Abstract
Background
Polyhexamethylene biguanide (PHMB) is an antiseptic polymer that is mainly used for cleaning hospitals and pools and combating Acantamoeba infection. Its fungicide activity was recently shown by its lethal effect on yeasts that contaminate the industrial ethanol process, and on the PE-2 strain of Saccharomyces cerevisiae, one of the main fermenting yeasts in Brazil. This pointed to the need to know the molecular mechanism that lay behind the cell resistance to this compound. In this study, we examined the factors involved in PHMB-cell interaction and the mechanisms that respond to the damage caused by this interaction. To achieve this, two research strategies were employed: the expression of some genes by RT-qPCR and the analysis of mutant strains.
Results
Cell Wall integrity (CWI) genes were induced in the PHMB-resistant Saccharomyces cerevisiae strain JP-1, although they are poorly expressed in the PHMB-sensitive Saccharomyces cerevisiae PE2 strain. This suggested that PHMB damages the glucan structure on the yeast cell wall. It was also confirmed by the observed sensitivity of the yeast deletion strains, Δslg1, Δrom2, Δmkk2, Δslt2, Δknr4, Δswi4 and Δswi4, which showed that the protein kinase C (PKC) regulatory mechanism is involved in the response and resistance to PHMB. The sensitivity of the Δhog1 mutant was also observed. Furthermore, the cytotoxicity assay and gene expression analysis showed that the part played by YAP1 and CTT1 genes in cell resistance to PHMB is unrelated to oxidative stress response. Thus, we suggested that Yap1p can play a role in cell wall maintenance by controlling the expression of the CWI genes.
Conclusion
The PHMB treatment of the yeast cells activates the PKC1/Slt2 (CWI) pathway. In addition, it is suggested that HOG1 and YAP1 can play a role in the regulation of CWI genes.
Background
Previous work has shown that polyhexamethylene biguanide (PHMB) was effective in selectively killing the yeasts that are regarded as contaminants of the ethanol fermentation process, with special attention being paid to Dekkera bruxellensis [1]. PHMB has been used as an antiseptic and disinfectant in medicine and industry, and its current applications include the following: impregnation of fabrics to inhibit microbial growth [2,3], water treatment [4], disinfection of a wide range of solid surfaces such as contact lenses [5], mouthwashing [6,7], the treatment of hatching eggs to prevent contamination by Salmonella [8,9], treatment against fungi [10] and Acanthamoeba [11-13]. Its commercial preparations consist of mixtures of polymeric biguanides of blocks with molecular structure [-CH2)6.NH.C(= NH).NH.C(= NH).NH-]n, where n may vary from 2 to 40 (average 11) and molecular mass ranging from 400 to 8000 [14].
The heterogeneity of the molecule is increased further by the presence of either amine, or cyanoguanidine or guanidine end-groups in any combination at the terminal positions of each chain. Nonetheless, the cationic nature of the guanidine group makes the molecule positively charged at physiological to low pH [15]. As a result, PHMB is highly adsorptive to anionic surfaces such as bacterial cell walls and its biocidal mechanism may include damage to the cell membrane [16]. At lower concentrations, it has been suggested that the bacteriostatic effects of PHMB may be partly due to a powerful cooperative interaction with (polyanionic) nucleic acids [17]. It was suggested that PHMB should interact with phospholipids of the Acanthamoeba cell membrane and thus cause changes in cell permeability [18,19], and this was further supported by work on E. coli [14]. The external layer of Saccharomyces cerevisiae and other yeast species plasma membrane is enhanced by phosphatidylcholine, ergosterol and sphingolipids [20]. Owing to the cationic nature of PHMB, its toxic effect may be mediated by its links to the negative phospholipids on the yeast cell surface. In the presence of PHMB, the homogeneous distribution of phospholipids that are usually linked to biological membranes, is transformed into a mosaic of individual phospholipid domains that produce fluid and liquid crystalline regions in the cell membrane [15]. By means of the whole-genome transcriptional profile, Allen et al [14] show that E. coli responds to bacteriostatic levels of PHMB by altering the expression of many of the genes involved at all levels of the cell ultra structure, i.e. the outer membrane, periplasm, inner membrane and cytoplasmic domains, but not the lipolysaccharide layer. There was also an alteration in the expression of genes associated with stresses such as acid resistance, alkali resistance, osmotic shock and cell-envelope perturbation.
As well as the fungicide activity in D. bruxellensis, Pichia galeiformes and Candida tropicalis, the yeast species isolated PHMB as a contaminant through ethanol fermentation, and this also differentially affected Saccharomyces cerevisiae industrial strains, for example the PE-2 strain was more affected than JP1 [1]. This result might impose constraints on the use of PHMB in controlling incidents of yeast contamination in industrial fermentations. Thus, the further steps towards developing industrial formulations of PHMB, must rely on determining the identification of biological activity and targets of PHMB on the yeast cells, and identifying the yeast mechanisms that respond to the damage caused by this biocide.
In this study, we have sought to understand the aspects of PHMB-cell interaction discussed above, by adopting two strategies: 1) testing the biocide effects of PHMB on the expression of yeast genes involved in the cell wall integrity mechanism (CWI) and 2) by testing yeast strains with mutations in genes involved in CWI and their oxidative stress response. The results strongly indicated that PHMB may act by destabilizing the yeast cell wall, thus inducing the cell wall integrity pathway response. Moreover, an account is given of the potential regulatory role of Yap1p on the expression of CWI genes.
Results and Discussion
PHMB treatment induces genes of the yeast Cell Wall Integrity (CWI) sensing mechanism
Our previous results showed that the lethal effect of PHMB on yeast cells was partly mitigated by trehalose [1], which is known to be a protective agent of the cell envelope [20]. Moreover, previous global gene expression analysis revealed that exposure of E. coli cells to PHMB induced the expression of genes involved in cell wall maintenance [14]. This led us to search for CWI genes in the yeast genome (Additional file 1). Moreover, recent studies have shown that some genes involved in trehalose metabolism are regulated by stress response elements (STRE) in their promoter region; [21], in view of this we have included some yeast genes containing STRE in their promoter in our analysis. From a list of selected genes (Additional file 1), we conducted a quantitative PCR analysis after cell exposure to PHMB and the results revealed that the CHS1, FKS1, GAS1, HSP150, KRE6, MSN2, MSN4, PKH1 and YLR194c genes were up-regulated in the PHMB-resistant JPI strain, while remaining unchanged in the PHMB-sensitive PE-2 strain (Figure 1). Moreover, CRZ1 and RLM1 genes were induced in JP1 and repressed in PE-2, while CIN5 and MNN9 did not change in JP1, but were repressed in PE-2. The expression of the SLT2 gene remained unchanged in both the industrial strains (Figure 1).
Figure 1.
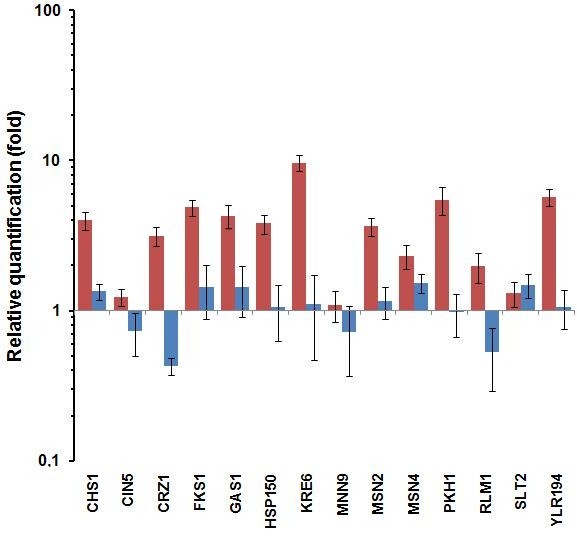
Relative expressions of the yeast genes involved in the cell wall integrity mechanism and in the general stress response. Relative Quantity represents the expression of the studied genes in the PHMB-treated cells (0.01%) over non-treated cells of the two industrial strains JP1 (red column) and PE-2 (blue column).
The products of the CHS1, GAS1, FKS1, HSP150, KRE6, PKH1 and YLR194c genes can be located either in the cell membrane or in the cell wall, and are mostly involved in the maintenance and remodeling of the cell envelope in response to environmental changes. The membrane-located chitin synthase 1, encoded by CHS1, acts on the turn-over of the chitin in the septum during the cell division [22]. The GAS1 gene product is a GPI-anchored protein located in the plasma membrane that acts by stretching the cover of β-1,3-glucan during the remodeling of the cell wall. Its expression is dependent on the SBF transcription factor, which comprises the Swi4p and Swi6p proteins [23]. The FKS1 gene encodes the catalytic sub-unit of the 1,3-β-D-glucan synthase complex. This complex is controlled by the regulatory subunit Rho1p, which in turn is activated by the nutrient sensing TOR pathway that regulates the activity of protein kinase C - Pkc1p, a key component of the Cell Wall Integrity (CWI) mechanism [21]. The Hsp150p protein is located in the cell wall and is secreted into the medium in response to heat or osmotic shock. Its gene is up-regulated under Calcofluor white and Zymolyase [24], heat shock [25] nitrogen limitation [26] treatments and by decreasing the β-1,3-glucan level in the cell wall [27]. The PKH1 gene encodes a phosphoinositol-dependent protein kinase, whose function overlaps Pkh2p, and is involved in maintaining the cell wall. This protein phosphorylates and activates Pkc1p [28]. The ORF YLR194c encodes for a putative GPI-attached protein and its expression is induced by cell wall stress or by mutation in the FKS1 gene [29]. The MNN9 gene encodes a β-1,6-mannosyltransferase involved in the transfer of mannose from UDP-mannose to oligosaccharides during the synthesis of the β-glucan bridge of the cell wall [30]. In addition, the KRE6 gene encodes a putative synthetase that acts on the synthesis of the β-1,6-glucan cover and mutation in this gene causes a 50% reduction in the synthesis of β-1,6-glucan in the cell wall [29]. The Kre6p protein was shown to be phosphorylated and genetically interact with components of the Pkc1p-mediated MAP kinase pathway [31] in order to regulate the β-1,6-glucan synthesis [32]. It was found that over-expression of the KRE6 gene offset the defect in the cell wall organization of the pkc1-mutant [28]. Thus, this gene could be a key to explain the difference in resistance of the two industrial strains to PHMB, since it was highly expressed in the PHMB-resistant JP1 strain (Figure 1).
Crz1p is a calcineurin-dependent zinc finger- type transcription factor that binds to the promoters of the FKS1 and CHS1 genes in response to calcofluor and calcium, respectively [23]. Both genes were found to be co-expressed, with a peak in G1, when the isotropic cell wall synthesis allowed daughter-cell expansion [23] and our results (Figure 1) corroborate that of the co-expression pattern. The lower expression level of the CRZ1 gene in PE-2 could explain the lack of CHS1 and FKS1 induction in this strain, and together with the low expression of KRE6, could produce the previously mentioned sensitive phenotype of the PE-2 strain to PHMB [1]. Moreover, these results suggest that the tolerance of the JP1 strain might be due to a more effective restoration of the cell envelope after it has been damaged by PHMB. This might be evidence that PHMB damages the glucan structure on the yeast cell wall.
Resistance to PHMB depends on the functionality of the PKC and HOG mechanisms
To test the hypothesis of cell wall damage, resistance to PHMB was evaluated in yeast strains with deletion in a series of CWI genes, including the genes involved in the HOG and PKC pathways (Additional file 2). The results of the cytotoxicity assay showed that deletion of the SLG1/WSC1, SLT2, ROM2, SWI4, SWI6 and KNR4 genes impaired the chances of cell survival to exposure to PHMB, while deletion of HOG1 and MKK2 genes only affected cell survival at higher PHMB concentration (Figure 2). The remaining mutants tested did not show altered sensitivity to this compound (data not shown). SLG1/WSC1 gene encodes one of the sensor-transducer proteins located in the cell membrane that activates the GDP/GTP exchange protein Rom2p in response to cell wall damage [28]. It was observed that the absence of this protein impairs the activation of Slt2p/Mpk1p kinase by heat shock [33]. The hypersensitivity of the Δslg1/wsc1 mutant to PHMB (Figure 2), together with the parental phenotype of yeast mutants for other sensors (Additional file 2), showed that Slg1p/Wsc1p is the main sensor for the damage caused by PHMB.
Figure 2.
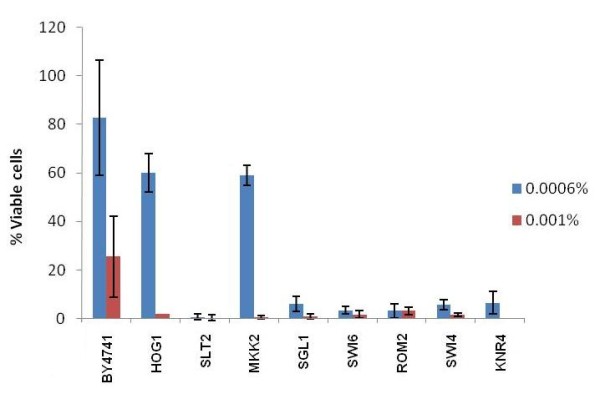
Cytotoxicity assay of yeast CWI mutants to different doses of PHMB. The percentage of viable cells after treatments refers to the number of CFU in treated samples over compared with non-treated samples of a given strain.
Following the PKC cascade, it was observed that the Δrom2 mutant was very sensitive to PHMB (Figure 2). The GTP/GDP-exchange factor Rom2p activates the Ras-like protein kinase Rho1p that triggers the PKC cascade by activating Pkc1p, which in turn activates Bck1p [23]. Bck1p, known as MAP kinase kinase kinase (MAPKKK), phosphorylates Mkk2p (MAPKK) in response to cell wall damage, which further phosphorylates Mpk1p/Slt2p (MAPK). The parental phenotype of the Δbck1 mutant and the intermediary sensitivity of the Δmkk2 mutant to PHMB (Figure 2) could be explained by the complementation by their paralogous Bck2p and Mkk1p, respectively.
Furthermore, the hyper-sensitivity of the Δmpk1/slt2 mutant (Figure 2) showed the importance of the SLT2 gene in PHMB resistance. Slt2p resides predominantly in the nucleus under non-stress conditions, but rapidly relocates to the cytoplasm in response to cell wall stress [34]. Once activated, Slt2p phosphorylates and activates the Rlm1p [23] transcription factor, as well as Swi4p [28]. As stated above, Swi4p and Swi6p form the SBF transcriptional factor that regulates the CWI genes, such as GAS1, FKS1 and SMI1/KNR4 [23]. Mutation of the SMI1/KNR4 gene increased the sensitivity of the yeast cells to PHMB (Figure 2). It was recommended that Smi1p/Knr4p should coordinate the cell cycle progression in connection with cell wall synthesis and this protein was shown to be essential for viability of the cell in the absence of a functional PKC1/Slt2 pathway [35]. A physical interaction between Knr4p and Slt2p was also described and it was proposed that such interaction modulate the Slt2p-dependent activation of Rlm1 and SBF transcriptional factors [36]. Activation of Rlm1p induces the expression of CHS1, FKS1, KRE6 and HSP150 [23], the same genes up-regulated in JP1 strain upon the PHMB treatment (Figure 1).
Finally, the Δhog1 mutation showed intermediate sensitivity to PHMB (Figure 2). It was suggested that at hyperosmotic shock, Hog1p activates Rlm1p that in turn regulates the subsequent expression of the SLT2 gene [37]. Thus, a cross-talk between HOG and PKC MAPK pathways should be established following the perturbation of the cell wall. The results of this research indicate that Hog1p may act as an amplifier of the sensing mechanism of the cell wall damage caused by PHMB, as has been recently described in the case of other cell wall damage [38]. Furthermore, double mutant analysis showed that there was a synergistic interplay between Hog1p and Yap1p in a resistance to arsenite stress, which suggests the existence of a genetic inter-dependence of both genes on the stress response [39]. Recent studies have shown that Hog1p is involved in cell wall remodeling by regulating the expression of EXG1 gene, which encodes the glucanase that affects the level of β-glucan [38]. This hypothesis supports the idea that PKC and HOG pathways converge to regulate the maintenance of the cell wall [38,40,41]. On the basis of this evidence, we suggest that PHMB may affect the β-glucan structure of the cell wall that is sensed by the PKC pathway, together with Hog1p.
The YAP1 gene is also involved in the cellular resistance to PHMB, but not through oxidative stress response
Following the induction of the MSN2 and MSN4 genes in the JP1 strain upon exposure to PHMB (Figure 1), we further screened the collection of deletion strains whose genes encode proteins involved in the complex general stress response mechanism (Additional file 2). Among these, the Δyap1 mutant showed a high degree of sensitivity to PHMB (Figure 3A, B). It was suggestive of the induction of oxidative damage to the cell membrane by PHMB. The Yap1p-mediated regulatory pathway that controls the oxidative stress adaptive response is activated by redox sensory mechanisms that detect changes in the intracellular redox balance caused by reactive oxygen species (ROS) and by oxidized thiols such as glutathione, thiorredoxin and SH-containing proteins [42]. Sulfhydryl groups, including non-protein (NP-SH), mostly represented by glutathione, and protein bound (PB-SH), are abundant in cells and can be oxidized by ROS [43]. Nevertheless, we did not observe only a small change in the mobilization of intracellular thiol groups when BY4741 or yap1 strains are submitted to PHMB for 72 hours, as it was observed after H2O2 reference treatment (Figure 4B). Moreover, no increase in the peroxidation of the membrane phospholipids was observed (data not shown). In addition, no other strain with deletion in oxidative stress genes was sensitive to PHMB, except the Δctt1 mutant (Figure 3B, 4A). The CTT1 gene encodes the cytosolic catalase T involved in the breakdown of hydrogen peroxide to oxygen and water, which is regulated by the oxidative stress transcription factors Yap1p and Skn7p and by the general stress response transcriptional complex Msn2/4p [44]. Winderickx et al. [45] found that oxidative stress-induced expression of CTT1 requires Yap1p even though this gene does not include a YRE motif in its promoter, although it contains a STRE motif [45]. Yap1p participates in the regulation of both antioxidant genes and several drug resistance genes [46-48], and this transcription factor regulates the genes that do not have YRE in their promoter regions [49,50]. Yap1p plays a role in regulating the expression of components of the STRE activation machinery, and as a result, the STRE-dependent gene expression, even though Yap1 does not bind STREs directly [49]. This suggests that YAP1 and CTT1 genes might be involved in the resistance to PHMB in a way that is not linked to the oxidative stress response pathway. There are some indications of the participation of the upper elements of the PKC pathway in the survival of cells when subjected to oxidizing agents such as diamide and hydrogen peroxide [51]. It is known that ethanol induces the expression of various stress responsive genes, such as CTT1, through the binding of the Msn2/4 transcriptional complex to their promoters. This complex is activated by Hog1p phophorylation that leads to the migration of Msn2p and Msn4p from the cytosol to the nucleus so that they can trigger the induction of the target genes [52]. This indicates that there is a connection between the CWI pathway (PKC-HOG) and the oxidative stress response in Saccharomyces cerevisiae, in which Ctt1p can act as a bridge. Very recently, Liu et al [53] found that SOD1 gene is involved in tolerance to cell wall damaging agents in yeast, which supports this connection between these pathways.
Figure 3.
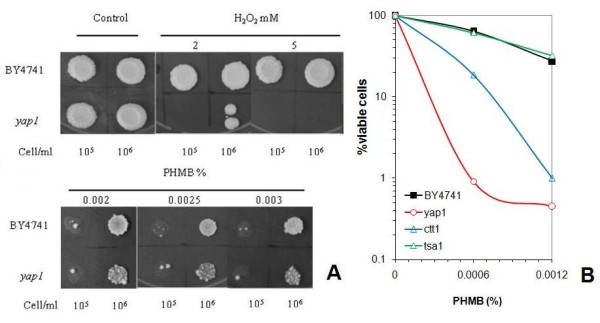
Cytotoxicity assay to PHMB of yeast strains with deletion in oxidative stress response genes. (A) Spot test assay of parental BY4741 and Δyap1 mutants to oxidizing compound H2O2 and to PHMB. (B) Dose-response cell survival in a PHMB assay of ctt1, tsa1 and yap1 mutant strains.
Figure 4.
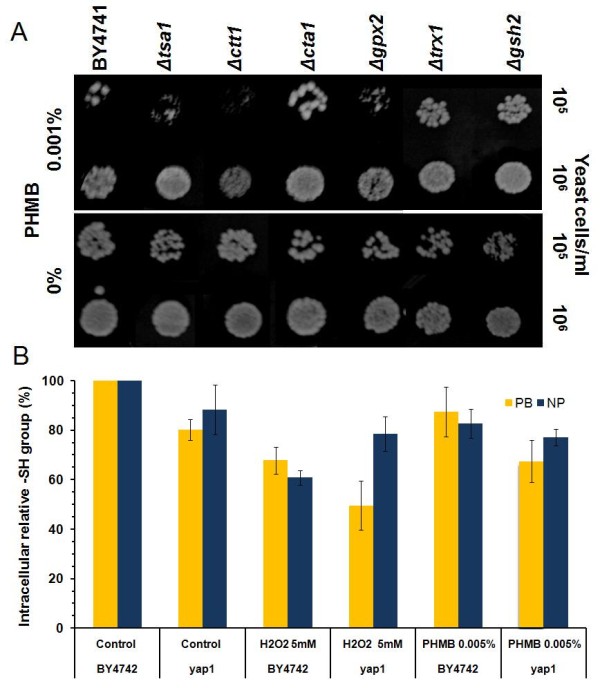
Cytotoxicity assay to PHMB of yeast strains with deletion in oxidative stress response genes. (A) Spot test assay in YPD plates in the presence and absence of PHMB. (B) Determination of relative sulfhydryl groups in soluble proteins (PB) and non-proteins (NP) of molecules in the cell extract of BY4741 parental yeast cells and Δyap1 mutant strains in the presence of 0.005% PHMB and 5 mM H2O2.
It was shown that Yap1p appears to be necessary to activate the SRP1 gene that encodes a cell wall protein [54]. The Δyap1 mutant was reported to be sensitive Congo Red [54], as we also observed (data not shown). Taken together, the evidence points to the involvement of Yap1p in the CWI mechanism.
Yap1p also regulates the expression of CWI genes
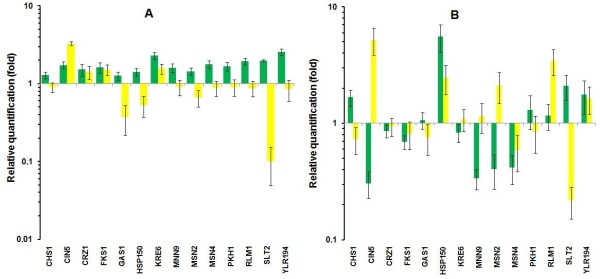
The possible involvement of Yap1p in the regulation of CWI genes was tested by measuring the expression in parental and Δyap1 strains of those genes when they were up-regulated by PHMB in the JP1 strain. The results showed that only KRE6 and YLR194c were slightly up-regulated by PHMB treatment in the parental BY4741 cells (Figure 5A), which could explain the high sensitivity of this laboratory strain compared to JP1 (data not shown). Two results were particularly relevant in the Δyap1 mutant. First, the expression of the SLT2 gene was down-regulated by a factor of ten (Figure 5A), which was not observed even in the PHMB-sensitive industrial PE-2 strain (Figure 1), and the slight expression of YLR194c was removed (Figure 5A). It is also noteworthy that the CIN5 gene showed up-regulation by a factor of 3.5 after the PHMB treatment (Figure 5A), while no change was observed for this gene in either of the industrial strains (Figure 1). It can be concluded that Yap1p also works in response to cell wall damage by controlling the expression of the genes at the lower part of the PKC cascade. It is known that Slt2p phosphorylates and activates the Rlm1p transcription factor, which in turn back-regulates the expression of the SLT2 gene itself [28]. In view of this, Yap1p could be involved in the regulation of both the RLM1 and SLT2 gene expression. This idea was strengthened by further observation which showed that the SLT2 gene was down-regulated in the Δyap1 mutant after heat shock treatment (Figure 5B). The behavior of the RLM1 gene was opposite to its expression, in which a significant over-expression was observed in the Δyap1 mutant after heat shock treatment (Figure 5B). Thus, we suggest that Yap1p should directly co-regulate the expression of the SLT2 gene in response to damage in the cell wall. As a result, this protein should work on the co-regulation of CWI genes. This co-regulation can amplify the signal for CWI gene expression. It was reported that activation of Slt2p by heat shock could occur in a PKC-independent manner [55]. Thus, we suggest that this regulation should occur via Yap1p. The presence of TF binding sites in the promoter region of the CWI genes was identified by an in silico analysis (Additional file 1 and Additional file 2). The fact that we did not observe the presence of the conserved Yap1-Responsive Element (YRE) motif at the SLT2 gene promoter does not impair its regulation by Yap1p. This suggestion is supported by experimental evidence that shows signs of the regulation of STRE-dependent genes by Yap1p, even though this protein does not recognize the STRE motif [49]. All these possible alternatives must be tested further.
Figure 5.
Relative Quantity of yeast genes involved in the cell wall integrity mechanism and the general stress response of parental BY4741 (green column) and Δyap1 (yellow column), strains in response to treatments with 0.005% PHMB (A) and heat shock at 42°C (B).
Conclusion
In this study, the up-regulation of a number of genes required for cell wall polymer synthesis and remodeling has been shown by RT-qPCR upon PHMB-treatment of PHMB-resistant Saccharomyces cerevisiae industrial JPI strain, but not in the PHMB-sensitive PE-2 strain. This finding indicates the involvement of the CWI pathway in the tolerance to the cell wall stress caused by this biocide. In addition, the up-regulation of the genes encoding Msn2/4 transcription factors indicates that the general stress response could also be involved in yeast cell response to PHMB. A cytotoxicity assay of the wild-type BY4741 strain and a series of mutant strains (with single deletion in CWI genes, in HOG1 and YAP1 and a number of other genes related to oxidative stress response) confirmed the involvement of the CWI pathway in the response to PHMB cell wall damage. Hog1p should act as one of the modulators of this response. In addition, the products of YAP1 and CTT1 genes also work on the resistance to PHMB in a way that does not depend on their participation in the oxidative stress response pathway. The fact that SLT2 gene expression was down-regulated after PHMB or heat shock treatments in the Δyap1 mutant indicates the potential regulatory role of Yap1p in the expression of CWI genes.
Methods
Strains and media
Industrial strains of Saccharomyces cerevisiae JP1 and PE-2 were described in advance [56]. The yeast mutant strains were taken from the EUROSCARF collection (Institute of Microbiology of the University of Frankfurt, Germany) or kindly provided by Prof Johan Thevelen (Catholic University of Leuven, Belgium) (Additional file 2). The cells were cultivated and maintained in YPD medium (1% yeast extract, 2% peptone, 2% glucose), with 2% agar for solid medium. The antifungal compound PHMB (polyhexamethyl biguanide) was provided by AEB Bioquímica Latino Americanas S/A (Brazil).
Citotoxicity tests
Yeast cells were pre-cultivated overnight in YPD at 30°C and re-inoculated in the same medium for the exponential growth phase (5 hours) or stationary growth phase (24 hours). The cells were collected by centrifugation, washed in 0.85% NaCl and re-suspended in 100 mM potassium phosphate buffer pH 7 to concentration of 2 × 107 cells/ml.The yeast cells were treated for 30 minutes in the presence of different concentrations of PHMB with agitation at 180 rpm, and adequately diluted and plated on YPD. After five days of incubation at 30°C, the number of viable cells after each treatment was calculated from the percentage of colony forming units using water-treated cells as the reference of 100% cell viability. The results represent the average of biological replicates with technical triplicates for each. Semi-quantitative spot-test assays were conducted with exponentially growing cells at 2 × 107 cells/ml in 100 mM potassium phosphate buffer pH 7. After carrying out appropriate dilutions, aliquots of 5 μl were spotted on YPD plates, supplemented with PHMB in different concentrations. The plates were incubated at 30°C for 72 hours.
Determination of sulfhydryl groups
Protein- bound sulfhydryl (PB-SH) levels were measured in accordance with the method of Sedlak and Lindsay [57], by subtracting the non- protein sulfhydryl (NP-SH) content from the total sulfhydryl (T-SH) content [43]. Cells of the BY4741 and Δ yap1 strains were grown on YPD plates in the presence of 0.5 mM H2O2, 0.005% PHMB and without treatment for 72 hours at 30°C. Approximately 109 cells were collected from each culture. Protein extracts were obtained in 0.02 M EDTA pH 4.7 with the addition of glass beads, followed by centrifugation at 17 900 g for 15 min. The T-SH concentrations were determined by absorption levels at 412 nm after incubating 200 μl aliquots of protein extracts supernatants with 780 μl of 0.2 M Tris pH 8.2 and 20 μl of 5 mM DTNB for 30 min. The (NP-SH) contents were determined in the supernatant, after protein precipitation with 5% trichloroacetic acid (final concentration) by incubating 450 μl supernatant, 900 μl 0.4 M Tris pH 8.9 and 26 μl 5 mM DTNB for 5 min. Absorbance was measured at 412 nm and the protein bound sulfhydryl (PB-SH) content was calculated by subtracting the NP-SH value from the T-SH content. The results are relative to the concentration of these groups in the control cells (100%) that were not exposed to any agent, and represent the average of three separate experiments.
Cell treatments for gene expression analysis
Two independent cultures of each strain of Saccharomyces cerevisiae were grown in YPD until the early stationary phase. The cultures were re-inoculated in fresh medium to an initial DO at 600 nm of 0.5 and split in two flaks for non-treated and PHMB-treated cells at 0.005% (for haploid laboratory strains) and 0.01% (for industrial strains). After incubation for one hour at 30°C with agitation, the cells were collected by centrifugation and immediately frozen in liquid nitrogen and stored at -80°C. For genes response to thermal shock, cells of BY4741 and Δyap1 strains prepared as above and test cell suspensions, were incubated for one hour 42°C, while the reference was incubated at 30°C. Following this, the yeast cells were collected and stored as above.
RNA extraction and cDNA Synthesis
For total RNA extraction, the frozen cells were suspended in 600 μl of AE buffer (50 mM sodium acetate, 10 mM EDTA, adjusted to pH 5.3 with acetic acid), 40 μl of 10% SDS and 600 μl phenol equilibrated to pH 4 (Invitrogen) and homogenized 15 seconds on vortex. Following incubation at 65°C for 10 minutes, with mixing every two minutes, and incubation for five minutes at 4°C, the lysate was centrifuged at 13,000 g for five min at 4°C. The aqueous phase was recovered to a new tube and extracted once with equal volume of phenol-chloroform (5:1) solution pH 5.3 and once with one volume of chloroform. Finally, lithium chloride was added to 2.5 M final concentration and total RNA was precipitated for 30 minutes at -20°C and recovered by centrifugation for 20 min at 13,000 g. The pellet was washed with 70% cold ethanol, dried at room temperature for 10 minutes, suspended in 25 μl of DEPC-treated de-ionized water and stored at -80°C until use. RNA quantification was performed by absorbance analysis at 260 nm (1 OD260 = 40 μg RNA/ml). The purity was analyzed by the absorbance ratio 260 nm/280 nm that ranged from 1.9 to 2.1. The integrity of RNA was verified by agarose gel electrophoresis and used the sharpness and intensity of bands corresponding to 26S and 18S rRNA as parameters. For the cDNA synthesis, one μg of total RNA was used for 40 μl of reverse transcription reaction with the kit ImProm-II ™ Reverse Transcription System Promega II (Promega, USA), following the manufacturer's instructions.
Quantitative RT-PCR (RT-qPCR)
The coding regions of the target genes were recovered from the Yeast Genome Database - SGD http://www.yeastgenome.org. The primer design was undertaken by the on-line Genscript Primer Design in advanced mode http://www.genscript.com/cgi-bin/tools/primer_genscript.cgi using the following parameters: the sizes of the primers between 17 and 25 bases, Tm value of 59°C and size of amplicons between 70 and 110 bp. The primer pairs were analyzed with a Netprimer tool to determine the formation of self-hybrids, duplex, hairpins and loops http://www.premierbiosoft.com/netprimer/netprlaunch/netprlaunch.html and select those with a ranking greater than 90%. The primer pairs were subjected to a matching analysis with coding regions of target genes http://www.blast.org and to PCR in sand http://genome.ucsc.edu/cgi-bin/hgPcr using the genome of S. cerevisiae as a template. The primers were synthesized by IDT Technologies (USA). Tests on Real-Time PCR were conducted in the ABI Prism 7300 (Applied Biosystems, Foster City, CA, USA) detection system, using the SYBR Green PCR Master Mix (Applied Biosystems) kit. The amplification conditions adopted were: initial step at 50°C for 2 min, 95°C for 10 minutes, and 40 cycles of 95°C for 15 sec, and 60 min for 1 min. To determine the degree of contamination by genomic DNA, the PCR reactions were carried out with RNA samples for each condition. The results produced no detectable amplification in any condition. The values of the Cq threshold cycle were given automatically for the independent amplification. Raw Cq values for all the samples were then plotted in Microsoft Excel 2007 worksheets to create a suitable input file for geNorm applet that complied with the User's Guide [58] The first step was to determine the expression stability for the candidate reference genes - PDC1, LEU4, ADK1, ADH3 and EFB1 (Additional file 3). ADK1 and EFB1 have been described as reference genes [59,60] and the others form a part of the laboratory reference genes collection. According to geNorm, at least two of them should be used for data normalization (pairwise variation equal to 0.11) (Additional file 4) and ADK1 and EFB1 were chosen on the basis of the M value (Additional file 4 and Additional file 5).
In silico gene promoter analysis
The sequences corresponding to -1000 to -1 nucleotide position of the target genes, were recovered in FAST format from the SGD database and used to search for TF binding sites and DNA motifs at the YEASTRACT (Yeast Search for Transcriptional Regulators And Consensus Tracking) database at http://www.yeastract.com/index.php[61] following the instructions in the tutorial, and at the Saccharomyces cerevisiae Promoter Database http://rulai.cshl.edu/SCPD.
Authors' contributions
CE carried out the cytotoxicity and molecular genetic studies and drafted the manuscript. RML conducted all the normalization analysis with the aid of geNorm applet for RT-qPCR. MAMJ conceived the study, and participated in its design and coordination as well as giving assistance in drafting the manuscript. All the authors read and approved the final manuscript.
Supplementary Material
Saccharomyces cerevisiae genes tested by RT-qPCR. List of yeast genes selected from the Saccharomyces Genome Database that were tested by RT-qPCR upon cell treatment with PHMB and heat shock. The presence of general stress DNA binding motifs (STRE) and the recognition sequence for the transcription factor Yap1p (YRE) in the promoter region of those genes are shown.
Mutant strains with deletion in genes involved in the Cell Wall Integrity (CWI) mechanism. List of BY4741-derivative mutant strains with deletion in genes involved in the Cell Wall Integrity (CWI) mechanism, including genes belonging to PKC1 and HOG pathways. The presence of general stress DNA binding motifs (STRE) and the recognition sequence for the transcription factor Yap1p (YRE) in the promoter region of those genes are shown.
Relative quantity of reference genes. Relative quantity of reference genes in the presence (test sample) or absence (reference sample) of PHMB and heat shock (HS) used as input data for geNorm analysis.
Selection of reference genes for RT-qPCR. Graphics of Average expression stability values of remaining control genes and Determination of the optimal number of control genes for normalization obtained from geNorm analysis of the reference genes tested.
Normalization factors calculation for RT-qPCR analysis. Summary of the normalization factors calculation of the reference genes ADK1 and EFB1 for the biological replicates (experiments 1 and 2) in the presence (test sample) or absence (reference sample) of PHMB and heat shock (HS) used as input data for geNorm analysis.
Contributor Information
Carolina Elsztein, Email: carolinaelsztein@yahoo.com.ar.
Rodrigo M de Lucena, Email: lucenarm@yahoo.com.br.
Marcos A de Morais, Jr, Email: marcos.morais@pq.cnpq.br.
Acknowledgements
This work was supported by grants from the Brazilian agencies FACEPE and CNPq. CE was awarded a PhD scholarship by CAPES and RML was awarded a PhD scholarship by FACEPE.
References
- Elsztein C, Scavuzzi de Menezes JA, Morais MA. Polyhexamethyl biguanide can eliminate contaminant yeasts from the fuel-ethanol fermentation process. J Ind Microbiol Biotechnol. 2008;35(9):987–973. doi: 10.1007/s10295-008-0371-4. [DOI] [PubMed] [Google Scholar]
- Cazzaniga A, Serralta V, Davis S, Orr R, Eaglstein W, Mertz PM. The effect of an antimicrobial gauze dressing impregnated with 0.2-percent polyhexamethylene biguanide as a barrier to prevent Pseudomonas aeruginosa wound invasion. Wounds. 2002;14(5):169–176. [Google Scholar]
- Payne JD, Kudner DW. A durable antiodor finish for cotton textiles. Text Chem Color. 1996;28:28–30. [Google Scholar]
- Kusnetsov JM, Tulkki AI, Ahonen HE, Martikainen PJ. The Efficacy of three prevention strategies against legionella in cooling water systems. J Appl Microbiol. 1997;82:763–768. doi: 10.1046/j.1365-2672.1997.00151.x. [DOI] [PubMed] [Google Scholar]
- Hiti K, Walochnik J, Haller-Schober EM, Faschinger C, Aspock H. The Viability of Acanthamoeba after exposure to a multipurpose disinfecting contact lens solution and two hydrogen peroxide systems. Br J Ophthalmol. 2002;86:144–146. doi: 10.1136/bjo.86.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin M, Welk A, Bernhardt O, Ruhnau M, Pitten FA, Kocher T, Kramer A. The Effect of a polyhexamethylene biguanide mouthrinse on bacterial counts and plaque. J Clin Periodontol. 2001;28:1121–1126. doi: 10.1034/j.1600-051X.2001.281206.x. [DOI] [PubMed] [Google Scholar]
- Rosin M, Welk A, Kocher T, Majic-Todt A, Kramer A, Pitten FA. The effect of a polyhexamethylene biguanide mouthrinse compared to an essential oil rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque re-growth. J Clin Periodontol. 2002;29:392–399. doi: 10.1034/j.1600-051X.2002.290503.x. [DOI] [PubMed] [Google Scholar]
- Cox NA, Bailey JS, Berrang ME. Bactericidal treatment of hatching eggs. I. Chemical immersion treatments and salmonella. J Appl Poult Res. 1998;7:347–350. [Google Scholar]
- Cox NA, Berrang ME, Buhr RJ, Bailey JS. Bactericidal treatment of hatching eggs. II. Use of chemical disinfectants with vacuum to reduce salmonella. J Appl Poult Res. 1999;8:321–326. [Google Scholar]
- Messick CR, Pendland SL, Moshirfar M, Fiscella RG, Losnedahl KJ, Schriever CA, Schreckenberger PC. In-vitro activity of polyheamethylene biguanide (PHMB) against fungal isolates associated with infective keratitis. J Antimicrob Chemother. 1999;44:297–298. doi: 10.1093/jac/44.2.297. [DOI] [PubMed] [Google Scholar]
- Donoso R, Mura JJ, Lopez M. Acanthamoeba keratitis treated with propamidine and polyhexamethyl biguanide(PHMB) Rev Med Chil. 2002;130:396–401. [PubMed] [Google Scholar]
- Gray JV, Ogas JP, Kamada Y, Stone M, Levin DE, Herskowitz I. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 1997;16:4924–4937. doi: 10.1093/emboj/16.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Madhavan HN. Development and application of an in vitro susceptibility test for Acanthamoeba species isolated from keratitis to polyhexamethylene biguanide and chlorhexidine. Cornea. 2002;21:203–205. doi: 10.1097/00003226-200203000-00016. [DOI] [PubMed] [Google Scholar]
- Allen MJ, White GF, Morby AP. The response of Escherichia coli to exposure to the biocide polyhexamethylene biguanide. Microbiology. 2006;152:989–1000. doi: 10.1099/mic.0.28643-0. [DOI] [PubMed] [Google Scholar]
- O'Malley LP, Collins NA, White GF. Biodegradability of end-groups of the biocide polyhexamethylene biguanide (PHMB) assessed using model compounds. J Ind Microbiol Biotechnol. 2006;33:677–684. doi: 10.1007/s10295-006-0103-6. [DOI] [PubMed] [Google Scholar]
- Broxton P, Woodcick PM, Heatley F, Gilbert P. Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J Appl Bacteriol. 1984;57:115–124. doi: 10.1111/j.1365-2672.1984.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Morby AP, White GF. Cooperativity in the binding of the cationic biocide polyhexamethylene biguanide to nucleic acids. Biochem Biophys Res Commun. 2004;318:397–404. doi: 10.1016/j.bbrc.2004.04.043. [DOI] [PubMed] [Google Scholar]
- Seal D. Treatment of Acanthamoeba keratitis. Expert Rev Anti Infect Ther. 2003;1:205–208. doi: 10.1586/14787210.1.2.205. [DOI] [PubMed] [Google Scholar]
- Khunkitti W, Hann AC, Lloyd D, Furr JR, Russell AD. X-ray microanalysis of chlorine and phosphorus content in biguanide- treated Acanthamoeba castellanii. J Appl Microbiol. 1999;86:453–459. doi: 10.1046/j.1365-2672.1999.00683.x. [DOI] [PubMed] [Google Scholar]
- Beney L, Gervais P. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl Microbiol Biotechnol. 2001;57:34–42. doi: 10.1007/s002530100754. [DOI] [PubMed] [Google Scholar]
- Li L, Ye YR, Pan L, Zhu Y, Zheng SP, Lin Y. The induction of trehalose and glycerol in Saccharomyces cerevisiae in response to various stresses. Biochem Biophys Res Commun. 2009;387:778–783. doi: 10.1016/j.bbrc.2009.07.113. [DOI] [PubMed] [Google Scholar]
- Cabib E, Mol PC, Shaw JA, Choi WJ. Biosynthesis of cell wall and septum during yeast growth. Arch Med Res. 1993;24:301–303. [PubMed] [Google Scholar]
- Lesage G, Bussey H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006;70:317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorsma A, Nobel HH, Riet BB, Bargmann B, Brul S, Hellingwerf KJ, Klis FM. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast. 2004;21:413–427. doi: 10.1002/yea.1109. [DOI] [PubMed] [Google Scholar]
- Yun DJ, Zhao Y, Pardo JM, Narasimhan ML, Damsz B, Lee H, Abad LR, D'Urzo MP, Hasegawa PM, Bressan RA. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc Natl Acad Sci USA. 1997;94:7082–7087. doi: 10.1073/pnas.94.13.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P, Simonen M, Uimari A, Teesalu T, Makarov M. Dual regulation by heat and nutrient stress of the yeast HSP150 gene encoding a secretory glycoprotein. Mol Gen Genet. 1993;239:273–280. doi: 10.1007/BF00281628. [DOI] [PubMed] [Google Scholar]
- Kapteyn JC, Van Egmond P, Sievi E, Van Den Ende H, Makarow M, Klis FM. The contribution of the O-glycosylated protein Pir2p/Hsp150 to the construction of the yeast cell wall in wild-type cells and b1,6-glucan-deficient mutants. Mol Microbiol. 1999;31:1835–1844. doi: 10.1046/j.1365-2958.1999.01320.x. [DOI] [PubMed] [Google Scholar]
- Levin DE. Cell Wall Integrity Signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima H, Yabuki N, Arisawa M, Hamada K, Kitada K. Up-regulation of genes encoding glycosylphosphatidylinositol (GPI)-attached proteins in response to cell wall damage caused by disruption of FKS1 in Saccharomyces cerevisiae. Mol Gen Genet. 2000;264:64–74. doi: 10.1007/s004380000285. [DOI] [PubMed] [Google Scholar]
- Jungmann J, Munro S. Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with alpha-1,6-mannosyltransferase activity. EMBO J. 1998;17:423–34. doi: 10.1093/emboj/17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T, Paravicini G, Payton MA, Bussey H. Characterization of the yeast (l-6)-β-glucan biosynthetic components, Kre6p and Sknlp, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J Cell Biol. 1994;127:567–579. doi: 10.1083/jcb.127.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinian S, Bussey H. B-1, 6-Glucan synthesis in Saccharomyces cerevisiae. Mol Microbiol. 2000;35:477–489. doi: 10.1046/j.1365-2958.2000.01713.x. [DOI] [PubMed] [Google Scholar]
- Jacoby JJ, Nilius SM, Heinisch JJ. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLG1 gene. Mol Gen Genet. 1998;258:148–155. doi: 10.1007/s004380050717. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Durand F, Dagkessamanskaia A, Martin-Yken H, Graille M, Van Tilbeurgh H, Uversky VN, François JM. Structure-function analysis of Knr4/SmiI, a newly member of intrinsically disordered proteins family, indispensable in the absence of a functional PKCI-SLT2 pathway in Saccharomyces cerevisiae. Yeast. 2008;25:563–576. doi: 10.1002/yea.1608. [DOI] [PubMed] [Google Scholar]
- Martin-Yken H, Dagkessamanskaia A, Basmaji F. The interaction of Slt2 MAP kinase with Knr4 is necessary for signalling through the cell wall integrity pathway in Saccharomyces cerevisiae. Mol Microbiol. 2003;49:23–35. doi: 10.1046/j.1365-2958.2003.03541.x. [DOI] [PubMed] [Google Scholar]
- Hahn JS, Thiele DJ. Regulation of the Saccharomyces cerevisiae Slt2 Kinase Pathway by the Stress-inducible Sdp1 Dual Specificity Phosphatase. J Biol Chem. 2002;277:21278–21284. doi: 10.1074/jbc.M202557200. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Peña JM, García R, Nombela C, Arroyo J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: a yeast dialogue between MAPK routes. Yeast. 2010;27:495–502. doi: 10.1002/yea.1792. [DOI] [PubMed] [Google Scholar]
- Thorsen M, Di Y, Tängemo C, Morillas M, Ahmadpour D, Van der Does C, Wagner A, Johansson E, Boman J, Posas F, Wysocki R, Tamás MJ. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol Biol Cell. 2006;17:4400–4410. doi: 10.1091/mbc.E06-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande V, Del Vescovo V, Militti C, Mangiapelo E, Frontali L, Negri R, Bianchi MM. Cesium chloride sensing and signaling in Saccharomyces cerevisiae: an interplay among the HOG and CWI MAPK pathways and the transcription factor Yaf9. FEMS Yeast Res. 2009;9:400–410. doi: 10.1111/j.1567-1364.2009.00486.x. [DOI] [PubMed] [Google Scholar]
- García R, Rodríguez-Peña JM, Bermejo C, Nombela C, Arroyo J. The HOG and CWI pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in S. cerevisiae. J Biol Chem. 2009;284:10901–10911. doi: 10.1074/jbc.M808693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero E, Ros J, Belli G, Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim Biophys Acta. 2008;1780:1217–1235. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Demasi AP, Pereira GA, Netto LES. Yeast oxidative stress response Influences of cytosolic thioredoxin peroxidase I and of the mitochondrial functional state. FEBS J. 2006;273:805–816. doi: 10.1111/j.1742-4658.2006.05116.x. [DOI] [PubMed] [Google Scholar]
- Toone WM, Jones N. Stress-activated signaling pathways in yeast. Genes Cells. 1998;3:485–498. doi: 10.1046/j.1365-2443.1998.00211.x. [DOI] [PubMed] [Google Scholar]
- Winderickx J, de Winde JH, Crauwels M, Hino A, Hohmann S, Van Dijck P, Thevelein JM. Regulation of genes encoding subunits of the trehalose synthase complex inSaccharomyces cerevisiae: novel variations of STRE-mediated transcription control? Mol Gen Genet. 1996;252:470–482. doi: 10.1007/BF02173013. [DOI] [PubMed] [Google Scholar]
- Alarco AM, Balan I, Talibi D, Mainville N, Raymond M. Ap-1 mediated multidrug resistance in Saccharmoyces cerevisiae requires FLR1 enconding a transporter of the major facilitator superfamily. J Biol Chem. 1997;272:19304–19313. doi: 10.1074/jbc.272.31.19304. [DOI] [PubMed] [Google Scholar]
- Nguyen DT, Alarco AM, Raymond M. Multiple Yap1p-binding sites mediate induction of the yeast major facilitator FLR1 gene in response to drugs, oxidants, and alkylating agents. J Biol Chem. 2001;276:1138–1145. doi: 10.1074/jbc.M008377200. [DOI] [PubMed] [Google Scholar]
- Wu A, Wemmie JA, Edgington NP, Goebl M, Guevara JL, Moye-Rowley WS. Yeast bZip proteins mediate pleiotropic drug and metal resistance. J Biol Chem. 1993;268:18850–19958. [PubMed] [Google Scholar]
- Gounalaki N, Thireos G. Yap1p, a yeast transcriptional activator that mediates multidrug resistance, regulates the metabolic stress response. EMBO J. 1994;13:4036–4041. doi: 10.1002/j.1460-2075.1994.tb06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Spector D, Godon C, Labarre J, Toledano MB. A new antioxidant with alkyl hydroperoxide defense properties in yeast. J Biol Chem. 1999;274:4537–4544. doi: 10.1074/jbc.274.8.4537. [DOI] [PubMed] [Google Scholar]
- Vilella F, Herrero E, Torres J, Torre-Ruiz MA. Pkc1 and the upstream element of the Cell Integrity Pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J Biol Chem. 2005;280:9149–9159. doi: 10.1074/jbc.M411062200. [DOI] [PubMed] [Google Scholar]
- Ding J, Huang X, Zhang L, Zhao N, Yang D, Zhang. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2009;85:253–263. doi: 10.1007/s00253-009-2223-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang X, Zhang Z. Cu,Zn-superoxide dismutase is required for cell wall structure and for tolerance to cell wall-perturbing agents in Saccharomyces cerevisiae. FEBS Lett. 2010;584:1245–1250. doi: 10.1016/j.febslet.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Bourdineaud JP, De Sampaio G, Lauquin GJ. A Rox1-independent hypoxic pathway in yeast. Antagonistic action of the repressor Ord1 and activator Yap1 for hypoxic expression of the SRP1/TIR1 gene. Mol Microbiol. 2000;38:879–90. doi: 10.1046/j.1365-2958.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- Harrison JC, Zyla TR, Bardes ESG. Stress-specific Activation Mechanisms for the "Cell Integrity" MAPK Pathway. J Biol Chem. 2004;279(4):2616–2622. doi: 10.1074/jbc.M306110200. [DOI] [PubMed] [Google Scholar]
- Silva-Filho EA, Melo HF, Antunes DF, Santos SKB, Resende AM, Simões DA, Morais MA Jr. Isolation by genetic and physiological characteristics of a fuel-ethanol fermentative Saccharomyces cerevisiae strain with potential for genetic manipulation. J Ind Microbiol Biotechnol. 2005;32:481–486. doi: 10.1007/s10295-005-0027-6. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein- bound and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):research0034.1–research0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermayr M, Oechsner U, Gregor K, Schroth GP, Bandlow W. Transcription initiation in vivo without classical transactivators: DNA kinks ¯anking the corepromoter of the housekeeping yeast adenylate kinase gene, AKY2, position nucleosomes and constitutively activate transcription. Nucl Acids Res. 2002;30(19):4199–4207. doi: 10.1093/nar/gkf551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield DA, Westwater C, Paulling EE, Nicholas PJ, Balish E. Detection of Candida albicans mRNA from Formalin-Fixed, Paraffin-Embedded Mouse Tissues by Nested Reverse Transcription-PCR. J Clin Microbiol. 2003;41(2):831–834. doi: 10.1128/JCM.41.2.831-834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro PT, Mendes N, Teixeira MC, D'Orey S, Tenreiro S, Mira N, Pais H, Francisco AP, Carvalho AM, Lourenço A, Sá-Correia I, Oliveira AL, Freitas AT. YEASTRACT-DISCOVERER: new tools to improve the analysis of transcriptional regulatory associations in Saccharomyces cerevisiae. Nucl Acids Res. 2008;36:132–136. doi: 10.1093/nar/gkn600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Saccharomyces cerevisiae genes tested by RT-qPCR. List of yeast genes selected from the Saccharomyces Genome Database that were tested by RT-qPCR upon cell treatment with PHMB and heat shock. The presence of general stress DNA binding motifs (STRE) and the recognition sequence for the transcription factor Yap1p (YRE) in the promoter region of those genes are shown.
Mutant strains with deletion in genes involved in the Cell Wall Integrity (CWI) mechanism. List of BY4741-derivative mutant strains with deletion in genes involved in the Cell Wall Integrity (CWI) mechanism, including genes belonging to PKC1 and HOG pathways. The presence of general stress DNA binding motifs (STRE) and the recognition sequence for the transcription factor Yap1p (YRE) in the promoter region of those genes are shown.
Relative quantity of reference genes. Relative quantity of reference genes in the presence (test sample) or absence (reference sample) of PHMB and heat shock (HS) used as input data for geNorm analysis.
Selection of reference genes for RT-qPCR. Graphics of Average expression stability values of remaining control genes and Determination of the optimal number of control genes for normalization obtained from geNorm analysis of the reference genes tested.
Normalization factors calculation for RT-qPCR analysis. Summary of the normalization factors calculation of the reference genes ADK1 and EFB1 for the biological replicates (experiments 1 and 2) in the presence (test sample) or absence (reference sample) of PHMB and heat shock (HS) used as input data for geNorm analysis.