Abstract
The insular cortex is implicated in general attention and in taste perception. The effect of selective attention to taste on insular responses may therefore reflect a general effect of attention or it may be (taste) modality specific. To distinguish between these 2 possibilities, we used functional magnetic resonance imaging to evaluate brain response to tastes and odors while subjects passively sampled the stimuli or performed a detection task. We found that trying to detect a taste (attention to taste) resulted in activation of the primary taste cortex (anterior and mid-dorsal insula) but not in the primary olfactory cortex (piriform). In contrast, trying to detect an odor (attention to odor) increased activity in primary olfactory but not primary gustatory cortex. However, we did identify a region of far anterior insular cortex that responded to both taste and odor “searches.” These results demonstrate modality-specific activation of primary taste cortex by attention to taste and primary olfactory cortex by attention to odor and rule out the possibility that either response reflects a general effect of attentional deployment. The findings also support the existence of a multimodal region in far anterior insular cortex that is sensitive to directed attention to taste and smell.
Keywords: fMRI, gustatory, multimodal, neuroimaging, olfactory
Introduction
Selective attention brings relevant aspects of the sensory world into focus in the service of goal-directed behavior (Posner 1980; Posner et al. 1980; Mesulam 1981). Wilhelm Wundt stated that such a process must have a physiological correlate of an “increase in nervous excitation” (Wundt 1874). Indeed, functional neuroimaging studies reveal that directing attention toward specific features of a stimulus is reflected in an increase of activity in the region of sensory cortex coding that feature (Corbetta et al. 1991; Kanwisher and Wojciulik 2000). Attentional modulation of sensory cortex can also be observed independently of sensory activation in advance of stimulus presentation or on “blank trials.” This effect has been termed a “baseline shift” and is thought to represent the modulation of early cortical regions to increase sensitivity to incoming sensory signals (Kanwisher and Wojciulik 2000). Accordingly, it has been shown that searching for a sound in silence (Voisin et al. 2006), a sight in an empty visual scene (Kastner et al. 1999; Hopfinger et al. 2000), or an odor in odorless air (Zelano et al. 2005) results in activation of the respective primary sensory cortical region.
Extending this work to the gustatory modality in a prior study, we showed that searching for a taste in a tasteless solution during a taste detection task results in increased activity in the mid-dorsal and anterior insula (Veldhuizen et al. 2007). However, although these areas correspond to early taste cortex (Pritchard et al. 1986; Small et al. 1999; Ogawa et al. 2005; Small 2010), these same regions of insular cortex are also thought to play a role in attentional orienting independent of sensory processes (Kincade et al. 2005; Corbetta et al. 2008; Kurth et al. 2010; Menon and Uddin 2010). The aim of the current study was to test whether this insular modulation during a taste search reflects a general effect of attention or selective attention to taste.
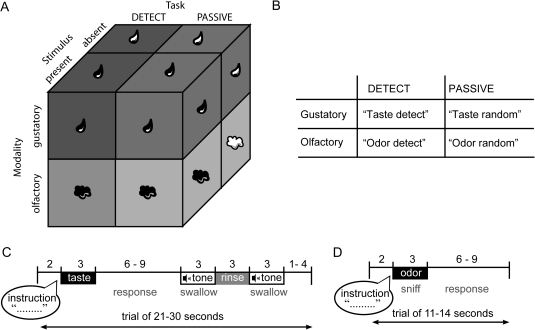
Toward this end, we used functional magnetic resonance imaging (fMRI) to measure brain response as subjects sampled tastes and odors and either performed a detection task or perceived the stimuli passively (Figure 1). The 2 × 2 × 2 factorial design we used has the within-subject factors: modality (smell or taste), task (detect or sample passively), and stimulus (present or absent). We predicted modality-specific baseline shifts such that trying to detect a taste in a tasteless solution (vs. passive sampling) would activate insular taste cortex but not piriform olfactory cortex, whereas trying to detect an odor in an odorless stream of air (vs. passive sampling) would activate piriform olfactory cortex but not insular taste cortex. Such a pattern of activity would be consistent with the interpretation that insular modulation represents selective attention to taste rather than a more general effect of attention. It would also rule out the possibility that the insular response reflects the greater effort that is required to perform a detection task compared with passively sampling stimuli because the effort in the olfactory and gustatory tasks should be roughly equivalent.
Figure 1.
Design and protocol. (A) Schematic of the 2 (modality) × 2 (task condition) × 2 (stimulus presence) factorial design. Stimuli could be olfactory (sniffed air from a nasal mask) or gustatory (dripped solutions onto the tongue from a taste manifold). Subjects were either performing a detection task (DETECT), during which they had to press a button to indicate they perceived a taste or an odor and another button when they perceived not taste or odor or they were randomly pressing a button without performing a detection task (PASSIVE). Half of the gustatory trials consisted of solutions containing a taste (sweet or sour; T+) and the other half of the trials were tasteless solution presentations (T−). Similarly, half of the olfactory trials contained an odor (chocolate-cookie or strawberry-and-cream; O+) and on the other half of the trials odorless air was presented (O−). (B) Schematic of the 4 possible instructions subjects received depending on modality and task. Hearing “taste detect” meant to expect a solution in the mouth, to probe for the presence of a taste, and to indicate with a buttonpress whether a taste was present. Hearing “odor detect” meant to sniff the air, to probe for the presence of an odor, and to indicate with a buttonpress whether an odor was present. Hearing “taste random” meant to expect a solution, but not perform a detection task and press either one of the left- and right-hand buttons randomly, and “sniff random” meant to sniff the air, but not to perform a detection task and press a button randomly. (C) Schematic of gustatory protocol. At the onset of each trial, the subject heard verbal auditory instructions, indicating which task to perform and to expect the delivery of a solution in the mouth. The subject then received the taste solution over 3 s. This was followed by a variable wait, during which the subject responded according to the instructions, followed by a cue to swallow the taste solution, a rinse, and a cue to swallow the rinse. A variable interval followed until the start of the next trial. (D) Schematic of olfactory protocol. At the onset of each trial, the subject heard verbal auditory instructions, indicating the subject to sniff and which task to perform. Immediately following this, the odor was delivered over 3 s. This was followed by a variable interval until the start of the next trial.
Materials and methods
Subjects
Thirteen right-handed subjects (11 women, 2 men, mean age 28.2 ± 6.4 years with a mean Edinburgh Handedness inventory score of 84; Oldfield 1971) gave informed consent to participate in our study that was approved by Yale University School of Medicine Human Investigation Committee. All subjects reported having no known taste, smell, neurological, or psychiatric disorder or claustrophobia.
Stimuli and delivery
Gustatory stimulation
Because water activates taste cortex (Frey and Petrides 1999; Zald and Pardo 2000) and has a taste (Bartoshuk et al. 1964), we used artificial saliva, rather than water, as our tasteless stimulus. We created this from a stock tasteless solution containing 2.5 mM sodium bicarbonate (Sigma, grade) and 25 mM potassium chloride (O'Doherty et al. 2001) dissolved in demineralized water. Three weaker versions were also prepared (at 25%, 50%, and 75% of the original concentration). Subjects sampled the solutions and selected the dilution that tasted most like nothing. Taste stimuli were a sweet taste solution (5.6 × 10−1 M sucrose, Sigma-Aldrich Inc.) and a sour taste solution (1.0 × 10−2 citric acid, Sigma-Aldrich) dissolved in demineralized water. Stimuli were all delivered as 0.5 mL of solution over 3 s from syringe pumps with a gustometer system that we described previously (Veldhuizen et al. 2007). In brief, this system consists of computer-controlled syringe pumps, which infuse liquids from syringes filled with taste solutions. The syringes connect to an fMRI-compatible custom-designed gustatory manifold via 25-foot length of Tygon beverage tubing. The gustatory manifold is made of Teflon and consists of ports, into which the beverage tubes are inserted, that funnel into narrower channels converging over a rounded ball situated at the center of the bottom of the manifold. When the pump is triggered, the liquid drips onto the ball and from there onto the tongue. This helps ensure that all liquids are received from the same location. The manifold is mounted on the MRI head coil and rests comfortably between the subject’s lips.
To measure swallowing and other laryngeal movement, expanding MR-compatible bellows were positioned over the subject’s thyroid cartilage (Martin et al. 2004). The bellows is connected to a valve that bleeds off residual air and is in turn connected to a spirometer/pressure transducer that feeds into an amplifier so that this signal can be digitally recorded at 100 Hz using Chart software version 5.5.1 (ADInstruments). We set a subject-specific threshold to filter out low-amplitude signals from the carotid pulse and then used the deviation of the amplitude of the remaining signal in either direction (above or below baseline) to quantify tongue movement. Overall tongue movement is calculated by averaging deviation of amplitude during each trial.
Olfactory stimulation
Two food odors were used, chocolate-cookie and strawberry-and-cream (6002335, 6106524 from Bell Labs Flavors and Fragrances, Inc.). Odors were presented by a custom-built MRI-compatible olfactometer programmed in Labview. The design of the olfactometer is based upon that described by Johnson and Sobel (2007), which was adapted from (Kobal 1981). A detailed description of the method of air-dilution olfactometry we used can be found in Small, Veldhuizen, et al. (2008). In brief, the olfactometer uses mass flow controllers to adjust the flow rate of compressed breathing air to 10 L/min. The humidity of the air is adjusted to 25% water vapor with a sparging humidifier. The olfactometer has separate channels for odorized and clean air. The air exits the odor wells into a mixing manifold (where dilution clean air may mix with odorized air) and then passes into one of two 25-foot Teflon tubes, where one channel is dedicated for always clean air and the other for odorized air. To prevent condensation, the temperature in the air tubes is heated by running watertubes (connected to a water recirculator, with water heated to 40 °C) alongside the air tubes in the trunk. The trunk terminates in a custom-built Teflon manifold that is placed upon the subject’s chest. This manifold also receives a vacuum line that serves to evacuate air from either the clean air or odor channel, thereby creating a closed loop so that odorized air does not contaminate the headspace. Because switching between odorized and clean air occurs very close to the subject’s nose, stimulus onset and offset is on the order of milliseconds. The subjects receive the air via a nasal mask. The mask is coupled to a pneumotachograph to measure airflow in the nose (Johnson and Sobel 2007). The pneumotachograph connects to a second spirometer, from which a signal is fed into the Powerlab amplifier and digitally recorded with Chart software. This allows us to record airflow simultaneous with scanning. We calculate sniff volume by averaging area under the curve for the signal during each trial.
Experimental design and procedure
All subjects first participated in a screening and training session in a mock scanner. The purpose of this session was to familiarize subjects with the tasks involved and to identify subjects that were uncomfortable with any part of the experimental procedure, for example, swallowing in supine position. Subjects were first presented with several variants of a tasteless solution and were required to choose the one that “tasted most like nothing.” Subjects then performed 2 runs of the experimental task in the fMRI simulator.
A long event-related design was used for both olfactory and gustatory delivery protocols and is depicted and described in Figure 1A. Neural response was assayed under 2 different conditions, DETECT and PASSIVE. Both conditions began with an auditory verbal instruction (2-s duration). The first part of the verbal instruction specified the modality in which the stimulus would be presented; for olfactory trials, this was the spoken word “odor” and for taste trials, the word was “taste.” Subjects were trained to sniff the air presented via the nasal mask when they heard odor, which was followed by the presentation of odorized or odorless air over 3 s. In contrast, when they heard the cue taste, they received a 3-s presentation of a taste or tasteless solution. In DETECT, the second part of the instruction was “detect.” During training, subjects had learned that this cue meant that they should perform a search by probing the stimulus for the presence of a taste or an odor, thereby engaging selective attention. If a taste or an odor was detected, they were to press button A. If no taste or odor was present, then they should press button B (with the button location counterbalanced across subjects). In PASSIVE, the second part of the instruction was “random” instead of “detect,” which indicated that they should not probe for the presence of tastes or odors and at random press either one of the buttons. After presentation of the stimulus, a window of time (6–9 s) followed during which the subject responded according to the instructions. This variable length of time is included in the design to aid in the deconvolution of event-specific responses (Friston et al. 1999). In all gustatory trials, a 3-s tone was played to cue the subject to swallow, a tasteless rinse of 3 s was presented, and a final swallow was cued, after which there was a variable interval (1–4 s) until the next trial.
Trials were blocked according to condition so that for the first half of a run, subjects perform one condition and the next half of the run they performed the other condition. The order of tasks within each run was counterbalanced, and the order of the runs was counterbalanced across subjects. In addition to the 2 conditions (DETECT and PASSIVE) and 2 modalities (taste and smell), the tastes and odors were either present or absent in their respective vehicle (i.e., air and liquid). This means that for the gustatory modality, there were taste (T+) or tasteless (T−) events and for the olfactory modality, there were odor (O+) or odorless (O−) events. There were equal numbers of these events and they were randomly interleaved within a block. This created 8 different event types: 1) DETECTT+, 2) DETECTT−, 3) PASSIVET+, 4) PASSIVET−, 5) DETECTO+, 6) DETECTO−, 7) PASSIVEO+, and 8) PASSIVEO−. Each run consisted of 28 trials and there were 6 runs performed over the course of the experiment. This resulted in 21 repeats for each event type for intertrial averaging to increased signal to noise.
After the scanning session, subjects rated the stimuli for several different attributes. Pleasantness was rated on a visual analogue line scale (VAS) of 100 mm with the label “most unpleasant sensation ever” at the left anchor point (−100), the label “neutral” in the middle (0), and the label “most pleasant sensation ever” at the right anchor point (+100) (Lawless and Heymann 1999). Edibility and familiarity were rated on similar VAS’s, with “not edible at all” and “not familiar at all” as the left anchor, “neutral” in the middle, and “very edible” and “very familiar on the right anchor. Subjective intensity was rated on a modified version of the general Labeled Magnitude Scale (gLMS) (Green et al. 1996). We used a vertical line-scale of 100 mm with the label “barely detectable” at the lower anchor and the label “strongest imaginable sensation” at the upper anchor. In between these labels, the following words were quasi-logarithmically spaced: “weak” (6 mm), “moderate” (17 mm), “strong” (35 mm), and “very strong” (53 mm).
fMRI acquisition
The images were acquired on a Siemens 3 T Trio magnetom scanner. Echoplanar imaging was used to measure the blood oxygenation level–dependent (BOLD) signal as an indication of cerebral brain activation. A susceptibility-weighted single-shot echoplanar method was used to image the regional distribution of the BOLD signal with time repetition (TR), 2000 ms; time echo (TE), 20 ms; flip angle, 90°; field of view (FOV), 220 mm; matrix, 64 × 64; slice thickness, 3 mm; and acquisition of 40 contiguous slices. Slices were acquired in an interleaved mode. The MR signal was allowed to equilibrate at the beginning of each functional run over 6 scans for a total of 12 s, which were then excluded from analysis. For a high-resolution anatomical scan, a T1-weighted 3D FLASH sequence was used (TR/TE, 2530/3.66 ms; flip angle, 20°; matrix, 256 × 256; 1-mm thick slices; FOV, 256; 176 slices).
fMRI data analysis
Data were preprocessed and analyzed using SPM5 software (Wellcome Department of Imaging Neuroscience, London, UK) (Friston et al. 1995; Worsley and Friston 1995) in MATLAB 7.3.0 (Mathworks, Inc.). The images were time-acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the mean. The images (anatomical and functional) were normalized to the standard MNI template brain implemented in SPM5. Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. We then detrended the fMRI time series. This method removes, at each voxel, any linear component matching the global signal (Macey et al. 2004). Functional images were smoothed with a full width at half maximum isotropic Gaussian kernel of 6 mm. For time series analysis on all subjects, a high-pass filter (128 s) was included in the design matrix (according to convention in SPM5) in order to remove low-frequency noise and slow drifts in the signal. Condition-specific effects at each voxel were estimated using the general linear model. The response to events was modeled by a canonical hemodynamic response function, including a temporal derivative to enable examination of differences in timing between various events (Henson et al. 2002). We defined our events of interest as mini blocks with onsets at the time of cue presentation (when subjects start directing their attention) with a duration of 1) 5 s plus the variable time window until the first swallow cue (which is the time until the stimulus is swallowed) for the gustatory conditions and 2) 5 s for olfactory conditions (which is the time until the olfactory stimulus is replaced by odorless air). The swallows and the rinses were modeled as events of no interest. SPM creates a statistical brain map of t values that indicates the probability of an event of interest to be significantly associated with neural response in each voxel using the theory of Gaussian random fields (Friston et al. 1995; Worsley and Friston 1995).
A random effects analysis was employed to take within- and between-subject variability into account and thus allows inferences to be drawn about the population. For this analysis, parameter estimate images from each event of interest for each subject were entered into a second-level 2 × 2 × 2 analysis of variance (ANOVA) as implemented in SPM5 with factors condition (DETECT or PASSIVE), modality (gustatory or olfactory), and stimulus presence (taste/odor present or taste/odor absent).
The analyses reported here focus on the following contrasts: 1) DETECT T− minus PASSIVE T−, which isolates attention to taste in the absence of gustatory stimulation and 2) DETECT O− minus PASSIVE O−, which isolates attention to odor in the absence of olfactory stimulation. T-maps of these contrasts were thresholded at Puncorrected = 0.005 and a cluster size ≥ 3 voxels. Peaks were considered significant at P < 0.05 corrected for multiple comparisons using the false discovery rate (FDR) across the whole brain for unpredicted peaks and across small volumes defined using coordinates from prior studies investigating the neural correlates of taste (anticipatory) attention (Veldhuizen et al. 2007; Small, Aschenbrenner, et al. 2008) and olfaction (Veldhuizen et al. 2010) as the centroids of a 10 mm sphere for predicted peaks. In addition, to determine if predicted results overlapped with our previous findings, we created a mask from our previous data set (Veldhuizen et al. 2007). This mask, as well as other masks created from the current data set (described below in the Results section), were thresholded at Puncorrected < 0.05 (according to convention in SPM5). To identify regions of overlapping responses to taste and to odor attention, we ran conjunction analyses with a conjunction null hypothesis. This statistic identifies voxels that are significantly activated in each of the individual contrasts included in the conjunction (Friston et al. 2005).
Results
Behavior
Subjective ratings of the stimuli
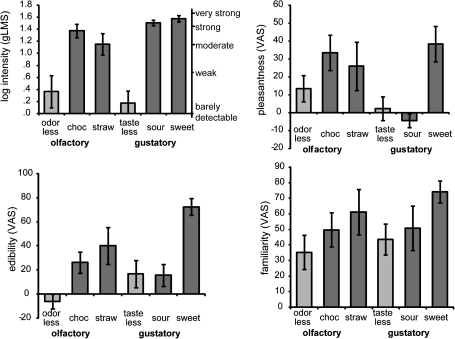
Intensity ratings made on the gLMS indicated that the tasteless solution was rated between barely detectable and weak in subjective intensity and the odorless air as weak (top panel Figure 2). The taste and odor stimuli were all rated close to “strong” in subjective intensity. We first ran a 3-way ANOVA on the log-transformed gLMS ratings within each of the modalities to assess the differences in intensity of the stimuli. For the olfactory stimuli, we observed a main effect of stimulus (F2,24 = 17.164, P < 0.001). Decomposition of the effects with post hoc paired comparisons (adjusted with a Bonferroni correction) showed that the odorless stimulus was perceived as less intense than chocolate or strawberry odor (P = 0.008 and P = 0.002) and that the chocolate and strawberry odors were perceived as equally intense (P = 0.105). We also observed a main effect of stimulus for the gustatory stimuli (F2,24 = 51.124, P < 0.001), with the tasteless stimulus perceived as less intense than either the sweet or sour taste (P’s < 0.001) and the sweet and sour taste perceived as similarly intense (P = 0.172).
Figure 2.
Perceptual ratings. Average perceived intensity (top left panel), pleasantness (top right panel), edibility (bottom row, left panel), and familiarity (bottom row, right panel) across subjects (±standard error of the mean) for the olfactory and gustatory stimuli. Odorless, odorless air; choc, chocolate-cookie; straw, strawberry-and-cream; tasteless, tasteless solution; sour, citric acid solution; and sweet, sucrose solution. We included the relative positions of the gLMS labels on the graph for perceived intensity.
Next, we collapsed the intensity ratings of chocolate and strawberry into one variable odor and of sweet and sour taste into one variable taste. We then ran a 2 × 2 within-subjects ANOVA with factors modality (gustatory vs. olfactory) and stimulus (vehicle vs. odor/taste) to test whether there were differences in intensity of the stimuli across modalities. There was no main effect of modality (F1,12 = 0.068, P = 0.799). There was a significant effect of stimulus (F1,12 = 50.677, P < 0.001), with the vehicles being perceived as less intense than the odor and taste stimuli (in line with the analysis reported above). We observed a trend for an interaction effect of modality and stimulus (F1,12 = 3.991, P = 0.069), which was driven by a higher intensity for the tastes compared with the odors (P = 0.044). These analyses indicate that the vehicles were perceived as less intense compared with the odors or tastes and that the tastes were perceived as stronger than the odors.
Average ratings on the pleasantness, familiarity, and edibility scales are all shown in Figure 2. Similar to the analyses for the intensity ratings as described above, we first ran a 3-way ANOVA within each of the modalities to assess the differences in pleasantness, familiarity, and edibility for the various stimuli. For the olfactory stimuli, we observed a main effect of stimulus on edibility (F2,24 = 7.833, P = 0.002) but not for pleasantness (F2,24 = 2.314, P = 0.120) or familiarity (F2,24 = 1.909, P = 0.170) ratings. Decomposition of the effect for edibility showed that the strawberry odor was judged as more edible than the odorless air (P = 0.002). The chocolate odor was not perceived as more edible than the strawberry odor (P = 0.955) but trended toward being more edible than the odorless air (P = 0.069). For the gustatory stimuli, we observed a main effect of stimulus on pleasantness (F2,26 = 5.072, P = 0.015) and edibility (F2,26 = 12.284, P < 0.001) but not familiarity (F2,26 = 1.832, P = 0.182) ratings. The tasteless stimulus was less pleasant than the sweet taste (P = 0.006) and the sour and the tasteless stimuli were judged as less edible than the sweet stimulus (P < 0.001 and P = 0.010).
Next, we evaluated the influence of modality on pleasantness, familiarity, and edibility ratings by collapsing chocolate and strawberry into one variable odor and sweet and sour taste into one variable taste. We then ran 2 × 2 within-subjects ANOVA with factors modality (gustatory vs. olfactory) and stimulus (vehicle vs. odor/taste). We observed no main effect of modality on pleasantness (F1,12 = 1.745, P = 0.211), familiarity (F1,12 = 0.555, P = 0.471), or edibility (F1,12 = 3.430, P = 0.089) ratings. There was a significant effect of stimulus for the pleasantness (F1,12 = 7.595, P = 0.017) and edibility (F1,12 = 35.119, P < 0.001) but not for the familiarity (F1,12 = 2.726), P = 0.125) ratings. In agreement with the prior analysis, the respective vehicles were judged as less pleasant and edible than the odors and tastes (in line with the analysis reported above). There were no significant interaction effects of modality and stimulus on pleasantness (F1,12 = 0.040, P = 0.846), familiarity (F1,12 = 0.006, P = 0.939), or edibility (F1,12 = 0.784, P = 0.393) ratings.
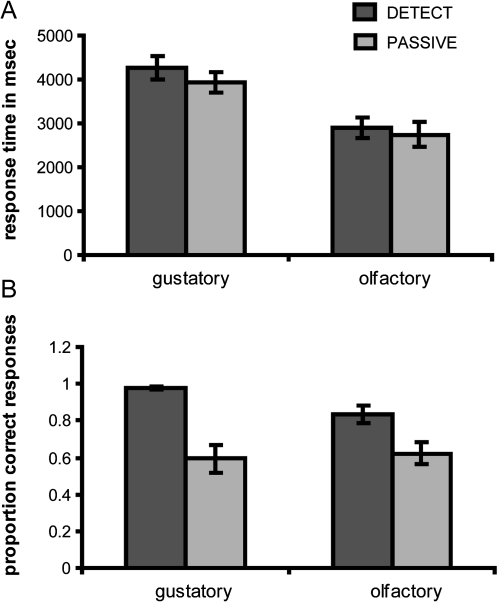
Response times and accuracy on the tasks performed in the scanner
Due to technical difficulties, we were unable to collect responses for one subject. Average response times for each modality and task are depicted in Figure 3A. A 2 × 2 within-subjects ANOVA with factors modality (gustatory or olfactory) and condition (DETECT or PASSIVE) showed a significant effect of modality; response times in the gustatory modality were slower than in the olfactory modality (F1,11 = 27.786, P < 0.001), possibly related to increased time for the stimulus to spread and bind to gustatory receptors in the oral cavity. There was also an effect of task: response times to task PASSIVE (i.e., random pressing) were faster than to task DETECT (F1,11 = 5.482, P = 0.039), consistent with the extra processing time required to engage selective attention and perform the detection task. There was no significant interaction effect.
Figure 3.
Response times and proportion correct responses. (A) Average response time (in milliseconds) across subjects (±standard error of the mean) for the detection task condition (dark gray bars) and the passive task condition (light gray bars) for the 2 modalities. (B) Average proportion correct responses across subjects (±standard error of the mean) for the detection task condition (dark gray bars) and the passive task condition (light gray bars) for the 2 modalities.
The average proportion of correct responses for each modality and task are depicted in Figure 2B. A 2 (modality) × 2 (task) within-subjects ANOVA on the proportion correct responses showed a significant effect of task; in both modalities, accuracy was lower in the PASSIVE condition (which was around chance level (0.61) compared with the DETECT task (proportion correct responses of 0.91) (F1,11 = 25.584, P = 0.000). This suggests that subjects followed the instructions and were pressing the buttons randomly during the PASSIVE condition and were performing a detection task during the DETECT condition.
We also observed an interaction effect between task and modality (F1,11 = 5.916, P = 0.033), indicating that more mistakes were made during the detection task for the olfactory compared with the gustatory modality. This suggests that the odor detection task was more difficult than the taste detection task. One possible reason for this discrepancy is that the odorless trials might have been mildly contaminated by an odorant. Although the average odorless intensity ratings did not differ from 0 (indicating no sensation) (Figure 2), inspection of individual data sets revealed that 4 subjects rated the odorless trials as between “moderate” and “strong” in intensity and made more than 25% incorrect responses. This means that the odorless trials may have been mildly contaminated for these subjects, possibly because the odor and odorized lines converge just before the nasal mask allowing for the possibility that odorized airflow during odor trials contaminated this section of the tube over the course of the experiment (ratings were collected after the entire experiment was completed). Therefore, it might have been more difficult to identify odorless trials as “blanks” compared with the tasteless trials. We therefore ran all imaging analyses twice: once with all subjects and once excluding the 4 subjects who made more than 25% incorrect responses on the odor trials. These parallel analyses produced nearly identical data. We therefore report the data from the entire group, and additional analyses with the 4 excluded subjects are reported in the Supplementary Material.
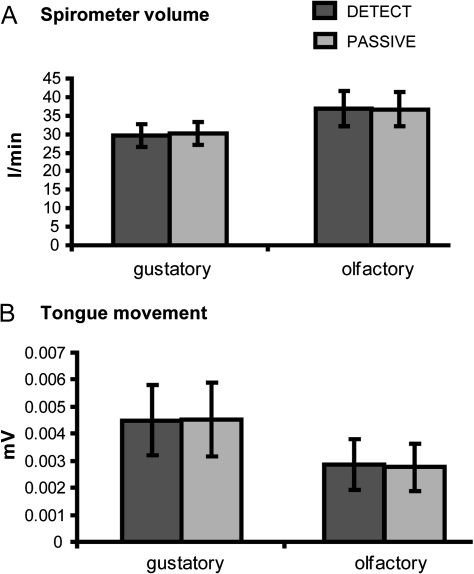
Tongue movement and sniff volume
It is possible that subjects sniff more or explore the oral cavity more during the detection tasks compared with the passive tasks. To rule out the possibility that differences in sniff volume during the olfactory conditions or in tongue movement during the gustatory conditions contributed to the observed differences in neural activity, we conducted a 2 (modality) × 2 (task) ANOVA on sniff volume and on tongue movement. Figure 4A shows the average spirometer volume per modality and task and as can be observed in this figure, there is less spirometer volume for the gustatory conditions compared with the olfactory conditions (F1,12 = 7.598, P = 0.017). We observed no difference in spirometer volume between task DETECT and PASSIVE. Inspection of Figure 4B also shows that there is less tongue movement during the olfactory conditions compared with the gustatory conditions (F1,12 = 11.463, P = 0.005) but no difference in tongue movement between the tasks DETECT and PASSIVE. These results suggest that differences in neural response observed between tasks cannot be contributed to behavioral differences in exploring the stimuli for the presence of a taste or odor.
Figure 4.
Spirometer volume and tongue movement. (A) Average spirometer volume (in l/min) across subjects (±standard error of the mean) for the detection task condition (dark gray bars) and the passive task condition (light gray bars) for the 2 modalities. (B) Average tongue movement (in microvolts) across subjects (±standard error of the mean) for the detection task condition (dark gray bars) and the passive task condition (light gray bars) for the 2 modalities.
Imaging results
Neural response during attention to taste
To isolate responses to attention to taste, we subtracted the PASSIVE task from the DETECT task during the tasteless trials. This resulted in activation in the left mid-dorsal insula and overlying Rolandic operculum and bilateral anterior insula and overlying frontal operculum (Table 1 and Figure 5). We next used the inclusive masking function to limit the regions reported to those areas present in the mask made from the prior data set (Veldhuizen et al. 2007). This procedure produced a peak in mid-dorsal insula (at −39 0 24) (see inset labeled “replication” in Figure 5) and left anterior insula. To test whether activity was specific to attention to taste and not merely a reflection of task difficulty or general attention, we masked (DETECT T− minus PASSIVE T−) exclusively with (DETECT O− minus PASSIVE O−). This isolated peaks that responded exclusively to baseline increases in attention to taste and not to baseline increases during attention to odor (Table 1). Notably, the bilateral clusters in the anterior insula/overlying operculum cluster remained significant (Figure 5), indicating that a significant effect of attention in this area is observed only when attention is directed to taste. To confirm that the areas responding to attention to taste also fall within cortical areas responding to sensory gustatory representation, we masked inclusively with (PASSIVE T+ minus PASSIVE T−). This analysis resulted in activity in bilateral anterior insula and overlying operculum (Table 1). We observed a trend for the peak in mid-dorsal insula in this inclusive mask (Z = 3.05).
Table 1.
Peak activations during attention to taste
| Region |
x, y, z coordinates MNI |
Cluster size in voxels | Z value | Z valuea | Z valueb | PFDR | ||
| DETECT T− minus PASSIVE T− | ||||||||
| Left anterior insula/frontal operculumabc | −36 | 27 | 6 | 68 | 4.00 | 4.00 | 4.00 | 0.003d |
| −42 | 36 | 3 | 3.10 | 3.10 | 3.10 | |||
| Right anterior insula/frontal operculum/caudal OFCbc | 33 | 27 | −3 | 24 | 3.12 | 2.80 | 0.036d | |
| 24 | 27 | −3 | 2.92 | 2.92 | ||||
| Left mid-dorsal insulaab | −39 | −9 | 27 | 8 | 3.07 | 3.07 | 3.07 | 0.036d |
| −39 | 0 | 24 | 2.80 | 2.80 | 2.80 | |||
Bold font indicates the maximally activated voxel in the cluster. Italics indicate that a peak falls under the same cluster as the preceding peak; T-map thresholded at Puncorrected = 0.005.
Cluster is also significant if masked inclusively by replication mask.
Cluster is also significant if masked exclusively by DETECT O− minus PASSIVE O−(attention to odor).
Cluster is also significant if masked inclusively by PASSIVE T+ minus PASSIVE T−(sensory taste representation).
Significant at PFDRcorrected = 0.05 across the region of interest.
Figure 5.
Neural responses in the insula and overlying operculum during attention to taste. Saggital and axial sections of the insula showing areas that respond to DETECT T− minus PASSIVE T−. The bar graphs show the percent signal change in the voxel responding maximally to DETECT T– (dark blue) PASSIVE T– (light blue) (averaged over subjects, ±standard error of the mean). Percent signal change to DETECT O– (red) and PASSIVE O– (orange) is also illustrated for this voxel. The asterisk denotes the conditions that differ significantly (DETECT T– and PASSIVE T–). The line graph displays the time course of the signal for DETECT T− (dark blue) and PASSIVE T– (light blue) in this area (parameter estimate in arbitrary units (±standard error of the mean), averaged over subjects). We note that the time course data and bar graphs may not correspond exactly because the bar graphs reflect data fitted to the canonical HRF, whereas the time courses are extracted using a finite impulse response model (Glascher 2009). Insets depict which areas also responded to DETECT T− minus PASSIVE T− in a previous study (labeled “replication”) and which areas show responses that are specific to DETECT T− minus PASSIVE T− and not to DETECT O− minus PASSIVE O− (labeled “detect T-specific”). The color bar depicts the scale of T− values in the contrast map. The map is thresholded at P < 0.005.
Neural response to attention to odor
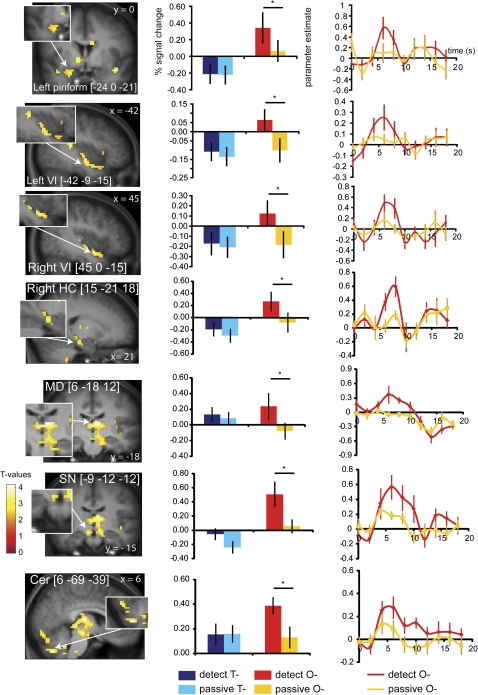
To test the prediction that searching for the presence of an odor activates piriform cortex (Zelano et al. 2005), we contrasted (DETECT O− minus PASSIVE O−). As predicted, this produced significant responses in the left piriform cortex (Figure 6), as well as a weaker nonsignificant activation in the same area of the right hemisphere ([27 3 −18], Z = 2.59). Additional peaks were observed in the ventral insula (Figure 6), anterior insula/frontal operculum, mediodorsal thalamus, right substantia nigra, right parahippocampal gyrus, and cerebellum (Table 2). To isolate those areas that responded exclusively to attention to odor and not to attention to taste, we masked (DETECT O− minus PASSIVE O−) exclusively with (DETECT T− minus PASSIVE T−). All the regions identified in the initial analyses survived the masked analysis (Table 2). To isolate those areas that also respond to sensory representation of olfaction, we masked (DETECT O− minus PASSIVE O−) inclusively with (PASSIVE O+ minus PASSIVE O−). Some of the regions identified in the original analysis survive this masked analysis, including left piriform cortex, right ventral insula, mediodorsal thalamus, right substantia nigra, and right parahippocampal gyrus (Table 2) but not anterior insula/frontal operculum, left ventral insula, and cerebellum. Note that we ran an extra analysis excluding the 4 subjects that made more than 25% incorrect responses in the olfactory detection task, which we report in Supplementary Table 1. We observed the same areas as listed in Table 2 (albeit at lower Z values due to the reduced number of subjects).
Figure 6.
Neural responses in the piriform cortex, ventral insula (VI), (para)hippocampal gyrus (HC), mediodorsal thalamus (MD), substantianigra (SN), and cerebellum (cer) during attention to odor. Coronal and sagittal sections show areas that respond to DETECT O− minus PASSIVE O−. The bar graphs next to the sections show the percent signal change in the voxel responding maximally to DETECT O– (red) PASSIVE O– (orange) (averaged over subjects, ±standard error of the mean) in the same area. Percent signal change to DETECT T– (dark blue) and PASSIVE T− (light blue) is also illustrated for this voxel. The asterisk denotes the conditions that differ significantly (DETECT O− and PASSIVE O−). The line graph next to the bar graph displays the time course of the signal for DETECT O– (red) and PASSIVE O– (orange) in this area (parameter estimate in arbitrary units [±standard error of the mean], averaged over subjects). Insets depict which areas show responses that are specific to DETECT O− minus PASSIVE O− and not to DETECT T− minus PASSIVE T−. The color bar depicts the scale of T− values in the contrast map. The map is thresholded at P < 0.005.
Table 2.
Peak activations during attention to odor
| Region |
x, y, z coordinates MNI |
Cluster size in voxels | Z value | Z valuea | PFDR | ||
| DETECT O− minus PASSIVE O− | |||||||
| Right (para)hippocampal gyrus/substantia nigra/mediodorsal thalamusab | 15 | −21 | −18 | 529 | 4.55 | 4.55 | 0.038c |
| 6 | −18 | 12 | 4.54 | 4.54 | |||
| −9 | −12 | −12 | 4.41 | 4.41 | |||
| Cerebelluma | 6 | −69 | −39 | 259 | 4.38 | 4.38 | 0.038c |
| 0 | −51 | −33 | 3.98 | 3.81 | |||
| −12 | −69 | −42 | 3.79 | 3.79 | |||
| Right ventral insula/caudal OFCab | 45 | 0 | −15 | 53 | 3.89 | 3.89 | 0.030d |
| 36 | 12 | −21 | 3.31 | 3.31 | |||
| 48 | 9 | −18 | 3.19 | 3.19 | |||
| Right anterior insula/frontal operculuma | 36 | 21 | 0 | 31 | 3.60 | 3.60 | 0.023d |
| 36 | 15 | −6 | 3.30 | 3.20 | |||
| Left ventral insula/caudal OFCa | −42 | −9 | −15 | 57 | 3.58 | 3.58 | 0.020d |
| −45 | −18 | 0 | 3.47 | 3.47 | |||
| −42 | 0 | −18 | 3.25 | ||||
| Left piriform/caudal OFCab | −24 | 0 | −21 | 42 | 3.26 | 3.26 | 0.015d |
| Left anterior insulaa | −39 | 18 | 6 | 11 | 2.91 | 2.67 | e |
Bold font indicates the maximally activated voxel in the cluster. Italics indicate that a peak falls under the same cluster as the preceding peak; T-map thresholded at Puncorrected = 0.005.
Cluster is also significant if masked exclusively by DETECT T− minus PASSIVE T−(attention to taste).
Cluster is also significant if masked inclusively by PASSIVE O+ minus PASSIVE O−(sensory olfactory stimulation).
Significant at PFDRcorrected = 0.05 across the whole brain.
Significant at PFDRcorrected = 0.05 across the region of interest.
Not significant corrected for multiple comparisons, but we chose to report this peak because it contributes to the peak in the conjuction analysis.
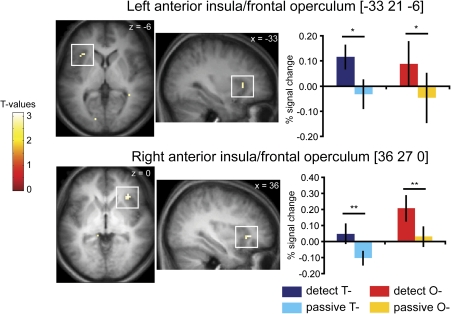
Overlap in gustatory and olfactory selective attention
To isolate regions sensitive to attention to taste and to smell, we performed a conjunction analysis (based on the conjunction null hypothesis) of (DETECTT− minus PASSIVET−) and (DETECTO− minus PASSIVEO−). We observed activity in the rostral-most section of the left anterior insular cortex, extending into overlying frontal operculum ([−33 21 6], Z = 2.73, cluster size = 6 voxels, PFDR = 0.003) and a trend for activity in this area in the right hemisphere ([36 27 0], Z = 3.02, cluster size = 8 voxels, PFDR = 0.083) (Figure 7). A less stringent conjunction analysis (based on the global null hypothesis) shows larger clusters with higher Z values ([36 27 0], Z = 4.51, cluster size = 36 voxels and [−39 18 6], Z = 4.10, cluster size = 93 voxels.
Figure 7.
Overlap in neural response in anterior insula and overlying frontal operculum during attention to taste and attention to odor. Axial and sagittal sections show areas that respond to DETECT T− minus PASSIVE T− and DETECT O− minus PASSIVE O−. The bar graphs show the percent signal change in the voxel responding maximally to DETECT T−(dark blue) PASSIVE T−(light blue) and DETECT O−(red) PASSIVE O−(orange) (averaged over subjects, ± standard error of the mean). The single and double asterisk denote the conditions that differ significantly and show a trend for significance, respectively. The color bar depicts the scale of T− values in the contrast map. The map is thresholded at P < 0.005.
Discussion
Replicating prior work, we demonstrate that attention to odor activates primary olfactory cortex and attention to taste activates primary gustatory cortex (Zelano et al. 2005; Veldhuizen et al. 2007; Plailly et al. 2008). Extending this work, we show that these responses are modality-specific; that is attending to taste activates taste but not olfactory cortex and attending to odor activates olfactory but not taste cortex. We also identified a region of far anterior dorsal insula and overlying operculum that is sensitive to attention to taste and to odor. These findings are consistent with suggestion that primary taste cortex is located in the posterior section of the anterior insular cortex (Small 2010) and that selective attention to taste modulates activity here. Our results also accord with Craig’s suggestion that the rostral-most region of anterior insular cortex is specialized for representing a more general awareness of our sensorium (Craig 2009).
Modality-specific effects:attention to taste
Attending to a specific taste quality can selectively increase sensitivity to an incoming sensory signal (Marks 2002). For example, Marks and Wheeler (1998) showed that directing one’s attention toward a taste increases detection sensitivity for that taste (Marks and Wheeler 1998). In that study, individual taste thresholds for sweet and sour taste were measured under 2 conditions. In one condition, subjects were cued to expect sweet taste. However, there was a 0.75 probability of receiving sucrose on a trial and a 0.25 probability of receiving the citric acid. In the other session, the subject was cued to expect sour taste, and the probability of receiving a sour taste was now 0.75 and 0.25 for sucrose. Thresholds for the taste that was attended were lower when attended regardless of the taste quality (Marks and Wheeler 1998).
Accordingly, in an earlier study, we showed that trying to detect a taste in a tasteless solution results in activation of the mid-dorsal insular cortex and suggested that this reflected the neural correlate of selective attention to taste (Veldhuizen et al. 2007). More specifically, we argued that this baseline shift might reflect the enhanced sensitivity of early taste cortex to incoming taste signals. However, one problem with that interpretation was that insular cortex activation is frequently observed in neuroimaging studies of attention that do not involve gustatory stimulation (Hopfinger et al. 2000; Johansen-Berg et al. 2000; Kincade et al. 2005; Voisin et al. 2006), leading to the suggestion that it plays a role in general attention (Kurth et al. 2010; Menon and Uddin 2010) and perhaps in consciousness (Craig 2009). In other words, it was unclear from our initial study whether the insular modulation reflected a general effect of attention or selective attention to taste.
The results from the current study support the later possibility. Using a similar paradigm, we again demonstrate activation in the mid-dorsal insula when subjects try to detect a taste in a tasteless solution. More importantly, we show that activation is not observed in this region when subjects try to detect odor. This indicates that attentional modulation in the mid-dorsal insula is specific to selective attention to taste. The result also rules out nonspecific effects like difficulty or effort as an explanation because the olfactory detection task was arguably more difficult that the taste detection task, as evidenced by the lower accuracy scores.
Attention to odors
Replicating earlier findings from Zelano et al. (2005), we show that trying to detect an odor results in activity in piriform cortex. Our data also align with those of Zelano et al. in showing that this response is specific to attention to odors. In their study, subjects rated the intensity of sounds and smells, whereas in our study, subjects attempted to detect tastes and odors. In both studies, piriform responses were observed only when subjects performed the olfactory task. In other words, when a subject attends to the presence or the intensity of an odor, but not a taste or a sound, piriform cortex modulation is observed.
In addition to the piriform cortex, we also observed activation of mediodorsal thalamus, right substantia nigra, right parahippocampal gyrus, cerebellum, and several regions of orbitofrontal cortex (OFC) in response to attending to odors but not to tastes. Several prior studies have implicated the mediodorsal thalamus and OFC in olfactory attention. The olfactory system misses the precortical thalamic relay that is obligatory in other sensory systems, axons from the olfactory epithelium travel directly to the olfactory bulb and from there to anterior olfactory nucleus, the olfactory tubercle, the piriform cortex, several amygdaloid subnuclei, and rostral entorhinal cortex (Price 1973; de Olmos et al. 1978; Turner et al. 1978). However, from these cortices, there are connections with the OFC (Tanabe et al. 1975; Carmichael et al. 1994), which is in turn is connected to the mediodorsal nucleus of the thalamus (Yarita et al. 1980; Price 1985; Cavada et al. 2000). Both of these regions have been implicated in odor attention and/or consciousness. In a recent study by Plailly et al. (2008), subjects were instructed to detect odors and/or tones under 2 conditions: attend to the odor and ignore the tones or ignore the odor and attend to the tones. They showed that the connection from piriform to OFC via the mediodorsal thalamus becomes stronger during attention to odor compared with attention to tones. They concluded that this would be consistent with a gating mechanism that passes behaviorally relevant information on to OFC for continued processing, which is consistent with the proposed dynamic role for the thalamus sensory processing in other modalities (Guillery and Sherman 2002). Sabri et al. (2005) compared counting oddball odors (attend odor) versus counting regular tones (ignore odor) with both odors and tones being presented equally often in both conditions. They observed activation of subgenual anterior cingulate cortex (ACC), caudomedial OFC when ignoring the odors, and right OFC when attending to odors. The activation of subgenual ACC and caudomedial OFC was interpreted as resulting from involuntarily attending to the odor, potentially because the oddball odors “pop out” of the stream of sensory information. The OFC has been suggested to play a role in higher order olfactory processing, including the encoding of odor reward value, odor discrimination, and integration with other sensory information (Gottfried 2006, 2010). In addition, a recent lesion study suggests odor awareness relies on the OFC (Li et al. 2010). Thus, the current findings add to the literature in establishing a role for mediodorsal thalamus and OFC in olfactory attention.
We also found that the cerebellum responded preferentially when subjects attended to odors. There are several prior reports of cerebellar activation to odors (Small et al. 1997; Sobel et al. 1998; Zatorre et al. 2000; Savic et al. 2000; Ferdon and Murphy 2003; Bensafi et al. 2008) and to attention to odors (Zelano et al. 2005). This is in line with the suggestion that the cerebellum plays a role in optimizing sniffing for sensory processing (Mainland and Sobel 2006) and in goal-directed attention (Courchesne and Greg 1997).
Finally, attending to odor also preferentially recruited the parahippocampal gyrus and substantia nigra. Both regions have been implicated in olfaction (Savic et al. 2000; Gottfried et al. 2004; Small, Veldhuizen, et al. 2008), however, neither is thought to play a direct role in attention. We therefore speculate that their recruitment in the current study may be explained by their involvement in related processes. The para-hippocampal gyrus plays a role in the retrieval of remembered odors (Gottfried et al. 2004). The odors used in the current study were clearly familiar. Therefore, it is possible that searching for a familiar odor reactivated para-hippocampal olfactory memory circuits. On the other hand, the substantia nigra contains dopamine neurons that play a critical role in reward learning (Schultz 2010). Here, the attention task required subjects to search for a pleasurable aroma and this may well have elicited expectation of the pleasant olfactory sensation thereby engaging reward circuits.
Overlapping representation
In addition to the modality-specific effects described above, we identified a region of rostral anterior insula that responded during attention to both tastes and odors. Activation in this region of insular cortex has been observed in a large number of studies and is associated with a variety of processes that include awareness of sensations, subjective feelings, decision making, anticipation, attention, and self-recognition, leading to the suggestion that this area plays a critical role in general consciousness (Craig 2009). Our findings are consistent with this possibility. However, it is also possible that the overlap is specific to taste and smell and reflects their common role in flavor processing. Prior work on olfactory and auditory attention did not assess overlap either because a no-attention baseline (Plailly et al. 2008) or a no-stimulus baseline (Sabri et al. 2005; Zelano et al. 2005) was absent in the design. It may also be important that here we assessed attention to orthonasal odor perception. It is conceivable that there might be more overlap between attention to retronasal olfaction and taste as both these sensory signals are perceived to originate from the mouth and are often experienced simultaneously and confused (Murphy et al. 1977; Murphy and Cain 1980; Rozin 1982; Ashkenazi and Marks 2004; Heilmann and Hummel 2004).
Summary
In summary, we found that trying to detect a taste in tasteless solution results in activation of the primary taste cortex but not in the primary piriform olfactory cortex, whereas trying to detect an odor in odorless air increased activity in piriform cortex but not in the anterior and mid-dorsal insula. In addition to these modality-specific effects of selective attention, an additional region of insular cortex was identified that responded to taste and odor searches. These results demonstrate both distinct and overlapping insular mechanisms for attention to taste and to odor.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was supported by a grant from the National Institutes of Health; National Institute on Deafness and Communication Disorders to D.M.S. [R01 DC006706].
Supplementary Material
References
- Ashkenazi A, Marks LE. Effect of endogenous attention on detection of weak gustatory and olfactory flavors. Percept Psychophys. 2004;66:596–608. doi: 10.3758/bf03194904. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, McBurney DH, Pfaffmann C. Taste of sodium chloride solutions after adaptation to sodium chloride: implications for the “water taste”. Science. 1964;143:967–968. doi: 10.1126/science.143.3609.967. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Iannilli E, Gerber J, Hummel T. Neural coding of stimulus concentration in the human olfactory and intranasal trigeminal systems. Neuroscience. 2008;154:832–838. doi: 10.1016/j.neuroscience.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Allen G Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- de Olmos J, Hardy H, Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- Ferdon S, Murphy C. The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage. 2003;20:12–21. doi: 10.1016/s1053-8119(03)00276-3. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Re-examination of the human taste region: a positron emission tomography study. Eur J Neurosci. 1999;11:2985–2988. doi: 10.1046/j.1460-9568.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RNA, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Glascher J. Visualization of group inference data in functional neuroimaging. Neuroinform. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. Smell: central nervous processing. In: Hummel T, Welge-Lüssen A, editors. Taste and smell an update. Basel (Switzerland): Karger; 2006. pp. 44–69. [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Smith AP, Rugg MD, Dolan RJ. Remembrance of odors past: human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Heilmann S, Hummel T. A new method for comparing orthonasal and retronasal olfaction. Behav Neurosci. 2004;118:412–419. doi: 10.1037/0735-7044.118.2.412. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Christensen V, Woolrich M, Matthews PM. Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport. 2000;11:1237–1241. doi: 10.1097/00001756-200004270-00019. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Sobel N. Methods for building an olfactometer with known concentration outcomes. J Neurosci Methods. 2007;160:231–245. doi: 10.1016/j.jneumeth.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: insights from brain imaging. Nat Rev Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal G. Pain-related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain. 1985;22(2):151–63. doi: 10.1016/0304-3959(85)90175-7. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox P, Laird A, Eickhoff S. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless HT, Heymann H. Sensory evaluation of food. Principles and practices. Gaithersburg (MD): Aspen Publisher, Inc; 1999. [Google Scholar]
- Li W, Lopez L, Osher J, Howard JD, Parrish TB, Gottfried JA. Right orbitofrontal cortex mediates conscious olfactory perception. Psychol Sci. 2010;21:1454–1463. doi: 10.1177/0956797610382121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Sens. 2006;31:181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- Marks LE. The role of attention in chemosensation. Food Qual Pref. 2002;14:147–155. [Google Scholar]
- Marks LE, Wheeler ME. Attention and the detectability of weak taste stimuli. Chem Senses. 1998;23:19–29. doi: 10.1093/chemse/23.1.19. [DOI] [PubMed] [Google Scholar]
- Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophys. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin L. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annu Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS. Taste and olfaction: independence vs interaction. Physiol Behav. 1980;24:601–605. doi: 10.1016/0031-9384(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM. Mutual action of taste and olfaction. Sens Processes. 1977;1:204–211. [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophys. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Wakita M, Hasegawa K, Kobayakawa T, Sakai N, Hirai T, Yamashita Y, Saito S. Functional MRI detection of activation in the primary gustatory cortices in humans. Chem Sens. 2005;30:583–592. doi: 10.1093/chemse/bji052. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Plailly J, Howard JD, Gitelman DR, Gottfried JA. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci. 2008;28:5257–5267. doi: 10.1523/JNEUROSCI.5607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Price JL. Beyond the primary olfactory cortex: olfactory-related areas in the neocortex, thalamus and hypothalamus. Chem Sens. 1985;10:239–258. [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Rozin P. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 1982;31:397–401. doi: 10.3758/bf03202667. [DOI] [PubMed] [Google Scholar]
- Sabri M, Radnovich AJ, Li TQ, Kareken DA. Neural correlates of olfactory change detection. Neuroimage. 2005;25:969–974. doi: 10.1016/j.neuroimage.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. Taste representation in the human insula. Brain Struct Funct. 2010;214:551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Small DM, Aschenbrenner K, Veldhuizen MG, Felsted JA. Sweet expectations: greater response in the anterior insula and midbrain to unexpected compared to expected eweet taste. Chem Sens. 2008;33:S163. [Google Scholar]
- Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. Flavor processing: more than the sum of its parts. Neuroreport. 1997;8:3913–3917. doi: 10.1097/00001756-199712220-00014. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57:786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JDE, Sullivan EV. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Yarita H, Iino M, Ooshima Y, Takagi SF. An olfactory projection area in orbitofrontal cortex of the monkey. J Neurophys. 1975;38:1269–1283. doi: 10.1152/jn.1975.38.5.1269. [DOI] [PubMed] [Google Scholar]
- Turner BH, Gupta KC, Mishkin M. The locus and cytoarchitecture of the projection areas of the olfactory bulb in Macaca mulatta. J Comp Neurol. 1978;177:381–396. doi: 10.1002/cne.901770303. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Sens. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM. The insular taste cortex contributes to odor quality coding. Front Hum Neurosci. 2010;4:58. doi: 10.3389/fnhum.2010.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin J, Bidet-Caulet A, Bertrand O, Fonlupt P. Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J Neurosci. 2006;26:273–278. doi: 10.1523/JNEUROSCI.2967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Wundt W. Principles of physiological psychology. London: Swan Sonnenschein & Co. Lim; 1874. [Google Scholar]
- Yarita H, Iino M, Tanabe T, Kogure S, Takagi SF. A transthalamic olfactory pathway to orbitofrontal cortex in the monkey. J Neurophys. 1980;43:69–85. doi: 10.1152/jn.1980.43.1.69. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chem Sens. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Rouby C. Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport. 2000;11:2711–2716. doi: 10.1097/00001756-200008210-00021. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.