Abstract
Chemokines, small pro-inflammatory chemoattractant cytokines that bind to specific G-protein coupled seven-span transmembrane receptors (GPCRs), are major regulators of cell trafficking and adhesion. The chemokine CXCL12 [also called stromal-derived factor-1 (SDF-1)] is an important α-chemokine that binds primarily to its cognate receptor CXCR4 and thus regulates the trafficking of normal and malignant cells. For many years it was believed that CXCR4 was the only receptor for CXCL12. Yet recent work has demonstrated that CXCL12 also binds to another seven-transmembrane span receptor called CXCR7. Our group and others have established critical roles for CXCR4 and CXCR7 on mediating tumor metastasis in several types of cancers, in addition to their contributions as biomarkers of tumor behavior as well as potential therapeutic targets. Here we review the current concepts regarding the role of CXCL12/CXCR4/CXCR7 axis activation, which regulates the pattern of tumor growth and metastatic spread to organs expressing high levels of CXCL12 to develop secondary tumors. We also summarize recent therapeutic approaches to target these receptors and/or their ligands.
1. Introduction
Chemokines are a superfamily of chemoattracting, cytokine-like proteins that bind to and activate a family of chemokine receptors. Over 50 chemokines have been identified, and they are divided into 4 families (CXC, CX3C, CC, and C) on the basis of the positions of 4 conserved cysteine residues [1].
Chemokine receptors are seven-transmemberane receptors coupled to G-proteins, all with their N-terminus outside the cell surface, three extracellular and three intracellular loops as well as a C-terminus in the cytoplasm. One of the intracellular loops of the chemokine receptors couples with heterotrimeric G-proteins, and that mediate ligand binding to the receptor which initiates a cascade of signal transduction events [2].
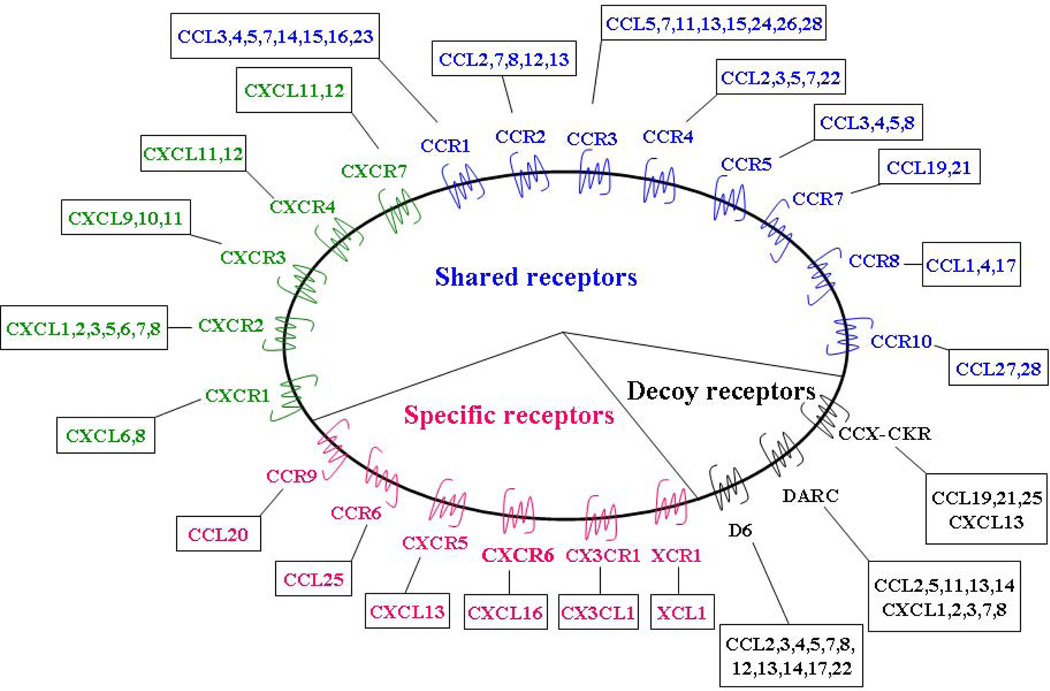
Most chemokine receptors are promiscuous as each can bind with high affinity to multiple chemokine ligands (CXCR, CCR, XCR, and CX3CR). As a result there is a high degree of redundancy in the chemokine family as multiple chemokines bind to the same receptor [3]. This feature may represent an essential feature for fine tuning of specific responses. In general, the CC receptors are more promiscuous than the CXC receptors [4]. Some chemokines bind to multiple receptors and some receptors in turn bind multiple chemokines, whereas certain chemokines interact with single receptor and some receptors bind only one chemokine. (Fig. 1).
Figure 1. Chemokine family and their cognate receptors.
Most chemokines can bind multiple receptors, and a single receptor can bind multiple chemokines. As shown in this case for most CC (green) and CXC (blue) chemokines. Decoy receptors (black) can also interact with multiple chemokines. By contrast, a minority of receptors (red) have only one ligand.
To date, at least 20 chemokine receptors (CCR1-11, CXCR1-7, XCR1, and CX3CR1) have been identified. Chemokines and their receptors are now known to play important roles in inflammation, infection, tissue injury, allergy, cardiovascular diseases, and malignant tumors [5].
One of the most intriguing and perhaps important roles that chemokines and the chemokine receptors have is in regulating metastasis. Here, chemokine receptors may potentially facilitate tumor dissemination at each of the key steps of metastasis, including adherence of tumor cells to endothelium, extravasation from blood vessels, metastatic colonization, angiogenesis, proliferation, and protection from the host response via activation of key survival pathways such as ERK/MAPK, PI-3K/Akt/mTOR, or Jak/STAT, et al [6–8]. In addition, it is increasingly recognized that chemokines play an important role in facilitating communication between cancer cells and non-neoplatic cells in the tumor microenvironment(TME), including endothelial cells and fibroblasts, promoting the infiltration, activation of neutrophils and tumor-associated macrophages (TAMs) within the TME [9,10]. In this review, we mainly focus on the roles of chemokines CXCL12 and its cognate receptors CXCR4 and CXCR7 as they pertain to cancer progression.
2. CXCL12, CXCR4 and CXCR7
2.1 CXCL12/SDF-1
Stromal-derived factor-1 (SDF-1 or CXCL12) is a CXC chemokine. It was first cloned from a bone marrow-derived stromal cell line and was later identified as a pre-B-cell growth stimulating factor (PBSF). CXCL12 is broadly expressed in a variety of tissue types where it acts as a potent chemoattractant for immature and mature hematopoietic cells [11,12]. CXCL12 has been identified as playing an important role in the homing of hematopoietic stem cells to the bone marrow and mediate the survival as well as the proliferation of human and murine progenitor cells [13–15]. CXCL12 has two major isoforms, α and β [16]. Both are derived from a single gene, while CXCL12β differs by an additional four amino acids (RLKM) at the C-terminal end due to alternative splicing [17]. CXCL12α is the predominant isoform secreted by marrow stromal cells and endothelial cells and is found in nearly all organs. The α-isoform secretion enhances tissue damage but undergoes rapid proteolysis in blood [18]. In contrast, the β-isoform is more resistant to blood-dependent degradation, stimulates angiogenesis and is present in highly vascularized organs such as liver, spleen, and kidneys [18]. The secretion of CXCL12 within or around injured tissues is a crucial event that may create a microenvironment which faciliates the homing of circulating endothelial tissue-committed stem cells (TCSCs) and the affected tissue resulting in organ regeneration or tissue repair [19].
Four additional human isoforms of CXCL12 have been reported, all with chemotactic activities [20]. They are derived from alternative splicing events sharing the same first three exons, but use different fourth exons, described as CXCL12γ (gamma), CXCL12δ (delta), CXCL12ε (epsilon) and CXCL12φ (phi) [20]. CXCL12γ is located in very active, less vascularized organs susceptible to infarction such as the heart and the brain [18]. CXCL12δ is predicted to be more than 50% longer than CXCL12α [20]. Whether the additional amino acids affect affinity or activity of this ligand for its receptor remains unclear.
In bone marrow, CXCL12, is mainly produced by osteoblasts lining the bone endosteum [21–23], and regulates the migration of CD34+ cells, where the chemoattractant activity is significantly inhibited by pretreatment of CD34+ cells with either neutralizing anti-CXCR4 antibodies or neutralizing CXCR4 peptides [22], suggesting the existence of CXCL12/CXCR4 functional binding on CD34+ cells. CXCL12 preferentially induced the migration of repopulating cells of SCID mice [24]. Consistently, human CD34+ cells are capable of repopulating the marrow of NOD/SCID mice with multilineage hematopoiesis [25]. Interestingly, DNA-damaging agents such as irradiation, cyclophosphamide, or 5-fluorouracil increase CXCL12 expression in both mouse marrow and in cultured cells [25,26]. The CXCL12 promoter contains two HIF-1α binding sites, and CXCL12 expression is enhanced in endothelial cells expressing HIF-1α [27]. Elevation of HIF-1α expression in either hypoxic or damaged tissues results in the elevation of CXCL12 levels which results in the chemoattraction of CXCR4+ tissue-committed stem cells (TCSCs) that participate in tissue regeneration [27].
2.2 CXCL12 receptors
2.2.1 CXCR4
CXCR4 is a highly conserved seven-span transmembrane GPCR that binds the ligand CXCL12α. CXCR4 has received considerable attention since it serves as a co-receptor for entry of T-tropic (X4) HIV viruses that target CD4+ T cells [28,29]. During development, CXCR4 is expressed in a broad range of tissues, including immune and the central nervous systems and can mediate migration of resting leukocytes and hematopoietic progenitors in response to CXCL12 functioning in a number of physiological processes [30–33]. In the immune system, CXCR4 is highly expressed by monocytes, B cells, and naïve T cells in peripheral blood as well as early hematopoietic progenitor cells in bone marrow [34]. Differential expression of CXCR4 in CD34+ progenitor cells may be involved in maintaining hematopoietic progenitor cells in the marrow and regulating stem cell trafficking [35]. Studies have demonstrated that CXCL12 / CXCR4 axis plays an important role in development. For example, the deficiency of this axis leads to circulatory, CNS, immune, and hematopoietic defects [36,37].
Once CXCL12 binds to CXCR4, the receptor forms a complex with the Gαi subunit G protein, resulting in inhibition of adenylyl cyclase–mediated cyclic adenosine monophosphate (cAMP) production and mobilization of intracellular calcium. Dissociation of the Gαi subunit from Gβγ leads to activation of multiple downstream targets, including ERK1/2, MAPK, JNK, and AKT effectors [38–41]. Ligand-stimulated chemotaxis is accompanied by cytoskeletal rearrangements, actin polymerization, polarization, pseudopodia formation, and integrin-dependent adhesion to endothelial cells and other biologic substrates.
To date, CXCR4 is one of the most common chemokine receptor that has been demonstrated to be over expressed in more over 23 human cancers, including breast cancer, ovarian cancer, melanoma, and prostate cancer (PCa) (as reviewed in [42]), Although CXCR4 is expressed in a broad array of tissues, CXCR4 expression is low or absent in many normal tissues, including breast [43] and ovary [44].
CXCR4 expression is up-regulated in malignant cells via several mechanisms. VEGF is a known inducer of CXCR4 expression, and it has been shown that HIF-1 acts upstream to induce VEGF [45]. HIF-1 is a heterodimeric transcription factor responsive to oxygen concentrations in tissues and has been shown to up-regulate CXCR4 expression. Thus, in hypoxic regions of expanding tumors, chemokine receptor levels might be increased to facilitate survival and escape from the primary tumor mass. In addition to facilitating distant metastasis, HIF-1 has been shown to induce CXCR4 in gliomas, leading to enhanced proliferation, resistance to apoptosis, and local invasion [45].
2.2.2 CXCR7/RDC-1
The concept of an exclusive interaction of CXCL12 with CXCR4 was challenged after it was noticed that murine fetal liver cells from CXCR4 knockout mice still may bind CXCL12 [46]. In addition, discrepancies were observed between CXCR4 expression and CXCL12 binding affinity in several human cancer cell lines [46]. These observations suggest a presence of another CXCL12 binding receptor. This receptor was recently identified and named CXCR7 (or RDC-1) [46,47], which was originally cloned on the basis of its homology with conserved domains of GPCRs [48]. The CXCR7/RDC-1 gene maps to mouse chromosome 1 and human chromosome 2, where the genes encoding CXCR1, CXCR2, and CXCR4 are located. CXCR7/RDC-1 shares homology with the viral gene ORF74 encoding a chemokine receptor that suggests that it may signal constitutively in the absence of ligand. In addition to CXCL12, CXCR7 binds with low affinity to the chemokine CXCL11 (I-TAC) [46]. CXCR7 expression has been found in T lymphocytes and during CXCL12-mediated chemotaxis [47]. CXCR7 expression is tightly regulated in B cell development and differentiation. The expression of CXCR7 correlates with the ability of B cells to differentiate into plasma cells upon activation, suggesting that CXCR7 is a marker for memory B cells, which are competent to become antibody secreting cells [49,50].
CXCR7 expression has been shown to be elevated in endothelial cells associated with tumors [51]. Membrane-associated CXCR7 is expressed on many tumor cell lines, on activated endothelial cells, and on fetal liver cells [46]. CXCR7 is expressed by the placenta [52] as well as in the vascular endothelium [53,54]. These characteristics suggest that like CXCR4, CXCR7 plays a role in regulating immunity, angiogenesis, stem cell trafficking, and mediating organ-specific metastases of cancer..
However, growing evidence has suggested that CXCR7 functions as a decoy receptor, which does not activate Gi pathways of a chemokine receptor that would result in GTP hydrolysis or calcium mobilization [46]. Yet CXCR7 significantly increases cell proliferation and elevates cellular adhesion property in other conditions [46,50,53,55]. Thus debate has arisen whether CXCR7 functions like GPCR mediating the signal transduction process [56]. As a result, several mechanisms underlying CXCR7 function have been proposed. One role that CXCR7 may play is to scavenge or sequester CXCL12α, thereby generating gradients of CXCL12α that lead to differential signaling by CXCR4 [57,58]. Another role for the receptor is that it may serve as a co-receptor for CXCR4 [59,60] and enhances CXCL12-mediate G protein signaling [59], as the two receptors form heterodimers in the context of overexpression in transiently transfected cells. These observations suggest that ligand binding to CXCR7 results in crosstalk with CXCR4 mediated by intracellular signaling molecules [61]. More recently, it has been demonstrated that CXCR7 interacts with β-arrestin in a ligand dependent manner [56,62,63]. CXCR7 can signal through β-arrestin and act as an endogenous β-arrestin-biased receptor, which suggests that other receptors that are currently thought to be orphans or decoys may also signal through non-G protein-mediated mechanisms [56].
2.3 CXCL12 and CXCR4/CXCR7 axis in cancers
2.3.1 Prostate cancer (PCa)
The binding of CXCL12 to CXCR4 initiates divergent signaling pathways downstream of ligand binding, which can lead to multiple responses. One mechanism as to how PCa utilizes the CXCR4 receptor is whether CXCL12 act as a growth factor? Kukreja et al. reported that CXCL12 treatment of PC3 human PCa cells lead to MEK, IKK and IκBα phosphorylation [64]. This leads to nuclear localization of NF-κB followed by transcription and expression of CXCR4. These data suggest that CXCL12α-induced expression of CXCR4 in PC3 cells is dependent on MEK/ERK signaling cascade and NF-κB activation which is critical for tumor cell survival. Further works are needed to explore the network of signaling pathways triggered by CXCL12/CXCR4 axis.
Previous works by our group and others have found that CXCL12/CXCR4 axis plays a central role in PCa progression. Sun et al. showed that the levels of CXCL12 in human and mouse tissues were higher in the preferable sites of metastasis for PCa cells (i.e. bone, liver, and kidney), compared with tissues rarely affected (i.e. lung, tongue, and eye) [65]. CXCL12 was localized to the metaphysis of the long bones, nearest the growth plate, but not in the center of bone marrow [65]. In addition, CXCL12 increased the adhesion of PCa cells to an endothelial cell monolayer and to immobilized fibronectin, laminin, and collagen [66,67], and to osteosarcoma cells [23]. This might occur through an up-regulation of α5 and β3 integrins [66]. In an in vivo metastasis model, neutralizing antibody against CXCR4 limited the extent of bone metastases and the antibody and blocking peptide of CXCR4 limited the growth of intraosseous prostate cancer cells after intratibial injections [65]. These data provided evidence that CXCL12/CXCR4 participates in localizing tumors to the bone marrow in PCa [23,65,68].
Darash-Yahana et al., demonstrated that PCa tumor-associated blood vessels and basal cell hyperplasia expressed CXCL12 [69]. Subcutaneous xenografts of PC3 cells that over expressed CXCR4 in a mouse model were demonstrated significantly greater blood vessel density, functionality, invasiveness of tumors into the surrounding tissues. Neutralizing the interactions of CXCL12/CXCR4 with antibodies against CXCR4 inhibited the CXCR4-dependent tumor growth and vascularization [69]. Moreover, CXCL12 was report to trigger an angiogenic switch, through the up-regulation of vascular endothelial growth factor (VEGF) and CXCL8, as demonstrated using CXCR4 siRNA [70]. Interestingly, CXCL12 down-regulated the glycolytic enzyme phosphoglycerate kinase 1, which was a negative regulator of VEGF and CXCL8 expression [71].
In the tumor microenvironment, cultured prostate cancer-associated fibroblasts (CAFs) expressing high levels of CD90 (hi) were isolated and analyzed for a tumor-promoting phenotype. Co-culture or conditioned medium from CD90(hi) cells increased CXCR4 expression in BPH-1 epithelial cells, at least in part due to TGF-beta, and protected BPH-1 cells from apoptosis [72]. These data suggest that CAFs may get involved in tumor progression.
Although expression of CXCR4 was found on almost all types of tissue-committed stem cells in the body [73], CXCR4 identified on PCa stem cells remains uncertain. One group reported that in the invasive front of pancreatic tumors, a distinct subpopulation of CD133+ CXCR4+ cancer stem cells was identified that determines the metastatic phenotype of the individual tumor [74]. Similarly, the role of CD133+ CXCR4+ and/or CXCR7+ PCa stem cells is not yet known. So far, functional CXCR7 was only identified on renal progenitor cells [75].
We recently reported that CXCR7 is highly expressed on human PCa cells [54]. Staining of high-density tissue microarrays demonstrated that CXCR7 expression at the protein level was greater on aggressive tumors. Furthermore, studies on established PCa cell lines revealed that CXCR7 regulated cell proliferation most likely because of the enhanced cell survival, adhesion, and chemotaxis. In addition, CXCR7 increased expression of proangiopoietic factors such as IL-8 and VEGF [54]. Interestingly, the CXCR7 levels are influenced by CXCR4 activation. Evidence was also found that CXCR7 signaling in PCa cell lines results in phosphorylation of serine/threonine kinase Akt. PCa cells over-expressing CXCR7 grew faster with a higher density of neovessels in an immunodeficient mouse model [54]. Indeed, because hypoxia induces CXCR7 by endothelial cells, and is expressed by tumor-associated vessels, the contribution of CXCR7 in neoangiogenesis and formation of tumor vasculature is likely. It is not clear however at this point how important the interaction between CXCL12 and CXCR7 is in chemoattraction of endothelial progenitors.
Furthermore, our group recently found that hypermethylated in cancer (HIC1), a suppressor gene, is a potential upstream target of CXCR7, which is generally silenced by hypermethylated in CpG islands in PCa. Luciferase promoter assays revealed that a functional conserved HiRE (HIC-responsive element) in PCa cells inversely regulated CXCR7 expression (unpublished data). These observations raise a possibility that alteration of HIC1 expression by epigenetic approach may inhibit CXCR7 level in cancer cells, which provide an optional proposal in cancer treatment.
2.3.2. Breast cancer
Muller et al. reported in breast cancer that CXCR4 and CXCL12 are central players in regulating metastasis by showing that normal breast tissues express little CXCR4, whereas breast neoplasms express high levels of CXCR4 [43]. CXCR4 signaling in response to CXCL12 mediates actin polymerization and pseudopodia formation, and subsequently induces chemotactic and invasive responses [43]. These data formed the basis of the hypothesis that malignant cells may employ chemokine receptors to migrate toward chemokine ligands expressed at common metastatic sites, such as the lungs, bone marrow, and lymph nodes. Indeed, CXCR4 appears to be one of a limited number of genes that are enriched in a subpopulation of metastatic breast cancer cells, as over expression of CXCR4 alone significantly increased numbers of bone metastases in vivo [76]. Supporting evidence for the hypothesis was demonstrated by Liang et al., as blocking CXCR4 expression by siRNAs decreased breast cancer cell invasion in an in vitro assay and inhibited metastasis in an animal model [77]. Interestingly, the CXCR4 carboxy-terminal domain (CTD) appears to play a major role in regulating receptor desensitization and down-regulation [78]. Whereas deletion of the C-terminal domain of CXCR4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells [78].
Elucidation of the underlying mechanisms of breast cancer invasion and metastasis focused on CXCR4 has resulted in several important observations. Ligand-binding studies indicate that the number and affinity of CXCR4 receptors are similar in nonmetastatic cells versus highly metastatic cells. In metastatic cells, CXCL12 binding to the Gαβγ/GDP protein complex leads to a GTP-for-GDP exchange, allowing Gαi to dissociate from the Gβγ subunit, leading to activation of ERK1/2, IκBα, JNK, Akt, p38 MAPK, and GSK-3αβ. In nonmetastatic cells, CXCR4 is able to independently form a complex with Gαi or Gβ subunits, but no Gαβγ heterotrimer could be associated with CXCR4 and, ultimately, Gβγ-dependent downstream signaling did not occur [79]. Although the molecular basis for the difference in G protein signaling in metastatic versus nonmetastatic cells remains to be elucidated, these studies have implications for clinical studies that are examining CXCR4 protein expression but not receptor function. As observed in breast cancer cell lines, detection of CXCR4 protein does not necessarily indicate CXCR4-mediated signaling [80].
There is increasing evidence that CXCR4 interacts with several growth factor receptor tyrosine kinases. Upon activating IGF-1R, IGF-1 was shown to transactivate CXCR4 signal transduction in metastatic MDA-MB-231 cells but not to activate nonmetastatic MCF-7 cells, even though both cell lines are positive for IGF-1R and CXCR4 [81]. Myofibroblasts associated with breast cancer, but not those in normal breast tissue, produce CXCL12 and enhance growth of tumors through mechanisms that include proliferation and survival of malignant cells and angiogenesis [9,82]. Specific alleles of CXCL12 are associated with an increases risk of breast cancer [83], and CXCL12 has been shown to transactivate Her2/neu, an established oncogene in breast cancer [84].
A role of CXCR7 in cell growth/survival was first indicated by an observation that CXCR7-transfected MDA-MB-435s cells expanded more rapidly in culture than the control cells [46]. Expression of CXCR7 confers a survival advantage to cells that becomes experimentally evident using tissue culture conditions that are suboptimal for cell growth [46]. In the same study, CXCR7 transfectant cells adhered to the activated human umbilical vein endothelial cell (HUVECs) markedly more than did the wild-type cells and even showed some adherence to unstimulated HUVECs [46]. Further data revealed that the HUVECs up-regulate CXCR7 expression after in vitro stimulation with TNF-α and IL-1β. Increased CXCR7 mRNA expression was also observed after HUVEC activation. Similar results were obtained using endothelial cells derived from lung, heart, and various other tissues [46]. Likewise, MDA-MB-435s CXCR7-transfected cells formed larger tumors than wild type or MDA-MB-435s vector-transfected control cells [53], In the mouse 4T1 cells animal model, CXCR7 expression dramatically enhances growth of cell-derived breast tumors [53]. Moreover, expression of CXCR7 on breast cancer cells enhances ability of these cells to seed and proliferate in lung metastases [53]. Another study has shown that high levels of CXCR7 in breast cancer facilitates cancer cells ability pass through the hemato-encephalic barrier, which lead to brain metastasis [85].
2.3.3 Lung cancer
Small cell lung cancer (SCLC) is an aggressive and rapidly metastasizing neoplasm with a high propensity for marrow involvement. Burger et al. reported that SCLC cells expressed high levels of functional CXCR4 receptors, and demonstrated CXCR4 activation induced migratory and invasive responses and adhesion to marrow stromal cells in a CXCR4- and integrin-dependent fashion [86,87]. CXCL12/CXCR4 axis is the important mediators for the adherence of SCLC cells to ECM proteins like fibronectin, collagen I and VCAM-1 [88]. CXCR4-induced adhesion of SCLC cells to marrow stromal cells protects against etoposide-induced apoptosis [88]. The CXCR4 antagonist T140 and its derivates blocks the CXCR4-mediated adhesion to ECM components and thereby altered SCLC chemosensitivity [88]. Collectively, these studies indicate that expression of CXCR4 by SCLC cells facilitates the activation of integrins that regulate the adhesion of tumor cells to the marrow microenvironment, which in turn confers drug resistance and facilitates tumor cell growth. Moreover, CXCR4 may direct the distinct metastatic pattern observed in patients with SCLC [9].
Neoplastic cells in non-small cell lung cancer (NSCLC) may also express CXCR4. Interestingly, Su et al. found that differential expression of CXCR4 correlated with metastatic potential in vitro and in vivo, which suggests that the movement of NSCLC from primary sites to metastatic nodes may be dependent on the levels of CXCR4 [89]. As seen in other tumors, hypoxia is able to induce a significant increase in the expression levels of CXCR4 on NSCLC cells through the VHL-HIF-1α pathway, supporting the notion that HIF-1α-mediated upregulation of CXCR4 may be a common response of tumor cells to hypoxia [90,91].
CXCR7 immunostaining in lung cancer has been shown in samples obtained from multiple patients with squamous cell carcinomas but also its expression can be seen occasionally in lung adenocarcinomas [53]. In vascular endothelium, CXCR7 has been identified on a large percentage of tumor vasculature from human malignancies [53]. CXCR7 is also detected on the surface of murine breast Lewis lung carcinoma (LLC) cell lines and RNAi targeting CXCR7 results in smaller tumor formation than control transfected cells [53]. Similarly, the use of a CXCR7 antagonist, CCX754, develops markedly smaller tumors in mice than those found in animals receiving vehicle only [46]. These data demonstrated the CXCR7 has pro-tumor properties in lung cancer. Recently studies have shown that higher expression of CXCR7 is related to early and metastatic recurrence in pathological stage I NSCLC [92]. In keeping with these observations in patients with higher levels of CXCR7 expression, the EGFR mutations are frequently observed [92].
2.3.4 Pancreatic adenocarcinoma
Pancreatic cancer is currently the fourth leading cause for cancer-related mortality. The CXCL12/CXCR4 axis also has an important role in the pancreatic cancer progression through tumor cell migration and angiogenesis [93–96]. Based on the phenomena that undetectable CXCL12 expression was found in all examined pancreatic cancer cell lines, but was identified in all pancreatic cancer tissue samples [95,96], Gao et al. hypothesized that CXCL12/CXCR4 axis functions through paracrine mechanism [97]. The CXCR4+ pancreatic stellate cells (PSCs)-conditioned media promoted the proliferation, migration and invasion of pancreatic cancer cells while CXCR4 antagonist AMD3100 significantly inhibited these promotive effects [97]. The relationship between CXCR7 and pancreatic carcinoma not been extensively explored yet one study suggests that patients with CXCR4low/CXCR7low tumor had a significantly shorter 5-year disease-free survival (DFS) and overall survival (OS) than patients with a CXCR7high/CXCR4high tumor [98].
As mentioned above, the expression of CXCR4 is found in almost all types of tissue-committed stem cells in the body. Indeed, in human pancreatic cancer tissue contains cancer stem cells defined by CD133 expression that are exclusively tumorigenic and highly resistant to standard chemotherapy. In the invasive front of pancreatic tumors, a distinct subpopulation of CD133+ CXCR4+ cancer stem cells had been identified that determines the metastatic phenotype of the individual tumor. Depletion of the cancer stem cell pool for these migrating cancer stem cells significantly reduces the metastatic phenotype of pancreatic tumors without affecting their tumorigenic potential, which indicates that a subpopulation of migrating CD133+ CXCR4+ cancer stem cells is essential for tumor metastasis [74]. These intriguing findings may suggest the potential therapeutic target for this aggressive disease.
2.3.5 Other malignancies
CXCR4 activation by CXCL12 induces migration and/or survival of neuronal and glial tumors, neuroblastoma cells, colorectal cancer, head and neck, brain, bladder, esophagealcancer, melanoma, renal cell cancer, ovarian cancer, and rhabdomyosarcoma (RMS) [99]. In ovarian cancer, CXCR4 is a dominant chemokine receptor in cancer tissues [100]. CXCL12 has a direct effect on the proliferation of ovarian tumor cells, which are stimulated to grow in vitro in the presence of CXCL12 and this pro-proliferative effect can be blocked with neutralizing antibody against CXCR4 or with inhibitor of CXCR4 [101,102].
Zhou et al. demonstrated that over expression of CXCR4 in glioblastoma cell lines enhanced their growth in soft agar, and expression of anti-sense CXCR4 reduces neurite outgrowth and cellular differentiation [103]. Treatment of the glioblastoma cell lines with antibody to CXCR4 or CXCL12 inhibited proliferation, a finding that suggests that CXCR4 gene is required to prevent apoptosis following serum withdrawal [103,104]. Similarly, systemic administration of CXCR4 antagonists inhibits the growth of intracranial glioblastoma and medulloblastoma xenografts by increasing apoptosis and decreasing the proliferation of tumor cells [105].
In patients with colorectal cancer and melanoma, CXCR4 expression in primary tumor cells correlates with recurrence, metastasis, and survival [106]. Interestingly, in these cancer patients, the rate of bone metastasis is not as high as that of prostate and breast cancers suggesting that CXCL12/CXCR4 may not be the only mechanism required for the establishment and localization of tumors to the marrow [91,107,108]. Moreover, in some instances CXCR4 may not be responsible for invasion but rather is critical for the outgrowth of micrometastases [109].
CXCR4 and CXCR7 were both identified in rhabdomyosarcoma (RMS) cells, which play overlapping and distinct roles in regulating metastatic behavior [110,111]. CXCR4 was more highly expressed on the more metastatic alveolar RMS (ARMS) cell lines than on the less metastatic lines derived from embryonal RMS (ERMS) [111], yet the expression of CXCR7 is inverse [110]. Although CXCL12 did not affect proliferation or survival of these cell lines through CXCR4 or CXCR7, migration, chemotaxis and adhesion was often observed [111]. In other findings, it was observed that hypoxia enhances both CXCR4 and CXCR7 promoter activity and receptor expression in ERMS cells [112]. Moreover, CXCR7 RMS cells responded to CXCL12 and CXCL11 in the presence of CXCR4 antagonists (T140, AMD3100). Animals injected with RMS cells which overexpressed CXCR7 experienced increased seeding efficiency of the tumor cells into bone marrow, whereas CXCR7 down-regulation showed had the opposite effect. Thus, both CXCL12/CXCR4 and CXCL12/CXCL11/CXCR7 axis are involved in the progression of RMS.
In contrast to solid tumors that invade into the marrow, most hematopoietic malignancies originate in the marrow. In this niche, hematopoietic malignancies cells are in close contact with marrow stromal cells that provide growth and survival signals through surface-bound or secreted factors. CXCL12/CXCR4 dependence has been demonstrated in acute leukemia, multiple myeloma, B-cell chronic lymphocytic leukemia, and cute myelogenous leukemia [113]. As a result, several studies are now underway to determine the therapeutic potential of CXCR4 antagonists in combination with other conventional therapies [102,114].
2.3.6 Tumor microenviroment (TME)
Tumors arise from cells that have sustained genetic mutations resulting in deregulation of normal growth control mechanisms [115]. Research focused on the origins of cancer has identified that genetic mutations within tumor cells have resulted in the concept that neoplastic progression is a cell-autonomous process. However, it has become increasingly apparent that the microenvironment influences many steps in tumor progression [116]. Not only do the cancer cells interact with each other, they are also influenced by the extracellular matrix (ECM) and other microenvironmental cells including fibroblasts, endothelial cells, and inflammatory cells [116,117].
More increasing evidence suggests that the stroma actively contributes to the growth and invasion of malignant tumors [118–122]. In further support of this notion, it was recently suggested that of CXCL12 may play a critical role as a chemoattractant in cancer development possibly at the level of the tumor niche [123,124]. The data suggests that both CXCL12 expression by fibroblasts and CXCR4 expression on tumor cells, within hypoxic areas of tumors, trigger tumor cell growth, motility and invasiveness. Importantly, CAFs, but not normal fibroblasts, stimulate tumor progression through CXCL12 secretion. Interestingly, Begley et al. have demonstrated that fibroblasts derived from older men, express more CXCL12 than younger men, suggesting that prostate stroma of older men may be “primed” for PCa growth [122,123]. At the same time, CXCL12 from CAFs may induce the recruitment of endothelial progenitors, which allow for tumor angiogenesis [124]. Targeted metastasis to the marrow or other sites of high CXCL12 expression involves CXCR4 activation on circulating tumor cells that “hijack” the CXCL12/CXCR4 axis for homing to microenvironments. CXCL12 gradients attract CXCR4-positive tumor cells to marrow niches where marrow stromal cells secrete high levels of CXCL12. As a consequence, tumor cells can invade adjacent tissues, resulting in bone destruction [69,122,125].
In addition, the stroma cells from specialized microenvironments actually modulate CXCR4 expression, which is responsible for tumorigenesis and tumor progression. Ao et al. indicated that elevated stromal TGF-β elicits epithelial CXCR4 expression, allowing stromal SDF-1 to activate Akt pathway in the epithelial cells, which might interact initially to contribute to tumor formation and to set up conditions that would predispose cells to further malignant progression. P-Akt can then contribute to a loss of the growth-inhibitory response to TGF-β by blocking Smad-dependent manner [126]. These findings suggest that relatively small changes in specific molecular signals can alter the manner in which cells see and respond to their microenvironment. It also indicates that both of TGF-β and CXCL12/SDF-1 pathways are linked paracrine arbiters of tumorigenesis in CAF-driven tumorigenesis in vivo [126]. Similarly, IL-5 and IFN-γ are released from stromal cells and act as differential mediators for CXCR4 expression in neuroblastoma (NB) cell lines, which is associated with tumor metastasis in vivo [127]. Obviously, the tumor and stroma cell interactions is truly reciprocal; while stroma cells may support tumors, tumor cells in turn modulate the microenvironments within which they inside. It is notable that cancer is a systemic disease, encompassing multiple components of tumor and stroma cells that are a prerequisite for tumor cell invasion and metastasis (Fig2).
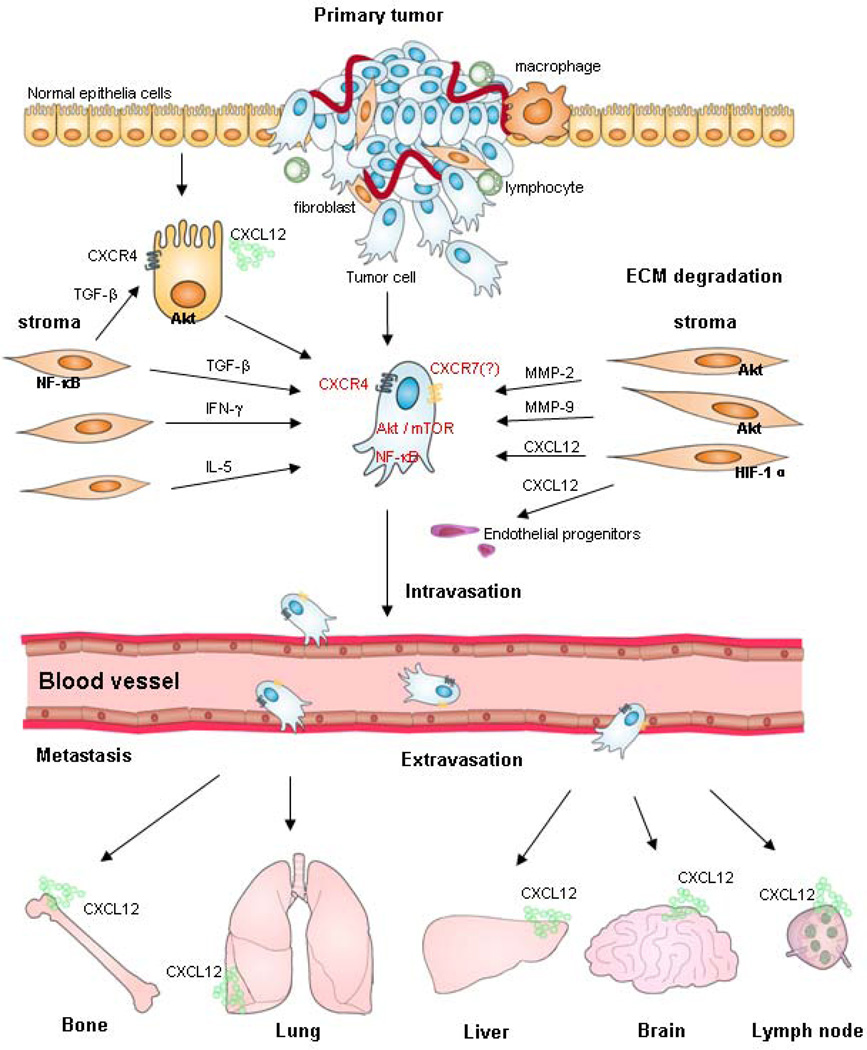
Figure 2. Schematic representation showing the role of microenvironment in tumor cell CXCR4 receptor activation in both the primary and metastatic sites.
Molecular cross-talks between stroma and tumor cells in microenviroment may upregulate CXCR4 expression, which involve in tumorigenesis, and tumor cell intravasation. By CXCL12 gradients chemoattrcting of organ secretion, CXCR4-positive tumor cells in circulation may be responsible for the process of extravasation, and organ-specific metastasis. Abbreviation: CXL12 (SDF-1), stroma-derived factor; TGF-β, transforming growth factor-β; ECM, extracellular matric; IFN-γ, interferon-γ; NF-κB, nuclear factor κB; MMP-2,9, matrix metalloproteinase-2,9.
3. Therapeutical targeting based on the chemokine axis
The CXCL12/CXCR4/CXCR7 axis is a potential target for therapeutics that blocks CXCL12/CXCR4 or CXCL12/CXCR7 interactions or which inhibit activities of downstream signaling. Currently, CXCR4 has been targeted by multiple antagonists, such as bicyclams (AMD3100), and T22, TN14003, CTCE-9908, ALX40-4C, which are analogues and peptides designed to the amino-terminal region of the chemokine, CXCL12.
Bicyclams are macrocyclic polyamines, were found to be potent and selective inhibitors of the X4 strains of HIV, a function that primarily depends on their affinity for CXCR4. Functional studies have shown that a strong and direct interaction occurs between CXCR4 and bicyclams without receptor internalization. This interaction inhibits CXCL12 binding and signaling through CXCR4 and is able to block the binding of the CXCR4 monoclonal antibodies at nanomolar concentrations. Bicyclams with an aromatic linker, for example, AMD3100, have also been demonstrated effectiveness in mobilization of CD34+ stem cells from the bone marrow for autologous transplantation in patient with leukemia or lymphoma and has fewer side effects than any current protocols [128]. At present AMD3100 is being clinical used as an orphan drug for these patients..
TN14003 is derived from amidating the carboxy terminus of T140 and by substituting basic residues with nonbasic polar amino acids to reduce the total-positive charges of the molecule. Liang et al. investigated the role of synthetic antagonist 14-mer peptide (TN14003) in inhibiting metastasis in an animal model [77]. Their data suggest that TN14003 is not only effective in limiting metastasis of breast cancer via inhibiting migration, but it may also prove useful as a diagnostic tool to identify CXCR4 receptor positive tumor cells in culture and tumors in paraffin embedded clinical samples. CTCE-9908 has shown both inhibition of the primary tumor and anti-metastatic effects in animal models of melanoma, osteosarcoma, breast, and prostate tumors[129–131]. In PCa, CTCE-9908 delivered intraperitoneally was also associated with inhibition of VEGF and angiogenesis, and reduced recruitment of myeloid host cells [130].
ALX40-4C, an oligocationic peptide, was originally developed to mimic the basic domain of the HIV-1 transactivation protein Tat in order to inhibit HIV-1 replication through inhibition of the Tat–Tar complex [132]. However, its main inhibitory effect is at the level of viral entry. This inhibition is due to its selective binding to the second extracellular loop of CXCR4 and abrogation of its use as a co-receptor for CXCR4-specific strains of HIV. ALX40-4C inhibits HIV-1 infection of T cell lines at nanomolar concentrations and also inhibits CXCL12 binding to CXCR4 at micromolar concentrations.
Based on observations as to the role of CXCR7 in progression and metastasis of several tumor types, therapies to block CXCR7 have been developed. Some small molecular inhibitors such as CCX733 or CCX266, siRNA, and blocking antibodies are already employed in experimental models in vitro and in vivo [61]. Yet the ability of CXCL12 to activate CXCR7 as well as CXCR4, raises some doubts as to whether the “selective blockage” of CXCR4 by T140 or AMD3100 without simultaneous blocking of CXCR7 will be effective. In fact, blockage of CXCR4 only partially inhibited responsiveness of tumor cells to CXCL12 gradients in several animal models [99,133,134]. Findings in our group also indicate that targeting of CXCR4 alone without simultaneous blockage of CXCR7 is likely to be an inefficient strategy for inhibiting CXCL12-mediated prometastatic responses of PCa cells (Unpublished data), suggesting that it may be more efficient to block some shared signaling molecules common to both receptors.
The development of a number of potent inhibitors of the CXCL12/CXCR4/CXCR7 axis, which have low toxicity, has opened the possibilities that investigations to disrupt this axis will have therapeutic benefit. While most studies are still in their infancy, there is great hope that agents that modulate the CXCL12/CXCR4 or CXCL12/CXCR7 axis will be useful in clinics. Importantly, the use of CXCR4/CXCL12 inhibitors in the treatment of cancer has produced some encouraging preclinical data. However, to be truly effective, a greater understanding of the role of CXCL12 and CXCR4 or CXCR7 in tumorigenesis and their other functions is required.
4. Conclusions
CXCL12 is primarily thought to regulate hematopoietic stem cell migration into and out of the bone marrow. CXCL12 is widely expressed in many tissues throughout development [135] and serves as a powerful chemoattractant for hematopoietic cells, where it facilitates their transmigration through endothelial cell barriers [136]. CXCL12 secretion by stomal cells in the tumor microenvironment is thought attract cancer cells via stimulation of the CXCR4 receptor that is up-regulated by tumor cells. CXCL12/CXCR4 activation regulates the pattern of metastatic spread with organs expressing high levels of CXCL12 developing secondary tumors (i.e., the bone marrow compartment). CXCL12 has a wide range of effects in regards to tumor development but the primary role of CXCL12 appears to be the facilitation of metastasis or mobilization of tumor cell, the recruitment of hematopoietic cells and perhaps the establishment of the cancer stem-like cell within the tumor microenvironment where high levels of CXCL12 recruit a highly tumorigenic population of tumor cells and promotes cell survival, proliferation, angiogenesis, and metastasis.
In contrast, CXCR7, the second CXCL12 receptor, may not induce Gαi-dependent calcium flux and receptor internalization. Similar to CXCR4, CXCR7 also serves as a co-receptor for several HIV strains and SIV, however, CXCR7 may not be crucial for trafficking of hematopoietic stem cells since its expression on CD34+ cells is very low. Notably, our group and others have demonstrated that CXCR7 is involved in tumor cell growth, survival, and metastasis in many several tumor types. It is highly expressed on tumor-associated vasculature and may have an important role in tumor neovascularization. Blockade of CXCR7 could potentially be employed simultaneously with CXCR4 blockage in the inhibition of CXCL12-dependent tumor progression and metastasis, which may offer the better therapeutic effect in cancer treatment.
Acknowledgements
We apologize to the many authors whose excellent work we could not cite owing to space limitation. Research in the authors’ laboratory is supported by National Natural funding of China (30973012, 81071747), National Key Program (973) for Basic Research of China (NO2010CB504300), Shanghai Education Committee Key Discipline and Specialties Foundation Project Number: J50208, and Shanghai Pujiang Program (10PJ1406400).
References
- 1.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16(3):663–673. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff RM. Chemokines and chemokine receptors: Standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, et al. Cxc chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer. 2007;14(4):1039–1052. doi: 10.1677/erc.1.01301. [DOI] [PubMed] [Google Scholar]
- 4.New DC, Wong YH. Cc chemokine receptor-coupled signalling pathways. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35(9):779–788. [PubMed] [Google Scholar]
- 5.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 6.Lazennec G, Richmond A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol Med. 16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keeley EC, Mehrad B, Strieter RM. Cxc chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 106:91–111. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruizinga RC, Bestebroer J, Berghuis P, de Haas CJ, Links TP, de Vries EG, et al. Role of chemokines and their receptors in cancer. Curr Pharm Des. 2009;15(29):3396–3416. doi: 10.2174/138161209789105081. [DOI] [PubMed] [Google Scholar]
- 9.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann TN, Burger M, Burger JA. The role of adhesion molecules and chemokine receptor cxcr4 (cd184) in small cell lung cancer. J Biol Regul Homeost Agents. 2004;18(2):126–130. [PubMed] [Google Scholar]
- 11.Secchiero P, Celeghini C, Cutroneo G, Di Baldassarre A, Rana R, Zauli G. Differential effects of stromal derived factor-1 alpha (sdf-1 alpha) on early and late stages of human megakaryocytic development. Anat Rec. 2000;260(2):141–147. doi: 10.1002/1097-0185(20001001)260:2<141::AID-AR40>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 binding to its chemokine receptor cxcr4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36(5):840–853. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11(3):303–311. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa J, Migita M, Ueda T, Fukazawa R, Adachi K, Ooue Y, et al. Dextran sulfate and stromal cell derived factor-1 promote cxcr4 expression and improve bone marrow homing efficiency of infused hematopoietic stem cells. J Nippon Med Sch. 2009;76(4):198–208. doi: 10.1272/jnms.76.198. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakou C, Rabin N, Pizzey A, Nathwani A, Yong K. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica. 2008;93(10):1457–1465. doi: 10.3324/haematol.12553. [DOI] [PubMed] [Google Scholar]
- 16.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: A cloning strategy for secreted proteins and type i membrane proteins. Science. 1993;261(5121):600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 17.Dettin M, Pasquato A, Scarinci C, Zanchetta M, De Rossi A, Di Bello C. Anti-hiv activity and conformational studies of peptides derived from the c-terminal sequence of sdf-1. J Med Chem. 2004;47(12):3058–3064. doi: 10.1021/jm031067a. [DOI] [PubMed] [Google Scholar]
- 18.Janowski M. Functional diversity of sdf-1 splicing variants. Cell Adh Migr. 2009;3(3):243–249. doi: 10.4161/cam.3.3.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucia M, Wojakowski W, Reca R, Machalinski B, Gozdzik J, Majka M, et al. The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an sdf-1-, hgf-, and lif-dependent manner. Arch Immunol Ther Exp (Warsz) 2006;54(2):121–135. doi: 10.1007/s00005-006-0015-1. [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, et al. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–179. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Neiva K, Sun YX, Taichman RS. The role of osteoblasts in regulating hematopoietic stem cell activity and tumor metastasis. Braz J Med Biol Res. 2005;38(10):1449–1454. doi: 10.1590/s0100-879x2005001000001. [DOI] [PubMed] [Google Scholar]
- 22.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of sdf-1 (cxcl12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38(4):497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/cxcr4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–1837. [PubMed] [Google Scholar]
- 24.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of nod/scid mice on cxcr4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 25.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106(11):1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-csf induces stem cell mobilization by decreasing bone marrow sdf-1 and up-regulating cxcr4. Nat Immunol. 2002;3(7):687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 27.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through hif-1 induction of sdf-1. Nat Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 28.Caruz A, Samsom M, Alonso JM, Alcami J, Baleux F, Virelizier JL, et al. Genomic organization and promoter characterization of human cxcr4 gene. FEBS Lett. 1998;426(2):271–278. doi: 10.1016/s0014-5793(98)00359-7. [DOI] [PubMed] [Google Scholar]
- 29.Gupta SK, Pillarisetti K. Cutting edge: Cxcr4-lo: Molecular cloning and functional expression of a novel human cxcr4 splice variant. J Immunol. 1999;163(5):2368–2372. [PubMed] [Google Scholar]
- 30.Wegner SA, Ehrenberg PK, Chang G, Dayhoff DE, Sleeker AL, Michael NL. Genomic organization and functional characterization of the chemokine receptor cxcr4, a major entry co-receptor for human immunodeficiency virus type 1. J Biol Chem. 1998;273(8):4754–4760. doi: 10.1074/jbc.273.8.4754. [DOI] [PubMed] [Google Scholar]
- 31.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor cxcr4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 32.Feil C, Augustin HG. Endothelial cells differentially express functional cxc-chemokine receptor-4 (cxcr-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun. 1998;247(1):38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- 33.Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, et al. Differential signalling of the chemokine receptor cxcr4 by stromal cell-derived factor 1 and the hiv glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12(1):117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 34.Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, et al. Expression of cxcr4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29(6):1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine sdf-1 is a chemoattractant for human cd34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of cd34+ progenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, et al. Guidance of primordial germ cell migration by the chemokine sdf-1. Cell. 2002;111(5):647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 37.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of b-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the cxc chemokine pbsf/sdf-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee RL, Westendorf J, Gold MR. Differential role of reactive oxygen species in the activation of mitogen-activated protein kinases and akt by key receptors on b-lymphocytes: Cd40, the b cell antigen receptor, and cxcr4. J Cell Commun Signal. 2007;1(1):33–43. doi: 10.1007/s12079-007-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu DY, Tang CH, Yeh WL, Wong KL, Lin CP, Chen YH, et al. Sdf-1alpha up-regulates interleukin-6 through cxcr4, pi3k/akt, erk, and nf-kappab-dependent pathway in microglia. Eur J Pharmacol. 2009;613(1–3):146–154. doi: 10.1016/j.ejphar.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Princen K, Hatse S, Vermeire K, De Clercq E, Schols D. Evaluation of sdf-1/cxcr4-induced ca2+ signaling by fluorometric imaging plate reader (flipr) and flow cytometry. Cytometry A. 2003;51(1):35–45. doi: 10.1002/cyto.a.10008. [DOI] [PubMed] [Google Scholar]
- 41.Roland J, Murphy BJ, Ahr B, Robert-Hebmann V, Delauzun V, Nye KE, et al. Role of the intracellular domains of cxcr4 in sdf-1-mediated signaling. Blood. 2003;101(2):399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- 42.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 43.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 44.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: A role for chemokine receptors? Cancer Res. 2001;61(13):4961–4965. [PubMed] [Google Scholar]
- 45.Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, et al. Hypoxia-inducible factor 1 and vegf upregulate cxcr4 in glioblastoma: Implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86(12):1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 46.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for sdf-1 and i-tac involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine sdf-1/cxcl12 binds to and signals through the orphan receptor rdc1 in t lymphocytes. J Biol Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 48.Libert F, Parmentier M, Lefort A, Dumont JE, Vassart G. Complete nucleotide sequence of a putative g protein coupled receptor: Rdc1. Nucleic Acids Res. 1990;18(7):1917. doi: 10.1093/nar/18.7.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SW, Brockbank SM, Mobbs ML, Le Good NJ, Soma-Haddrick S, Heuze AJ, et al. The orphan g-protein coupled receptor rdc1: Evidence for a role in chondrocyte hypertrophy and articular cartilage matrix turnover. Osteoarthritis Cartilage. 2006;14(6):597–608. doi: 10.1016/j.joca.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Raggo C, Ruhl R, McAllister S, Koon H, Dezube BJ, Fruh K, et al. Novel cellular genes essential for transformation of endothelial cells by kaposi's sarcoma-associated herpesvirus. Cancer Res. 2005;65(12):5084–5095. doi: 10.1158/0008-5472.CAN-04-2822. [DOI] [PubMed] [Google Scholar]
- 51.Martinez A, Kapas S, Miller MJ, Ward Y, Cuttitta F. Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology. 2000;141(1):406–411. doi: 10.1210/endo.141.1.7261. [DOI] [PubMed] [Google Scholar]
- 52.Tripathi V, Verma R, Dinda A, Malhotra N, Kaur J, Luthra K. Differential expression of rdc1/cxcr7 in the human placenta. J Clin Immunol. 2009;29(3):379–386. doi: 10.1007/s10875-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 53.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, et al. Cxcr7 (rdc1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104(40):15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Shiozawa Y, Wang Y, Jung Y, Pienta KJ, Mehra R, et al. The role of cxcr7/rdc1 as a chemokine receptor for cxcl12/sdf-1 in prostate cancer. J Biol Chem. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 55.Begley LA, MacDonald JW, Day ML, Macoska JA. Cxcl12 activates a robust transcriptional response in human prostate epithelial cells. J Biol Chem. 2007;282(37):26767–26774. doi: 10.1074/jbc.M700440200. [DOI] [PubMed] [Google Scholar]
- 56.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin- but not g protein-mediated signaling by the "Decoy" Receptor cxcr7. Proc Natl Acad Sci U S A. 107(2):628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132(3):463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 58.Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: Antagonistic interactions between the chemokine receptors cxcr4 and cxcr7/rdc1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. Cxcr7 heterodimerizes with cxcr4 and regulates cxcl12-mediated g protein signaling. Blood. 2009;113(24):6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 60.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second cxcl12/sdf-1 receptor, cxcr7. Proc Natl Acad Sci U S A. 2007;104(37):14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A, et al. A crosstalk between intracellular cxcr7 and cxcr4 involved in rapid cxcl12-triggered integrin activation but not in chemokine-triggered motility of human t lymphocytes and cd34+ cells. J Leukoc Biol. 2008;84(4):1130–1140. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- 62.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. Amd3100 is a cxcr7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75(5):1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 63.Luker KE, Gupta M, Steele JM, Foerster BR, Luker GD. Imaging ligand-dependent activation of cxcr7. Neoplasia. 2009;11(10):1022–1035. doi: 10.1593/neo.09724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandis AZ, Cherla RP, Chernock RD, Ganju RK. Cxcr4/ccr5 down-modulation and chemotaxis are regulated by the proteasome pathway. J Biol Chem. 2002;277(20):18111–18117. doi: 10.1074/jbc.M200750200. [DOI] [PubMed] [Google Scholar]
- 65.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K, et al. Skeletal localization and neutralization of the sdf-1(cxcl12)/cxcr4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20(2):318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 66.Engl T, Relja B, Marian D, Blumenberg C, Muller I, Beecken WD, et al. Cxcr4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8(4):290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of cxcr4 expression in pc-3 cells by stromal-derived factor-1alpha (cxcl12) increases endothelial adhesion and transendothelial migration: Role of mek/erk signaling pathway-dependent nf-kappab activation. Cancer Res. 2005;65(21):9891–9898. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]
- 68.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, et al. Expression of cxcr4 and cxcl12 (sdf-1) in human prostate cancers (pca) in vivo. J Cell Biochem. 2003;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 69.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, et al. Role of high expression levels of cxcr4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18(11):1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS. Diverse signaling pathways through the sdf-1/cxcr4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17(12):1578–1592. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Dai J, Jung Y, Wei CL, Wang Y, Havens AM, et al. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2007;67(1):149–159. doi: 10.1158/0008-5472.CAN-06-2971. [DOI] [PubMed] [Google Scholar]
- 72.Zhao H, Peehl DM. Tumor-promoting phenotype of cd90hi prostate cancer-associated fibroblasts. Prostate. 2009;69(9):991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the sdf-1-cxcr4 axis by the third complement component (c3)--implications for trafficking of cxcr4+ stem cells. Exp Hematol. 2006;34(8):986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, et al. Essential but differential role for cxcr4 and cxcr7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205(2):479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 77.Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of cxcr4 blocks breast cancer metastasis. Cancer Res. 2005;65(3):967–971. [PMC free article] [PubMed] [Google Scholar]
- 78.Ueda Y, Neel NF, Schutyser E, Raman D, Richmond A. Deletion of the cooh-terminal domain of cxc chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res. 2006;66(11):5665–5675. doi: 10.1158/0008-5472.CAN-05-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holland JD, Kochetkova M, Akekawatchai C, Dottore M, Lopez A, McColl SR. Differential functional activation of chemokine receptor cxcr4 is mediated by g proteins in breast cancer cells. Cancer Res. 2006;66(8):4117–4124. doi: 10.1158/0008-5472.CAN-05-1631. [DOI] [PubMed] [Google Scholar]
- 80.Fulton AM. The chemokine receptors cxcr4 and cxcr3 in cancer. Curr Oncol Rep. 2009;11(2):125–131. doi: 10.1007/s11912-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 81.Akekawatchai C, Holland JD, Kochetkova M, Wallace JC, McColl SR. Transactivation of cxcr4 by the insulin-like growth factor-1 receptor (igf-1r) in human mda-mb-231 breast cancer epithelial cells. J Biol Chem. 2005;280(48):39701–39708. doi: 10.1074/jbc.M509829200. [DOI] [PubMed] [Google Scholar]
- 82.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated sdf-1/cxcl12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 83.Razmkhah M, Talei AR, Doroudchi M, Khalili-Azad T, Ghaderi A. Stromal cell-derived factor-1 (sdf-1) alleles and susceptibility to breast carcinoma. Cancer Lett. 2005;225(2):261–266. doi: 10.1016/j.canlet.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 84.Cabioglu N, Summy J, Miller C, Parikh NU, Sahin AA, Tuzlali S, et al. Cxcl-12/stromal cell-derived factor-1alpha transactivates her2-neu in breast cancer cells by a novel pathway involving src kinase activation. Cancer Res. 2005;65(15):6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 85.Salmaggi A, Maderna E, Calatozzolo C, Gaviani P, Canazza A, Milanesi I, et al. Cxcl12, cxcr4 and cxcr7 expression in brain metastases. Cancer Biol Ther. 2009;8(17):1608–1614. doi: 10.4161/cbt.8.17.9202. [DOI] [PubMed] [Google Scholar]
- 86.Burger JA, Kipps TJ. Cxcr4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 87.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, et al. Functional expression of cxcr4 (cd184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22(50):8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 88.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. Cxcr4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (sclc) cells. Oncogene. 2005;24(27):4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 89.Su LP, Zhang JP, Xu HB, Chen J, Wang Y, Xiong SD. [the role of cxcr4 in lung cancer metastasis and its possible mechanism] Zhonghua Yi Xue Za Zhi. 2005;85(17):1190–1194. [PubMed] [Google Scholar]
- 90.Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through cxcr4 and c-kit in small cell lung cancer cells. Cancer Res. 2002;62(21):6304–6311. [PubMed] [Google Scholar]
- 91.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, et al. Epidermal growth factor and hypoxia-induced expression of cxc chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/pten/akt/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem. 2005;280(23):22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 92.Iwakiri S, Mino N, Takahashi T, Sonobe M, Nagai S, Okubo K, et al. Higher expression of chemokine receptor cxcr7 is linked to early and metastatic recurrence in pathological stage i nonsmall cell lung cancer. Cancer. 2009;115(11):2580–2593. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 93.Billadeau DD, Chatterjee S, Bramati P, Sreekumar R, Shah V, Hedin K, et al. Characterization of the cxcr4 signaling in pancreatic cancer cells. Int J Gastrointest Cancer. 2006;37(4):110–119. doi: 10.1007/s12029-007-0011-7. [DOI] [PubMed] [Google Scholar]
- 94.Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, et al. Cxcr4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3(1):29–37. [PubMed] [Google Scholar]
- 95.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, et al. Expression of stromal cell-derived factor 1 and cxcr4 ligand receptor system in pancreatic cancer: A possible role for tumor progression. Clin Cancer Res. 2000;6(9):3530–3535. [PubMed] [Google Scholar]
- 96.Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional cxcr4. Cancer Res. 2004;64(22):8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- 97.Gao Z, Wang X, Wu K, Zhao Y, Hu G. Pancreatic stellate cells increase the invasion of human pancreatic cancer cells through the stromal cell-derived factor-1/cxcr4 axis. Pancreatology. 10(2–3):186–193. doi: 10.1159/000236012. [DOI] [PubMed] [Google Scholar]
- 98.Marechal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, et al. High expression of cxcr4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. 2009;100(9):1444–1451. doi: 10.1038/sj.bjc.6605020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, et al. Both hepatocyte growth factor (hgf) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only hgf enhances their resistance to radiochemotherapy. Cancer Res. 2003;63(22):7926–7935. [PubMed] [Google Scholar]
- 100.Balkwill F. The significance of cancer cell expression of the chemokine receptor cxcr4. Semin Cancer Biol. 2004;14(3):171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 101.Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S, et al. Cxcr4 neutralization, a novel therapeutic approach for non-hodgkin's lymphoma. Cancer Res. 2002;62(11):3106–3112. [PubMed] [Google Scholar]
- 102.Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, et al. Multiple actions of the chemokine cxcl12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62(20):5930–5938. [PubMed] [Google Scholar]
- 103.Zhou Y, Larsen PH, Hao C, Yong VW. Cxcr4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277(51):49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 104.Rubin JB, Kung AL, Klein RS, Chan JA, Sun Y, Schmidt K, et al. A small-molecule antagonist of cxcr4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100(23):13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sehgal A, Keener C, Boynton AL, Warrick J, Murphy GP. Cxcr-4, a chemokine receptor, is overexpressed in and required for proliferation of glioblastoma tumor cells. J Surg Oncol. 1998;69(2):99–104. doi: 10.1002/(sici)1096-9098(199810)69:2<99::aid-jso10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, et al. Chemokine receptor cxcr4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg. 2006;244(1):113–120. doi: 10.1097/01.sla.0000217690.65909.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, et al. A possible role for cxcr4 and its ligand, the cxc chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167(8):4747–4757. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 108.Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, et al. Expression of cxcr4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11(5):1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 109.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor cxcr4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63(13):3833–3839. [PubMed] [Google Scholar]
- 110.Grymula K, Tarnowski M, Wysoczynski M, Drukala J, Barr FG, Ratajczak J, et al. Overlapping and distinct role of cxcr7-sdf-1/itac and cxcr4-sdf-1 axes in regulating metastatic behavior of human rhabdomyosarcomas. Int J Cancer. doi: 10.1002/ijc.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, et al. Cxcr4-sdf-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100(7):2597–2606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 112.Tarnowski M, Grymula K, Reca R, Jankowski K, Maksym R, Tarnowska J, et al. Regulation of expression of stromal-derived factor-1 receptors: Cxcr4 and cxcr7 in human rhabdomyosarcomas. Mol Cancer Res. 8(1):1–14. doi: 10.1158/1541-7786.MCR-09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell vla-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9(9):1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 114.Sanz-Rodriguez F, Hidalgo A, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates vla-4 integrin-mediated multiple myeloma cell adhesion to cs-1/fibronectin and vcam-1. Blood. 2001;97(2):346–351. doi: 10.1182/blood.v97.2.346. [DOI] [PubMed] [Google Scholar]
- 115.Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 116.Horgan K, Jones DL, Mansel RE. Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg. 1987;74(3):227–229. doi: 10.1002/bjs.1800740326. [DOI] [PubMed] [Google Scholar]
- 117.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95(2):859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clarke MF, Fuller M. Stem cells and cancer: Two faces of eve. Cell. 2006;124(6):1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 119.Li L, Neaves WB. Normal stem cells and cancer stem cells: The niche matters. Cancer Res. 2006;66(9):4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 120.Polyak K, Hahn WC. Roots and stems: Stem cells in cancer. Nat Med. 2006;12(3):296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- 121.Rak J. Is cancer stem cell a cell, or a multicellular unit capable of inducing angiogenesis? Med Hypotheses. 2006;66(3):601–604. doi: 10.1016/j.mehy.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 122.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 123.Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA. Cxcl12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell. 2005;4(6):291–298. doi: 10.1111/j.1474-9726.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 124.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. Vegfr1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Houshmand P, Zlotnik A. Targeting tumor cells. Curr Opin Cell Biol. 2003;15(5):640–644. doi: 10.1016/s0955-0674(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 126.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 127.Zhang L, Yeger H, Das B, Irwin MS, Baruchel S. Tissue microenvironment modulates cxcr4 expression and tumor metastasis in neuroblastoma. Neoplasia. 2007;9(1):36–46. doi: 10.1593/neo.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, et al. Plerixafor (amd3100) and granulocyte colony-stimulating factor (g-csf) mobilize different cd34+ cell populations based on global gene and microrna expression signatures. Blood. 2009;114(12):2530–2541. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim SY, Lee CH, Midura BV, Yeung C, Mendoza A, Hong SH, et al. Inhibition of the cxcr4/cxcl12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008;25(3):201–211. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Porvasnik S, Sakamoto N, Kusmartsev S, Eruslanov E, Kim WJ, Cao W, et al. Effects of cxcr4 antagonist ctce-9908 on prostate tumor growth. Prostate. 2009;69(13):1460–1469. doi: 10.1002/pros.21008. [DOI] [PubMed] [Google Scholar]
- 131.Richert MM, Vaidya KS, Mills CN, Wong D, Korz W, Hurst DR, et al. Inhibition of cxcr4 by ctce-9908 inhibits breast cancer metastasis to lung and bone. Oncol Rep. 2009;21(3):761–767. [PubMed] [Google Scholar]
- 132.Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, et al. High-level expression of chemokine cxcl16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. 2007;67(10):4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- 133.Wysoczynski M, Kucia M, Ratajczak J, Ratajczak MZ. Cleavage fragments of the third complement component (c3) enhance stromal derived factor-1 (sdf-1)-mediated platelet production during reactive postbleeding thrombocytosis. Leukemia. 2007;21(5):973–982. doi: 10.1038/sj.leu.2404629. [DOI] [PubMed] [Google Scholar]
- 134.Wysoczynski M, Miekus K, Jankowski K, Wanzeck J, Bertolone S, Janowska-Wieczorek A, et al. Leukemia inhibitory factor: A newly identified metastatic factor in rhabdomyosarcomas. Cancer Res. 2007;67(5):2131–2140. doi: 10.1158/0008-5472.CAN-06-1021. [DOI] [PubMed] [Google Scholar]
- 135.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine sdf-1 and its receptor, cxcr4. Dev Biol. 1999;213(2):442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 136.Mohle R, Moore MA, Nachman RL, Rafii S. Transendothelial migration of cd34+ and mature hematopoietic cells: An in vitro study using a human bone marrow endothelial cell line. Blood. 1997;89(1):72–80. [PubMed] [Google Scholar]