Abstract
Female Sprague–Dawley rats display considerable variability in their preference for the artificial sweetener sucralose over water. While some rats can be classified as sucralose preferrers (SP), as they prefer sucralose across a broad range of concentrations, others can be classified as sucralose avoiders (SA), as they avoid sucralose at concentrations above 0.1 g/L. Here, we expand on a previous report of this phenomenon by demonstrating, in a series of 2-bottle 24-h preference tests involving water and an ascending series of sucralose concentrations, that this variability in sucralose preference is robust across sex, stage of the estrous cycle, and 2 rat strains (Long–Evans and Sprague–Dawley). In a second experiment involving a large sample of rats (n = 50), we established that the ratio of SP to SA is approximately 35–65%. This bimodal behavioral response to sucralose appears to be driven by taste because rats display a similar bimodal licking response to a range of sucralose solutions presented during brief-access tests. Finally, we have shown that sucralose avoidance is extremely robust as 23-h water-deprived SA continue to avoid sucralose in 1-h single-bottle intake tests. Based on their reduced licking responses to sucralose during brief-access (taste driven) tests, and the fact that their distaste for sucralose cannot be overcome by the motivation to rehydrate, we conclude that SA detect a negative taste quality of sucralose that SP are relatively insensitive to.
Keywords: artificial sweeteners, individual differences, sex differences, strain differences, taste preferences
Introduction
Nonnutritive sweeteners have become popular alternatives to natural sugars. It has been demonstrated, however, that a shift from appetitive to rejection responses occurs as the concentration of some artificial sweeteners is increased. For example, concentration-related changes in the taste profile of saccharin have been observed in a variety of species (Collier 1962; Morrison and Jessup 1977a, 1977b; Hoover 1980; Schiffman et al. 1995; Smith 2000; Smith and Sclafani 2002), and humans report that, in addition to saccharin, stevioside, and acesulfame K taste bitter at high concentrations (Schiffman et al. 1995). These shifts in taste perception appear to be related to the activation of bitter-taste receptors and/or the transient receptor potential vanilloid-1 (TRPV1) receptor by high concentrations of artificial sweeteners resulting in an aversive taste component commonly described as bitter or metallic (Kuhn et al. 2004; Riera et al. 2008).
Sucralose is a trichlorinated sweetener that is derived from sucrose but reportedly 600 times sweeter than sucrose on a per weight basis (Knight 1994). It is currently marketed as the primary sweetening agent in SPLENDA. Despite its growing popularity in the commercial market, there are limited reports of sucralose's taste profile or acceptance in humans. In a study that examined sweet and bitter ratings of a large number of natural sugars and artificial sweeteners in trained tasters, sucralose was reported to taste predominately sweet with a concentration-independent bitter taste that exceeded sucrose but was less than that reported for saccharin, stevioside, and acesulfame K (Schiffman et al. 1995).
Currently, only a handful of studies have examined the behavioral response to sucralose in rodents. In one study, preference for sucralose increased as a function of increasing sucralose concentration in male mice subjected to a series of 2-bottle preference tests involving water and an ascending series of sucralose concentrations (Bachmanov et al. 2001). A different behavioral profile has begun to emerge in rats. In a study by Sclafani and Clare (2004), female rats displayed a weak concentration–independent preference for water over sucralose (0.25–4 g/L) in 2-bottle preference tests, although considerable individual variability in the preference scores was noted. A closer examination of the data revealed that 50% of the female rats preferred all but the highest sucralose concentrations (preference scores ranging from 62% to 78%), whereas the remaining female rats strongly avoided all sucralose concentrations (preference scores ranging from 11% to 21%). In this same study, only 20% of male rats were reported to prefer sucralose over water in a single 2-bottle preference test involving water and 0.5 g/L sucralose (Sclafani and Clare 2004). Similarly, a subsequent study found that a group of male rats displayed indifference and then preference for water over sucralose across an ascending series of sucralose concentrations (0.0003–10 g/L) (Bello and Hajnal 2005), and only 23% of these male rats preferred 0.5 g/L sucralose over water. Taken together, these 2 studies suggest that the rat's preference for sucralose may be sexually dimorphic. Finally, preference for sucralose has also been examined in high (HiS)- and low (LoS)-saccharin preferring rats. While the majority of HiS rats preferred sucralose to water at all concentrations (0.01–0.1 g/L), the majority of LoS rats avoided sucralose at all but the lowest concentration (Dess et al. 2009). Thus, the saccharin taste profile of HiS and LoS rats appears to generalize to sucralose suggesting that the natural variation characterized by the bimodal distribution of sucralose preference may mirror some aspects of the HiS and LoS breeding lines.
Taken together, the available data in rats reveal an interesting bimodal behavioral response to sucralose. That is, it appears as though rats can be classified as either sucralose preferrers (SP) or sucralose avoiders (SA) on the basis of their behavioral responses during 2-bottle preference tests involving water and an ascending series of sucralose concentrations (Sclafani and Clare 2004). The existing data provide some evidence that preference for sucralose may be sexually dimorphic (Sclafani and Clare 2004; Bello and Hajnal 2005), but no study to date has systematically tested this hypothesis despite numerous reports of sex differences in the intake of, and preference for, both natural and artificial sweeteners (Valenstein et al. 1967; Wade and Zucker 1969; Curtis et al. 2004; Atchley et al. 2005). Thus, one goal of the present study was to determine whether this highly variable response to sucralose is influenced by sex or hormonal status in cycling female rats. A second goal of this study was to determine whether preference for sucralose is reliable across rat strain because previous studies have demonstrated differences in the preference for artificial sweeteners among various rodent strains (e.g., Pothion et al. 2004; Lush 2010). Of particular relevance to the current study are reports that male B6 mice (but not male 129 mice) display variability in their preference for aspartame, ranging from indifference to preference (Bachmanov et al. 2001). In another study, one species of Peromyscus mice (Peromyscus aztecus) displayed considerable variability in the preference for quinine solutions ranging from avoidance to indifference to preference, whereas another species (Peromyscus melanotis) displayed only avoidance responses (Glendinning 1993). Our third goal was to determine whether the bimodal behavioral response to sucralose is driven by taste and whether it is robust across multiple behavioral testing paradigms including brief-access tests and single-bottle intake tests.
Materials and methods
Animals and housing
Male and female Long–Evans rats and male Sprague–Dawley rats were obtained from Charles River Breeding Laboratory (body weights 200–225 g at study onset). Rats were individually housed in plastic tub cages equipped with 2 drip-resistant bottles containing ball-tip spouts. Rats were adapted to the cages for at least 1 week prior to data collection. At study onset, rats were given free access to powdered chow (Purina 5001) and deionized water unless otherwise noted. Testing rooms were maintained at 22 °C with a 12:12 h light–dark cycle (dark onset 1400 h). Animal usage and experimental protocols were approved by the Florida State University Institutional Animal Care and Use Committee.
Taste solutions
Taste solutions were prepared by dissolving various concentrations of sucralose (Tate & Lyle) in deionized water. Sucralose solutions (0.0001, 0.001, 0.01, 0.1, 0.25, 0.5, 1.0, and 2.0 g/L) were presented either in an ascending series (Experiments 1, 2, and 4) or in random order (Experiment 3). These particular sucralose concentrations were chosen to be consistent with previous studies (Sclafani and Clare 2004; Bello and Hajnal 2005) and to extend the low end of the concentration ranges that have been examined previously.
Procedure
Experiment 1
To determine whether sucralose preference is influenced by sex, male, and female Long–Evans rats (n = 22 per sex) were given a series of 24-h 2-bottle preference tests between deionized water and an ascending series of sucralose solutions (0.0001, 0.001, 0.01, 0.25, 0.5, 1.0, or 2.0 g/L). Each concentration of sucralose was presented for 2 days before testing the next concentration in the series for a total of 14 days. Water and sucralose intakes were recorded daily and bottle position was alternated each day.
To examine any possible role of endogenous estradiol, preference for sucralose was also monitored at different stages of the estrous cycle in a group of Long–Evans female rats (n = 9). Stage of the estrous cycle (diestrus 1, diestrus 2, proestrus, or estrus) was determined by examining the appearance of vaginal cytology samples obtained daily by inserting the tip of a moistened cotton swab into the rat's vaginal canal. The resulting samples were transferred to glass microscope slides and examined under low magnification (4×). Cycle stage labels were assigned to the 24-h period ending at the time of sampling (1000 h) as described previously (Eckel 2004; Becker et al. 2005). Rats received the same series of 24-h 2-bottle preference tests as described above, except that they received each sucralose concentration for 4 consecutive days, coincident with 1 estrous cycle.
In order to explore the ubiquity of the bimodal preference profile, we examined whether sucralose preference is influenced by rat strain. Long–Evans and Sprague–Dawley male rats (n = 16 and 15, respectively) were presented with increasing concentrations of sucralose in a series of 24-h 2-bottle preference tests as described above.
Experiment 2
Previous studies have reported that about 20% of male rats can be classified as SP. This conclusion is limited, however, by the single concentration of sucralose tested in one study (Sclafani and Clare 2004) and by the small sample size in the other study (Bello and Hajnal 2005). Here, we sought to establish the ratio of SP to SA by exposing a total of 50 male Long–Evans rats to a series of 24-h 2-bottle preference tests involving water and an ascending series of sucralose concentrations (0.001, 0.01, 0.1, and 1.0 g/L). This abbreviated concentration curve (relative to Experiment 1) was chosen to include 2 sucralose concentrations that are typically preferred by all rats and 2 concentrations of sucralose that split the rat sample into SP and SA. Each concentration of sucralose was presented for 2 days for a total of 8 test days. Water and sucralose intakes were recorded daily and bottle position was alternated each day.
Experiment 3
In order to determine whether the bimodal behavioral response to sucralose is limited to 24-h 2-bottle preference tests, male Long–Evans rats (n = 16) received brief-access tests to varying concentrations of sucralose solutions in a Davis rig (Davis MS80 Rig; Dilog Instruments and Systems). This behavioral testing apparatus consists of a plastic chamber with an opening that allows access to 1 of 8 spill-proof glass drinking tubes positioned on a sliding platform. A mechanical shutter opens and closes to allow the rat access to each of the 8 tubes for a user-specified length of time. A computer controls the movement of the platform, which determines the order of tube presentations and the opening and closing of the shutter, which determines the length of access to a tube and the interval between tube presentations. Each individual lick is detected by a contact lickometer and recorded on a computer via DavisPro collection software (Dilog Instruments and Systems).
Throughout training and testing, rats were maintained on a 23-h water-deprivation schedule in order to ensure that SA would lick the higher concentrations of sucralose. Training consisted of 2 sessions. During the first session, rats were placed in the test cage and given 15-min access to one bottle of water and allowed to drink freely. During the second session, rats were given 60 s to initiate licking to water. After the first lick, each tube was available for 30 s before moving on to the next tube and a total of 8 tubes were presented 3 times each during a single session. All tubes contained water, but the shutter and platform were active to familiarize the rats with the testing procedure.
During testing, each of the 8 tubes contained either water or varying concentrations of sucralose solutions (0.0001, 0.001, 0.01, 0.25, 0.5, 1.0, or 2.0 g/L). Each rat was given 60 s to initiate licking. If a lick was recorded during this interval, the clock was reset and the rat was given 30 s to lick the solution. At the end of this 30-s session or after 60 s if no licks were recorded, the shutter would close for 10 s before presenting the next tube. Each tube was presented a total of 3 times per test session in randomized blocks. This paradigm was repeated daily for 5 days. Upon completing all of the licking trials, rats were classified as either SP or SA on the basis of their licking responses at the highest sucralose concentration (2.0 g/L). At this concentration, individual rats either emitted many (>120) licks per session or very few licks per session (<30) and were classified as either SP or SA, respectively. The licking profiles of SP and SA were then compared across the entire sucralose concentration curve. Because this initial analysis appeared to reveal a possible contrast effect between low and high sucralose concentrations (e.g., licking to water and low sucralose concentrations among SA was higher than predicted), a follow-up study was conducted in which all rats were retested with water and the 2 lowest concentrations of sucralose presented 5 times per test session in randomized blocks for a total of 3 days.
Following the completion of Davis-rig testing, rats were given the same series of 2-bottle preference tests as described in Experiment 2 in order to provide an independent classification of the rats as either SP or SA. This was done in order to determine whether the sucralose preference/avoidance profile assessed via the brief-access test predicted the rats' categorization as either SP or SA via 2-bottle preference tests.
Experiment 4
Previous research suggests that at high concentrations of sucralose (1–4 g/L), rats categorized as SA display strong avoidance for sucralose and consume water almost exclusively (Sclafani and Clare 2004). However, rats under these test conditions are fluid replete and given the choice to consume either water or sucralose. In order to assess the robustness of this sucralose avoidance, a group of 18 male Long–Evans rats were categorized as either SP (n = 6) or SA (n = 12) using the series of 2-bottle preference tests described in Experiment 2. Rats were then adapted to a 23-h water-deprivation schedule for 5 days. Following adaptation, rats were given 1-h access to one bottle containing 0.001, 0.01, 0.1, or 1.0 g/L sucralose every other day, in an ascending order. Based on a concern that SA would not consume enough of the sucralose solutions to adequately rehydrate on test days, each sucralose test day was followed by a water test day in which rats received 1-h access to one bottle containing water.
Data analysis
For experiments involving 2-bottle preference tests, intakes of water and each concentration of sucralose were monitored daily and then averaged across the 2 or 4 test days. Average preference for each concentration of sucralose was calculated by dividing average sucralose intake by average total fluid (sucralose plus water) intake and expressing the scores in percent. Rats were classified as SP if they displayed a preference (>50%) on at least 5 of the 7 sucralose concentrations (Experiment 1) or 3 of the 4 sucralose concentrations (Experiments 2–4). The remaining rats were classified as SA. Preference scores and fluid intakes were analyzed by 2- or 3-factor mixed-design analyses of variance (ANOVAs) with sex, strain, and/or SP/SA group as between-subjects factors and estrous stage and/or concentration as within-subjects factors, as appropriate.
The number of licks for water and each concentration of sucralose obtained during Davis-rig testing was averaged across the 3 daily trials and the 5 test sessions (i.e., data represent an average of up to 15 presentations per concentration). As we were interested in the rats' orosensory driven behavior, in the rare instance where an animal did not make a single lick to a tube, that zero was not included in the average as it could not be the result of a taste-guided behavior. The mean number of licks measured in the Davis rig and the mean intakes (g) for one-bottle tests were analyzed by 2-factor mixed-design (SP/SA group × concentration) ANOVAs. Significant main or interactive effects (P < 0.05) were examined using Tukey's honestly significant difference test.
Results
Experiment 1
Effects of sex and stage of the estrous cycle
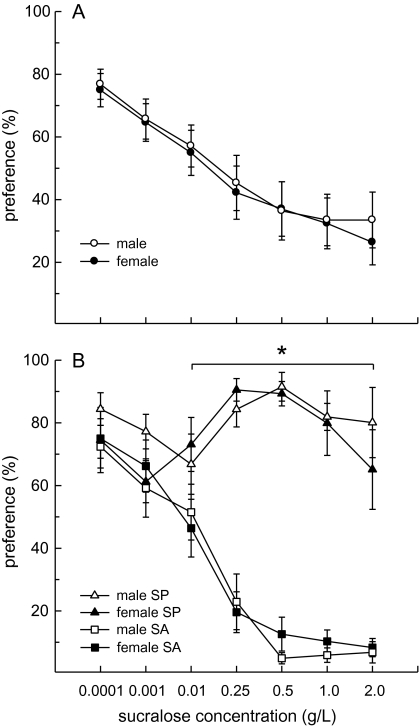
Overall, male and female rats displayed a comparable decrease in sucralose preference as the concentration of sucralose increased (F6,252 = 13.97, P < 0.0001, Figure 1A). At low (0.0001–0.01 g/L) concentrations, rats displayed a moderate preference for sucralose. However, at higher (0.25–2 g/L) concentrations, rats displayed a mild sucralose avoidance. Individual rats were then classified as SP or SA, based on their individual preference curves. While the number of SP was slightly higher in males (9 of 22) than in females (7 of 22) and the number of SA was slightly higher in females (15 of 22) than in males [(13 of 22), these sex differences were not reliable (χ2 = 0.39), not significant (n.s.)]. Overall, a greater proportion of rats were categorized as SA rather than SP (64% vs. 36%, respectively).
Figure 1.
Preference for sucralose across a range of concentrations was similar in male and female Long–Evans rats. Data are mean (±SEM) preference scores (sucralose intake divided by total fluid intake, expressed as percentages). (A) Prior to categorization as either SP or SA, the rats displayed a reduction in their preference for sucralose over water as the concentration of sucralose was increased. This effect was similar in male and female rats. (B) SP preferred sucralose over water at all concentrations. In contrast, SA displayed indifference to 0.01 g/L sucralose and then preferred water over sucralose at concentrations of 0.25 g/L and higher. The preference curves of SP and SA were similar in both sexes. *Male and female SP > male and female SA, Ps < 0.05.
Additional analyses of the preference scores revealed an interactive effect of SP/SA group and concentration (F6,240 = 23.79, P < 0.0001, Figure 1B). Both SP and SA preferred sucralose to water at the 2 lowest concentrations. A significant SP/SA group difference in preference scores first emerged at the 0.01 g/L sucralose concentration and this group difference strengthened as the concentration of sucralose increased (Ps < 0.05). Sucralose preference peaked at the 0.5 g/L concentration in SP and sucralose avoidance was strongest at the 2.0 g/L concentration in SA. No sex differences in sucralose preference were detected among the groups of SP or SA.
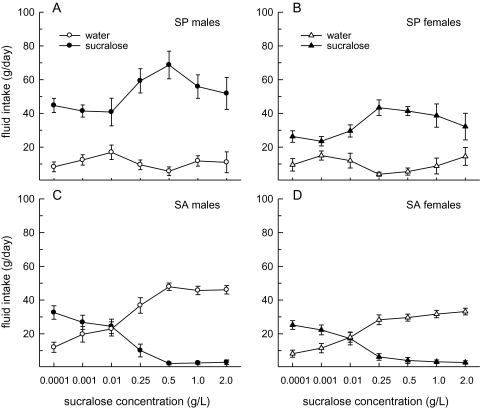
Examination of fluid intake revealed that all SP consumed more sucralose than water across all 2-bottle preference tests (Figure 2A,B). Among SP, sucralose intake was greater in males than in females (F1,13 = 6.89, P < 0.05) and varied by concentration (F6,78 = 8.41, P < 0.0001), with the highest intakes occurring in the 0.25–1.0 g/L range in both sexes (Ps < 0.05). In comparison, water intake was consistently low and similar in males and females. As a result, total fluid intake among SP mirrored their sucralose intake. Males consumed more fluid than females (F1,13 = 10.22, P < 0.01) and total fluid intake was influenced by sucralose concentration (F6,78 = 12.98, P < 0.0001), with peak fluid intakes occurring when 0.25–1.0 g/L sucralose was available (Ps < 0.05).
Figure 2.
Sex differences in sucralose intake and water intake were detected among SP and SA, respectively. Data are mean (±SEM) intakes of water and sucralose in male and female SP and SA. (A, B) Male SP consumed more sucralose than female SP. In addition, sucralose intake increased as a function of increasing sucralose concentration in both male and female SP. (C, D) Male SA consumed more water than female SA. In addition, water intake increased and sucralose intake decreased as a function of increasing sucralose concentration in both sexes.
Examination of fluid intake among SA revealed that all SA consumed more water than sucralose when sucralose concentrations exceeded 0.01 g/L (Figure 2C,D). Among SA, sucralose intake decreased as a function of increasing concentration (F6,162 = 42.63, P < 0.0001), with lowest intakes occurring in the 0.5–2 g/L sucralose range (Ps < 0.05). This effect did not differ in males and females. Water intake was greater in males than in females (F1,162 = 14.51, P < 0.001) and varied by concentration (F6,162 = 47.49, P < 0.0001), with highest water intakes occurring in both sexes when 0.25–2 g/L sucralose was available (Ps < 0.05). Although total fluid intake was greater in males than in females (F1, 27 = 39.17, P < 0.0001), their pattern of fluid intake remained stable across the sucralose concentration curves in both sexes.
Sucralose preference was not influenced by stage of the estrous cycle in cycling female rats. As was observed in females tested without regard to cycle stage, cycle-synchronized rats displayed a decrease in sucralose preference as a function of increasing sucralose concentration (F6,144 = 7.22, P < 0.0001). This preference curve was not influenced by either a main or interactive effect of cycle stage (F3,144 = 1.29, F18,144 = 1.11, respectively, n.s.). As such, data were collapsed across stage of the estrous cycle. Prior to categorization as SP or SA, female rats displayed a preference for the 0.0001 and 0.001 g/L concentrations (83.7 ± 7.3 and 74.3 ± 10.5, respectively), indifference at the 0.01 g/L concentration (58.3 ± 12.3), and avoidance at the 0.25–2.0 g/L concentrations (range in avoidance scores: 38.4 ± 13.7–24.1 ± 11.0). Following categorization as SP or SA, the sucralose preference cures in females were similar to that observed in male rats (data not shown).
Effects of strain
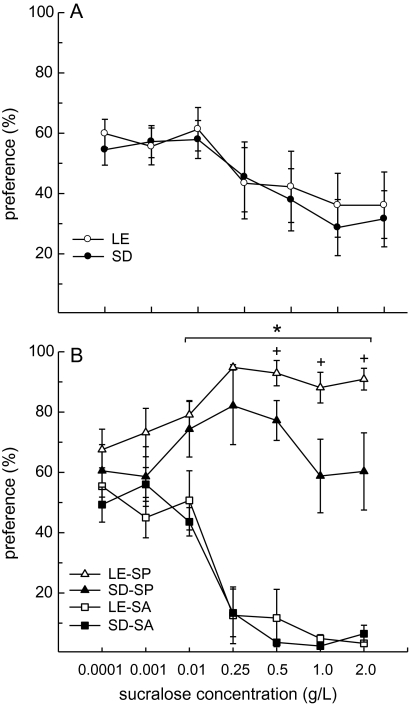
Prior to categorization as either SP or SA, male Long–Evans and Sprague–Dawley rats displayed a comparable decrease in sucralose preference as the concentration of sucralose increased (F6,162 = 7.41, P < 0.0001, Figure 3A). At low (0.0001–0.01 g/L) concentrations, both strains displayed a moderate preference for sucralose. At higher (0.25–2 g/L) concentrations, both strains displayed a weak-to-moderate avoidance of sucralose. A breakdown of these data by SP/SA group revealed that the proportion of SP (6 of 16) to SA (10 of 16) in Long–Evans and the proportion of SP (7 of 15) to SA (8 of 15) in Sprague–Dawley rats was not significantly different (χ2 = 0.37, n.s.). Overall, a greater proportion of both strains were categorized as SA rather than SP (58% vs. 42%, respectively). Further analysis of the preference scores revealed an interactive effect of SP/SA group and concentration (F6,162 = 19.16, P < 0.0001, Figure 3B). Overall, rats preferred sucralose to water at the 2 lowest concentrations. A significant SP/SA group difference first emerged at the 0.01 g/L sucralose concentration and this group difference became more pronounced as the concentration of sucralose increased (Ps < 0.05). Sucralose preference peaked at the 0.25 g/L sucralose concentration in SP of both strains and reached a nadir in the 1.0–2.0 g/L concentration range in SA of both strains. A main effect of rat strain was also detected (F1,162 = 4.61, P < 0.05). This strain difference was driven by the greater preference scores in Long–Evans SP, relative to Sprague–Dawley SP, at the 3 highest concentrations of sucralose (Ps < 0.05).
Figure 3.
Preference for sucralose across a range of concentrations was similar in male Long–Evans (LE) and Sprague–Dawley (SD) rats. Data are mean (±SEM) preference scores (sucralose intake divided by total fluid intake, expressed as percentages). (A) Prior to categorization as either SP or SA, the rats displayed a reduction in their preference for sucralose over water as the concentration of sucralose was increased. This effect was similar in LE and SD rats. (B) SP preferred sucralose over water at all concentrations. This preference for sucralose was greater in LE rats, relative to SD rats, at concentrations of sucralose ≥ 0.5 g/L. In contrast, SA displayed indifference to 0.01 g/L sucralose and preferred water over sucralose at concentrations of 0.25 g/L and higher. No strain differences were detected among SA. *LE and SD SP > LE and SD SA, Ps < 0.05. +LE SP > SD SP, Ps < 0.05.
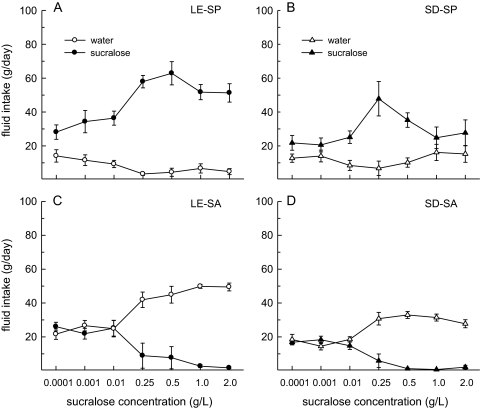
Examination of fluid intake revealed that all SP consumed more sucralose than water across all 2-bottle tests (Figure 4A,B). Among SP, sucralose intake was greater in the Long–Evans than Sprague–Dawley rats (F1,66 = 15.81, P < 0.01) and influenced by concentration (F6,66 = 7.76, P < 0.0001), with sucralose intakes peaking in the 0.25–0.5 g/L range in both strains. Water intake remained low in both strains of SP. As a result, total fluid intake mirrored sucralose intake. Long–Evans SP consumed more total fluid than Sprague–Dawley SP (F1,66 = 9.83, P < 0.001) and total intakes were influenced by concentration (F6,66 = 9.78, P < 0.0001), with peak fluid intakes occurring when 0.25 and 0.5 g/L sucralose was available.
Figure 4.
Strain differences in sucralose intake and water intake were detected among SP and SA, respectively. Data are mean (±SEM) intakes of water and sucralose in male Long–Evans (LE) and Sprague–Dawley (SD) SP and SA. (A, B) LE SP consumed more sucralose than SD SP. In addition, sucralose intake increased as a function of increasing sucralose concentration in both strains of SP. (C, D) LE SA consumed more water than SD SA. In addition, water intake increased and sucralose intake decreased as a function of increasing sucralose concentration in both strains.
SA consumed more water than sucralose at concentrations greater than 0.01 g/L (Figure 4C,D). SA decreased their sucralose intake as a function of increasing concentration (F6,96 = 15.85, P < 0.0001), with lowest intakes occurring in the 0.25–2 g/L range in both strains. Water intake increased as a function of increasing sucralose concentration (F6,96 = 21.43, P < 0.0001) and Long–Evans rats consumed more water than Sprague–Dawley rats (F1,96 = 20.35, P < 0.001). The differences in water intake between the 2 strains drove an interactive effect of SP/SA group and concentration on total fluid intake (F6,96 = 2.76, P < 0.05), with Long–Evans rats consuming significantly more fluid than Sprague–Dawley rats as sucralose concentration increased.
Experiment 2
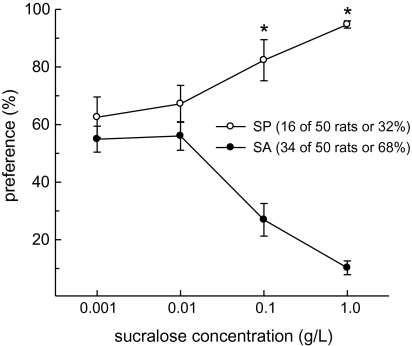
The use of an abbreviated (relative to Experiment 1) 2-bottle preference testing paradigm involving water and an ascending series of 4 sucralose concentrations revealed that 16 of the 50 rats tested (32%) were SP (i.e., they preferred at least 3 of the 4 sucralose concentrations tested) and 34 of the 50 rats tested (68%) were SA (i.e., they avoided sucralose solutions that exceeded 0.01 g/L) (Figure 5). As in Experiment 1, sucralose preference was influenced by an interactive effect of SP/SA group and concentration (F3,144 = 30.7, P < 0.001). Preference for sucralose increased among SP and decreased among SA, as a function of increasing sucralose concentration such that SP/SA group differences were detected at 0.1 and 1 g/L (Ps < 0.05).
Figure 5.
Approximately twice as many male Long–Evans rats were classified as SA, relative to SP, on the basis of their behavioral responses to 2-bottle 24-h preference tests involving water and an ascending series of sucralose concentrations. Data are mean (±SEM) preference scores (sucralose intake divided by total fluid intake, expressed as percentages). Preference for sucralose increased among SP and decreased among SA, as a function of increasing sucralose concentration. Group differences were detected at the 2 highest sucralose concentrations. *SP > SA, Ps < 0.001.
Experiment 3
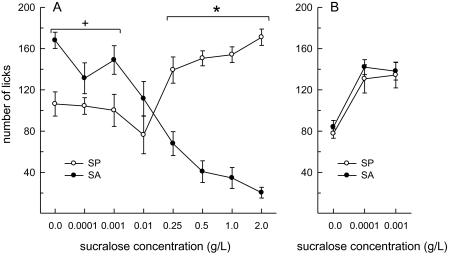
Based on the nonoverlapping licking responses to the highest concentration of sucralose during brief-access testing in the Davis rig, rats were classified as SP (emitted more than 120 licks per 30 s) or SA (emitted fewer than 30 licks per 30-s test session). An examination of the number of licks across the sucralose concentration curve revealed an interactive effect of SP/SA group and concentration (F7,98 = 31.79, P < 0.0001) (Figure 6A). While the number of licks to the 4 highest concentrations of sucralose was greater in SP, relative to SA, the numbers of licks to water and the 2 lowest concentrations of sucralose were greater in SA, relative to SP (Ps < 0.05). The latter finding was unexpected based on previous studies involving 2-bottle preference tests in which SP/SA group differences were not apparent at low concentrations of sucralose. We reasoned, therefore, that the latter finding may have been driven by a contrast effect between high and low sucralose concentrations in both SA and SP. To examine this hypothesis, rats were retested using the first 3 solutions in the series (0, 0.0001, and 0.001 g/L sucralose). This analysis revealed a main effect of concentration (F2,28 = 49.76, P < 0.001) (Figure 6B). The 2 sucralose solutions elicited a greater licking response than water (Ps < 0.05), and this effect was similar in SP and SA. No main or interactive effects of group were detected. To further ensure that the decrease in licking by SA to higher concentrations of sucralose was not a result of malaise or learning, we compared each SA's licking response with the first presentation of each concentration on the first day of testing to their averaged response across all 5 days. At no concentration did the SA's licking responses differ between time points (F1,16 = 2.94, n.s.).
Figure 6.
Sucralose-elicited licking behavior during brief (30 s) access tests differs in SA and SP. Data are mean (±SEM) number of licks/30-s presentation. Rats were classified as either SP or SA on the basis of their bimodal behavioral responses to the highest concentration of sucralose. (A) SP licked more and SA licked less, as a function of increasing sucralose concentration. SA licked more than SP to the 3 lowest concentrations and SP licked more than SA to the 4 highest concentrations. (B) A retest of the licking curves generated by the 3 lowest concentrations revealed no differences between SA and SP, suggesting that the earlier group differences at these concentrations were the result of a contrast effect across between low and high sucralose concentrations across the full range of concentrations tested in (A). +SA > SP, Ps < 0.05. *SP > SA, Ps < 0.05.
Following testing in the Davis rig, rats were reclassified as SP or SA on the basis of their sucralose preference as tested via a series of 24-h 2-bottle preference tests for water versus 4 concentrations of sucralose (0.001, 0.01, 0.1, and 1 g/L). The classification via both behavioral tests was identical. That is, the 7 rats classified as SP in the Davis rig were also classified as SP via a 2-bottle preference paradigm (mean preference score of 80 and 89 at the 2 highest concentrations, respectively). Similarly, the 9 rats classified as SA in the Davis rig were also classified as SA via a 2-bottle preference paradigm (mean preference score of 31 and 26 at the 2 highest concentrations, respectively).
Experiment 4
When given the choice between water and sucralose solutions exceeding 0.01 g/L, SA consume water almost exclusively (Sclafani and Clare 2004; Bello and Hajnal 2005). To test the robustness of this phenomenon, male rats were categorized as either SP or SA and then adapted to a 23 h/day water-deprivation schedule. These highly motivated rats were then given a series of brief (1 h) access one-bottle intake tests involving water and an ascending series of sucralose solutions. Under these conditions, fluid intake was influenced by an interactive effect of SP/SA group and sucralose concentration (F4,64 = 21.09, P < 0.0001) (Table 1). Intakes of water and the 2 lowest (0.001 and 0.01 g/L) concentrations of sucralose were similar between SP and SA. However, at the 2 highest (0.1 and 1 g/L) concentrations of sucralose, SA consumed less than SP (Ps < 0.05). In addition, SP consumed greater amounts of 0.1 and 1 g/L sucralose, relative to water, whereas SA consumed less 1 g/L sucralose, relative to water (Ps < 0.05).
Table 1.
One-bottle intakes of water and 2 concentrations of sucralose in fluid-restricted SP and SA
| Concentration of sucralose (g/L) |
|||||
| 0 | 0.001 | 0.01 | 0.1 | 1.0 | |
| SP | 17.9 ± 0.5 g | 21.6 ± 1.0 g | 16.4 ± 0.5 g | 24.0 ± 1.1 gb | 23.5 ± 1.4 gb |
| SA | 16.9 ± 0.9 g | 21.0 ± 1.0 g | 17.3 ± 1.0 g | 20.2 ± 1.0 ga | 9.1 ± 1.2 gac |
Data are presented as mean ± SEM. SP and SA were maintained on a 23-h fluid-deprivation schedule and then given daily, 1-h one-bottle intake tests involving an ascending series of sucralose solutions dissolved in water.
Intakes of 0.1 and 1.0 g/L sucralose were reduced in SA, relative to SP, P < 0.05.
Among SP, 0.1 and 1.0 g/L sucralose intake was greater than water (0 g/L) intake, P < 0.05.
Among SA, water (0 g/L) intake exceeded 1.0 g/L sucralose intake, P < 0.01.
Discussion
Sclafani and Clare (2004) provided the first demonstration of a bimodal behavioral response to sucralose in rats. Using a series of 2-bottle preference tests, they reported that individual rats could be classified as either SP (i.e., they preferred sucralose to water over a range [0.25–4 g/L] of sucralose concentrations) or SA (i.e., they displayed a profound avoidance of sucralose across the same range). This highly variable response appears unique to sucralose, as similar, natural variability has not been reported in the preferences for other sweeteners. Our current findings extend the existing literature examining sucralose preference in rats (Sclafani and Clare 2004; Bello and Hajnal 2005; Dess et al. 2009) by demonstrating that the bimodal behavioral response to sucralose is robust across sex, 2 rat strains, and multiple testing paradigms.
The perception of, and subsequent preference for, a variety of sweet tastants is influenced by estradiol. For example, the number of licks elicited by dilute (0.025 M) sucrose solutions during brief-access tests is decreased by both endogenous and exogenous estradiol in female rats (Curtis et al. 2004; Atchley et al. 2005). This reduced behavioral response is likely mediated by estradiol's ability to increase the detection threshold (i.e., reduce sensitivity) for sucrose in female rats (Curtis et al. 2005). On the other hand, female rats display greater preference than male rats for more concentrated sweet solutions including glucose and saccharin (Valenstein et al. 1967; Wade and Zucker 1969; Zucker 1969). Taken together, these data suggest that preference for sucralose may also be sexually dimorphic, with males displaying greater preference at near-threshold concentrations and females displaying greater preference at high concentrations. However, our direct examination of sucralose preference in male and female rats failed to support this hypothesis. Preference for sucralose was virtually identical in males and females both prior to and after their classification as either SP or SA (Figure 1), the proportion of SP to SA (33% and 66%, respectively) was similar in males and females, and sucralose preference was not influenced by stage of the estrous cycle. Thus, previous reports of sex differences in the preference for natural and artificial sweeteners do not appear to extend to sucralose, at least throughout the concentration range tested here. The reasons for this are unclear but may be related to our concentration range and/or the possibility that sucralose may have an aversive component that may have obscured any sex differences. It should be noted, however, that we failed to see a sex difference among SP, which appear to be relatively insensitive to any aversive properties of sucralose.
In the present study, we found that only ∼33% of both males and females could be classified as SP. This finding is not consistent with a previous report that female rats were more than twice as likely to be classified as SP than male rats (∼50% vs. 20%, respectively) (Sclafani and Clare 2004). These inconsistent findings are likely the result of methodological differences. Here, males and females were subjected to identical testing protocols. However, in a previous study (Sclafani and Clare 2004), females were tested across a series of increasing sucralose concentrations, whereas males were tested at a single concentration of sucralose (0.5 g/L), which may have biased the results toward a greater number of SA. Our additional finding that ∼35% of a large group (n = 50) of male rats were classified as SA (Figure 6), provides further evidence that the true ratio of SP to SA is ∼35–65%. Finally, the previous study reporting the ratio of SP to SA in male and female rats involved Sprague–Dawley rats (Sclafani and Clare 2004), whereas our study included both Long–Evans and Sprague–Dawley rats. While a strain difference may have contributed to the discrepant findings, this remains unlikely because our direct comparison of these 2 strains yielded few differences as discussed in greater detail below. The only other study to examine the influence of sex on sucralose preference involved LoS and HiS rats. Similar to our current findings, the authors found neither sex differences in sucralose preference nor sex differences in the ratio of SP to SA in these rats selectively bred for their saccharin preference (Dess et al. 2009). Taken together, the available data suggest that sucralose preference is not sexually dimorphic in the rat.
Although our series of 2-bottle preference tests failed to yield any sex differences in sucralose preference, we did observe sex differences in both sucralose and water intakes that were dependent upon the rats' SP/SA categorization. Males consumed more sucralose than females, however, this effect was only observed among SP (Figure 2A,B). Males also consumed more water than females, however, this effect was only observed among SA (Figure 2C,D). Because total fluid intake was comprised mostly of sucralose in SP and water in SA, it is likely that the sex differences in sucralose and water intake were driven by sex differences in fluid intake because male rats typically consume more fluid than female rats.
Previous studies of the rat's behavioral response to sucralose are limited to the Sprague–Dawley strain (Sclafani and Clare 2004; Bello and Hajnal 2005; Dess et al. 2009). Here, we provide the first evidence that a bimodal behavioral response to sucralose also exists in Long–Evans rats. This finding extends a previous report that preference for saccharin and sucrose are similar in these 2 rat strains (Tordoff et al. 2008). Further examination of the preference scores among individual rats revealed that the proportion of SP to SA was similar in both strains, suggesting that the bimodal response to sucralose is a robust phenomenon. We did, however, detect a strain difference among SP in which Long–Evans rats displayed greater preference for, and consumption of, the 3 highest concentrations of sucralose, relative to Sprague–Dawley rats (Figure 4B). This strain difference may be related to the greater variability in sucralose intake in Sprague–Dawley SP, relative to Long–Evans SP. Specifically, 2 of the 7 Sprague–Dawley SP consumed low amounts of the 2 highest sucralose concentrations but very high amounts of all other sucralose concentrations. As such, these 2 rats met our criterion for classification of SP (preference for sucralose at 5 of 7 concentrations), but their low sucralose intakes and preference scores at the upper end of the concentration curve drove down the overall means of the Sprague–Dawley SP group. In contrast, similar variability in sucralose intakes and preference scores were not observed in Long–Evans SP. In a previous report, consumption of a 5% glucose solution was greater in Long–Evans rats, relative to Sprague–Dawley rats (Fregly and Rowland 1992). This raises the possibility that an increase in the avidity for sweet-tasting solutions in Long–Evans rats may drive the more uniformly increased sucralose preference observed in Long–Evans SP, relative to Sprague–Dawley SP. Additional research investigating strain differences in preference for sweet tastants are required to test this hypothesis directly.
While the mechanism underlying the bimodal behavioral response to sucralose is unknown, we speculate that it may be related to a differential sensitivity to the orosensory effects of sucralose. The fact that SA display avoidance, rather than indifference, raises the possibility that sucralose may have an aversive taste component that is differentially sensed by SA and SP. Indeed, it is well established that some artificial sweeteners have a bitter aftertaste (e.g., Hoover 1980; Schiffman et al. 1995) and chlorinated sugars in particular (of which sucralose is one) are often reported to have a bitter-taste quality (Shamil et al. 1987). While humans report that the bitter aftertaste of sucralose is about one-third that of saccharin, acesulfame K, and stevioside, they also report that sucralose has a bitter taste that is more than 4 times that of sucrose (Schiffman et al. 1995). As such, ongoing research in our lab is investigating the hypothesis that SA are more sensitive than SP to a bitter-taste quality of sucralose. Another possibility is that sucralose may have a metallic aftertaste that SA are more sensitive to. Previous studies have shown that other artificial sweeteners that are reported to have bitter and/or metallic aftertastes (e.g., saccharin and acesulfame K) activate the TRPV1 receptor, which is also activated by metallic tasting salts (Riera et al. 2007) and contributes to the reduced preference for various artificial sweeteners in mice (Riera et al. 2008, 2009). Finally, we cannot exclude the possibility that SA may have heightened sensitivity to the postingestive consequences of sucralose. This hypothesis is based on studies suggesting that sweet and bitter-taste receptors in the gut may have physiological and/or behavioral effects (Hofer et al. 1996; Mace et al. 2007; Sutherland et al. 2007; Egan and Margolskee 2008; Glendinning et al. 2008; Hao et al. 2008). Indeed, intragastric infusions of concentrated sucralose (16 g/L) have been shown to decrease fluid intake in mice (Sclafani et al. 2010). Prior to the current study, investigations of the bimodal behavioral response to sucralose have been limited to 24-h 2-bottle preference tests, which would produce both orosensory and postingestive consequences. As such, we utilized a behavioral testing paradigm in the present study that minimizes postingestive feedback in order to assess the importance of the taste of sucralose in driving the bimodal behavioral response to sucralose in rats.
In a brief-access test like the one employed here, a solution's orosensory properties drive an animal's affective unconditioned licking response. Rats given brief access to concentrations of sucralose presented at random in our initial Davis-rig experiment produced patterns of licking that partially modeled the bimodal behavioral responses obtained in 2-bottle preference tests here and in a previous study (Sclafani and Clare 2004). Interestingly, we found an interaction effect in which SP showed reduced licking to low concentrations of sucralose (0–0.001 g/L) and increased licking to high concentrations of sucralose (0.25–2 g/L), relative to SA (Figure 6A). The licking behavior we measured at high concentrations of sucralose is consistent with findings involving 2-bottle preference studies here and in previous studies (Sclafani and Clare 2004; Bello and Hajnal 2005). Moreover, the licking pattern of SP suggests that they found the sucralose solutions more palatable as the concentration increased, whereas the licking pattern of SA suggests that they found the sucralose solutions less palatable as the concentration increased. Because postingestive feedback is minimized during these brief-access taste tests, we conclude that taste is more important than the possible postingestive effects of sucralose in driving avoidance in SA at high sucralose concentrations. One caveat to this conclusion is that it is based on averaged licking responses across 5 days of testing. Thus, it is possible that if SA experienced any aversive postingestive consequences on day 1 of testing, this could have driven down their licking behavior on subsequent testing days. In order to explore this notion, we compared the licking responses of SA on the first presentation of each concentration with those on each of the 4 subsequent testing days. At no concentration did the number of licks differ across days and there was no tendency for SA to decrease the number of licks across the 5-day testing period. In fact, on the first day of testing, SA could be differentiated from SP after a single 30-s presentation of 2 g/L sucralose, suggesting a rapid robust difference in the behavioral response to this stimulus. Taken together, these additional observations are not compatible with the notion that a conditioned postingestive effect of sucralose may have contributed to the reduced licking of SA, relative to SP. Thus, these findings strengthen our conclusion that it is the orosensory, rather than postingestive, effects of sucralose that contribute to sucralose avoidance in SA.
We were surprised by the group differences among SP and SA in the number of licks at the low end of the sucralose concentration curve, as a similar relationship has not been detected in 2-bottle preference tests. In the brief-access test, sucralose concentrations were presented at random to reduce the possibility of contrast effects across the range of sucralose concentrations. Despite this precaution, we decided that a follow-up study was necessary to investigate whether contrast effects between low and high sucralose concentrations were responsible for the SP/SA group differences at the low end of the sucralose concentration curve. We hypothesized that SP may have emitted fewer licks to water and the low concentrations of sucralose due to the availability of higher more palatable concentrations of sucralose and/or that SA may have emitted more licks to water and the low concentrations of sucralose as they found the highest sucralose concentrations less palatable. To test this hypothesis, rats received brief access to water and the 2 lowest (0.0001 and 0.001 g/L) sucralose concentrations from the first curve, presented at random. In support of the presence of contrast effects, we failed to detect any differences between SP and SA under these conditions (Figure 6B). Taken together, these experiments suggest that the hedonic evaluation of the 2 lowest concentrations of sucralose is similar in SP and SA, consistent with findings of similar preference scores among SP and SA within this same concentration range, as assessed in longer-term 2-bottle preference tests. In comparison, the divergent licking responses of SP and SA at the higher sucralose concentrations suggest that the 2 groups differ in their hedonic evaluation of these sucralose concentrations and that these group differences are likely mediated by differences in taste perception.
The results of the present study, together with previous reports (Sclafani and Clare 2004; Bello and Hajnal 2005), provide compelling evidence in support of the 2-bottle preference test as a highly reliable method for distinguishing between SP and SA. It is not known, however, whether this classification holds up under alternative conditions in which rats are highly motivated to consume fluids. In order to address this question, fluid-deprived SP and SA were given a series of 1-bottle tests containing an ascending series of sucralose concentrations. Despite their highly motivated state, SA continued to consume less of the 2 highest concentrations of sucralose (0.1 and 1 g/L), relative to SP. At the highest sucralose concentration, SA not only consumed less fluid than SP, but they also consumed significantly less fluid relative to their own baseline water intake. In comparison, SP consumed more of the 2 highest sucralose concentrations, relative to their baseline water intake (Table 1). These findings demonstrate that the avoidance demonstrated by SA in the 2-bottle test is a powerful enough phenomenon to reduce intake while the rat is fluid deprived. These findings, together with the findings of our brief-access tests, suggest that SA detect a negative taste quality in sucralose that is not detected by SP.
Understanding natural variation in taste perception has become increasingly valuable in describing human variation as we learn more about how these differences interact with other behaviors. For example, there is a growing literature suggesting that the ability to taste the bitter compound 6-n-propylthiouracil (PROP) covaries with perception of other tastants in humans (Bartoshuk 1979; Chang et al. 2006; Hayes et al. 2007) and there is evidence that this variation may also affect food choice (Akella et al. 1997; Keller et al. 2002; Keller and Tepper 2004; Garcia-Bailo et al. 2009). Less obvious connections have been made between variations in taste perception and high-risk behaviors. Rats that have been selectively bred for increased avidity for sweet-tasting solutions also demonstrate increased impulsivity, increased cocaine self-administration, and increased ethanol intake (Dess et al. 1998; Perry et al. 2007; Anker et al. 2008). In addition, rats bred for high and low preference for saccharin have served as models for the study of drug abuse and, conversely, rats bred for increased intake of drugs and alcohol demonstrate an increased avidity for a number of sweet-tasting substances (reviewed in Carroll et al. 2008; Kampov-Polevoy et al. 1999). In the present study, we have shown that there is a natural and robust variation in the taste perception of rats. That rats can be easily categorized as either SP or SA across sex, strain, testing paradigm, and fluid state suggest that the rat's bimodal behavioral response to sucralose is the result of a highly consistent set of behaviors rather than subtle individual differences in intake. Such extreme natural variation in taste preference has rarely been reported in rats. Additional work is required to determine whether SA and SP will serve as an animal model for examining differences in taste sensitivity, but we are hopeful as the ability to utilize natural variation within the rat has the potential to increase our understanding of the involvement of taste in guiding ingestive behavior with implications for clinical nutrition, weight management, and the development of chronic diseases.
Funding
This work was supported by the National Institutes of Health [grant numbers R01 DK073936 to L.A.E. and T32 DC000044 to G.C.L. and A-M.T.].
Acknowledgments
We thank Chris Carballo and Michelle Bales for their excellent technical assistance as well as Tate and Lyle for providing sucralose.
References
- Akella GD, Henderson SA, Drewnowski A. Sensory acceptance of Japanese green tea and soy products is linked to genetic sensitivity to 6-n-propylthiouracil. Nutr Cancer. 1997;29:146–151. doi: 10.1080/01635589709514616. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–629. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- Atchley DP, Weaver KL, Eckel LA. Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats. Physiol Behav. 2005;86:265–271. doi: 10.1016/j.physbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 1979;205:934–935. doi: 10.1126/science.472717. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KB, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bello NT, Hajnal A. Male rats show an indifference-avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutr Res. 2005;25:693–699. doi: 10.1016/j.nutres.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Chang W-I, Chung J-W, Kim Y-K, Chung S-C, Kho H-S. The relationship between phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) taster status and taste thresholds for sucrose and quinine. Arch Oral Biol. 2006;51:427–432. doi: 10.1016/j.archoralbio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Collier G. Some properties of saccharin as a reinforcer. J Exp Psychol. 1962;64:184–191. doi: 10.1037/h0048795. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav. 2004;80:657–664. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Stratford JM, Contreras RJ. Estrogen increases the taste threshold for sucrose in rats. Physiol Behav. 2005;86:281–286. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Chapman CD, Monroe D. Consumption of SC45647 and sucralose by rats selectively bred for high and low saccharin intake. Chem Senses. 2009;34:211–220. doi: 10.1093/chemse/bjn078. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: an indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8:78–81. doi: 10.1124/mi.8.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly MJ, Rowland NE. Comparison of preference thresholds for NaCl solution in rats of the Sprague-Dawley and Long-Evans strains. Physiol Behav. 1992;51:915–918. doi: 10.1016/0031-9384(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Garcia-Bailo B, Toguri C, Eny KM, El-Sohemy A. Genetic variation in taste and its influence on food selection. J Integr Biol. 2009;13:69–80. doi: 10.1089/omi.2008.0031. [DOI] [PubMed] [Google Scholar]
- Glendinning JI. Preference and aversion for deterrent chemicals in two species of Peromyscus mouse. Physiol Behav. 1993;54:141–150. doi: 10.1016/0031-9384(93)90056-l. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav. 2008;93:757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Sternini C, Raybould HE. Role of CCK-1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2008;294:R33–R38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chem Senses. 2007;32:225–236. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of a-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. Saccharin—bitter aftertaste? N Engl J Med. 1980;302:573–575. doi: 10.1056/NEJM198003063021009. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38:3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Keller KL, Tepper BJ. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obes Res. 2004;12:904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- Knight I. The development and application of sucralose, a new high-intensity sweetener. Can J Physiol Pharmacol. 1994;72:435–439. doi: 10.1139/y94-063. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice: VI. Saccharin, acesulfame, dulcin, and sucrose. Genet Res. 2010;12:366–371. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, Kellet GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GR, Jessup A. A dual taste for saccharin in the rat. II Taste change following alloxan injection. Chem Senses Flavor. 1977a;2:395–400. [Google Scholar]
- Morrison GR, Jessup A. Does saccharin have a dual taste for the rat? In: Weiffenbach JM, editor. Taste and development: the genesis of sweet preference. Washington (DC): U.S. Government Printing Office; 1977b. [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Riera CE, Vogel H, Simon SA, Damak S, le Coutre J. The capsaicin receptor participates in artificial sweetener aversion. Biochem Biophys Res Commun. 2008;376:653–657. doi: 10.1016/j.bbrc.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Riera CE, Vogel H, Simon SA, Damak S, le Coutre J. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J Neurosci. 2009;29:2654–2662. doi: 10.1523/JNEUROSCI.4694-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera CE, Vogel H, Simon SA, le Coutre J. Artificial sweeteners and salts producing metallic taste sensation activate TRPV1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R626–R634. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a functional of concentration. Brain Res Bull. 1995;36:503–515. doi: 10.1016/0361-9230(94)00225-p. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Clare RA. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 2004;29:523–528. doi: 10.1093/chemse/bjh055. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamil S, Birch GG, Mathlouti M, Clifford MN. Apparent specific volumes and tastes of cyclamates, other sulfamates, saccharins and acesulfame sweeteners. Chem Senses. 1987;12:397–409. [Google Scholar]
- Smith JC. Microstructure of the rat's intake of food, sucrose and saccharin in 24-h tests. Neurosci Biobehav Rev. 2000;24:199–212. doi: 10.1016/s0149-7634(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Smith JC, Sclafani A. Saccharin as a sugar substitute revisited. Appetite. 2002;38:155–160. doi: 10.1006/appe.2001.0467. [DOI] [PubMed] [Google Scholar]
- Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–G1428. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler P. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Sex differences in taste preferences for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- Zucker I. Hormonal determinants of sex differences in saccharin preference, food intake, and body weight. Physiol Behav. 1969;4:595–602. [Google Scholar]