Abstract
There is increasing concern about abuse of propofol, a widely-used surgical anesthetic and sedative that is currently not a controlled substance. The purpose of the present study was to establish a rat model of the psychoactive effect of sub-anesthetic doses of propofol that could be useful for confirming abuse liability and studying mechanisms of propofol abuse. Sprague-Dawley rats were trained to discriminate propofol (10 mg/kg, i.p.) from vehicle (2% methylcellulose). Carisoprodol (100 mg/kg), chlordiazepoxide (10 mg/kg) and dizocilpine (0.1 mg/kg) were tested for substitution for the discriminative-stimulus effects of propofol (10 mg/kg), whereas pentylenetetrazol (10 mg/kg) was tested for antagonism of the discriminative-stimulus effects. Propofol (10 mg/kg) was tested for substitution in rats trained to discriminate carisoprodol from vehicle. Carisoprodol produced 59% propofol-appropriate responding, chlordiazepoxide 65%, and dizocilpine 34%. Pentylenetetrazol decreased propofol-appropriate responding to 41%. Propofol produced 52% carisoprodol-appropriate responding. Mortality rate during training of 10 mg/kg propofol was 38%. Post-mortem examination revealed cardiovascular abnormalities similar to those observed in propofol-infusion syndrome in humans. The results demonstrate that propofol can be trained as a discriminative stimulus. Its discriminative-stimulus effects were more similar to compounds promoting GABA-A receptor activity than to a compound inhibiting NMDA receptor activity. Because propofol has discriminative-stimulus effects similar to known drugs of abuse and occasions a high mortality rate, its potential for continued abuse is of particular concern.
Keywords: discrimination learning, stimulus generalization, propofol, GABA-A receptor, rat
Introduction
Propofol has attracted increasing concern about its safety and abuse potential, and a recent review (Wilson et al., 2010) concluded that it should be scheduled as a controlled substance at the Federal level. Animal studies have tended to confirm the abuse potential of propofol, though the results have been equivocal. Propofol can induce conditioned place preference (Pain et al., 1996, 1997) and was self-administered by some subjects in studies of rats and baboons (Weerts et al., 1999; LeSage et al., 2000). On the other hand, a study using mice reported that propofol was not self-administered (Blokhina et al., 2004). In a human study, 6 of 12 people chose to receive propofol, and preference was directly related to whether subjects reported pleasant subjective effects following propofol administration (Zacny et al., 1993).
The purpose of these studies was to determine whether non-sedating doses of propofol produce a discriminative stimulus and to assess potential pharmacological mechanisms for this effect. Propofol has not previously been trained as a discriminative stimulus, though a drug discrimination approach (Colpaert, 1999) was thought to be particularly applicable in light of self-administration studies that did not show unequivocal evidence that propofol was reinforcing in all subjects, and the observation that the subjective qualities of propofol appear to determine drug taking in humans (Zacny et al., 1993). The locomotor depressant effects of propofol were first studied in mice for identification of appropriate training doses in the propofol discrimination studies.
Method
Subjects
Male Sprague-Dawley rats were obtained from Harlan-Sprague Dawley (Indianapolis, IN). All rats were housed individually and were maintained on a 12:12 light/dark cycle. Body weights were maintained at 320-350 g by limiting food to 20 g/day. Water was freely available in the home cages. Male Swiss-Webster mice were obtained from Harlan at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group-housed in cages on a 12-/12-h light/dark cycle and were allowed free access to food and water. All housing and procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity
A Digiscan apparatus (model RXYZCM-16; Omnitech Electronics, Columbus, OH) was used to measure ambulation within clear acrylic testing chambers (40.5× 40.5×30.5 cm) that were housed in dimly lit sound-attenuating chambers. Separate groups of eight mice received either vehicle (2% methylcellulose) or propofol (3, 10 or 30 mg/kg) by i.p. injection immediately before testing, and ambulation counts were recorded for 1 h.
Drug Discrimination Procedures
Two-lever test chambers (Coulbourn Instruments, Allentown, PA), interfaces and computers programmed in MED-PC IV (Med Associates, East Fairfield, VT) were used for the operation of the chambers and collection of data.
Groups of 16 rats were used for propofol discrimination training, one group at 5 mg/kg and another at 10 mg/kg. An additional group of rats was trained to discriminate carisoprodol (100 mg/kg) from vehicle (2% methylcellulose). Food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available under a fixed-ratio 10 schedule of reinforcement when responding occurred on the injection-appropriate lever. Before each training session, the rats received an i.p. injection of either vehicle (2% methylcellulose) or training drug. Twenty minutes following injection, the rats were placed in the experimental chamber for a 10-min session during which they could earn up to 20 food pellets. Subjects qualified for testing when they achieved 85% injection-appropriate responding for both the first reinforcer and total session in nine of ten training sessions.
Carisoprodol (100 mg/kg, 20 min pretreatment), chlordiazepoxide (10 mg/kg, 15 min) and dizocilpine (0.1 mg/kg, 15 min) were tested for substitution for propofol. Propofol was tested for substitution in carisoprodol-trained rats. The ability of pentylenetetrazol (10 mg/kg, 25 min) to block the discriminative-stimulus effects of propofol was also tested.
Drugs
All compounds were obtained from Sigma-Aldrich (St. Louis, MO) and were administered intraperitoneally in a volume of 1 ml/kg. Propofol and carisoprodol were suspended in 2% methylcellulose. Chlordiazepoxide, dizocilpine and pentylenetetrazol were dissolved in 0.9% saline.
Data Analysis
Ambulation counts within 10-min periods were considered in a 4 × 6 ANOVA [Treatment × Time (repeated)]. The 10 to 40-min time period was selected for analysis of dose-response data, because this was the period in which locomotor depression occurred. Ambulation counts from 10 to 40 min at each dose were compared with the vehicle control using individual F tests, within the Treatment × Time interaction.
Drug-appropriate responding and response rate data were analyzed by one-way, repeated-measures ANOVA. Individual doses were compared with the vehicle control using individual F tests. Full substitution was defined as >80% drug-appropriate responding and partial substitution as ≥40% and <80% drug-appropriate responding and not statistically different from the training drug. Survival data were analyzed using a Tarone-Ware X2 test. Mortality in three groups of 16 rats (10 mg/kg propofol group, 5 mg/kg propofol group and carisoprodol group) was compared only during the training phase and testing of the training drug dose effect (125 days). After this time, the three groups were tested with different drugs at different time points, which would potentially confound sources of mortality. The criterion for significance in all analyses was set a priori at p<0.05.
Results
Locomotor activity
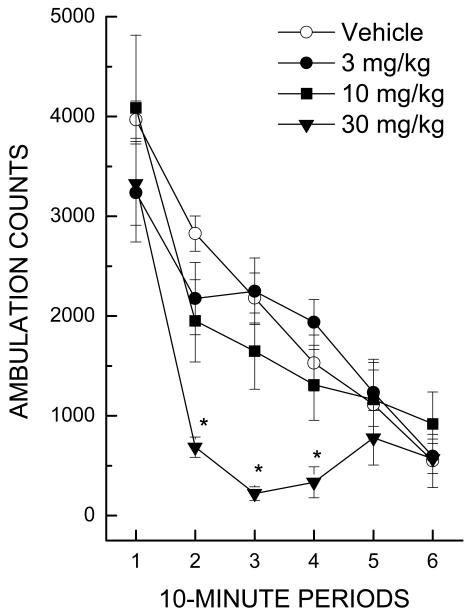
The 30 mg/kg dose of propofol yielded depression of locomotor activity beginning after 10 min and lasting approximately 30 min (Figure 1). Maximal depressant effects were evident during the period from 20-30 min following injection, and activity had returned to baseline after 40 min [F(15,135)=3.94, p<.001; Treatment × Time].
Figure 1.
Effect of propofol on horizontal activity counts/10 min as a function of dose and time interval during a 60-min session. Individual comparisons with the vehicle group within the 10- to 40-min time period confirmed significant depression only for the 30 mg/kg dose (* indicates p< 0.05).
Discrimination
Drug-lever responding remained at chance levels for the group receiving 5 mg/kg propofol for 44 training sessions (22 drug and 22 vehicle), so the training dose was increased to 10 mg/kg. Most subjects in both groups reached the training criterion within 60 to 70 sessions. The GABA-A receptor positive modulators carisoprodol and chlordiazepoxide both partially substituted for the discriminative-stimulus effects of propofol (Table 1). Dizocilpine failed to substitute for the discriminative-stimulus effects of propofol, but decreased response rate [F(3,18)=4.96, NS]. The training dose of propofol did not depress rate of responding during training or in the subsequent substitution/antagonism tests. The GABA-A receptor antagonist pentylenetetrazol partially blocked the discriminative-stimulus effects of propofol without altering response rate [F(1,1)=0.03, NS]. Propofol partially substituted in carisoprodol-trained rats without altering response rate [F(1,5)=3.11, NS]. Higher doses were not used because they depressed response rates.
Table 1.
Maximum percent drug-appropriate responding (DAR) of each test compound and effects on rate of responding at that dose.
| Training Drug |
Test Compound | Dose (mg/kg) |
N Tes |
%DAR | Rate resp/s |
|---|---|---|---|---|---|
| Propofol | Vehicle | 0 | 8/8 | 15.2±12.3 | 0.882±0.115 |
| Propofol | Propofol | 1 | 8/8 | 35.6±17.4 | 1.03±0.070 |
| Propofol | Propofol | 2.5 | 8/8 | 35.3±17.1 | 1.122±0.129 |
| Propofol | Propofol | 5 | 8/8 | 46.6±16.4 | 1.225±0.126 |
| Propofol | Propofol | 10 | 7/7 | 90.7±8.3 | 0.992±0.067 |
| Propofol | Chlordiazepoxide | 10 | 5/5 | 64.8±21.4* | 1.296±0.224 |
| Propofol | Carisoprodol | 100 | 5/5 | 59.5±24.3* | 1.210±0.210 |
| Propofol | Dizocilpine | 0.1 | 5/5 | 34.1±17.0 | 0.376±0.216† |
| Propofol | Propofol + Pentylenetetrazol | 10 | 5/5 | 32.2±20.4 | 0.941±0.273 |
|
| |||||
| Carisoprodol | Vehicle | 0 | 6/6 | 1.2±0.8 | 1.171±0.192 |
| Carisoprodol | Carisoprodol | 100 | 6/6 | 99.2±0.8 | 1.139±0.279 |
| Carisoprodol | Propofol | 10 | 4/6 | 51.6±27.6* | 0.617±0.225 |
N Test = number of rats which completed the first fixed ratio / total number tested. resp/s = responses per second.
shows values that reached the criteria for partial substitution (≥40% and <80% drug-appropriate responding and not statistically different from the training drug).
shows response rates different from vehicle control.
Survival
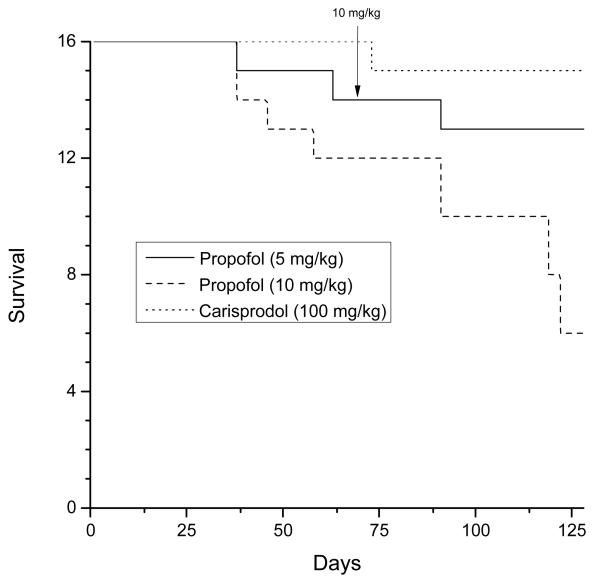
Substantial lethality was observed during these experiments. Three of sixteen rats died during training in the 5 mg/kg group. Ten of sixteen rats died in the 10 mg/kg group, whereas only one of thirty-two rats died during training in the carisoprodol-training group. A Tarone-Ware test indicated a significant effect [X2(2)= 8.38, p<0.02]. Additional rats died following training, such that only 8 of 32 propofol-trained rats completed testing. Accordingly, it was possible to test only single doses of each test compound in small groups of the surviving rats. Necropsy noted cardiomyocyte degeneration with inflammatory infiltrates and mineralization, and multifocal infiltrates of the foamy microphages in heart, spleen and kidney. The lung showed diffuse congestion of the vessels and edematous multifocal alveoli consistent with cardiovascular collapse.
Discussion
The current studies used drug-discrimination training to establish a rat model of the psychoactive effects of sub-anesthetic doses of propofol. Doses up to 10 mg/kg failed to produce significant sedation in locomotor activity studies in mice. Rats subsequently trained at this dose learned to discriminate propofol from saline and showed no evidence of sedation, as reflected in their rates of responding. A 5 mg/kg dose was not discriminable by the rats in the present study, possibly indicating a relatively narrow dose range for a “non-sedative” discriminative effect. The results are in accordance with other studies showing that centrally acting anesthetics are discriminable (e.g., Shelton, 2010; Shelton and Nicholson, 2010).
Extensive testing of the pharmacological mechanisms of the propofol discriminative stimulus was not possible due to the high mortality produced by propofol. Compounds acting via GABA or NMDA receptors were tested at selected doses that yielded maximal effects in previous drug discrimination studies without suppressing response rates: carisoprodol (100 mg/kg), chlordiazepoxide (10 mg/kg), pentylenetetrazol (10 mg/kg) and dizocilpine (0.1 mg/kg) (Gatch et al., 2005; Gatch and Pratt, 2006; Gonzalez et al., 2009). The propofol discriminative stimulus was most similar to that of carisoprodol, a barbiturate-like compound (Gonzalez et al., 2009) and to chlordiazepoxide, a benzodiazepine. Pentylenetetrazol, a GABA-A receptor antagonist, partially blocked the discriminative-stimulus effects of propofol. These findings are in agreement with earlier reports that propofol acts at GABA-A receptors (Concas et al., 1991; Peduto et al., 1991), and has discriminative-stimulus effects similar to muscimol (Jones and Balster, 1998), although propofol does not substitute for midazolam (Ator, 2003).
Although these findings suggest that the discriminative-stimulus effects of propofol are mediated in part by the GABA-A receptor, propofol also binds to dopamine D2 and NMDA receptors (Schulte et al., 2000). The NMDA receptor antagonist dizocilpine produced only 34% propofol-appropriate responding in the present study. However, propofol modulated the effects of NMDA receptors in other studies (Orser et al., 1995; Li et al., 2008), so further testing may reveal a glutamatergic component of the propofol discriminative stimulus.
The drug-discrimination training in this study exposed the rats only intermittently to non-sedating doses of propofol, yet produced considerable mortality over time. The mortality was dose-dependent, as 10 mg/kg propofol produced substantially more mortality than 5 mg/kg; however, switching to 10 mg/kg did not appear to increase mortality. The reason for this is not clear, but it is possible that gradually increasing the dose confers tolerance to the lethal effects. The propofol-associated mortality was not related to the vehicle (2% methylcellulose) or to training of a sedative drug, per se, as the comparison group was matched on these characteristics.
It seems noteworthy that the cardiac myopathy and lung lesions observed in association with intermittent propofol at 10 mg/kg are consistent with abnormalities observed in propofol-infusion syndrome (PRIS), an often-fatal complication of extended propofol infusion in humans (Vasile et al., 2003; Zaccheo and Bucher, 2008; Riezzo et al., 2009). PRIS is characterized by cardiac and renal failure, myopathies of both cardiac and skeletal muscle, and metabolic acidosis. Myocytic degeneration in PRIS has been linked to the ability of propofol to inhibit mitochondrial energy production in heart and skeletal muscle, particularly when combined with glucocorticoids or catecholamines (Vasile et al., 2003; Zaccheo and Bucher, 2008). Because PRIS has been reported mainly in critically-ill patients requiring prolonged sedation, the causes of mortality observed in the current study may differ. However, it is clear that abuse of propofol in humans is associated with a substantial risk of mortality (Wischmeyer et al., 2007), and at least one case of PRIS has been reported (Riezzo et al., 2009). Further, even though short-term exposure to propofol produces neuroprotection, long-term exposure leads to increasing neurodegeneration in rat neuronal cultures (Berns et al., 2009). It may be significant that it took approximately a month before mortality occurred in the present study. Mortality was not reported in the conditioned place preference (Pain et al., 1996, 1997), or self-administration (Weerts et al., 1999; LeSage et al., 2000; Blokhina et al., 2004) studies. It is possible that in these investigations, propofol was not administered over a long enough period of time for significant problems to develop or warrant reporting.
Given that propofol produces a discriminable stimulus similar to known drugs of abuse, establishes a conditioned place preference (Pain et al., 1996, 1997), and can be self-administered (Weerts et al., 1999; LeSage et al., 2000; Blokhina et al., 2004), there is evidence confirming its abuse liability in animal models. This evidence, coupled with its apparent toxicity when administered repeatedly, suggest that the potential for continued abuse of propofol should be of particular concern.
Figure 2.
Effect of propofol on survival as a function of time. Each group started with 16 rats. The carisoprodol group was selected to act as a control because it was being trained at approximately the same time and because carisoprodol is suspended in the same vehicle as propofol (2% methylcellulose). The arrow indicates when the Propofol 5 mg/kg group was switched to 10 mg/kg.
Acknowledgments
Funding was provided by the National Institute on Drug Abuse (NIH N01DA-7-8872).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ator NA. Selectivity in generalization to GABAergic drugs in midazolam-trained baboons. Pharmacol Biochem Behav. 2003;75:435–445. doi: 10.1016/s0091-3057(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Berns M, Seeberg L, Schmidt M, Kerner T. High-dose propofol triggers short-term neuroprotection and long-term neurodegeneration in primary neuronal cultures from rat embryos. J Int Med Res. 2009;37:680–688. doi: 10.1177/147323000903700311. [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Dravolina OA, Bespalov AY, Balster RL, Zvartau EE. Intravenous self-administration of abused solvents and anesthetics in mice. Eur J Pharmacol. 2004;485:211–218. doi: 10.1016/j.ejphar.2003.11.068. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacology Biochemistry and Behavior. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Concas A, Santoro G, Serra M, Sanna E, Biggio G. Neurochemical action of the general anaesthetic propofol on the chloride channel coupled with GABAA receptors. Brain Res. 1991;542:225–232. doi: 10.1016/0006-8993(91)91571-h. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Pratt R. Ethanol modulates the discriminative stimulus effects of methamphetamine. In: Toolaney GH, editor. New Research on Methamphetamine Abuse. Nova Science Publishers, Inc.; Hauppauge, NY: 2006. [Google Scholar]
- Gatch MB, Selvig M, Forster MJ. GABAergic modulation of the discriminative stimulus effects of methamphetamine. Behav. Pharmacol. 2005;16:261–266. doi: 10.1097/01.fbp.0000166464.68186.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LA, Gatch MB, Taylor CM, Bell-Horner CL, Forster MJ, Dillon GH. Carisoprodol-mediated modulation of GABAA receptors: in vitro and in vivo studies. J Pharmacol Exp Ther. 2009;329:827–837. doi: 10.1124/jpet.109.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Balster RL. Muscimol-like discriminative stimulus effects of GABA agonists in rats. Pharmacology Biochemistry and Behavior. 1998;59:319–326. doi: 10.1016/s0091-3057(97)00413-9. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Stafford D, Glowa JR. Abuse liability of the anesthetic propofol: self-administration of propofol in rats under fixed-ratio schedules of drug delivery. Psychopharmacology (Berl) 2000;153:148–154. doi: 10.1007/s002130000430. [DOI] [PubMed] [Google Scholar]
- Li KY, Xiao C, Xiong M, Delphin E, Ye JH. Nanomolar propofol stimulates glutamate transmission to dopamine neurons: a possible mechanism of abuse potential? J Pharmacol Exp Ther. 2008;325:165–174. doi: 10.1124/jpet.107.132472. [DOI] [PubMed] [Google Scholar]
- Orser BA, Bertlik M, Wang LY, MacDonald JF. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmocol. 1995;116:1761–1768. doi: 10.1111/j.1476-5381.1995.tb16660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain L, Oberling P, Sandner G, Di Scala G. Effect of propofol on affective state as assessed by place conditioning paradigm in rats. Anesthesiology. 1996;85:121–128. doi: 10.1097/00000542-199607000-00017. [DOI] [PubMed] [Google Scholar]
- Pain L, Oberling P, Sandner G, Di Scala G. Effect of midazolam on propofol-induced positive affective state assessed by place conditioning in rats. Anesthesiology. 1997;87:935–943. doi: 10.1097/00000542-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Peduto VA, Concas A, Santoro G, Biggio G, Gessa GL. Biochemical and biophysiological evidence that propofol enhances GABAergic transmission in the rat brain. Anesthesiology. 1991;75:1000–1009. doi: 10.1097/00000542-199112000-00012. [DOI] [PubMed] [Google Scholar]
- Riezzo I, Centini F, Neri M, Rossi G, Spanoudaki E, Turillazzi E, Fineschi V. Brugada-like EKG pattern and myocardial effects in a chronic propofol abuser. Clin Toxicol (Phila) 2009;47:258–363. doi: 10.1080/15563650902887842. [DOI] [PubMed] [Google Scholar]
- Schulte D, Callado LF, Davidson C, Phillips PE, Roewer N, Schulte am Esch J, Stamford JA. Propofol decreases stimulated dopamine release in the rat nucleus accumbens by a mechanism independent of dopamine D2, GABAA and NMDA receptors. Br J Anaesth. 2000;84:250–253. doi: 10.1093/oxfordjournals.bja.a013413. [DOI] [PubMed] [Google Scholar]
- Shelton KL. Pharmacological characterization of the discriminative stimulus of inhaled 1,1,1-trichloroethane. J Pharmacol Exp Ther. 2010;333:612–620. doi: 10.1124/jpet.109.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL, Nicholson KL. GABA(A) positive modulator and NMDA antagonist-like discriminative stimulus effects of isoflurane vapor in mice. Psychopharmacology (Berl) 2010;212:559–569. doi: 10.1007/s00213-010-1979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29:1417–1425. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Griffiths RR. Comparison of the intravenous reinforcing effects of propofol and methohexital in baboons. Drug Alcohol Depend. 1999;57:51–60. doi: 10.1016/s0376-8716(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Wilson C, Canning P, Caravati EM. The abuse potential of propofol. Clin Toxicol (Phila) 2010;48:165–170. doi: 10.3109/15563651003757954. [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE, Johnson BR, Wilson JE, Dingmann C, Bachman HM, Roller E, Tran ZV, Henthorn TK. A survey of propofol abuse in academic anesthesia programs. Anesth Analg. 2007;105:1066–1071. doi: 10.1213/01.ane.0000270215.86253.30. [DOI] [PubMed] [Google Scholar]
- Zaccheo MM, Bucher DH. Propofol infusion syndrome: a rare complication with potentially fatal results. Crit Care Nurse. 2008;28:18–26. quiz 27. [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Thompson W, Apfelbaum JL. Propofol at a subanesthetic dose may have abuse potential in healthy volunteers. Anesth Analg. 1993;77:544–552. doi: 10.1213/00000539-199309000-00020. [DOI] [PubMed] [Google Scholar]