phase decline of HCV viral load of ≥ 1 log decline along with ≥ 0.3 log decline (demarcated by dotted lines) have higher rates of SVR as compared to other patients (n= 7/9, 78% SVR vs. n=9/32, 28% SVR respectively).
Overall median HCV RNA reduction between Day 0 and Day 28 of treatment (Figure 1B) was greater in NVS than CHIV patients (p=0.003). First and second phase HCV kinetics, as well as the correlation between the two were similar (Figure 1C) during combination HCV therapy in NVSs and CHIV patients (p > 0.05). Average decline in HCV viral load during phase one and two was higher in patients achieving SVR (phase one HCV VL decline: 1.28 logs vs. 0.53 logs (p=0.0007), phase two HCV VL decline: 0.39 logs vs. 0.23 logs in SVR vs. no SVR respectively (p=0.05).
Discussion
We found that NVSs have better virologic outcomes when treated with HCV therapy using peg-IFN/RBV when compared to that observed with chronic ARV-treated HIV/HCV coinfected patients with similar CD4 T cell counts. Preservation of host immunity by natural control of HIV probably enables these individuals to respond better to immune based therapy and clear HCV. In this regard, HIV infected NVSs seem to have SVR rates similar to those reported among HCV monoinfected patients. Finally, ARV treated chronic HIV infected patients seem to have lower response to immune based therapy despite having CD4 T cell recovery with maximal suppression of HIV replication.
Previously, we have shown that early HCV viral kinetics for PegIFN have excellent predictive ability for SVR in HIV/HCV co-infected patients 5. In this study, early viral kinetics were again predictive of SVR, suggesting early control of HCV replication is vital in eradication of HCV. Furthermore, we found that NVSs have better clinically relevant early virologic outcomes for HCV therapy using peg-IFN/RBV when compared to that observed with chronic ARV-treated HIV/HCV coinfected patients with similar CD4 T cell counts. These early virologic end points (RVR, cEVR) have been shown to be associated with SVR in larger clinical trials.
Our data demonstrates that spontaneous immune control of HIV in NVSs likely enables these individuals to respond better to immune based therapy and clear HCV. HIV infection decreases responsiveness to HCV treatment using IFN probably because host immune response to IFN-α is blunted 3, related to ongoing residual HIV viremia. In NVSs, the natural control of HIV replication to extremely low levels likely enables the immune system to respond to IFN-α better. Although some studies have shown better HLA B*57 restricted cellular responses associated with control of HIV and HCV6, another did not demonstrate a clinical benefit in either control of HCV replication or response to HCV treatment among NVSs 7. However, increased spontaneous clearance of HCV in NVSs as compared to CHIV patients has also been described 8, and others have found increased HCV-specific CD8 9 and CD4 10 T-cell responses in NVSs as compared to HIV/HCV progressors. These latter findings support the increased HCV virologic response to HCV therapy in NVS patients seen in our cohort.
Overall, our findings support the hypothesis that in HIV/HCV coinfected patients, baseline immune status is a major determinant in responding to HCV therapy using peg-IFN/RBV. While, immune reconstitution is important in eradicating HCV, it appears that natural preservation of CD4 T cell counts as well as intrinsic control of HIV replication in NVSs is superior to control of HIV replication using ART.
A larger prospective treatment study is needed to validate our findings, however, such studies are difficult given the low prevalence of NVSs coinfected with HCV.
Acknowledgments
Funding: This research was supported in whole by the Intramural Research Program of the NIH [National Institute of Allergy and Infectious Diseases and NIH Clinical Center].
Footnotes
Conflict of Interest Statement: Dr. Mani Subramanian is presently an employee at Gilead but was employed at Human Genome Sciences at the time of the study. All other authors have no conflicts of interest to report.
Publisher's Disclaimer: Disclaimer The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA. 2010 Jul 14;304(2):194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 2.Jagannathan P, Osborne CM, Royce C, et al. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J Virol. 2009 Mar;83(6):2728–2742. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lempicki RA, Polis MA, Yang J, et al. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV-coinfected persons. J Infect Dis. 2006 Apr 15;193(8):1172–1177. doi: 10.1086/501365. [DOI] [PubMed] [Google Scholar]
- 4.Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004 Jul 29;351(5):451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avidan NU, Goldstein D, Rozenberg L, et al. Hepatitis C viral kinetics during treatment with peg IFN-alpha-2b in HIV/HCV coinfected patients as a function of baseline CD4+ T-cell counts. J Acquir Immune Defic Syndr. 2009 Dec 1;52(4):452–458. doi: 10.1097/QAI.0b013e3181be7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim AY, Kuntzen T, Timm J, et al. Spontaneous control of HCV is associated with expression of HLA-B 57 and preservation of targeted epitopes. Gastroenterology. 2011 Feb;140(2):686–696. e681. doi: 10.1053/j.gastro.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro P, Laguno M, Nomdedeu M, et al. Clinicoimmunological progression and response to treatment of long-term nonprogressor HIV-hepatitis C virus-coinfected patients. AIDS Res Hum Retroviruses. 2007 Jul;23(7):863–867. doi: 10.1089/aid.2006.0251. [DOI] [PubMed] [Google Scholar]
- 8.Sajadi MM, Shakeri N, Talwani R, Redfield RR. Hepatitis C infection in HIV-1 natural viral suppressors. AIDS. 2010 Jul 17;24(11):1689–1695. doi: 10.1097/QAD.0b013e32833a2a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdez H, Carlson NL, Post AB, et al. HIV long-term non-progressors maintain brisk CD8 T cell responses to other viral antigens. AIDS. 2002 May 24;16(8):1113–1118. doi: 10.1097/00002030-200205240-00004. [DOI] [PubMed] [Google Scholar]
- 10.Alatrakchi N, Di Martino V, Thibault V, Autran B. Strong CD4 Th1 responses to HIV and hepatitis C virus in HIV-infected long-term non-progressors co-infected with hepatitis C virus. AIDS. 2002 Mar 29;16(5):713–717. doi: 10.1097/00002030-200203290-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez VD, Falconer K, Blom KG, et al. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol. 2009 Nov;83(21):11407–11411. doi: 10.1128/JVI.01211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]