Abstract
Objective
To compare the effects of a 4-week high–saturated fat/high–glycemic index (HIGH) diet with a low–saturated fat/low–glycemic index (LOW) diet on insulin and lipid metabolism, cerebrospinal fluid (CSF) markers of Alzheimer disease, and cognition for healthy adults and adults with amnestic mild cognitive impairment (aMCI).
Design
Randomized controlled trial.
Setting
Veterans Affairs Medical Center clinical research unit.
Participants
Forty-nine older adults (20 healthy adults with a mean [SD] age of 69.3 [7.4] years and 29 adults with aMCI with a mean [SD] age of 67.6 [6.8] years).
Intervention
Participants received the HIGH diet (fat, 45% [saturated fat, >25%]; carbohydrates, 35%–40% [glycemic index, >70]; and protein, 15%–20%) or the LOW diet (fat, 25%; [saturated fat, <7%]; carbohydrates, 55%–60% [glycemic index, <5]; and protein, 15%–20%) for 4 weeks. Cognitive tests, an oral glucose tolerance test, and lumbar puncture were conducted at baseline and during the fourth week of the diet.
Main Outcome Measures
The CSF concentrations of β-amyloid (Aβ42 and Aβ40), tau protein, insulin, F2-isoprostanes, and apolipoprotein E, plasma lipids and insulin, and measures of cognition.
Results
For the aMCI group, the LOW diet increased CSF Aβ42 concentrations, contrary to the pathologic pattern of lowered CSF Aβ42 typically observed in Alzheimer disease. The LOW diet had the opposite effect for healthy adults, ie, decreasing CSF Aβ42, whereas the HIGH diet increased CSF Aβ42. The CSF apolipoprotein E concentration was increased by the LOW diet and decreased by the HIGH diet for both groups. For the aMCI group, the CSF insulin concentration increased with the LOW diet, but the HIGH diet lowered the CSF insulin concentration for healthy adults. The HIGH diet increased and the LOW diet decreased plasma lipids, insulin, and CSF F2-isoprostane concentrations. Delayed visual memory improved for both groups after completion of 4 weeks of the LOW diet.
Conclusion
Our results suggest that diet may be a powerful environmental factor that modulates Alzheimer disease risk through its effects on central nervous system concentrations of Aβ42, lipoproteins, oxidative stress, and insulin.
Obesity, type 2 diabetes mellitus (DM2), cardiovascular disease, and hypercholesterolemia are established risk factors for pathologic brain aging that have been linked to underlying insulin resistance (the inability of insulin to perform its normal functions in target tissues).1 These conditions have increased substantially in prevalence partly due to increased caloric intake of saturated fat and simple carbohydrates.2–4 This consumption pattern may raise the risk of aging-related cognitive impairment and Alzheimer disease (AD); although review of the burgeoning and complex literature regarding this topic shows inconsistencies, several recent epidemiologic reviews suggest that saturated fat intake increases the risk of AD or cognitive impairment, whereas reduced saturated fat and increased intake of monounsaturated and polyunsaturated fats has protective effects.5–8 Despite these associations, clinical trials of specific fatty acids, such as docosahexaenoic acid, in adults with AD have produced disappointing results.9 Such results may have occurred because rather than individual dietary components, dietary patterns or combinations of nutrients must be considered when assessing diet effects on AD risk and pathophysiologic changes. This possibility is suported by epidemiologic findings that dietary patterns consisting of high intake of fruits and vegetables, unsaturated fatty acids, and fish and low intake of saturated fats, especially those derived from beef and dairy, are associated with a reduced risk of AD or its presumed prodrome, amnestic mild cognitive impairment (aMCI).10–12 Similar dietary patterns, consisting of high intake of saturated fats and simple carbohydrates, also have been associated with DM2 and insulin resistance, which are known risk factors for AD.13
Thus, a more promising approach to the study of dietary factors in AD might entail the use of whole-diet interventions, which have greater ecologic validity and preserve the nutritional milieu in which fat and carbohydrate consumption occurs. Animal models have examined the effects of diet intervention on AD pathophysiologic changes and have shown that high–saturated fat or high-sucrose diets modify processing of the amyloid precursor protein, from which the synaptotoxic β-amyloid (Aβ) peptide is produced, increase Aβ-related cerebrovascular disturbance, and reduce brain insulin signaling and expression of the Aβ-clearing protease, insulin-degrading enzyme.14,15 Controlled human studies of whole-diet effects on brain tissue are rare, and to our knowledge, no study has examined the effects of dietary intervention on cerebrospinal fluid (CSF) AD biomarkers. This important area of study might elucidate the early effects of diet on AD pathogenesis and implicate diet as a critical environmental factor in the AD causal pathway.
Thus, we compared the effects of a 4-week diet that mimics the high–saturated fat/high–simple carbohydrate (HIGH) composition of the macronutrient pattern associated with DM2 and insulin resistance with a low–saturated fat/low–simple carbohydrate (LOW) diet for healthy older adults and adults with aMCI. Both diets were isocaloric with the normal intake of participants, revealing the effects of dietary macronutrient composition independent of weight change. On the basis of previous work in animal models, we hypothesized that the HIGH diet would have negative effects and the LOW diet would have positive effects on the primary outcome measure, CSF Aβ42 concentrations. Secondary measures included CSF Aβ40, tau protein, insulin, apolipoprotein E (APOE), the oxidative stress marker F2-isoprostane, peripheral metabolic indexes, and cognition. We observed beneficial effects of the LOW diet on CSF Aβ and other biomarkers, whereas the HIGH diet moved CSF biomarkers in a direction that may characterize presymptomatic AD. Our results suggest that diet may be a powerful modulator of AD risk.
METHODS
STUDY PARTICIPANTS
The Human Subjects Review Committees of the University of Washington and the Veterans Affairs Puget Sound Health Care System approved the study, and written informed consent was obtained from all participants. Forty-nine adults participated, including 20 healthy control individuals (mean [SD] age, 69.3 [7.4] years) and 29 adults with aMCI (mean [SD] age, 67.6 [6.8] years), a disorder thought to represent prodromal AD.16 The target sample size was based on effect sizes drawn from a previous study17 in which central nervous system (CNS) insulin concentrations were raised experimentally in adults with aMCI, given that we anticipated that diet would induce a similar effect. All prospective participants underwent a comprehensive neuropsychological battery. Those whose delayed memory scores deviated 1.5 SDs or more from an estimate of their premorbid ability were considered for the diagnosis of aMCI (single or multiple domain), which was determined by expert consensus using all available cognitive and demographic-medical data, per published criteria.18 All study participants were free of major psychiatric disorders, alcoholism, neurologic disorders other than aMCI, renal or hepatic disease, DM2, chronic obstructive pulmonary disease, and unstable cardiac disease. Participants were not taking cholesterol-lowering medications.
PROCEDURE
Study participants were randomized to receive the HIGH (n=24) or LOW diet (n=25). Participants and all study personnel involved in data collection were masked to treatment assignment. Caloric needs to maintain prestudy weight were calculated by averaging the Mifflin–St. Jeor and Harris-Benedict equations, adjusted for physical activity, and rounding up to the nearest 200-calorie diet level.19,20 Cognitive testing, oral glucose tolerance testing, blood collection, and lumbar puncture were performed before and in the fourth week of the diet intervention.
DIET INTERVENTION
All food was delivered to the homes of participants twice weekly. Menus were designed by a research nutritionist and analyzed by ProNutra software (VioCare Inc, Princeton, New Jersey) to ensure adherence to macronutrient targets. The glycemic index was calculated to index simple carbohydrate content.21 The HIGH diet included 45% fat (saturated fat, 25%), 35% to 40% carbohydrates (glycemic index, >70), and 15% to 20% protein. The LOW diet included 25% fat (saturated fat,<7%), 55% to 60% carbohydrates (glycemic index,<55), and 15% to 20% protein. Study participants recorded all food consumed each day to assess adherence. The number of nonadherent incidents was small and comparable among groups (mean incidents per week ranged from 1.23 to 1.80 per group).
COGNITIVE PROTOCOL
Study participants completed tests of immediate and delayed memory (story recall, word list, and the Brief Visuospatial Memory Test), executive function (Trail-Making Test, part B; Stroop test/interference condition; and Verbal Fluency Test), and motor speed (Trail-Making Test, part A and Stroop test/matching condition), as previously described.17,22,23 Different but comparable versions of cognitive tests were administered before and after 4 weeks of dietary intervention.
ORAL GLUCOSE TOLERANCE TESTING
After obtaining a fasting blood sample through an intravenous catheter, participants drank a 75-g glucose solution, and additional samples were drawn at 15, 60, and 120 minutes. The integrated area under the curve (AUC) for insulin estimates insulin exposure and sensitivity, with higher values characteristic of hyperinsulinemia and lower insulin sensitivity, and AUC glucose reflects glucose tolerance.24
INSULIN, GLUCOSE, AND BLOOD LIPIDS
Insulin and glucose levels were measured, as previously described.25,26 The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated from fasting glucose and insulin concentrations, with greater values indicating greater insulin resistance.27 Cholesterol levels were measured using the enzymatic colorimetric Roche Cobas c 501 assay (F. Hoffmann–La Roche Ltd, Basel, Switzerland). Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated using Friedewald's formula.28
LUMBAR PUNCTURE
After a 12-hour fast, an intravenous catheter was inserted, and the L4–5 interspace was infiltrated with 1% lidocaine as local anesthesia. Using a 24-gauge Sprott spinal needle, 30 mL of CSF was withdrawn into sterile syringes, aliquoted into prechilled polyethylene tubes, frozen immediately with dry ice, and stored at −70°C until assay.
AD BIOMARKERS
The CSF Aβ42, tau protein, and phosphorylated tau (p-tau) were measured with the immunoassay INNO-BIA AlzBio3 (Innogenetics NV, Gent, Belgium). The CSF Aβ40 was measured by sandwich enzyme-linked immunosorbent assays, as previously described.29 The limit of detection was 15 pg/mL. Apolipoprotein E was measured using sandwich enzyme-linked immunosorbent assays; plates were coated using the mouse monoclonal antibody E276 paired with biotinylated mouse monoclonal E887 (MabTech AB, Nacka Strand, Sweden). The limit of detection for APOE was 150 pg/mL.
STATISTICAL ANALYSIS
All participants completed the study. Only a few data points were missing (<2% overall). Missing data were imputed using linear regression. If baseline and posttreatment values were missing, as with some assays because of poor venous or CSF access, the participant was dropped from the analysis. Based on previous work in animal models, we hypothesized that diet would modulate concentrations of the primary outcome measure, CSF Aβ42. Secondary measures included CSF Aβ40, tau protein, p-tau, insulin, APOE, F2-isoprostanes, peripheral metabolic markers, and cognition. Each biomarker was subjected to an omnibus repeated-measures analysis of variance (ANOVA) (Proc GLM; SAS statistical software, version 9.2; SAS Institute Inc, Cary, North Carolina) with time (baseline vs week 4) as the repeated factor and diet intervention (HIGH or LOW) and diagnostic group (healthy or aMCI) as the between-subjects factors. If a significant time × diet × diagnosis interaction was observed, change scores (week 4 - baseline) were compared using 2-tailed t tests. Two measures (HOMA-IR and F2-isoprostanes) required log transformations to normalize distributions.
Cognitive tests were grouped into 4 domains: immediate memory (immediate story recall, list recall, and visual memory), delayed memory (delayed recall of the preceding 3 tests), executive function (Trail-Making Test, part B; Stroop test/interference condition; and Verbal Fluency Test), and motor speed (Trail-Making Test, part A and Stroop test/matching condition). Outcome measures for each domain were subjected to omnibus, multivariate, and repeated-measures ANOVA; significant interactions were examined by subjecting constituent test scores to repeated-measures, univariate ANOVAs and t tests, as described herein. Age, baseline body mass index, educational level, and APOE-ε4 status (ε4 allele present or absent) were included as covariates in all biomarker and cognitive analyses. Inclusion of these covariates did not affect any result significantly.
RESULTS
Baseline demographic and dietary variables were comparable among the groups (Table 1), except that healthy controls in the HIGH group tended to have higher baseline body mass indexes than healthy participants in the LOW group (P=.07). As noted, baseline body mass index was included as a covariate in all analyses and did not affect results. Mean change scores with SEMs obtained after significant omnibus repeated-measures ANOVAs are presented in Figure 1 (metabolic indexes) and Figure 2 (CSF markers and cognition); prediet and postdiet means (SEMs) for all analyzed variables are included in eTable 1 (metabolic indexes) (http://www.archneurol.com) and eTable 2 (CSF markers and cognition).
Table 1.
Baseline Demographics and Prestudy Diet Composition as Determined From a 3-Day Food Intake Recorda
| Healthy Control Individuals |
aMCI Patients |
|||
|---|---|---|---|---|
| Variable | LOW (n=11) | HIGH (n=9) | LOW (n=14) | HIGH (n=15) |
| Sex, No. | ||||
| Male | 4 | 3 | 7 | 9 |
| Female | 7 | 6 | 7 | 6 |
| Age, y | 69.7 (8.0) | 68.8 (7.0) | 67.1 (6.8) | 68.1 (6.9) |
| Educational level, y | 13.5 (1.8) | 15.7 (2.2) | 15.6 (2.3) | 14.9 (2.2) |
| BMIb | 26.4 (2.6)b | 29.5 (4.5)b | 27.4 (3.8) | 27.5 (3.4) |
| Modified MMSE score | 96.6 (2.6) | 97.8 (2.8) | 95.0 (5.0) | 93.1 (4.4) |
| Caloric intake, kcal | 2012.8 (744.4) | 1955.7 (209.5) | 2191.1 (836.2) | 2101.5 (357.6) |
| % of Total kcal | ||||
| Protein | 16.2 (3.9) | 17.2 (3.0) | 17.4 (2.3) | 16.4 (3.3) |
| Carbohydrate | 46.7 (6.9) | 48.5 (7.1) | 46.3 (6.6) | 49.7 (4.4) |
| Total fat | 33.4 (6.5) | 33.4 (7.8) | 34.3 (5.9) | 31.7 (5.1) |
| Saturated fat | 10.2 (3.4) | 12.0 (4.7) | 12.6 (4.5) | 11.4 (2.6) |
Abbreviations: aMCI, amnestic mild cognitive impairment; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HIGH, high saturated fat/high glycemic index; LOW, low saturated fat/low glycemic index; MMSE, Mini-Mental State Examination.
Data are expressed as mean (SEM) unless otherwise indicated.
Healthy controls ingesting the HIGH diet tended to have higher baseline BMI values than healthy controls ingesting the LOW diet (P=.07).
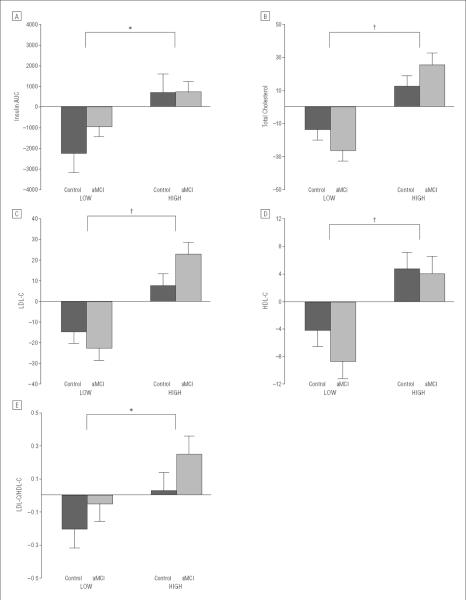
Figure 1.
Mean change from baseline (value at week 4 – baseline value) with standard errors for insulin concentration area under the curve (AUC) during testing for oral glucose tolerance (P=.01) (A), total cholesterol (B), low-density lipoprotein cholesterol (LDL-C) (C), high-density lipoprotein cholesterol (HDL-C) (D), and LDL-C/HDL-C ratio (E). The high–saturated fat/high–glycemic index (HIGH) diet raised and the low–saturated fat/low–glycemic index (LOW) diet lowered insulin concentration AUC for the healthy control and amnestic mild cognitive impairment (aMCI) groups (time×diet interaction, P=.01). Total cholesterol and LDL-C concentrations were increased with the HIGH diet and decreased with the LOW diet (time×diet, P <.001), with 2-fold greater effects noted for the aMCI group (time×diet×diagnosis, P=.04 and P=.049, respectively). The HDL-C and LDL-C/HDL-C ratio decreased with the LOW and increased with the HIGH diet interventions (time×diet, P<.001 and P=.048, respectively). Means (SEMs) at baseline and week 4 for all outcome variables are presented in eTable 1; no baseline difference was observed among groups for any variable. *P≤.05. †P≤.001.
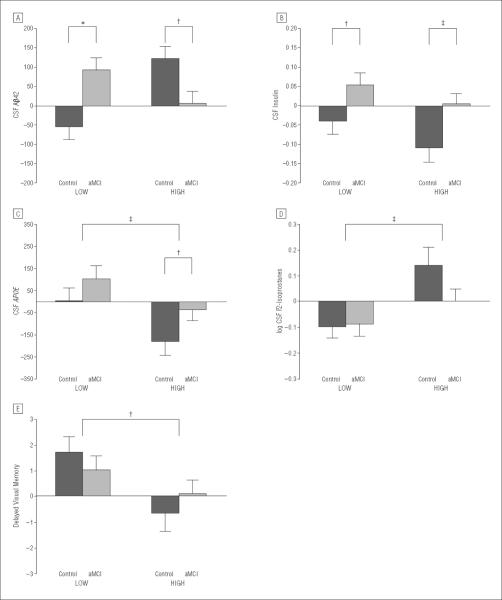
Figure 2.
Mean change from baseline (value at week 4 – baseline value) with standard errors for cerebrospinal fluid (CSF) concentrations of β-amyloid 42 (Aβ42) (A), insulin (B), apolipoprotein E (APOE) (C), log F2-isoprostanes (D), and delayed visual memory (E). The CSF Aβ42 concentration decreased for healthy adults and increased for adults with amnestic mild cognitive impairment (aMCI) after 4 weeks of consuming the low–saturated fat/low–glycemic index (LOW) diet and increased for healthy adults after the high–saturated fat/high–glycemic index (HIGH) diet (group×diet×week interaction, P<.001; LOW diet, healthy control vs aMCI group change scores, P*lt;.001; HIGH diet, healthy control vs aMCI group change scores, P=.05). Diet intervention affected CSF insulin concentrations (time×diet, P=.03; LOW diet, exploratory analyses, healthy control vs aMCI group change scores, P=.04; HIGH diet, healthy control vs aMCI group change scores, P=.01). Concentrations of CSF APOE were increased by the LOW diet and decreased by the HIGH diet for the healthy and aMCI groups (time×diet, P=.006). The CSF F2-isoprostane concentrations were reduced by the LOW diet for both groups and increased by the HIGH diet for the healthy control group (time×diet, P=.01). A trend was noted for differences between groups (time×diet×diagnosis, P=.10); in exploratory analyses, both groups showed lowered F2-isoprostane concentrations in the LOW diet, but only healthy adults showed increased concentrations in the HIGH condition (HIGH diet, healthy control vs aMCI group change scores, P=.04). The healthy control and aMCI groups showed improved delayed visual recall after the LOW diet (time × diet, P=.04). Raw means with SEM are presented in eTable 2; no baseline differences were observed among groups. *P≤.001; †P≤.05; and ‡P≤.01.
DIET INTERVENTION–MODULATED INSULIN AND LIPID METABOLISM
Metabolic indexes were examined to verify that the diets achieved their targeted modulation of insulin concentrations, insulin resistance, and lipid concentrations. For the healthy and aMCI groups, the HIGH diet increased and the LOW diet reduced insulin AUC (Figure 1A; time × diet interaction, P=.01). Glucose AUC was not affected by diet. The HOMA-IR values also tended to be modulated by diet for both groups (time × diet, P=.06), with increasing values indicative of greater insulin resistance observed in the HIGH condition and lower values in the LOW condition. Weight was unchanged by diet intervention for each diagnostic group.
The magnitude of diet effects on total cholesterol differed between the 2 diagnostic groups (Figure 1B; time × diet × diagnosis interaction, P=.04); although the HIGH diet increased cholesterol concentrations and the LOW diet lowered those concentrations for both groups (time × diet, P<.001), adults with aMCI showed nearly 2-fold greater changes compared with healthy adults. Change scores for the 2 diagnostic groups did not differ significantly in either diet condition, indicating that the overall time × diet × diagnosis interaction was produced by the combined effect of lowered and increased cholesterol concentrations across the 2 diet conditions. This pattern was mirrored for LDL-C (Figure 1C; time × diet × diagnosis, P=.048; time × diet, P<.001; no differences between healthy control vs aMCI group change scores). High-density lipoprotein cholesterol, regarded as a protective factor, also increased with the HIGH diet (likely due to the increase in monounsaturated and polyunsaturated fats that accompanied saturated fats in foods included in this diet) and decreased with the LOW diet (likely due to the overall reduction in dietary fat; Figure 1D; time × diet, P<.001). However, the ratio of LDL-C to high-density lipoprotein cholesterol, a cardiovascular risk index that is elevated in insulin resistance,30 was increased by the HIGH diet and lowered by the LOW diet for both groups (Figure 1E; time × diet, P=.04).
AD BIOMARKER RESPONSE TO DIET
The diet intervention had striking effects on the primary outcome measure, CSF Aβ42 concentrations, with different patterns observed for the healthy control and aMCI groups (time × diet × diagnosis interaction, P<.001). The LOW diet increased CSF Aβ42 for the aMCI group but decreased CSF Aβ42 for the healthy control group (Figure 2A; healthy control vs aMCI group change scores, P<.001). The HIGH diet increased CSF Aβ42 for healthy adults, but aMCI group concentrations were virtually unchanged (Figure 2A; healthy control vs aMCI group change scores, P=.05). No effects were observed for CSF Aβ40, tau protein, or p-tau.
The diet intervention also affected CSF insulin concentrations (Figure 2B; time × diet interaction, P=.03). The CSF insulin concentration increased in the LOW diet group and decreased in the HIGH diet group; although no interaction with diagnosis was observed, exploratory inspection of change scores showed that in the LOW diet, CSF insulin increases were restricted to the aMCI group (healthy control vs aMCI group change scores, P=.04) but in the HIGH diet, CSF insulin decreases were restricted to the healthy group (healthy control vs aMCI group change scores, P=.01).
Next, we examined diet effects on CSF APOE because of its important role in Aβ clearance.31,32 Concentrations of APOE were increased by the LOW diet and decreased by the HIGH diet (Figure 2C; time × diet interaction, P=.01). As noted, this pattern was unaffected by adjustments for APOE-ε4 carriage or any other covariate.
F2-isoprostanes are quantitative biomarkers of CNS free radical injury that are elevated in AD patients.33 The LOW diet reduced and the HIGH diet increased CSF F2-isoprostane concentrations, with a trend noted for different effects for the healthy and aMCI groups (Figure 2D; time × diet interaction, P=.001; time × diet × diagnosis, P=.10). Both groups showed lowered F2-isoprostane concentrations with the LOW diet, but an exploratory comparison revealed that only healthy adults showed increased concentrations with the HIGH diet (healthy control vs aMCI group change scores in the HIGH condition, P=.04).
EFFECT OF LOW DIET ON DELAYED MEMORY
Diet affected delayed memory differently for the 3 constituent measures of this domain (multiple ANOVA test × time × diet interaction, P=.05). The healthy control and aMCI groups showed improved delayed visual recall with the LOW diet (Figure 2E; time × diet, P=.04), but other delayed memory measures did not change significantly. No diet-related changes were observed for immediate memory, executive, or motor speed domains.
COMMENT
Our diet interventions successfully modulated insulin and lipid metabolism, allowing us to examine the effects of diet-induced metabolic changes on AD biomarkers. For healthy adults, the HIGH diet moved CSF biomarkers in a direction that may characterize a presymptomatic stage of AD before plaque deposition, increasing total Aβ42 and F2-isoprostane concentrations and lowering insulin concentrations. The AD biomarkers were unaffected by the HIGH diet for adults with aMCI, possibly because more extreme intervention is needed to exacerbate already-extant pathologic processes. However, the aMCI and healthy control groups showed beneficial effects of the LOW diet, including improved Aβ42 profiles, reduced F2-isoprostane concentrations, increased APOE, and improved memory. A summary of diet effects on CNS variables is presented in Table 2.
Table 2.
Summary of Diet Effects on Cerebrospinal Fluid Analytesa
| Healthy Control Individuals |
aMCI Patients |
|||
|---|---|---|---|---|
| Variable | LOW | HIGH | LOW | HIGH |
| β-Amyloid 42 | ↓ | ↑ | ↑ | NC |
| F2-isoprostanes | ↓ | ↑ | ↓ | NC |
| Insulin | NC | ↓ | ↑ | NC |
| Apolipoprotein E | ↑ | ↓ | ↑ | ↓ |
Abbreviations: aMCI, amnestic mild cognitive impairment; HIGH, high–saturated fat/high–glycemic index; LOW, low–saturated fat/low–glycemic index; NC, no change.
Downward arrows indicate decreased concentrations; upward arrows, increased concentrations.
DIET EFFECTS ON AD BIOMARKERS
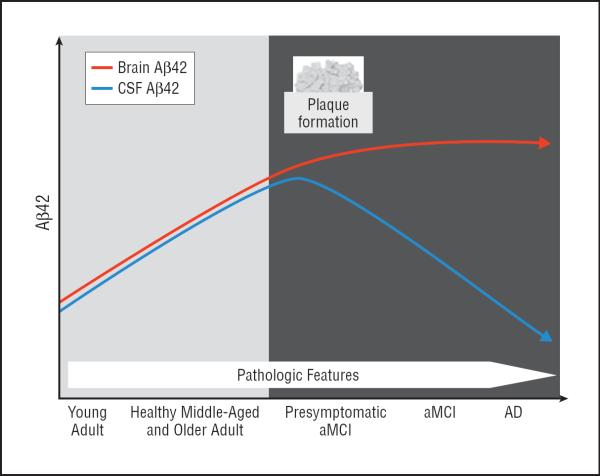
Dietary intervention had a remarkable effect on CSF Aβ42 concentrations. We predicted that the HIGH diet would induce stressors that would change CSF Aβ42 in a direction consistent with amplified AD pathophysiologic changes, whereas the LOW diet would suppress these stressors, thereby producing opposing changes in CSF Aβ42. Our results supported this prediction. Of importance, however, the pattern of diet-induced change differed between the healthy control and aMCI groups. We speculate that these patterns derive from disease stage–dependent differences in the trajectory of CSF Aβ42 and we propose a model of this trajectory that spans young adult age, healthy middle and older adult age, presymptomatic aMCI, and symptomatic aMCI and AD. According to this model (Figure 3), brain CSF Aβ42 concentrations rise with age to the point of fibrillar Aβ (plaque) deposition. Around the time Aβ deposition occurs in presymptomatic disease, CSF concentrations reach a tipping point and begin to decline, followed by the onset of symptomatic aMCI and AD.
Figure 3.
Model of hypothetical trajectory of brain and cerebrospinal fluid (CSF) β-amyloid 42 (Aβ42) accumulation with increasing Alzheimer disease (AD) symptoms and pathologic features.
Evidence of a tipping point in CSF Aβ42 concentration that corresponds with initiation of brain Aβ deposition is seen in studies of transgenic mice.34–36 Conclusive evidence of a tipping point model of CSF Aβ42 in humans is limited by the lack of longitudinal data spanning the continuum from healthy young adult age through the onset of AD. In large cross-sectional studies,37,38 however, total CSF Aβ42 concentrations increase from age 20 years until age 50 to 60 years in healthy adults. A decrease in CSF Aβ42 in presymptomatic aMCI patients is supported by findings that decreased Aβ42 during a 4-year period in healthy adults predicts future cognitive decline and that reduced CSF Aβ42 is associated with fibrillar Aβ deposition, even in cognitively healthy adults.39,40 A similar pattern has been reported in plasma Aβ42.41 Taken together, these findings suggest a stage of presymptomatic disease in which brain Aβ deposition begins and CSF Aβ42 decreases. Regarding changes during symptomatic stages, several studies38 have documented that CSF Aβ42 declines with clinical disease onset. Additional longitudinal evidence supporting this model is provided by studies of individuals with Down syndrome, who commonly develop neuropathologic features of AD with older age. These studies42,43 document increased CSF Aβ42 early in life with later decreases around the age at which plaque deposition occurs. Given converging data from animal and human studies, this tipping point model seems to be a reasonable description of changes in CSF Aβ42 trajectory that occur with aging and AD pathogenesis, although it may not apply to all adults with AD.
Using this model as a framework, our results showed that the HIGH diet increased CSF Aβ42 concentrations for healthy adults, potentially moving them closer to the tipping point. Conversely, the LOW diet lowered CSF Aβ42 for this group, moving concentrations away from the tipping point. For the aMCI group (who, in our model, have already passed the tipping point), the LOW diet increased CSF Aβ42, moving concentrations back toward the normal end of the continuum. The CSF Aβ42 concentrations for the aMCI group were unaffected by the HIGH diet, perhaps because existing disease was not exacerbated by our short-term intervention.
DIET-MODULATED PERIPHERAL INSULIN AND LIPID METABOLISM
A key finding of our study was that dietary macronutrient manipulation for 1 month modulated the metabolic profile of participants even in the absence of weight change, affecting insulin exposure, insulin sensitivity, and lipid metabolism for the healthy control and aMCI groups. Of interest, diet effects on total cholesterol and LDL-C were greater for the aMCI group. Many studies have documented lipid abnormalities in AD. Elevations in LDL-C and total cholesterol concentrations have been demonstrated in early AD,44 with cholesterol increases occurring in conjunction with greater β-amyloid disease.45 Whether modulation of lipid metabolism directly affects brain function and AD is controversial. For example, cholesterol does not cross an intact blood-brain barrier but may cross an impaired one.46 Diets high in saturated fat impair blood-brain barrier function; in a rodent model, evidence suggested that high-saturated fat diets may allow delivery of cholesterol and metabolites or Aβ complexed with lipoproteins from the periphery to the CNS.15
MARKERS OF OXIDATIVE STRESS AND DIET RESPONSE
The CSF F2-isoprostanes are quantitative biomarkers of free radical injury that reflect oxidative damage to the CNS.33 Dietary fat modulates brain concentrations of F2-isoprostanes in rodent models.47 In AD and perhaps in aMCI or latent-stage disease, F2-isoprostanes are increased; furthermore, they increase with normal aging and thus may reflect cumulative oxidative stress.48–50 The LOW diet reduced F2-isoprostanes for both groups, but the HIGH diet increased concentrations only for healthy adults, similar to the pattern observed for CSF Aβ42; these analyses were exploratory, however, and thus must be interpreted with caution. Synchronous increases in concentrations of CSF Aβ42 and F2-isoprostanes were observed previously in healthy adults when hyperinsulinemia was induced experimentally.26 Also, F2-isoprostane concentrations were elevated in cognitively normal adults who had abnormal AD biomarker profiles.49 Taken together, these results suggest that Aβ or forces that modulate Aβ increases oxidative stress and F2-isoprostane concentrations.
MODULATION OF CSF APOE AND INSULIN BY DIET INTERVENTION
The APOE concentrations were increased by the LOW diet and decreased by the HIGH diet. Despite extensive study, no consensus exists as to whether increasing APOE would favorably influence AD pathophysiologic changes. The finding that the LOW diet improved memory and the AD bio-marker profile for the aMCI group, as well as that it increased APOE, suggests that APOE increases are beneficial. However, factors other than totalAPOEconcentrations, such as the degree of APOE lipidation and other mechanisms correlated with increasedAPOE, may be responsible for memory and biomarker changes. One such mechanism may be diet-related modulation of adenosine triphosphate–binding cassette transporter 1 concentrations or activity. Adenosine triphosphate–binding cassette transporter1–mediatedAPOE secretion and lipidation modulate Aβ clearance via proteases such as the insulin-degrading enzyme.51
Reduced CNS insulin and insulin-signaling markers have been reported in AD.52,53 Insulin plays an important role in many brain functions relevant to AD, including participation in synapse formation and maintenance, Aβ regulation, tau protein phosphorylation, neurotransmitter modulation, and glucose use.54 Insulin crosses the blood-brain barrier via a saturable, receptor-mediated transport system.55 Brain insulin transport and signaling are compromised by persistent hyperinsulinemia and high-fat or high-fructose feeding in in vivo canine and rodent models.56,57 Consistent with those reports, consumption of the HIGH diet lowered CSF insulin concentrations for healthy adults, although these results must be considered exploratory and thus interpreted with caution. This reduction may promote AD, given previous findings that a high-fat diet reduced brain insulin signaling and insulin-degrading enzyme, increasing β-amyloid disease in Tg2576 mice.58 Conversely, exploratory analyses indicated that CSF insulin increased after consumption of the LOW diet for the aMCI group. Restoration of normal insulin concentrations and activity may have beneficial effects, such as protection against synaptotoxicity by oligomeric Aβ.59
IMPROVEMENT IN DELAYED MEMORY
Delayed memory, a hallmark cognitive deficit in aMCI and AD, was improved by the LOW diet. The precise mechanisms underlying this effect and its specificity to visual memory are unclear, but dietary modulation affects memory in animal models.8 We did not observe reduced cognitive performance for either group consuming the HIGH diet, perhaps because longer periods of exposure or weight gain are needed to manifest negative effects.
STUDY LIMITATIONS
Our study had several limitations that may affect its generalizability. The diet intervention was designed to investigate the effects of weight-stable macronutrient manipulation; weight change may produce quantitatively or qualitatively different results. Similarly, because our study was designed to mimic the dietary pattern that promotes DM2 and insulin resistance, we manipulated the amount and type of fats and carbohydrates; thus, our results may reflect changes in any of these characteristics. The length of time participants consumed the HIGH diet was restricted because of safety considerations; longer exposure may be needed to observe changes in cognition and other end points. Prospective participants with hyperlipidemia or statin use were excluded from the study, which likely increased the difficulty of detecting diet-related effects. Because of the intensive nature of the study, the sample size was relatively small, which may have affected our power to detect changes in more variable end points. Similarly, a number of analyses were conducted, although requirement of a significant omnibus repeated-measures ANOVA before post hoc testing should mitigate the occurrence of type I error. Notably, despite the inclusion of unusually healthy participants and the small sample size, we observed significant effects in key bio-marker and metabolic end points.
In conclusion, our study supports further investigation into the possibility that consumption of a diet high in saturated fat and simple carbohydrates may contribute to pathologic processes in the brain that increase the risk of AD. Conversely, diets low in saturated fat and simple carbohydrates may offer protection against AD and enhance brain health; we observed improvements in bio-marker profiles and delayed visual memory in participants consuming this type of diet. Using this human experimental model, our results provide converging support for recent epidemiologic investigations of dietary pattern and AD risk and for animal studies of diet effects on AD. Taken together, these studies suggest that the therapeutic effects of longer-term dietary intervention may be a promising avenue of exploration. In addition, identification of the pathophysiologic changes underlying dietary effects may reveal important therapeutic targets that can be modulated through targeted dietary or pharmacologic intervention.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by grant R37 AG-10880 from the National Institute on Aging to Dr Craft, grant P50 AG-05136 to Dr Montine, and grant 5T32 AG-000258 to Dr Postupna and supported by funding from the Nancy and Buster Alvord Endowment to Dr Montine. This article results from work supported by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
Footnotes
Financial Disclosure: None reported.
Online-Only Material: The eTables are available at http://www.archneurol.com.
REFERENCES
- 1.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68(suppl 2):S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 3.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91(3):502–509. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Olendzki BC, Hafner AR, et al. Low-carbohydrate and high-fat intake among adult patients with poorly controlled type 2 diabetes mellitus. Nutrition. 2006;22(11–12):1129–1136. doi: 10.1016/j.nut.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunnane SC, Plourde M, Pifferi F, Bégin M, Féart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer's disease. Prog Lipid Res. 2009;48(5):239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Solfrizzi V, Frisardi V, Capurso C, et al. Dietary fatty acids in dementia and predementia syndromes: epidemiological evidence and possible underlying mechanisms. Ageing Res Rev. 2010;9(2):184–199. doi: 10.1016/j.arr.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Panza F, Frisardi V, Capurso C, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21(3):691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- 8.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, et al. ω-3 Fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD Study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 10.Féart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia [published correction appears in JAMA. 2009;302(22):2436] JAMA. 2009;302(6):638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010;67(6):699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44(7):805–817. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder JE, Richardson JC, Virley DJ. Dietary manipulation and caloric restriction in the development of mouse models relevant to neurological diseases. Biochim Biophys Acta. 2010;1802(10):840–846. doi: 10.1016/j.bbadis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Takechi R, Galloway S, Pallebage-Gamarallage MMS, Lam V, Mamo JCL. Dietary fats, cerebrovasculature integrity and Alzheimer's disease risk. Prog Lipid Res. 2010;49(2):159–170. doi: 10.1016/j.plipres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier S, Reisberg B, Zaudig M, et al. International Psychogeriatric Association Expert Conference on Mild Cognitive Impairment. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 17.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates β-amyloid in early AD [published correction appears in Neurology. 2008;71(11):866] Neurology. 2008;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Carnegie Institution of Washington; Washington, DC: 1919. [Google Scholar]
- 20.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 21.Wolever TMS, Yang M, Zeng XY, Atkinson F, Brand-Miller JC. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am J Clin Nutr. 2006;83(6):1306–1312. doi: 10.1093/ajcn/83.6.1306. [DOI] [PubMed] [Google Scholar]
- 22.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedict RH. Effects of using same- versus alternate-form memory tests during short-interval repeated assessments in multiple sclerosis. J Int Neuropsychol Soc. 2005;11(6):727–736. doi: 10.1017/S1355617705050782. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care. 2004;27(8):1998–2002. doi: 10.2337/diacare.27.8.1998. [DOI] [PubMed] [Google Scholar]
- 25.Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 1996;17(1):123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 26.Fishel MA, Watson GS, Montine TJ, et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005;62(10):1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 29.Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid β proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57(1):100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 30.Ingelsson E, Schaefer EJ, Contois JH, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298(7):776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 31.Martins IJ, Berger T, Sharman MJ, Verdile G, Fuller SJ, Martins RN. Cholesterol metabolism and transport in the pathogenesis of Alzheimer's disease. J Neurochem. 2009;111(6):1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 32.Wahrle SE, Jiang H, Parsadanian M, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118(2):671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free radical–mediated damage to brain in Alzheimer's disease and its transgenic mouse models. Free Radic Biol Med. 2008;45(3):219–230. doi: 10.1016/j.freeradbiomed.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeMattos RB, Bales KR, Parsadanian M, et al. Plaque-associated disruption of CSF and plasma amyloid-β (Aβ) equilibrium in a mouse model of Alzheimer's disease. J Neurochem. 2002;81(2):229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 35.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21(2):372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Herukka S-K, Minkeviciene R, van Groen T, Tanila H. Longitudinal observation on CSF Aβ42 levels in young to middle-aged amyloid precursor protein/presenilin-1 doubly transgenic mice. Neurobiol Dis. 2004;17(3):516–523. doi: 10.1016/j.nbd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Peskind ER, Li G, Shofer J, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid β-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63(7):936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- 38.Shoji M, Kanai M, Matsubara E, et al. The levels of cerebrospinal fluid Aβ40 and Aβ42(43) are regulated age-dependently. Neurobiol Aging. 2001;22(2):209–215. doi: 10.1016/s0197-4580(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 39.Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67(2):217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- 40.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 41.Cosentino SA, Stern Y, Sokolov E, et al. Plasma ß-amyloid and cognitive decline. Arch Neurol. 2010;67(12):1485–1490. doi: 10.1001/archneurol.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Englund H, Annerén G, Gustafsson J, et al. Increase in β-amyloid levels in cerebrospinal fluid of children with Down syndrome. Dement Geriatr Cogn Disord. 2007;24(5):369–374. doi: 10.1159/000109215. [DOI] [PubMed] [Google Scholar]
- 43.Tapiola T, Soininen H, Pirttilä T. CSF tau and Aβ42 levels in patients with Down's syndrome. Neurology. 2001;56(7):979–980. doi: 10.1212/wnl.56.7.979. [DOI] [PubMed] [Google Scholar]
- 44.Kuo Y-M, Emmerling MR, Bisgaier CL, et al. Elevated low-density lipoprotein in Alzheimer's disease correlates with brain Aβ 1–42 levels. Biochem Biophys Res Commun. 1998;252(3):711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 45.Pappolla MA, Bryant-Thomas TK, Herbert D, et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61(2):199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- 46.Shobab LA, Hsiung G-YR, Feldman HH. Cholesterol in Alzheimer's disease. Lancet Neurol. 2005;4(12):841–852. doi: 10.1016/S1474-4422(05)70248-9. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Moreno C, Dorfman SE, Lichtenstein AH, Martín A. Dietary fat type affects vitamins C and E and biomarkers of oxidative status in peripheral and brain tissues of golden Syrian hamsters. J Nutr. 2004;134(3):655–660. doi: 10.1093/jn/134.3.655. [DOI] [PubMed] [Google Scholar]
- 48.Praticò D, Clark CM, Liun F, Rokach J, Lee VY-M, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59(6):972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 49.Montine TJ, Peskind ER, Quinn JF, et al. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer's disease as identified by biomarkers. Neuromolecular Med. 2011;13(1):37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66(3):315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Q, Lee CYD, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 53.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50(1):164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 54.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18(9):1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 56.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49(9):1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 57.Mielke JG, Taghibiglou C, Liu L, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem. 2005;93(6):1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004;24(49):11120–11126. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Felice FG, Vieira MNN, Bomfim TR, et al. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc Natl Acad Sci U S A. 2009;106(6):1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.