Abstract
The face adaptation effect, as described by M. A. Webster and O. H. MacLin (1999), is a robust perceptual shift in the appearance of faces after a brief adaptation period. For example, prolonged exposure to Asian faces causes a Eurasian face to appear distinctly Caucasian. This adaptation effect has been documented for general configural effects, as well as for the facial properties of gender, ethnicity, expression, and identity. We began by replicating the finding that adaptation to ethnicity, gender, and a combination of both features induces selective shifts in category appearance. We then investigated whether this adaptation has perceptual consequences beyond a shift in the perceived category boundary by measuring the effects of adaptation on RSVP, spatial search, and discrimination tasks. Adaptation had no discernable effect on performance for any of these tasks.
Keywords: adaptation, detection, discrimination, visual search
Introduction
Face adaptation effects were first described by Webster and MacLin (1999) using a configural face processing paradigm; after brief exposure to an expanded face, a normal face appears contracted. Webster, Kaping, Mizokami, and Duhamel (2004) and others (Leopold, O’Toole, Vetter, & Blanz, 2001; Ng, Ciaramitaro, Anstis, Boynton, & Fine, 2006; Rhodes, Jeffery, Watson, Clifford, & Nakayama, 2003; Webster & MacLin, 1999) have since shown that this adaptation technique also results in shifts in categorical boundaries for facial properties such as gender, ethnicity, identity, and attractiveness. For example, after adapting to female faces for a few minutes, observers perceive a gender-neutral face as male.
Face adaptation also seems to result in changes in the neural response. Reduced fMRI responses are found after adaptation to individual faces (Loffler, Yourganov, Wilkinson, & Wilson, 2005; Rotshtein, Henson, Treves, Driver, & Dolan, 2005; Winston, Henson, Fine-Goulden, & Dolan, 2004) and after adaptation to properties such as gender and ethnicity (Ng et al., 2006) within the fusiform gyrus, inferior occipital gyrus, and cingulate gyrus.
A simplistic explanation for these category shifts and reductions in fMRI response is that adaptation either reduces the responsivity of mechanisms tuned for the adapting category or shifts the selectivity of these mechanisms away from the category boundary (Grill-Spector & Malach, 2001). Changes such as these should have perceptual consequences beyond a category shift. In this paper, we examine whether face adaptation (to the properties of ethnicity and gender) affects either visual search or face discrimination performance.
In Experiment 1, we demonstrate that robust face adaptation effects are found using our stimuli and paradigm. We measured (for example) the probability that Asian morphed faces that varied in their maleness would be reported as appearing male, before and after adaptation to female Asian faces. A subset of these data has been reported elsewhere (Ng et al., 2006).
Having established that our adaptation paradigm resulted in robust category shifts, we then tested the effects of adaptation on three tasks. In Experiment 2, we used a visual search task and found that adaptation had no effect on the ability to locate a face belonging to an unadapted category. In Experiment 3, we used an RSVP task and found, similarly, that adaptation had no effect on the ability to detect a briefly presented face. In Experiment 4, we used a discrimination task and found that adaptation had no effect on the ability to make fine discriminations along a category boundary. From this, we conclude that while adaptation to a category of face stimuli can affect category boundaries, face adaptation does not play an obvious role in other every day tasks such as finding a face of a specific gender or ethnicity in a crowd.
General methods
Subjects
A total of 98 subjects gave informed consent to participate in these experiments, which were approved by the internal review boards either at The Salk Institute for Biological Studies or at the University of California, San Diego. All subjects, ages 18–30 years of age, had normal or corrected-to-normal vision. Subjects received either money or course credit for their participation. A separate cohort of subjects participated in each experiment. Across all experiments, seven subjects were not included in data analysis because they failed to complete the experiments.
Stimuli
Stimuli consisted of frontal-view gray-scale face images of Asian (A), Caucasian (C), male (M), and female (F) faces of neutral expression. Face images were taken from the Ekman 1976 face set and the Cohn–Kanade AU-Coded Facial Expression Database; additional face images were photographs of students and staff of UCSD and The Salk Institute. The unmorphed face image set contained 88 exemplars, 22 faces for each category.
Morph images were created by morphing a pair of face images (MorphMan, version 4.0; STOIK Imaging, Moscow, Russia) varying (for example) from male to female. Each morph continuum contained 50 images ranging from fully male to fully female. Ten morph continuums were created for each morphing dimension (i.e., 10 sets of Asian male–female morphs, 10 sets of Caucasian male– female morphs, and so on).
Stimuli were presented at a viewing distance of 57 cm on a Sony computer monitor using MATLAB and the COGENT toolbox on a Dell desktop computer. Stimuli subtended 6.8 degrees of visual angle in Experiments 1, 2, and 4, and 4.1 degrees of visual angle in Experiment 3.
Experiment 1: Category shifts as a result of adaptation
Webster et al. (2004) demonstrated that after adapting to a set of female faces, a previously neutral face appears male, and vice versa. Here, we replicated this experiment to demonstrate that adaptation causes a shift in categorical perception. We also extended the paradigm in order to examine whether mechanisms selective for gender and ethnicity are singly tuned (selective for gender or ethnicity) or jointly tuned (selective for both gender and ethnicity).
In the classic McCollough (1965) contingent adaptation paradigm, adaptation to red vertical gratings and green horizontal gratings makes horizontal gratings look pinkish and vertical gratings look greenish. This contingent aftereffect is traditionally attributed to the selective adaptation of neurons that respond to both color and orientation (Stromeyer, Kranda, & Sternheim, 1978). However, as described below, contingent adaptation effects can also be explained in terms of increasing representational efficiency by de-correlating features that are correlated in the environment (Barlow, 1991; Barlow, & Földiák, 1989). Contingent adaptation paradigms have previously been used to examine the mechanisms underlying face perception. Contingent adaptation effects have been measured for the features of gender, identity, eye-spacing, and masculinity (Little, DeBruine, & Jones, 2005) and orientation, gender, and configuration (Rhodes et al., 2004).
Methods
Subjects performed a 2AFC judgment on morph face images. Each subject was tested in 4 conditions over 8 testing sessions: (1) baseline (no adaptation); (2) local adaptation; (3) remote adaptation; and (4) contingent adaptation. Each testing session was separated by at least one day. In a given session, subjects either judged whether face images appeared Asian or Caucasian (for A/C morphs) or male or female (for M/F morphs). Subjects were given as much time as they needed to make each judgment and were instructed to maintain central fixation throughout the testing session.
Local adaptation
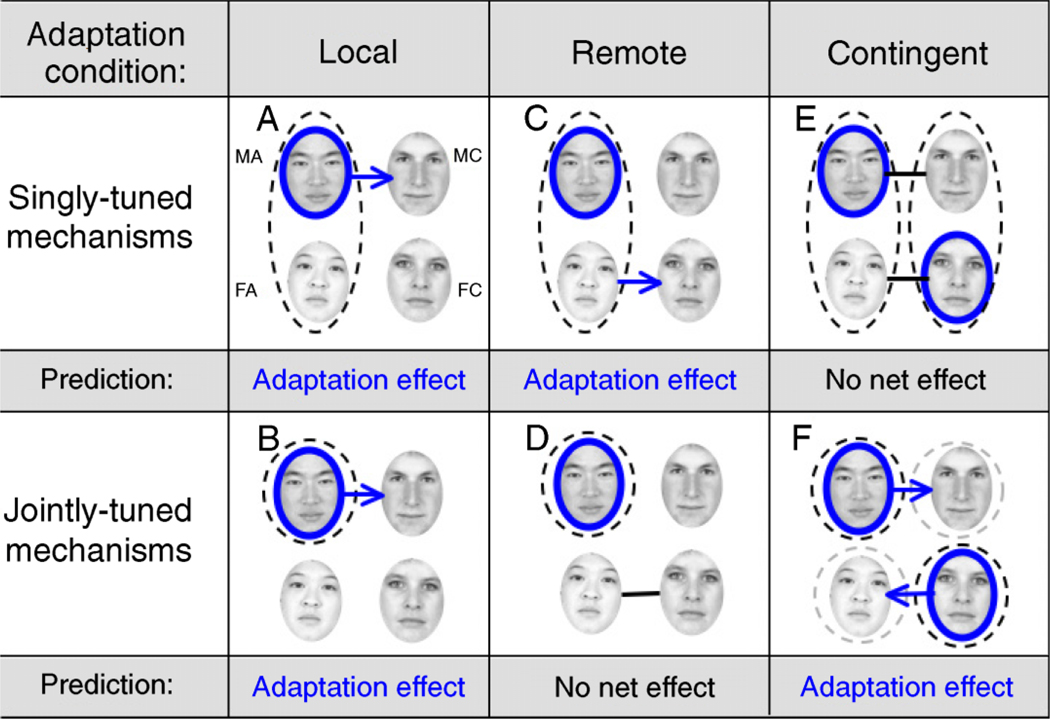
This was a replication of the Webster et al. (2004) experiment. Subjects were adapted to, for example, MA faces and performed one of two judgment tasks: (a) a gender (male or female) judgment on Asian faces morphed between male and female or (b) an ethnicity judgment for male faces morphed between Asian and Caucasian. In both cases, one end-point of the morph continuum was always exactly the same as the adapting face category (male Asian in the example shown in Figure 1), while the other endpoint of the continuum shared a single feature (ethnicity or gender) with the adapting category (male in the example shown in Figure 1). An adaptation effect, measured as a shift in the subjective midpoint, was expected regardless of whether mechanisms are singly or jointly tuned for ethnicity and gender (Figure 1A and 1B).
Figure 1.
Experiment 1 procedure and predictions. The four face categories are represented in each panel: MA, MC, FA, and FC faces in the top left, top right, bottom left, and bottom right, respectively. For all conditions, a single example is described in which subjects were first adapted to MA faces (blue circle). Jointly tuned (dashed circles) and singly tuned (dashed ovals) mechanisms that would be expected to be adapted during the task are shown. (A) Local adaptation for singly tuned mechanisms. Subjects discriminated the ethnicity of A/C male morphs. Mechanisms selective for Asian faces would be adapted (black dashed oval) while mechanisms selective for Caucasian faces would remain unadapted. We predict adaptation effects (blue arrow) whereby male faces appear more Caucasian. (B) Local adaptation for jointly tuned mechanisms. Mechanisms selective for MA faces would be adapted while mechanisms selective for MC faces would be unadapted; thus, we again predict an adaptation effect. (C) Remote adaptation for singly tuned mechanisms. Subjects discriminated the ethnicity of A/C female morphs. The adapted singly tuned mechanism is unselective for gender; however, adaptation would transfer to ethnicity discriminations of female faces. (D) Remote adaptation for jointly tuned mechanisms. Both mechanisms selective for FA and FC mediating the ethnicity discrimination are unadapted; thus, we predict no net effect (black line). (E) Contingent adaptation for singly tuned mechanisms. Subjects were adapted to MA and FC faces. Subjects then made ethnicity discriminations on A/C male morphs and A/C female morphs. Singly tuned mechanisms selective for both Asian and Caucasian faces would be adapted, resulting in no net effect. (F) Contingent adaptation for jointly tuned mechanisms. Mechanisms selective for FA and MC faces would remain unadapted, resulting in an adaptation effect.
Remote adaptation
A subject adapted to, for example, MA faces was tested with two possible morph continuums: (a) a gender judgment for Caucasian faces morphed between male and female or (b) an ethnicity judgment for female faces morphed between Asian and Caucasian. In both cases, one of the end-points of the continuum shared a single feature (ethnicity or gender) with the adapting face category while the other end-point of the continuum did not share any feature with the adapting face. Shifts in the subjective midpoint were expected if mechanisms were singly tuned, but not if they were jointly tuned (Figure 1A and 1D).
Contingent adaptation
Subjects were adapted to two face categories in random alternation. For example, observers might be adapted to both MA and FC faces. The adaptation face categories were always “opposites,” i.e., differed in both gender and ethnicity. Observers then performed one of two judgment tasks: (a) a gender judgment on morphs between FA and MA and FC and MC faces; or (b) an ethnicity judgment on morphs between FA and FC and MA and MC faces. Shifts in the subjective midpoint were expected if mechanisms were jointly tuned, but not if they were singly tuned (Figure 1E and 1F).
All possible combinations of adapting and test categories were tested in a counterbalanced and randomized design. Each testing session was separated by at least one day. In a given session, subjects were only adapted to a single adapting stimulus or, in the case of the contingent condition, one pair of adapting stimuli (e.g., MA and FC) and performed either gender or ethnicity judgments. Subjects were given as much time as they needed to make each judgment and responded with a key press.
Sessions 1 and 2 were baseline tests that measured responses to gender and ethnicity judgments without adaptation. The order of baseline tasks was randomized across subjects. In baseline conditions, there was no initial adaptation period and blanks replaced face images during the top up periods.
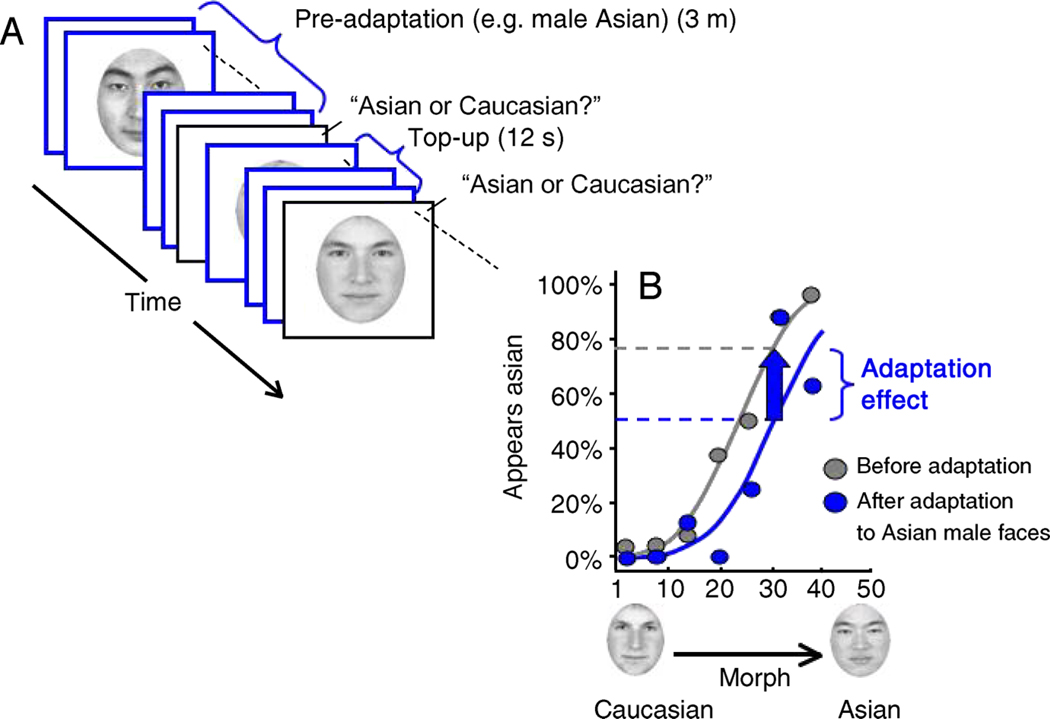
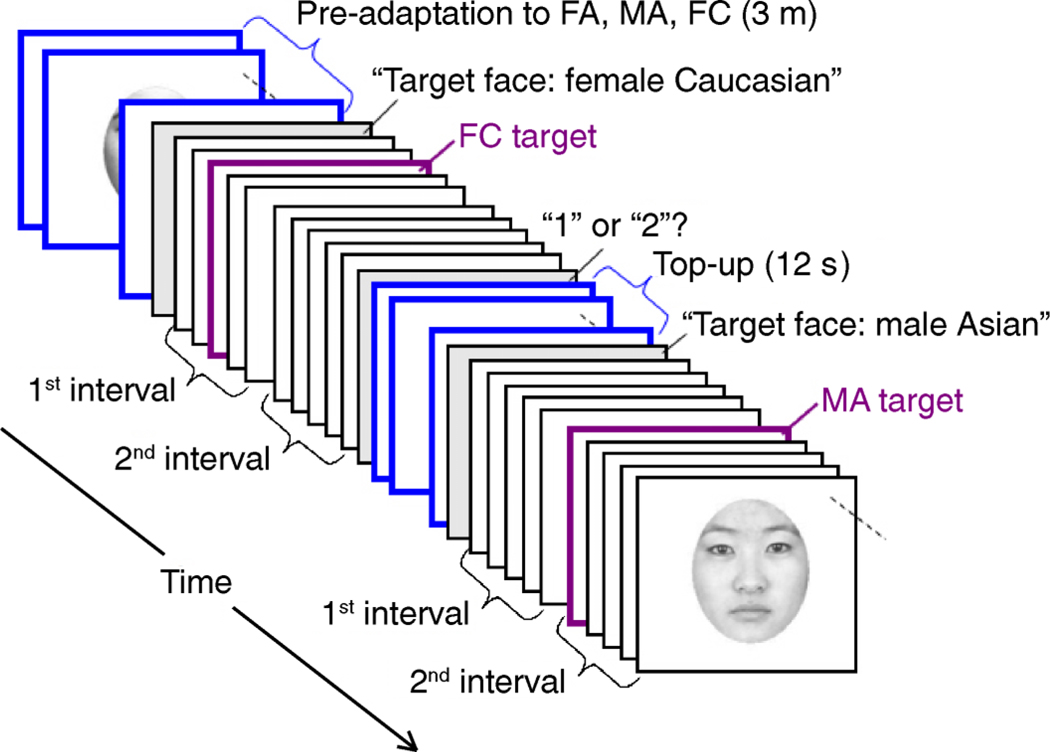
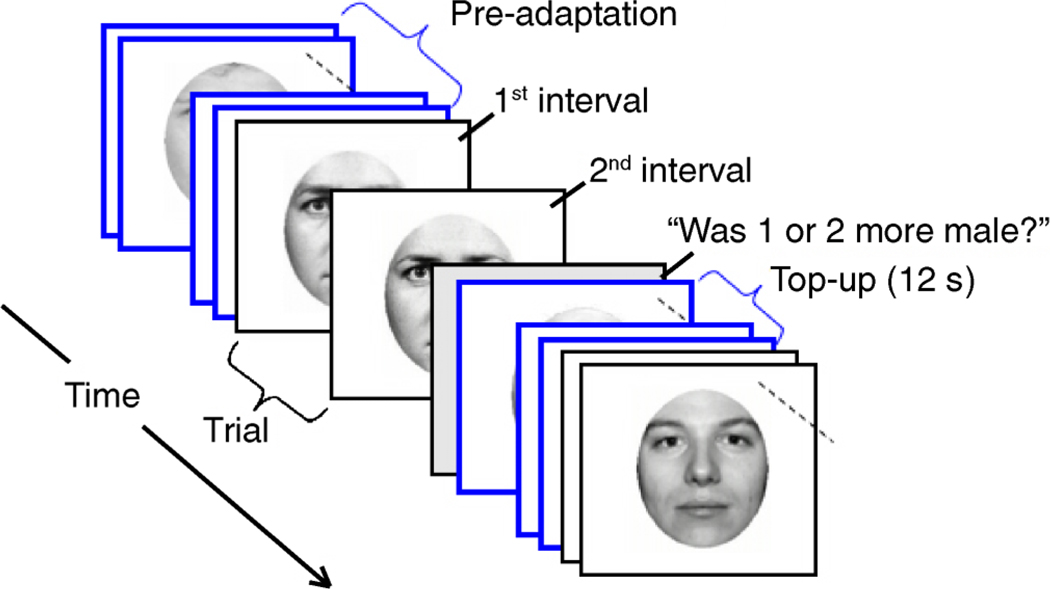
The remaining 6 sessions were adaptation conditions tested in random order. In each adaptation session, observers were pre-adapted for 3 min and were then “topped-up” with a 12-s re-adaptation period after each trial (Figure 2A).
Figure 2.
Experiment 1 design and an example psychometric function for an example of the local adaptation condition (as described in Figure 1A). (A) Subjects performed a 2AFC classification of morphed face images. There was an initial 3-min adaptation period and a 12-s top-up period between each trial. (B) The x-axis represents the morph continuum; the y-axis represents the percentage of time that the subject (Subject 1) judged that face as appearing Asian. We measured the shift in the psychometric function along the y-axis by interpolating (blue dotted line) the post-adaptation psychometric function to find the morph (No. 31) that was seen as Asian on 50% of the trials and then interpolated again (vertical arrow) to find that the percentage of trials this morph was seen as Asian before adaptation was 79% (gray dotted line); this yields a 29% adaptation effect.
During adaptation, subjects viewed (while maintaining fixation) a series of faces (1 s/image) from one or two of the 4 face categories (FA, FC, MA, MC). In the local and remote adaptation conditions, subjects were adapted with faces drawn from one category; while in the contingent adaptation condition, subjects were adapted to two categories (differing in both gender and ethnicity). To equalize the amount of adaptation for a given category over time, we used a blank screen as the ‘second category’ in both local and remote adaptation conditions. There were 96 trials per session and each session lasted approximately 40 min.
Responses were averaged across all possible morphs and a psychometric function (cumulative normal) was fit to each subject’s responses. See Figure 2B for an example; the x-axis represents the morph continuum and the y-axis represents the percentage of time the subject judged the morph as appearing Asian. We interpolated the psychometric function to find the morph that was seen as being Asian 50% of the time (and Caucasian the other 50% of the time). This point in the psychometric function is referred to as the subjective midpoint.
Changes in the categorical boundary were then quantified by measuring shifts in the subjective midpoint away from the adaptor category. After adapting to a MA face, for example, we expected to see a shift in the subjective midpoint when observers were asked to judge the ethnicity of a morph varying between Asian and Caucasian male. To quantify the shift in the subjective midpoint, we measured the difference between the pre- and post-adaptation subjective midpoints along the y-axis since the x-axis (morph continuum) is not necessarily linear (methods described in Ng et al., 2006). These shifts along the y-axis were averaged over multiple replications of the same experimental condition for each subject. We measured the shift in this subjective midpoint under local, remote, and contingent adaptation conditions.
Results
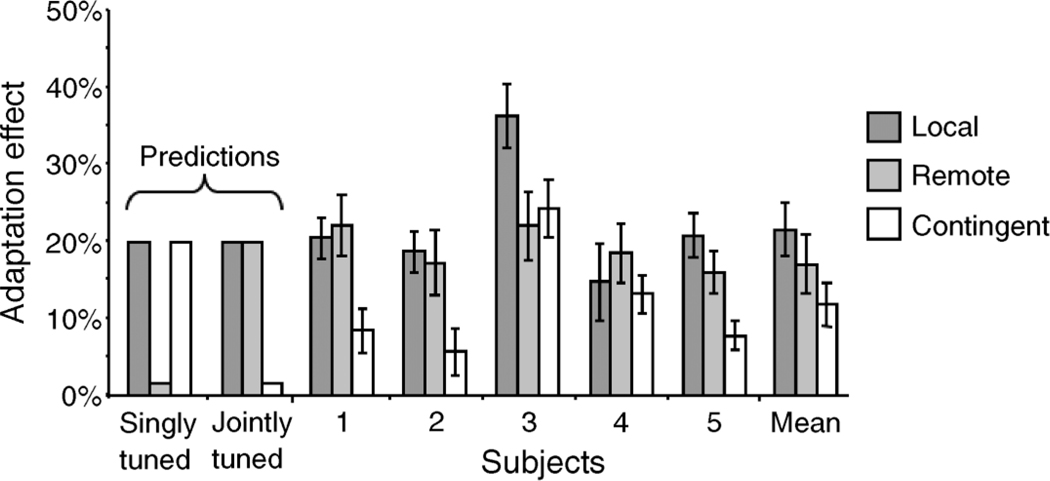
The mean adaptation effect for all 5 subjects is shown in Figure 3. We found significant adaptation effects for all subjects under all three adaptation conditions (p < .05). The average adaptation effect across all subjects for local, remote, and contingent adaptation was 21.54% (SEM ±3.47), 16.96% (SEM ±3.86), and 11.74% (SEM ±2.77), respectively.
Figure 3.
Experiment 1 predictions and results. Categorical boundary shifts were found in all subjects in all adaptation conditions. Error bars for individual subjects are calculated across all repeats within a condition. The error bar for the mean response is calculated across subjects.
Adaptation effects averaged across subjects were also significant (local: t(94) = 12.8586, p < .0001; remote t(100) = 11.2775, p < .0001; contingent: t(296) = 8.3715, p < .0001). As might be expected, we found greater adaptation in the local condition than in the remote or the contingent condition, though this result was only significant for the contingent condition (Tukey–Kramer, p < .05). There was also greater adaptation in the remote condition than the contingent condition (Tukey–Kramer, p < .05).
These results are consistent with a model in which the local adaptation effect is driven by both singly and jointly tuned mechanisms, adaptation in the remote condition is driven by singly tuned mechanisms, and adaptation in the contingent condition is driven by jointly tuned mechanisms. The presence of adaptation under all three conditions is therefore consistent with the existence of both singly and jointly tuned mechanisms.
However, it is also possible to explain our results without assuming that adaptation occurs within mechanisms tuned for the properties of gender and ethnicity. The quality of “femaleness” does not consist of a single feature. Rather, femaleness is represented by a variety of cues that are fairly unreliable individually but tend to be highly correlated with each other within female faces (rounder cheeks, larger eyes, etc.). According to “visual coding” theories, adaptation does not adapt individual mechanisms tuned for each of the individual underlying cues but rather results in adaptation to the conjunction of these features along axes whose orientations best de-correlate these features. Visual coding models predict opponent coding along dimensions such as gender and ethnicity, even though these qualities are clearly delineated by a complex multiplicity of cues (Barlow, 1991; Barlow, & Földiák, 1989).
According to “visual coding” models, local adaptation effects might be due to adaptation across the many cues that are associated (for example) with femaleness. Remote and contingent adaptation effects would be due to adaptation acting to de-correlate features (e.g., Asianness and femaleness), which were presented in a correlated manner during the adaptation period (Barlow, 1991; Barlow & Földiák, 1989).
We did not see a difference in category boundaries as a function of the gender or ethnicity of the subject being tested. However, we collected data from only 5 subjects in this experiment (2 female Asians, 2 Caucasian males, 1 Caucasian female). All subjects were UCSD students. It has previously been shown that the ethnicity of subjects (as opposed to their previous experience with different ethnic groups) does not have a significant effect on the position of perceived category boundaries (Webster et al., 2004). In any case, our measurement of adaptation shifts should be relatively robust to differences in the absolute position of the category boundary across subjects.
Experiment 2: Spatial search
You may find that in the photo montage of Figure 4 you can identify the woman more quickly than you can find the man in the red tie. It seems as if the distinctive feature of femaleness “pops out” from the men surrounding her. This phenomenon is consistent with the notion that adaptation to a particular face category may result in a reduction of the response to the adapted face category and thereby make a face belonging to a novel face category relatively more detectable.
Figure 4.
An example of how qualities such as gender or ethnicity can result in a face being distinctive in a crowd. Copyright permission granted by “skeptical brotha” (skepticalbrotha@yahoo.com).
Consistent with the notion that adaptation is capable of changing the detectability of stimuli, some early studies examining the effects of adaptation on low-level features have found increased detection thresholds after adaptation to spatial frequency (Blakemore & Campbell, 1969; Blakemore & Nachmias, 1971), orientation (Clifford, Wyatt, Arnold, Smith, & Wenderoth, 2001), and speed (Clifford & Wenderoth, 1999). In the color domain, adaptation has been observed to have selective effects on color detection thresholds, and these selective effects have been used to examine the selectivity of chromatic mechanisms (Macleod & von der Twer, 2003; Thornton & Pugh, 1983). For many tasks and stimuli, however, adaptation has little or no effect on detection thresholds (also see General discussion).
A reduction in response due to adaptation might be expected to affect search as well as detection. Studies examining search using low-level features such as orientation, shape, and color have shown that stimuli tend to “pop-out” when they are of higher luminance (Dawson & Thibodeau, 1998; Theeuwes, 1995), contrast (Nothdurft, 1993) or chromatic saturation than distractors (D’Zmura & Mangalick, 1994; Treisman & Gelade, 1980).
Adaptation has previously been shown to affect search in the color domain (McDermott, Mulligan, Bebis, & Webster, 2006; Webster, Raker, & Malkoc, 1998). McDermott et al. (2006) examined subjects’ ability to detect a target ellipse of variable color presented at a random location on a dense background of ellipses that varied along either the LvsM or SvsLM cardinal axes. Observers adapted by viewing a rapid succession of backgrounds drawn from one color axis and then searched for a target on a background from the same or different color axis. Targets were located more quickly on the background axis that observers were pre-exposed to, confirming that pre-exposure can improve search efficiency for stimuli that differ from the background.
Here we asked subjects to find a face belonging to a particular category among distractors after adaptation to either the target or a distractor category.
Experiment 2A: Gender and ethnicity search after adaptation to three out of the four face categories
Methods
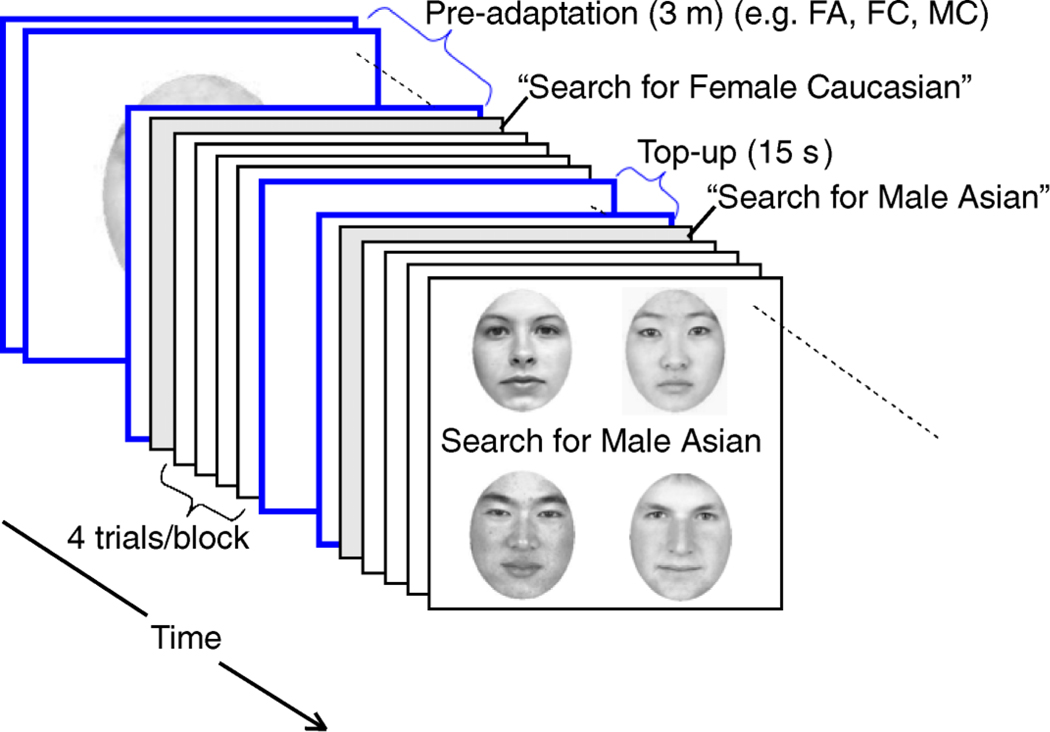
The experimental paradigm is shown in Figure 5. Subjects pre-adapted for 3-min to three of the four adaptation categories, for example MA, MC, and FA. On each trial, an auditory tone indicated the onset of the trial and four faces (one from each of the four categories) appeared in the corners of the computer screen, equidistant from the center. Subjects were instructed to report the location of the target face category as quickly as possible. The target face category, which could be any one of the four categories, randomly changed every 4 trials. The spatial location of each face category was randomized. Both response times and accuracy were recorded.
Figure 5.
Experiment 2 design. Subjects were pre-adapted to faces from 3 categories and adaptation was topped-up between each block of 4 trials. The target face category was cued before each block. A face from each of the 4 categories appeared on each trial.
A 15-s top-up adaptation period (1 image/s) was interposed between each block of four trials. During pre-adaptation and this top-up adaptation, subjects were instructed to fixate on a central fixation spot. At the end of this top-up adaptation period the instruction “Search for Male Asian” (for example) was displayed on the screen for 500 ms to inform the subjects of the target category for the next block.
Subjects were told to freely view the screen during test trials and to locate the target as quickly and accurately as possible. Each of the four spatial locations was identified with a unique key: top left = “A,” bottom left = “Z,” top right = “K,” bottom right = “M.” Positive and negative feedback were given after every trial via a change in the color and size of the fixation spot. Once the target was identified, the stimuli disappeared and subjects automatically moved onto the next trial or top-up period.
Given a test period of approximately 1.5 s (the average across subjects), subjects were exposed to adaptors for approximately 90% of the testing period (excluding the pre-adapt period). The extent of adaptation was therefore similar to the amount of adaptor exposure (approximately 85% of the testing period) in Experiment 1.
Each subject participated in two testing sessions, separated by 1 or 2 days. The only difference between the two testing sessions was in the choice of the categories that were used for adaptation. For example, an observer might be adapted to FA, FC, and MA faces in Session 1, resulting in FA as the most adapted category and MC as the least adapted category, FC and MA would be adapted at an intermediate level. This observer would then be adapted to MC, FC, and MA in Session 2. Here, the most adapted category would be MC, the least adapted category would be FA, and FC and MA would again be adapted at an intermediate level. Across subjects, all possible combinations of adaptor conditions were tested in a counterbalanced design. Each subject carried out a total of 800 trials across both sessions.
Results
Response times
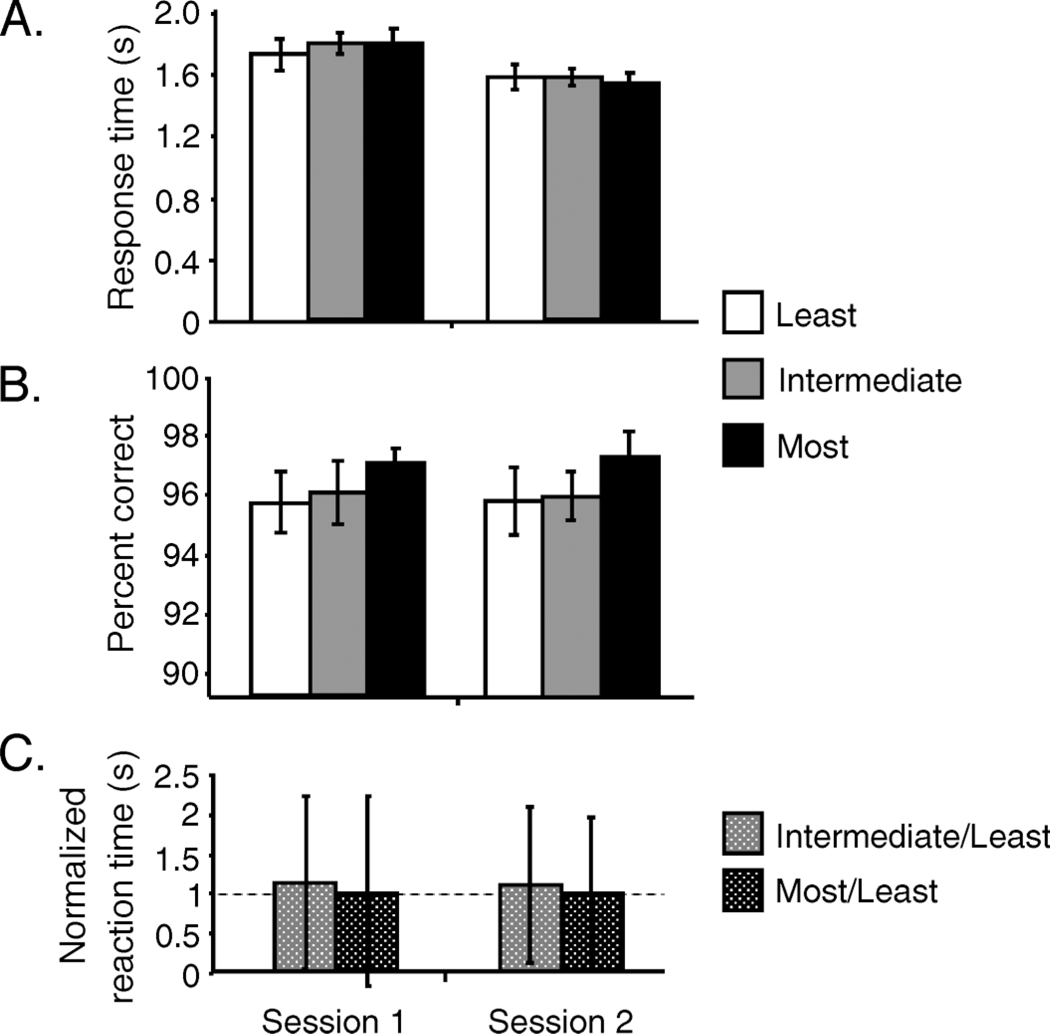
Figure 6A shows the effect of adaptation on the average amount of time it took subjects to search for the least, intermediate, and most adapted face categories for the first and second sessions (data from the two face categories that were adapted at an intermediate level were collapsed for each subject).
Figure 6.
Experiment 2A results. (A) Average response times for search of the least (white), intermediate (gray), and most (black) adapted target face categories across subjects. A significant decrease in response times from Sessions 1 to 2 can be attributed to practice effects (p < .0014). (B) There was no significant difference in percent correct across adaptation levels and sessions. (C) Normalized reaction times show no significant difference from 1 (dotted line), indicating no effect of the extent of adaptation on reaction time.
A comparison of search times between sessions (collapsing across adaptation levels) showed significant learning; subjects required significantly less search time in the second session (F(1, 70) = 11.12, p < .0014), presumably due to practice effects. However, we did not find that the amount of adaptation affected the time required to search for a target face category, for either session (Session 1: F(2, 33) = 0.1952, p = .8236; Session 2: F(2, 33) = 0.1280, p = .8803). There was no interaction between practice effects and the adaptation condition (F(,2, 2) = 1.2969, p = .2800).
Accuracy
Percent correct for each adaptation condition is shown for each session in Figure 6B. The percent correct averaged across subjects for least, intermediate, and most adapted face categories in Session 1 was 95.74% (SEM ±1.05), 96.06% (SEM ±1.06), and 97.03% (SEM ±0.52), respectively; in Session 2, 95.83% (SEM ±1.13), 95.92% (SEM ±0.84), and 97.25% (SEM ±0.83), respectively. Accuracy was not significantly different between the different adaptation levels for either session (Session 1: F(2, 33) = 0.5446, p = .5852; Session 2: F(2, 33) = 0.7113, p = .4984) or across sessions (F(5, 66) = 0.5059, p = .7708). In all conditions, performance was near ceiling since subjects were given as long as they needed to identify the location of the target face (this was also the case in Experiments 2B and 2C).
Normalized data
To reduce variance due to inter-subject variability in search times, we normalized search times observed for intermediate and high levels of adaptation based on the search time required for the least adapted condition, as shown in Figure 6C. Values greater than 1 indicate that search was faster than for the least adapted condition; values less than 1 indicate that search was slower than for the least adapted condition. Normalized search times were not significantly different from 1, suggesting that the adaptation condition had no effect on search time (Session 1: normalized intermediate, t(11) = 1.4451, p = .1763; normalized most, t(11) = 2.0436, p = .0657; Session 2: normalized intermediate, t(11) = 0.0555. p = .9568; normalized most, t(11) = 1.5318, p = .1538).
Experiment 2B: Gender search after adaptation to a single face category
One concern about our failure to find an improvement after adaptation in Experiment 2A was that subjects adapted to three face categories rather than to one or two face categories, as in Experiment 1. The results from Experiment 1 suggest that the strongest category shifts are found when adapting to a single face category. We therefore repeated Experiment 2A, using a procedure that allowed us to adapt along a single dimension.
Methods
The procedure was identical to that in Experiment 2A, except that all faces were Caucasian. Subjects were adapted to either male or female faces and were asked to identify the location of either the male or the female face. Only two face images were presented, one male and one female, on either side of the fixation spot. The location of each face type was random. The left and right spatial locations were identified using keys “Q” and “P,” respectively.
Each subject participated in two testing sessions, separated by 1 to 3 days. Subjects adapted to (for example) female face images in Session 1 and male face images in Session 2, or vice versa; the order was counterbalanced. Given an average test period of 500 ms, subjects were exposed to adaptors for approximately 95% of the testing period (excluding the pre-adapt period). Each subject carried out 800 trials across both sessions.
Results
Response times
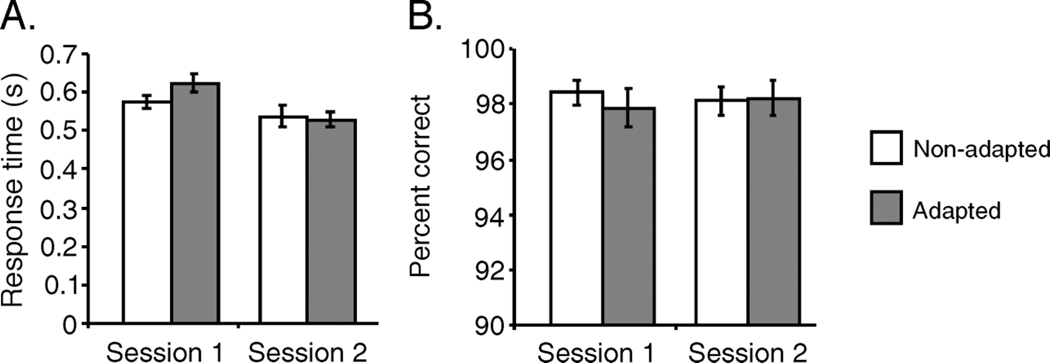
Figure 7A shows the effect of adaptation on the average amount of time it took each subject to search for the nonadapted and adapted face category. Response times were not significantly different for nonadapted compared to adapted face categories in either session (Session 1: t(9) = 1.4108, p = .9040; Session 2: t(9) = 1.2392, p = .1233). We again found a significant decrease in response times from Sessions 1 to 2, which can be attributed to practice effects (t(19), p <. 0001).
Figure 7.
Experiment 2B results. (A) Average response times across all subjects for search of the nonadapted (white) and adapted (gray) face categories by session. (B) Average percent correct across subjects.
Accuracy
The effect of adaptation on accuracy is shown in Figure 7B. In Session 1, the average percent correct across subjects for the adapted face category was 97.85% (SEM ±0.68), and the percent correct for the nonadapted face category was 98.40% (SEM ±0.45), respectively. In Session 2, the average percent correct for the adapted category was 98.20% (SEM ±0.62) and 98.10% (SEM ±0.50) for the nonadapted category. There was no significant difference in accuracy across conditions for either session (F(3, 36) = 0.1803, p = .9091).
Experiment 2C: Gender and ethnicity search after adaptation to a single face category
One concern in Experiment 2B is that it was possible for subjects to adopt a strategy of looking only to a single location (e.g., to the right of fixation). The gender of this single face would provide all the information needed to carry out the task. We therefore carried out the following experiment where subjects were adapted to a single face category but then had to identify a target face among three distractors.
Methods
In this experiment, the adaptation protocol was identical to Experiment 2B: subjects pre-adapted for 3 min to a single face category—either female or male Caucasian. The task was identical to Experiment 2A: subjects were instructed to locate a target face among a display of four faces (one from each category) as quickly and as accurately as possible. The target face belonged to one of the four possible categories (MA, MC, FA, FC) and switched randomly after each block of four trials. Both response times and accuracy were recorded. To maintain a similar amount of overall adaptation as in Experiments 1 and 2A, the top-up adaptation period lasted 10 s.
Each subject participated in two testing sessions, separated by 1 or 2 days. If an observer was adapted to FC faces in Session 1, he/she was adapted to MC faces in Session 2. The order of the adapting category was randomized across subjects. Each subject carried out a total of 800 trials across both sessions.
Results
Response times
The average response times (collapsing across adaptation conditions) for Sessions 1 and 2 are 1.5946 ± .0395 and 1.6310 ± .0684 s, respectively; a comparison shows no significant difference (F(1, 46) = 0.2128, p = .6467), unlike in Experiment 2A where there was a significant decrease in reaction time in the second session.
Like Experiments 2A and 2B, we did not find an effect of adaptation on the time required to search for a target face category. This was the case for both sessions (Session 1: F(2, 23) = 0.1126, p = .8940; Session 2: F(2, 23) = 0.0938, p = .9108).
Accuracy
The percent correct averaged across subjects for searching the least, intermediate, and most adapted face categories in Session 1 was 97.00% (SEM ±1.55), 96.00% (SEM ±0.96), and 96.33% (SEM ±1.58), respectively; in Session 2, 94.67% (SEM ±1.26), 96.50% (SEM ±0.57), and 96.50% (SEM ±0.99), respectively. Accuracy was not significantly different between the different adaptation levels for either session (Session 1: F(2, 23) = 0.1559, p = .8566; Session 2: F(2, 23) = 1.3253, p = .2871); or across sessions (F(2, 47) = 0.1232, p = .8844).
Normalized data
Normalized search times were not significantly different from 1, suggesting that the extent of adaptation had no effect on search time (Session 1: normalized intermediate, t(11) = 0.8364, p = .4207; normalized most, (5) = 2.8435, p = .0361; Session 2: normalized intermediate, t(11) = 0.1805. p = .8601; normalized most, t(5) = 0.8580, p = .4301).
Experiment 3: RSVP search
In Experiment 2, adaptation stimuli were presented centrally, and the target and distractors were presented in the periphery (though subjects were only asked to fixate during adaptation, not during search). One concern was that our failure to find an effect of adaptation on performance might be due to adaptation effects failing to transfer across retinal or spatial position.
Face selective cells are generally thought to be non-retinotopic (Haxby, Hoffman, & Gobbini, 2000; Tsao, Freiwald, Tootell, & Livingstone, 2006), and face adaptation effects have been found to transfer across retinal position (Kovacs, Zimmer, Harza, Antal, & Vidnyanszky, 2005), size (Webster & MacLin, 1999; Zhao & Chubb, 2001), and orientation (Watson & Clifford, 2003). However, in most of these examples, transfer of adaptation was not complete. It is not clear whether this reduction in the adaptation effect is due to some adaptation occurring within low-level mechanisms or due to some selectivity to retinal position, size, or orientation within higher level mechanisms that may or may not be dependent on context (Rolls & Baylis, 1986; Webster, Werner, & Field, 2005).
A second possible explanation for our failure to find an effect of adaptation in Experiment 2B (gender search after adaptation to a single face category) might have been that subjects were using a search strategy (e.g., fixating each face in turn) that reduced the effects of adaptation.
We therefore examined the effects of adaptation on a RSVP task. There is considerable overlap in the brain regions activated by RSVP tasks and spatial search tasks, suggesting that they rely, at least in part, on similar processes (Coull & Nobre, 1998). It has also been shown that a high contrast stimulus will “pop-out” among low contrast distractors, suggesting that if adaptation does serve to reduce neural responses to adapted stimuli then we might expect to see adaptation effects in a RSVP task (Fiser & Fine, 2000).
Experiment 3A: RSVP after adaptation to three out of the four face categories
Methods
The experimental paradigm is shown in Figure 8. Each subject participated in two testing sessions, separated by 1 or 2 days.
Figure 8.
Experiment 3A design. Subjects were pre-adapted to faces from 3 categories and topped-up between each trial. The target category was cued before the onset of a trial. One of the two intervals contained the target face. An auditory tone indicated the onset of each of the two RSVP intervals. In Session 1 (baseline), there was no pre-adaptation, and phase-scrambled face images (from the appropriate face category that would be used as adaptors during the second session) replaced adapting face images during the shortened 4 s pseudo top-up period.
The first session was designed to establish a baseline rate of RSVP presentation for each subject, without adaptation. In the first testing session, each trial consisted of two intervals in which a series of face images were presented in rapid succession (RSVP). Before each block of 70 trials, subjects were told that their task was to identify which of the two intervals contained a face image belonging to the target category, for example, FC. Each interval contained 5 face images; in one interval, all 5 of the face images were distractors, and in the other interval, one of the five face images belonged to the target face category. The order of these intervals was randomized. Each interval was followed by a 500-ms phase-scrambled face image.
An auditory tone at the end of the second interval alerted the observer to make a forced-choice response: “1” or “2,” to indicate whether the target appeared in the first or second interval. A correct answer was followed by a decrease in fixation spot size plus a high tone; an incorrect answer was followed by an increase in fixation spot size plus a low tone.
Subjects were given as long as needed to make a response. During this first testing session, the presentation rate for each face varied across 7 speeds using a method of constant stimuli. We then fit responses as a function of presentation rate with a cumulative normal psychometric function and interpolated to find the speed at which the observer achieved 65% correct performance. A total of 280 baseline trials (4 blocks of 70 trials) were carried out for each subject.
In the second session, each block of trials began by pre-adapting subjects using an initial 3-min adaptation period. The only difference between the pre-adaptation procedure from that used in Experiment 1 was that in this experiment, subjects were adapted to three (rather than one or two) face categories. For example, a subject might be adapted to FA, MC, and MA faces, presented in pseudorandom alternation. For each individual subject, the same 3 categories were used as adaptors throughout the entire session.
After this 3-min adaptation period, subjects moved to the testing phase where they again had to identify whether the target category appeared in the first or second interval. In this second session, the rate of presentation was held constant at a rate for which the subject had performed at 65% correct during the first, baseline (no adaptation) session.
The target face either belonged to the least or most adapted category. Preceding each block of 35 test trials was a cue indicating the target category. The target category alternated between the least adapted category and the most adapted category; if a subject was pre-adapted to FA, MC, and MA faces, then the least and most adapted categories would be FC and MA, respectively. Distractors belonged to categories other than the adapting category. The identities of adaptors were always different from the identities of the target and distractors. The two intervals of the test period lasted approximately 2 s, subjects were therefore exposed to adaptors for approximately 83% of the testing period (excluding the pre-adapt period).
The target category alternated across each of the 4 blocks of 35 trials, with half the subjects being tested on the least adapted category for blocks 1 and 3, and the other half in blocks 2 and 4. Across subjects, all possible combinations of adaptors and test faces were used in a counterbalanced design. A total of 140 adaptation trials (4 blocks of 35 trials) were carried out for each subject.
Results
Of the seventeen subjects that participated, four subjects’ data were discarded. For three of these subjects, fits to the baseline psychometric functions were too poor to obtain a speed threshold for 65% correct performance. A fourth subject performed at ceiling on the baseline experiment (our fastest possible presentation rate was 67 ms/image), leaving no room for improvement in the adaptation portion of the experiment (Session 2).
We were interested in determining whether adaptation would improve subjects’ performance in this RSVP search task when the target belonged to the least, as compared to the most adapted face category.
In the adaptation phase of the experiment (Session 2), the average percent correct for the least and most adapted face category was 64.67% (SEM ±2.85) and 66.68% (SEM ±2.27), respectively (collapsed across all categories). Performance was not significantly different between the most and the least adapted category (t(12) = 0.6953, p = .5001, 2-tailed dependent t-test). In fact, adaptation seemed to have no effect on performance on the RSVP task. Performance for the most and least adapted categories were not significantly different from 65% correct, the performance level predicted by the baseline experiment (most adapted category: t(12) = 0.7399, p = .2368; least adapted category t(12) = 0.1157, p = .5451, 1-tailed dependent t-test).
Experiment 3B: RSVP after adaptation to a single face category
Here we examined whether we might find adaptation effects after adapting to a single face category. The results from Experiment 1 suggest that the strongest category shifts are found when adapting to a single face category.
Methods
Each subject participated in two testing sessions, each separated by 1 or 2 days. The method used in Session 1 was identical to that used in Session 1 of Experiment 3A, where we obtained the presentation rate at which 65% correct performance is achieved.
However, in Session 2, each subject was adapted to only one of the four face categories. As in Experiment 3A, the initial adaptation period in Session 2 lasted 3 min, and there was a 12-s top-up period between each trial. The target face belonged to either the most adapted or least adapted category. For example, subjects pre-adapted to FA faces were tested on FA (most adapted) and MC (least adapted) targets. The order of adapting and target categories was randomized across subjects. A total of 140 adaptation trials (4 blocks of 35 trials) were carried out for each subject.
Results
Of the nine subjects that participated, two subjects’ data were discarded after the first session because fits to the baseline psychometric functions were too poor to obtain a speed threshold for 65% correct performance. Another two subjects failed to complete the second testing session.
In the adaptation phase of the experiment (Session 2), the average percent correct for searching for the least and most adapted category was 68.64% (SEM ±2.88) and 63.14% (SEM ±3.42), respectively (collapsed across all categories). Performance was not significantly different between the least and most adapted face categories (t(4) = 0.8742, p = .4314, 2-tailed dependent t-test). In fact, as in Experiment 3A, adaptation seemed to have no effect on performance on the RSVP task. Performance for least and most adapted face categories was not significantly different from 65% correct, the performance level predicted by the baseline experiment (least adapted category t(4) = 0.5426, p = .3081; most adapted category: t(4) = 1.2628, p = .1376, 1-tailed dependent t-test).
Experiment 4: Discrimination
As described in the Introduction, one possible explanation for the category shifts and reductions in fMRI response after adaptation (as shown by various groups; Fang, Murray, & He, 2007; Grill-Spector & Malach, 2001; Loffler et al., 2005; Winston et al., 2004; and replicated in Experiment 1) is a reduction in the responsivity of mechanisms tuned for the adapting category or a shift in their selectivity away from the category boundary.
Shifts or reductions in responsivity might be expected to have an effect on subjects’ ability to discriminate faces. Several groups have found that orientation adaptation affects orientation discrimination (Clifford et al., 2001; Dragoi, Sharma, Miller, & Sur, 2002; Regan & Beverly, 1985; though see Westheimer & Gee, 2002). Under certain regimes, lightness and contrast adaptation are capable of affecting contrast discrimination (Abbonizio, Langley, & Clifford, 2002; Barlow, 1969; Greenlee & Heitger, 1988), although these adaptation effects are by no means universal (Abbonizio et al., 2002; Foley & Chen, 1997; Määttänen & Koenderink, 1991; Ross, Speed, & Morgan, 1993) (also see General discussion).
Experiment 4A: Discrimination near the gender-neutral boundary after adaptation to male or female faces
Experiment 4 examines whether face adaptation might have any effect on performance discriminating face stimuli.
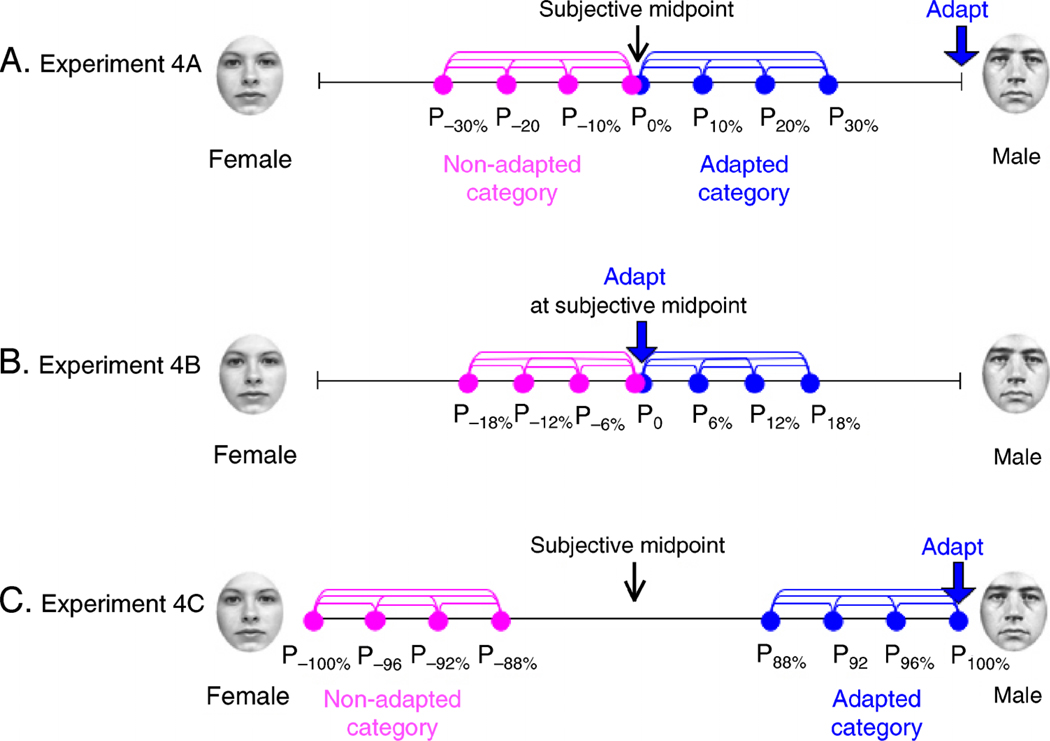
In Experiment 4A, we adapted subjects to fully male or female faces and tested discrimination performance near the category boundary, see Figure 9A.
Figure 9.
Experiment 4 design. (A) In Experiment 4A, subjects adapted to (for example) male faces then discriminated faces ranging from the midpoint to slightly female (nonadapted category condition) or from the midpoint to slight male (adapted category condition. (B) In Experiment 4B, subjects adapted to gender-neutral faces then discriminated faces on either side of the midpoint. (C) In Experiment 4C, subjects adapted to (for example) male faces then discriminated faces ranging from female to slightly less female (nonadapted category) or from male to slightly less male (adapted category).
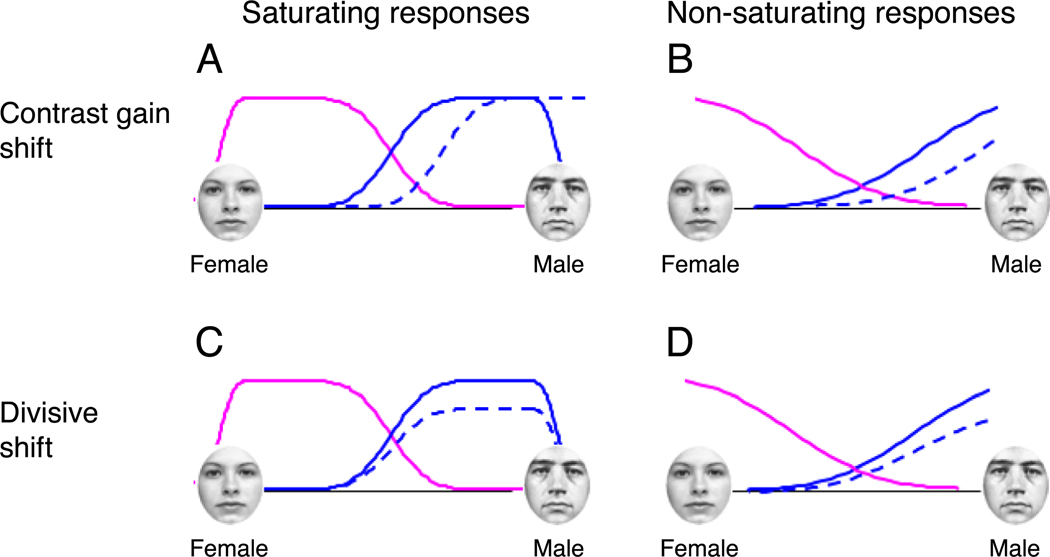
One possible explanation for our failure to find significant adaptation effects in Experiments 2 and 3 might be that face mechanisms have a saturated response for faces that are near the extreme of each category dimension (i.e., faces that are fully male, female, Asian, Caucasian). If responses are saturated for fully male and female faces and adaptation has the effect of a shift in selectivity, then adaptation might not affect the response to fully male and female faces, see Figure 14A. However, such a shift in selectivity as a result of adaptation to fully male or female faces should have an effect on discrimination performance for faces near the category midpoint.
Figure 14.
Possible response functions before (solid lines) and after (blue dotted lines) adaptation to male faces. (A) Responses saturate for fully male and female faces and adaptation results in a horizontal shift analogous to contrast gain control. (B) Responses are not yet saturated for fully male and female faces and adaptation results in a horizontal shift. (C) Responses saturate for fully male and female faces and adaptation results in a divisive shift. (D) Responses are not yet saturated for fully male and female faces and adaptation results in a divisive shift.
Methods
Each subject participated in two testing sessions, separated by 1 to 3 days.
In a pre-testing stage of the first session, we obtained each subjects’ individual perceptual categorical boundary between Caucasian male and Caucasian female for the 10 morph continuums used in the experiment. Subjects were presented with a pseudo-random series of morphed images and reported whether they appeared female or male by pressing “F” or “M,” respectively, for each image. A subset of the morphed images from each of the 10 gender morph continuums was shown; spaced evenly between being entirely male and being entirely female. The morph appearing male 50% of the time, averaged across all 10 morph continuums, was defined to be the categorical boundary for each observer.
Subjects were then randomly assigned in a counterbalanced manner, to either an adapted category or nonadapted category condition. In the adapted category condition, test images belonged to the same category as the adapting images. For example, subjects adapted to male faces who were assigned to the adapted category condition would, for example, be asked to discriminate which of following two morphed images appeared more male: P0 (the categorical boundary) or P+10% (slightly more male); see Figure 9A. If a subject were assigned to the nonadapted category condition he/she would be asked to discriminate which of the following two morphed images is more male: P0 (the categorical boundary) or P−10% (slightly more female); see Figure 9A.
Four positions along each half of the gender continuum were tested, each separated by 10% of the distance along the entire male–female continuum. The zero point, “P0,” is the subject’s category boundary. The remaining points are denoted as “P±10%,” P±20%,” and “P±30%,” sampling the space between the category boundary and faces that are 30% of the way towards the end-points of the continuum (note that this morph x-axis is not linear). This resulted in six possible discrimination comparisons for each condition. Subjects adapted to male faces in the adapted category condition were tested on P0 vs. P10%/P20%/P30%, P10% vs. P20%/P30%, and P20% vs. P30%; subjects adapted to male faces in the nonadapted category condition were tested on P0 vs. P−10%/P−20%/P−30%, P−10% vs. P−20%/ P−30%, and P−20% vs. P−30% (Figure 9A).
The testing stage of the first (baseline) session did not have an initial adaptation period, see Figure 10. Each trial began with a message on the screen that instructed subjects to advance to a trial by pressing the spacebar. Two test images then appeared in rapid succession, at a rate of 500 ms per image. Each image was followed by a 250-ms phase-scrambled image. All faces were Caucasian, and the pair of test images presented in the two intervals within a trial always belonged to the same individual morph continuum. The order in which the test images appeared within a trial was random. A different set of 5 morph continuums was used in each session.
Figure 10.
Experiment 4 design. (A) Gender morph continuum. Subjects adapted to male faces, for example, discriminate faces on either side of their subjective midpoint: the nonadapted or adapted category. Subjects were pre-adapted and topped-up between each trial (baseline testing excluded the pre-adaptation period and substituted the top-up period with shorter duration of phase-scrambled face images). Each test face image is followed with a mask.
On each trial, subjects reported whether the face image in the first or the second interval had appeared more male by pressing “1” or “2,” respectively. Each trial was followed by a pseudo-adaptation top-up period consisting of 4 s of phase-scrambled images presented at a rate of 1 image/s. (These phase-scrambled images were created using the appropriate face category images that would be used as adaptors during the second test session). Positive and negative feedback were given via a change in the size and color of the fixation spot. After every 4 blocks of 90 trials, subjects were shown their percent correct and wrote this value down. This provided subjects with additional feedback and introduced a mandatory rest period. Subjects carried out 360 trials in each of the two sessions.
In the second session, subjects were pre-adapted for 3 min to either male or female Caucasian faces. Adaptation was topped-up for 8 s before each trial, using the same procedure as in Experiment 3B. On each trial, a message on the screen instructed subjects to advance to a trial by pressing the spacebar after the adaptation period. Subjects then were asked to perform the same discrimination task (for example, which interval contained a more male face) as was carried out during the previous baseline session. Given a test period of 1 s, subjects were exposed to adaptors for approximately 87.5% of the testing period (excluding the pre-adapt period). Each subject carried out a total of 720 test trials across both sessions. All possible combinations of discrimination comparisons were tested in a counterbalanced design.
Results
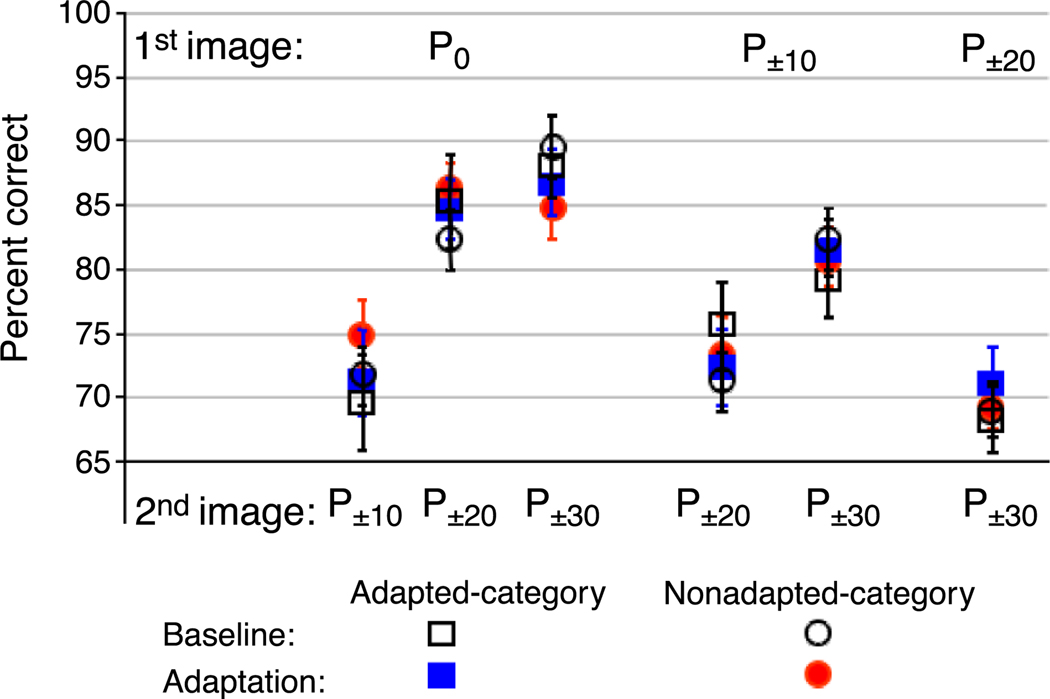
Figure 11 shows performance before and after adaptation for each discrimination comparison. The blue squares represent adapted category performance (if adaptation was to male face images, then discriminations were carried out between morph images that were slightly male); the gray circles represent nonadapted category performance (if adaptation was to male face images, then discriminations were carried out between morph images that were slightly female).
Figure 11.
Experiment 4A results. Performance before and after adaptation for each discrimination comparison across all subjects. Percent correct is shown for adapted category (squares) and nonadapted category (circles) discriminations, before (open black symbols) and after (filled colored symbols) adaptation.
Our choice of 10% step sizes was chosen on the basis of pilot data. Step sizes were chosen to represent the smallest perceptual change possible while remaining within a reasonably steep region (between 60% and 85% correct) of the psychometric function presumed to underlie discrimination performance as a function of morph difference.
Interestingly, performance was not significantly different between P0 vs. P±10%, P±10% vs. P±20%, and P±20% vs. P±30% (all comparisons, p > .1). Given that the x-axis was determined arbitrarily by the MorphMan software, there was no reason to assume that discrimination performance would be similar for morph pairs separated by 10% along the x-axis. The similarity of performance across morph pairs separated by a fixed distance in morph space (this observation also held true of the data in Experiments 4B and 4C) suggests that morph steps were, in fact, approximately linear in terms of perceptual difference.
As expected, subjects found it easier to discriminate which of two images was more male when the morph images to be discriminated were further separated along the morph continuum, e.g., performance for P0 vs. P30% and P0 vs. P20% was better than for P0 vs. P10%. This pattern of results was observed for all conditions and was also observed in Experiments 4B and 4C.
As would be expected, during the first baseline session in which no adaptation occurred, there was no significant difference in performance between adapted category (open black squares) and nonadapted category (open black circles) performance, across any of the discrimination comparisons (P0 vs. P±10%: t(18) = 1.110, p = .2817; P0 vs. P±20%: t(18) = 0.028, p = .9780; P0 vs. P±30%: t(18) = 0.444, p = .6623; P±10% vs. P±20%: t(18) = 1.035, p = .3142; P±10% vs. P±30%: t(18) = 0.031, p = .9754; P±20% vs. P±30%: t(18) = 0.361, p = .7226).
For the adapted category condition, there was no difference in performance between the baseline no-adapt condition (open black squares) and performance during the second adaptation session (filled blue squares) across any of the discrimination comparisons (P0 vs. P10%: t(9) = 0.0396, p = .9692; P0 vs. P20%: t(9) = 0.8327, p = .4266; P0 vs. P30%: t(9) = 0.1.170, p = .2771; P10% vs. P20%: t(9) = 0.6680, p = .5209; P10% vs. P30%: t(9) = 0.0441, p = .9658; P20% vs. P30%: t(9) = 1.119, p = .2950).
Similarly, for the nonadapted category condition, there was no difference in performance between the baseline no-adapt condition (open black circles) and performance during the second adaptation session (filled red circles) across any of the discrimination comparisons (P0 vs. P−10%: t(9) = 1.1507, p = .2795; P0 vs. P−20%: t(9) = 0.0826, p = .9360; P0 vs. P−30%: t(9) = 1.1765, p = .2696; P−10% vs. P−20%: t(9) = 0.2696; P−10% vs. P−30%: t(9) = 0.6585, p = .5267; P−20% vs. P−30%: t(9) = 0.1651, p = .8725).
Finally, during the second adaptation session there was no difference in performance between the adapted category (filled blue squares) and the nonadapted category (filled red circles), across any of the discrimination comparisons (P0 vs. P±10%: t(18) = 0.1080, p = .9150; P0 vs. P±20%: t(18) = 0.8710, p = .3953; P0 vs. P±30%: t(18) = 0.556, p = .5848; P±10% vs. P±20%: t(18) = 0.2560, p = .8009; P±10% vs. P±30%: t(18) = 0.6930, p = .4973; P±20% vs. P±30%: t(18) = 0.715, p = .4840).
Adapted category accuracy
The average percent correct before and after adaptation was as follows for the adapted category: P0 vs. P10%: 70.42% (SEM ±2.61) and 70.29% (SEM ±2.92), t(9) = 0.0396, p = .4846 ; P0 vs. P20%: 81.96% (SEM ±2.62) and 84.61% (SEM ±2.45), t(9) = 0.8327, p = .7867; P0 vs. P30%: 89.41%(SEM ±2.49) and 84.67%(SEM ±2.48), t(9) = 1.1570, p = .1385; P10% vs. P20%: 71.10% (SEM ±2.34), 73.24% (SEM ±2.90), t(9) = 0.6680, p = .7395; P10% vs. P30%: 82.35% (SEM ±2.11) and 82.48% (SEM ±2.07), p = .5171; P20% vs. P30%: 70.21% (SEM ±3.04) and 73.84% (SEM ±1.92), t(9) = 0.8525.
Nonadapted category accuracy
The average percent correct before and after adaptation was as follows for the nonadapted category: P0 vs. P−10%: 65.95% (SEM ±3.06) and 70.67% (SEM ±1.91), t(9) = 1.1507, p = .1397; P0 vs. P−20%: 81.85% (SEM ±3.35) and 81.67% (SEM ±2.32), t(9) = 0.0826, p = .5320; P0 vs. P−30%: 87.85% (SEM ±2.47) and 86.67% (SEM ±2.59), t(9) = 0.4501, p = .6683; P−10% vs. P−20%: 75.40% (SEM ±3.44) and 72.17% (SEM ±3.03), t(9) = 1.1765, p = .8652; P−10% vs. P−30: 82.21% (SEM ±−3.61) and 84.50% (SEM ±2.06), t(9) = 0.6585. p = .2634; P−20% vs. P−30: 71.88% (SEM ±3.48) and 71.33% (SEM ±2.94), t(9) = 0.1651, p = .5637.
Experiment 4B: Discrimination near the gender-neutral boundary after adaptation to gender-neutral faces
In the color domain, selective effects of adaptation on discrimination thresholds provide a well-established tool for examining the selectivity of chromatic mechanisms (Thornton & Pugh, 1983). Indeed, it has been suggested that our exquisite ability to make fine discrimination judgments for chromatically neutral stimuli may be a result of adapting to the statistics of natural scenes, where most stimuli are chromatically unsaturated (Macleod & von der Twer, 2003). According to this model, we might expect better performance discriminating gender-neutral faces after adaptation to gender-neutral faces. We tested this in Experiment 4B.
Methods
Methods were nearly identical to those used in Experiment 4A. The main difference was that we adapted subjects with faces that fell on each individual subject’s perceptual category midpoint and the morphs used to test discrimination were spaced slightly closer together on the morph continuum (Figure 9B).
In the pre-testing of the first session, we once again obtained each subject’s individual perceptual categorical boundary between male and female.
In the testing stage of the first session, we again tested four positions along the gender continuum; however, these points were separated by a smaller distance of 6% along the entire male–female continuum (as compared to 10% in Experiment 4A). Again, the zero point, “P0,” is the subject’s category boundary. Because we always adapted using faces at the category boundary, we defined the remaining testing points as “P±6%,” P±12%,” and “P±18%,” where a slightly female morph was described as P−18%, and a slightly male morph was described as P+18%. Data were collapsed across slightly male and slight female comparisons.
The second stage of Session 1 was once again a baseline (no adaptation) discrimination task, as in Experiment 4A. However, in the second session, subjects were pre-adapted for 3 min to gender-neutral faces. We once again used an 8-s top-up period between each trial. On each trial, subjects reported whether the face in the first or second interval was more male. Each subject carried out a total of 720 test trials across both sessions. All possible combinations of discrimination comparisons were tested in a counterbalanced design.
Results
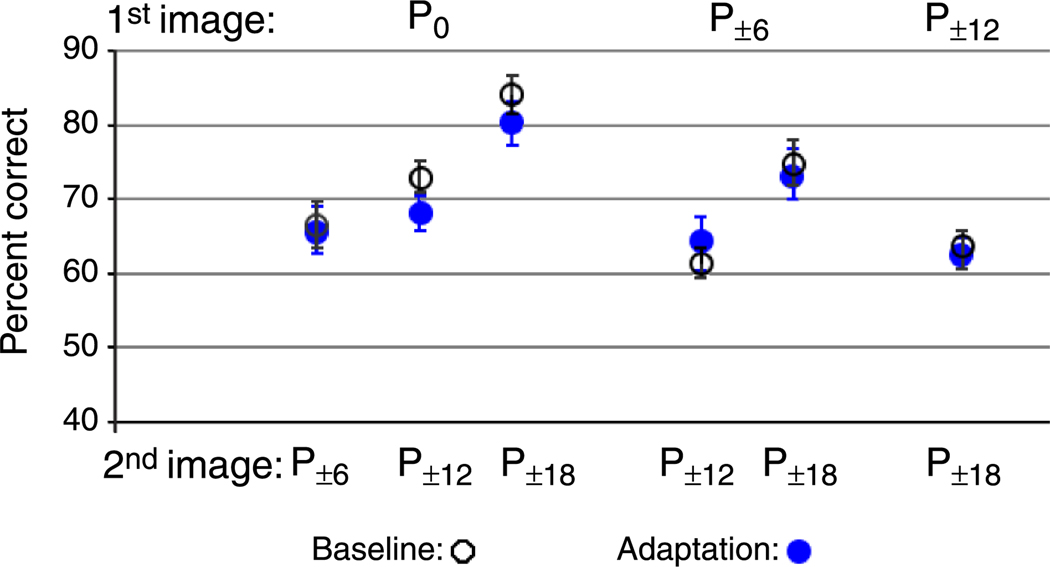
There was no significant difference in discrimination performance before and after adaptation for any of the possible comparisons P0 vs. P±6%: t(7) = 0.1520, p = .8839; P0 vs. P±9%: t(7) = 1.557, p = .1634; P0 vs. P±12%: t(7) = 0.827, p = .4353; P±6% vs. P±9%: t(7) = 0.672, p = .5234; P±6% vs. P±12%: t(7) = 0.147, p = .6566; P±9% vs. P±12%: t(7) = 0.147, p = .8874 (Figure 12).
Figure 12.
Experiment 4B results. Discrimination performance around the gender boundary before (open black circles) and after (filled blue circles) adaptation to gender-neutral faces.
Accuracy
The average percent correct before and after adaptation, as seen the Figure 12, is as follows: P0 vs. P±6%: 66.25% (SEM ±3.13) and 65.65% (SEM ±3.14); P0 vs. P±9%: 72.50% (SEM ±2.36) and 84.61% (SEM ±2.54); P0 vs. P±12%: 83.75% (SEM ±2.61) and 79.94% (SEM ±2.95); P±6% vs. P±9%: 61.04% (SEM ±2.02) and 63.81% (SEM ±3.64); P±6% vs. P±12%: 74.58% (SEM ±3.15) and 73.05% (SEM ±3.32); P±9% vs. P±12%: 62.92% (SEM ±2.65) and 62.49% (SEM ±2.13).
Experiment 4C: Discrimination of female and male faces after adaptation to male or female faces
In Experiment 4B, we found that adaptation to gender-neutral faces did not affect discrimination performance for gender-neutral faces. However, gender-neutral face stimuli are not common in daily life, making it unlikely that face mechanisms are tuned to have maximum discriminative sensitivity for gender-neutral faces.
In Experiment 4C, we examined the effects of adaptation to male or female faces on discriminations for fully male and female faces. If adaptation serves to increase discriminative sensitivity (Macleod & von der Twer, 2003), then we would expect better performance discriminating male faces after adaptation to male faces and better performance discriminating female faces after adaptation to female faces. Such adaptation effects might be a possible substrate for the “other race” effect in which people seem to be better at making fine discriminations within faces that belong to a familiar ethnic group (Bothwell, Brigham, & Malpass, 1989; Walker & Tanaka, 2003).
Methods
Once again, methods were nearly identical to the methods in Experiments 4A and 4B. However, in this experiment, the four tested positions along the gender continuum were positioned at the ends of the male–female continuum (Figure 9C). Fully female and fully male faces are denoted as “P−100%” and “P100%,” respectively. The points on the continuum that we tested included fully female and fully male faces as well as P±96%, P±92%, and P±88%.
In Session 1, subjects participated in a baseline (no adaptation) discrimination task, as in Experiments 4A and 4B. In the second session, subjects were pre-adapted for 3-min to either female or male faces. Nonadapted category subjects were either adapted to female faces and tested on male faces (P88%, P92%, P96%, and P100%) or were adapted to male faces and tested on female faces (P−88%, P−92%, P−96%, and P−100%). Adapted category subjects were adapted to female faces and tested on P−88%, P−92%, P−96%, and P−100% or were adapted to male faces and tested on P88%, P92%, P96%, and P100%.
In the second session, subjects were pre-adapted for 3 min, and top-adaptation periods of 8 s were interleaved between each trial. On each trial subjects reported whether the face in the first or second interval was more male, as in Experiments 4A and 4B. Each subject carried out a total of 720 test trials across both sessions. All possible combinations of discrimination comparisons were tested in a counterbalanced design.
Results
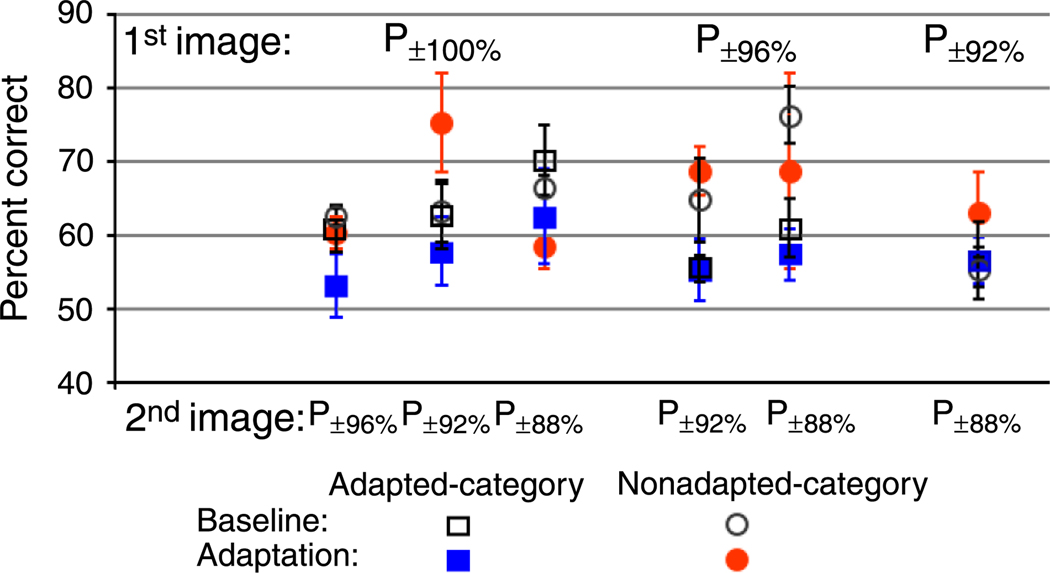
Figure 13 shows performance with and without adaptation in adapted and nonadapted category conditions across all subjects. As expected, during the first baseline session in which no adaptation occurred, there was no significant difference in performance between adapted category (open black squares) and nonadapted category (open black circles) performance across any of the discrimination comparisons (P±100% vs. P±96%: t(6) = 2.88, p = .7828; P±100% vs. P±92%: t(6) = 0.028, p = .9784; P±100% vs. P ± 88%: t(6) = 0.298, p = .7760; P±96% vs. P±92%: t(6) = 1.168, p = .2872; P±96% vs. P ± 88%: t(6) = 2.086, p = .0821; P±92% vs. P ± 88%: t(6) = 0.099, p = .9244).
Figure 13.
Experiment 4C results. Discrimination performance around the endpoints of the male–female morph continuum before (open black symbols) and after (filled colored symbols) adaptation to fully male or fully female faces.
For the adapted category condition, there was no difference in performance between the baseline no-adapt condition (open black squares) and performance during the second session that included adaptation (filled blue squares) across any of the discrimination comparisons (P±100% vs. P±96%: t(9) = 1.475, p = .1743; P±100% vs. P±92%: t(9) = 0.765, p = .4640; P±100% vs. P ± 88%: t(9) = 0.951, p = .3662; P±96% vs. P±92%: t(9) = 0.002, p = .9982; P±96% vs. P ± 88%: t(9) = 0.650, p = .5317; P±92% vs. P ± 88%: t(9) = 0.099, p = .9824).
Similarly, for the nonadapted category condition, there was no difference in performance between the baseline no-adapt condition (open black circles) and performance during the second session that included adaptation (filled red circles) across any of the discrimination comparisons (P±100% vs. P±96%: t(2) = 2.00, p = .1835; P±100% vs. P±92%: t(2) = 0.617, p = .600; P±100% vs. P±88%: t(2) = 0.397, p = .7295; P±96% vs. P±92%: t(2) = 0.934, p = .4488; P±96% vs. P±88%: t(2) = 0.560, p = .6318; P±92% vs. P±88%: t(2) = 0.456, p = .6931).
Finally, during the second adaptation session there was no difference in performance between the adapted category (filled blue squares) and the nonadapted category (filled red circles) across any of the discrimination comparisons (P±100% vs. P±96%: t(5) = 0.990, p = .3677; P±100% vs. P±92%: t(5) = 1.323, p = .2432; P±100% vs. P±88%: t(5) = 0.921, p = .3995; P±96% vs. P±92%: t(5) = 0.944, p = .3887; P±96% vs. P±88%: t(5) = 1.218, p = .2777; P±92% vs. P±88%: t(5) = 0.628, p = .5576).
Nonadapted category accuracy
The average percent correct before and after adaptation was as follows for the nonadapted category: P±100% vs. P±92%: 62.22% (SEM ±1.11) and 60% (SEM ±2.15); P±100% vs. P±96%: 62.75% (SEM ±3.92) and 75% (SEM ±6.67); P±100% vs. P±88%: 66.47% (SEM ±1.35) and 58.33% (SEM ±3.33); P±96% vs. P±92%: 64.42% (SEM ±5.59) and 68.33% (SEM ±3.33); P±96% vs. P±88%: 76.11% (SEM ±3.89) and 68.33% (SEM ±13.33); P±92% vs. P±88%: 55.28% (SEM ±2.80) and 62.50% (SEM ±5.83).
Adapted category accuracy
The average percent correct before and after adaptation was as follows for the adapted category: P±100% vs. P±92%: 60.56% (SEM ±3.15) and 52.83% (SEM ±4.33); P±100% vs. P±96%: 62.5% (SEM ±4.65) and 57.46% (SEM ±4.57); P±100% vs. P±88%: 69.72% (SEM ±4.86) and 62.21% (SEM ±6.42); P±96% vs. P±92%: 55.00% (SEM ±4.17) and 55.01% (SEM ±1.82); P±96% vs. P±88%: 60.56% (SEM ±3.96) and 57.00% (SEM ±3.63); P±92% vs. P±88%: 56.27% (SEM ±5.20) and 56.22% (SEM ±3.26).
General discussion
Like other groups (Ng et al., 2006; Rhodes et al., 2003; Webster et al., 2004; Webster & MacLin, 1999), we find that face adaptation results in dramatic shifts in the categorical appearance of faces. However, we failed to find any evidence that similar adaptation protocols resulted in any change in spatial search, temporal search, or discrimination performance.
The robust shifts in category appearance found in Experiment 1 suggest that our failure to find an effect of adaptation on the other tasks was not due to a failure to produce adequate adaptation. Nor is it likely that adaptation effects were masked by variability in our data. Measurements in all three sets of experiments were remarkably reliable, yet there was no indication in any of the experiments of a trend that simply failed to reach significance.
One potential explanation for the failure of adaptation to affect search, RSVP, or discrimination performance is that shifts in category boundaries may not be due to either a shift or a compression of response curves, but rather to a shift in the criterion for where the boundary is. According to this model, it has been argued that the role of adaptation for higher level stimuli may not be to increase sensitivity for perceptual judgments but rather to adjust the boundaries of our perceptual categories to match the distribution of the environment around us (Webster et al., 2005). However, attributing adaptation effects as a criterion shift does not explain why such strong fMRI adaptation is found for face stimuli. Strong adaptation effects have been found across relatively large regions of cortex for both individual faces (Gauthier et al., 2000; Loffler et al., 2005; Rotshtein et al., 2005; Winston et al., 2004) and categories of faces (Ng et al., 2006), after relatively short periods of adaptation, with adapted faces producing less BOLD response than nonadapted faces.
Figure 14 is a schematic of a subset of some example possible response properties of mechanisms tuned for femaleness and maleness, before and after adaptation to male faces. Here, we have assumed that mechanisms are tuned for “maleness” and “femaleness.” However, an analogous argument would also hold if adaptation occurred within multiple individual cues (rounder cheeks, larger eyes, etc.) associated with gender and ethnicity.
Figure 14A and 14C show response functions that saturate for fully male and female faces, similar to the response profiles presumed to underlie chromatic opponency. However, unlike chromatic stimuli, faces tend not to cluster near the midpoint of category boundariesV gender-neutral and Eurasian faces are relatively rare. As a result, if the response slopes of face selective mechanisms are designed to maximize discrimination abilities for common encountered stimuli, then we might expect response profiles more like those of Figure 14B and 14D, which maximize discrimination ability for stimuli near the end points of the continuum.
Interestingly, if response profiles maximize sensitivity to commonly encountered stimuli one might expect nonsaturating response profiles for gender and ethnicity (Figure 14B or 14D) and saturating response profiles (Figure 14A or 14C) for expression. Faces expressing extreme happiness or anger are less commonly experienced than those expressing gentle contentment or a mild peevishness. It should be noted that another group has failed to find any effect of adaptation on expression discrimination (Pallett & MacLeod, 2007) using a paradigm somewhat similar to our Experiment 4A.
A reduction in response magnitude as a consequence of adaptation would be expected to affect search and RSVP tasks, while changes in the response slope as a result of adaptation would be expected to affect discrimination performance. According to the models of Figure 14, we would not necessarily expect adaptation effects across all experiments.
In the case of Figure 14A, responses are saturated for fully male and female faces and adaptation shifts responses along the x-axis. Because this shift does not reduce the response to fully male faces, this model would not predict adaptation effects in Experiments 2, 3, or 4C. However, the adaptation shift does result in a change of slope near the category boundary, predicting an effect on discrimination performance for faces near the category midpoint, as in Experiments 4A and 4B.
In the case of Figure 14B, responses are not fully saturated at the endpoints of the continuum and adaptation shifts selectivity. For fully male faces, adaptation reduces the overall response as well increasing the slope. Near the category boundary, we see a reduction in slope. We should therefore have seen adaptation effects across all experiments.
In the case of Figure 14C, responses are saturated near the endpoints of the continuum, and adaptation is modeled by a divisive shift. We do see a change in overall response for fully male faces, but without a change in response slope. We also see a change in slope at the category boundary and would therefore expect adaptation effects in all experiments except Experiment 4C.
Finally, in Figure 14D, responses are not fully saturated at the endpoints of the continuum and adaptation is modeled by a divisive shift. Adaptation to male faces changes both the overall response and the slope for fully male faces. Adaptation also changes the slope at the category boundary. We would therefore expect adaptation effects across all experiments.
The size of the adaptation effect on discrimination performance is related to the difference in slope before and after adaptation. In some cases (depending on the exact response curve that is assumed), we would expect relatively small effects on discrimination performance. However, in none of the discrimination experiments did we see any indication of adaptation effects that were simply failing to reach significance.
The strongest adaptation effects would be expected within regimes where responses to nonadapted stimuli are saturated, whereas responses to adapted stimuli are below the saturation level (see the response to almost-male faces in Figure 14A). Consistent with this prediction, particularly strong effects of adaptation can be found within certain luminance regimes (Ohzawa, Sclar, & Freeman, 1985; Whittle, 1992). Responses to contrast show far less saturation than for luminance, and effects of adaptation on contrast discrimination tend to be comparatively weak (e.g., Greenlee & Heitger, 1988; Regan & Beverly, 1985; see also Webster et al., 2005). According to the model of Figure 14B, where face selective mechanisms are unsaturated for fully male and female faces and adaptation results in a shift along the x-axis, we might have obtained stronger adaptation effects if we had used hyper-male or hyper-female faces as the end-points of our morph continuum.
Of course the models described above are only a simplified subset of all possible models of adaptation. However, even when other models are considered it remains difficult to generate a plausible explanation that can explain large shifts in category boundaries and fMRI responses, yet no discernable effect on performance in any of the search or discrimination tasks that we carried out.
The most likely explanation for our findings may be that discrimination and search performance is in fact determined by our subjects’ ability to discriminate low-level features (such as orientation and contrast) rather than their ability to represent faces. Given that faces are highly over-learned, there may be a super-abundance of representational capacity devoted to face processing. Indeed, a surprising amount of visual cortex is devoted to face processingVsimilar to the amount of cortex in V1 (Dougherty et al., 2003; Kanwisher, McDermott, & Chun, 1997). Given that face stimuli are so heavily over-represented, it seems plausible that limits in discrimination and search performance might be mainly due to signal-to-noise and/or limits in representational capacity at lower levels of processing.
Low-level adaptation may possibly explain why Watson, Rhodes, and Clifford (2006) has found that adaptation to an “average Asian face” improves the ability to identify learned Asian individuals, and adapting to an “average Caucasian face” improves the ability to identify learned Caucasian individuals. Watson et al.’s task required identifying a learned individual compared to a single baseline “average face.” Consequently, adaptation to low-level properties may have enhanced low-level differences between the “average face” and the learned individual faces. In our experiment, adaptor and test faces always had different identities, thus reducing (though not entirely excluding) the potential for low-level adaptation.
Our findings show that adaptation to naturalistic face stimuli is unlikely to have a powerful effect on either discrimination or search performance under natural conditions. This suggests that the functional role of adaptation may be to adjust the boundaries of our perceptual categories to match the distribution of the environment around us (Webster et al., 2005) rather than to improve performance on these tasks.
Acknowledgments
Many thanks to Donald I. A. MacLeod, Horace B. Barlow, and Michael A. Webster for helping us interpret our lack of positive results. This research was supported by National Institutes of Health Grants EY-12925 (GMB), EY-014645 (IF).
Footnotes
Commercial relationships: none.
Contributor Information
Minna Ng, Email: mng@ucsd.edu, Department of Psychology, UCSD, San Diego, CA, USA.
Geoffrey M. Boynton, Email: gboynton@u.washington.edu, Department of Psychology, University of Washington, Seattle, WA, USA.
Ione Fine, Email: ionefine@u.washington.edu, Department of Psychology, University of Washington, Seattle, WA, USA.
References
- Abbonizio G, Langley K, Clifford CW. Contrast adaptation may enhance contrast discrimination. Spatial Vision. 2002;16:45–58. doi: 10.1163/15685680260433904. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Pattern recognition and the responses of sensory neurons. Annals of the New York Academy of Sciences. 1969;156:872–881. doi: 10.1111/j.1749-6632.1969.tb14019.x. [DOI] [PubMed] [Google Scholar]
- Barlow HB. A theory about the functional role and synaptic mechanism of after-effects. In: Blakemore C, editor. Vision: Coding and efficiency. Cambridge: Cambridge University Press; 1991. pp. 363–375. [Google Scholar]
- Barlow HB, Földiák P. Adaptation and decorrelation in the cortex. In: Durbin R, Miall C, Mitchiso G, editors. The computing neuron. Boston: Addison-Wesley; 1989. pp. 54–72. [Google Scholar]
- Blakemore C, Campbell FW. Adaptation to spatial stimuli. The Journal of Physiology. 1969;200 [PubMed] [Google Scholar]
- Blakemore C, Nachmias J. The orientation specificity of two visual after-effects. The Journal of Physiology. 1971;213:157–174. doi: 10.1113/jphysiol.1971.sp009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell RK, Brigham JC, Malpass RS. Cross-racial identification. Personality and Social Psychology Bulletin. 1989;15:19–25. [Google Scholar]
- Clifford CW, Wenderoth P. Adaptation to temporal modulation can enhance differential speed sensitivity. Vision Research. 1999;39:4324–4332. doi: 10.1016/s0042-6989(99)00151-0. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Wyatt AM, Arnold DH, Smith ST, Wenderoth P. Orthogonal adaptation improves orientation discrimination. Vision Research. 2001;41:151–159. doi: 10.1016/s0042-6989(00)00248-0. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Thibodeau MH. The effect of adapting luminance on the latency of visual search. Acta Psychologica. 1998;99:115–139. doi: 10.1016/s0001-6918(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. Journal of Vision. 2003;3(10):1, 586–598. doi: 10.1167/3.10.1. http://journalofvision.org/3/10/1/ [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nature Neuroscience. 2002;5:883–891. doi: 10.1038/nn900. [DOI] [PubMed] [Google Scholar]
- D’Zmura M, Mangalick A. Detection of contrary chromatic change. Journal of the Optical Society of America A, Optics, Image Science and Vision. 1994;11:543–546. doi: 10.1364/josaa.11.000543. [DOI] [PubMed] [Google Scholar]
- Fang F, Murray SO, He S. Duration-dependent FMRI adaptation and distributed viewer-centered face representation in human visual cortex. Cerebral Cortex. 2007;17:1402–1411. doi: 10.1093/cercor/bhl053. [DOI] [PubMed] [Google Scholar]
- Fiser J, Fine I. Temporal characteristics of fast contrast adaptation in high level object identification tasks. Paper presented at the IOVS.2000. [Google Scholar]
- Foley JM, Chen CC. Analysis of the effect of pattern adaptation on pattern pedestal effects: A two-process model. Vision Research. 1997;37:2779–2788. doi: 10.1016/s0042-6989(97)00081-3. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Moylan J, Skudlarski P, Gore JC, Anderson AW. The fusiform “face area” is part of a network that processes faces at the individual level. Journal of Cognitive Neuroscience. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- Greenlee MW, Heitger F. The functional role of contrast adaptation. Vision Research. 1988;28:791–797. doi: 10.1016/0042-6989(88)90026-0. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G, Zimmer M, Harza I, Antal A, Vidnyanszky Z. Position-specificity of facial adaptation. Neuroreport. 2005;16:1945–1949. doi: 10.1097/01.wnr.0000187635.76127.bc. [DOI] [PubMed] [Google Scholar]
- Leopold DA, O’Toole AJ, Vetter T, Blanz V. Prototype-referenced shape encoding revealed by high-level aftereffects. Nature Neuroscience. 2001;4:89–94. doi: 10.1038/82947. [DOI] [PubMed] [Google Scholar]
- Little AC, DeBruine LM, Jones BC. Sex-contingent face after-effects suggest distinct neural populations code male and female faces. Proceedings of the Royal Society of London B: Biological Sciences. 2005;272:2283–2287. doi: 10.1098/rspb.2005.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler G, Yourganov G, Wilkinson F, Wilson HR. fMRI evidence for the neural representation of faces. Nature Neuroscience. 2005;8:1386–1390. doi: 10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- Määttänen LM, Koenderink JJ. Contrast adaptation and contrast gain control. Experimental Brain Research. 1991;87:205–212. doi: 10.1007/BF00228521. [DOI] [PubMed] [Google Scholar]
- Macleod DI, von der Twer T. The Pleistochrome: Optimal opponent codes for natural colours. In: Mausfeld R, Heyer D, editors. Colour perception: Mind and the physical world. London: Oxford University Press; 2003. [Google Scholar]
- McCollough C. Color adaptation of edge-detectors in the human visual system. Science. 1965;149:1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- McDermott K, Mulligan JB, Bebis G, Webster MA. Visual search and eye movements in novel and familiar contexts. Proceedings of SPIE. 2006;6057:104–115. [Google Scholar]
- Ng M, Ciaramitaro VM, Anstis S, Boynton GM, Fine I. Selectivity for the configural cues that identify the gender, ethnicity, and identity of faces in human cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19552–19557. doi: 10.1073/pnas.0605358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurft HC. The conspicuousness of orientation and motion contrast. Spatial Vision. 1993;7:341–363. doi: 10.1163/156856893x00487. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat’s visual system. Journal of Neurophysiology. 1985;54:651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- Pallett PM, MacLeod DI. Face discrimination does not rely on configural information; Paper presented at the Vision Science Society; Sarasota. 2007. [Google Scholar]
- Regan D, Beverly KI. Postadaptation orientation discrimination. Journal of the Optical Society of America A, Optics and Image Science. 1985;2:147–155. doi: 10.1364/josaa.2.000147. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Watson TL, Clifford CW, Nakayama K. Fitting the mind to the world: Face adaptation and attractiveness aftereffects. Psychological Science. 2003;14:558–566. doi: 10.1046/j.0956-7976.2003.psci_1465.x. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Watson TL, Jaquet E, Winkler C, Clifford CW. Orientation-contingent face aftereffects and implications for face-coding mechanisms. Current Biology. 2004;14:2119–2123. doi: 10.1016/j.cub.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Baylis GC. Size and contrast have only small effects on the responses to faces of neurons in the cortex of the superior temporal sulcus of the monkey. Experimental Brain Research. 1986;65:38–48. doi: 10.1007/BF00243828. [DOI] [PubMed] [Google Scholar]
- Ross J, Speed HD, Morgan MJ. The effects of adaptation and masking on incremental thresholds for contrast. Vision Research. 1993;33:2051–2056. doi: 10.1016/0042-6989(93)90003-f. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nature Neuroscience. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Stromeyer CF, Kranda K, III, Sternheim CE. Selective chromatic adaptation at different spatial frequencies. Vision Research. 1978;18:427–437. doi: 10.1016/0042-6989(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Abrupt luminance change pops out; abrupt color change does not. Perception & Psychophysics. 1995;57:637–644. doi: 10.3758/bf03213269. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Pugh EN., Jr Red/green color opponency at detection threshold. Science. 1983;219:191–193. doi: 10.1126/science.6849131. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PM, Tanaka JW. An encoding advantage for own-race versus other-race faces. Perception. 2003;32:1117–1125. doi: 10.1068/p5098. [DOI] [PubMed] [Google Scholar]
- Watson T, Rhodes G, Clifford CWG. Improved facial identity recognition following adaptation; Paper presented at the Vision Science Society; Sarasota. 2006. [Google Scholar]
- Watson TL, Clifford CW. Pulling faces: An investigation of the face-distortion aftereffect. Perception. 2003;32:1109–1116. doi: 10.1068/p5082. [DOI] [PubMed] [Google Scholar]
- Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature. 2004;428:557–561. doi: 10.1038/nature02420. [DOI] [PubMed] [Google Scholar]
- Webster MA, MacLin OH. Figural aftereffects in the perception of faces. Psychonomic Bulletin and Review. 1999;6:647–653. doi: 10.3758/bf03212974. [DOI] [PubMed] [Google Scholar]
- Webster MA, Raker VE, Malkoc G. Visual search and natural color distributions. Proceedings of SPIE, 3299. 1998:498–509. [Google Scholar]
- Webster MA, Werner JS, Field DJ. Adaptation and the phenomenology of perception. In: Clifford CW, Rhodes G, editors. Fitting the mind to the world: Adaptation and after-effects in high-level vision. vol. 2. London: Oxford University Press; 2005. pp. 241–280. [Google Scholar]
- Westheimer G, Gee A. Orthogonal adaptation and orientation discrimination. Vision Research. 2002;42:2339–2343. doi: 10.1016/s0042-6989(02)00192-x. [DOI] [PubMed] [Google Scholar]
- Whittle P. Brightness, discriminability and the “crispening effect.”. Vision Research. 1992;32:1493–1507. doi: 10.1016/0042-6989(92)90205-w. [DOI] [PubMed] [Google Scholar]
- Winston JS, Henson RN, Fine-Goulden MR, Dolan RJ. fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. Journal of Neurophysiology. 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chubb C. The size-tuning of the face-distortion after-effect. Vision Research. 2001;41:2979–2994. doi: 10.1016/s0042-6989(01)00202-4. [DOI] [PubMed] [Google Scholar]