Summary
When embedded in adjacent distractors, a target becomes more difficult to perceive. The neural mechanism for this ubiquitous visual crowding effect remains unresolved [1, 2]. Stimuli presented on opposite sides of the vertical meridian initially project to different hemispheres, whereas stimuli with the same spatial distance but presented to one side of the vertical meridian project to the same hemisphere. Dissociation between visual spatial distance and cortical distance can also be found in V2 and V3 (quadrant representations of the visual hemifield) along the horizontal meridian. In the current study, we observed a strong crowding effect from spatially adjacent distractors with either Gabor or letter targets presented near the vertical or horizontal meridian. Interestingly, for a target presented near the vertical meridian, a distractor from the same side of the meridian (cortically near) had a significantly stronger crowding effect compared with an equidistant distractor presented on the opposite side (cortically remote). No such meridian modulation was observed across the horizontal meridian. These results constrain the cortical locus of the crowding effect to a stage in which left and right visual spaces are represented discontinuously but the upper and lower visual fields are represented continuously, likely beyond the early retinotopic areas.
Results and Discussion
Functional properties of crowding have been extensively investigated, including the spatial extent of crowding, spatial positional asymmetries of crowding, and its distinction from ordinary masking [1, 2]. Crowding is a cortical phenomenon because it occurs even when the target and distractors are presented dichoptically [3–7] and the orientation-specific adaptation effect also largely survives crowding [8]. Although the exact neuronal mechanism of the crowding effect remains unresolved, several theories are available, including that crowding occurs due to the pooling of the target and distractors by the large peripheral receptive fields of cortical area V1 [9]; that crowding occurs at a stage beyond feature detection in which abnormal integration of information from both the target and distractors impairs the discriminability of the target but preserves its visibility [10]; and that the crowding effect is primarily due to the poor resolution of spatial attention [8, 11]. For all of these hypotheses, it is critically important to localize the neural site of the crowding effect in order to understand this phenomenon.
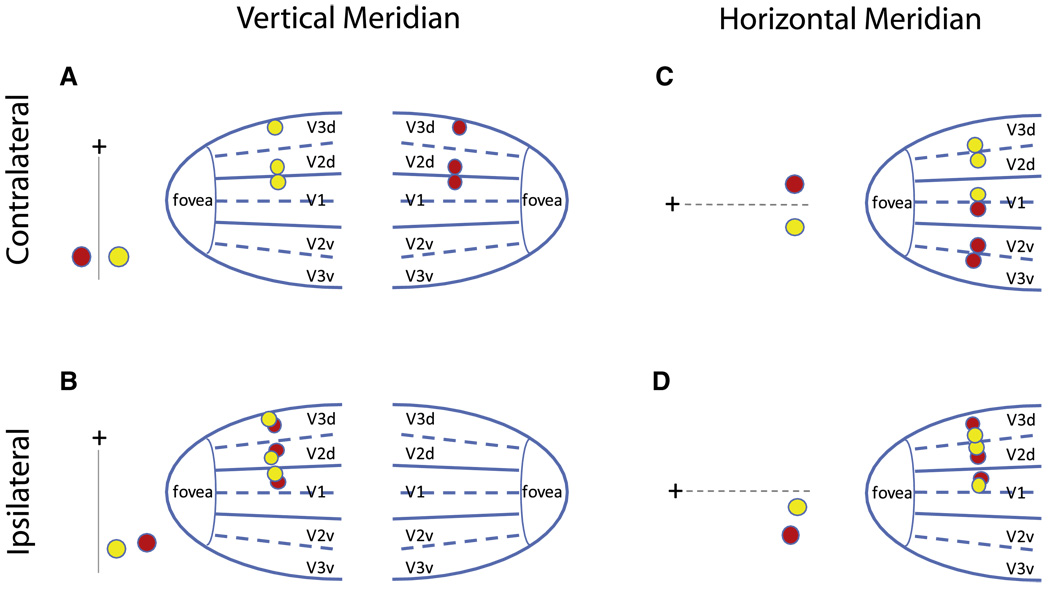
In the current study, in order to investigate the neural site of the crowding effect, we took advantage of the dissociation between visual spatial distance and cortical distance. Visual space is represented retinotopically in visual cortical areas but with a small number of discontinuities. There is the division of the hemifield representation between the two hemispheres—most early visual cortices in each hemisphere represent the contralateral visual hemifield. Although each hemifield is represented continuously in V1, in V2 and V3, the upper and lower visual quadrants are further divided and separately represented in the ventral and dorsal projections [12]. Thus, two visual stimuli presented next to each other on opposite sides of the vertical meridian (VM) project to the left and right primary and other early visual cortices, cortically far from each other (Figure 1A); whereas, two stimuli presented next to each other on the same side of the VM project to the same contralateral visual cortex, cortically adjacent to each other (Figure 1B). Two stimuli presented near the horizontal meridian (HM) are represented close to each other in V1 but could be represented far from each other in V2 and V3 if they are on opposite sides of the HM (Figures 1C–1D; see also Supplemental Experimental Procedures available online).
Figure 1. Schematic Representations of the Dissociation between Visual Spatial Distance and Cortical Distance.
(A) Two visual stimuli (shown as a red and a yellow dot) presented next to each other on opposite sides of the vertical meridian (VM) project to the left and right primary and other early visual cortices, cortically far from each other.
(B) Two stimuli presented next to each other on the same side of the VM project to the same contralateral visual cortex, cortically adjacent to each other.
(C and D) Two stimuli presented near the horizontal meridian (HM) are represented close to each other in V1, but could be represented either far from (C) or close to (D) each other in V2 and V3, depending upon whether they are on the opposite or the same side of the HM.
Target-Distractor Interaction across the Vertical Meridian
We manipulated the cortical distance between the target and distractor by presenting them either on the same side or on opposite sides of the VM in the lower peripheral visual field while maintaining an identical spatial distance between the target and distractor in two conditions, and we measured observers’ performance on target detection anddiscrimination.
Target Detection Not Affected by Distractors
Crowding typically affects target discrimination rather than detection [10]. By using a Gabor patch as the target and a plaid of identical size as the distractor, we measured the contrast threshold for detecting the target near the VM under three different conditions: target alone, with an ipsilateral distractor, or with a contralateral distractor (see Experimental Procedures for details). Results showed that the contrast thresholds for these three conditions were 17.7% ± 1.7%, 19.9% ± 2.3%, and 19.1 ± 2.8% (mean 6 SEM), without significant difference between them [F(2,14) = 1.77, p > 0.2] (Figure 2A). The observation that target detection was not impaired gave us confidence in the subsequent experiments to attribute the potential detrimental effect from distractors on target discrimination to crowding rather than the reduced target visibility.
Figure 2. Target-Distractor Interaction across the Vertical Meridian.
(A) Contrast threshold for target detection under three conditions (target alone, with a contralateral distractor, with an ipsilateral distractor). There was no significant difference across conditions on target detection.
(B) Percentage of correct responses for orientation discrimination under the same three conditions. Both ipsilateral and contralateral distractors induced significant crowding effects compared with the target alone condition. Further, the ipsilateral distractor induced a significantly stronger crowding effect than the contralateral distractor.
(C) Results from the letter identification task were consistent with those from the orientation discrimination experiment.
Error bars represent ± SEM. *p < 0.05, **p < 0.01.
Stronger Crowding with Ipsilateral Than with Contralateral Distractor Near the Vertical Meridian Orientation Discrimination
Following the detection experiment, the Gabor target and the plaid distractor with the same parameters and spatial arrangements were used in the orientation discrimination experiment. Observers were asked to identify the target orientation (±5°) under the same three conditions (target alone, distractor ipsilateral, or distractor contralateral to the target relative to the VM). The percentage of correct responses served as the dependant variable. Results showed a significant main effect for the test conditions [F(2,14) = 46.3, p < 0.001] (Figure 2B). The target alone condition had the best performance (93.4% ± 0.8%). Both ipsilateral and contralateral distractors induced highly significant crowding effects (82.8% ± 1.8% for the ipsilateral condition and 88.9% ± 1.4% for the contralateral condition; p < 0.01 for both conditions compared with the target alone condition). Interestingly, there was also a significant difference between the two crowded conditions (ipsilateral versus contralateral, p < 0.002). Observers performed better when the distractor was presented on the opposite side of the VM (i.e., a different hemifield) as the target than when the distractor was presented on the same side of the VM. In other words, an ipsilateral distractor (cortically adjacent) had a significantly stronger crowding effect on the target than did a contralateral distractor (cortically remote). The pattern of results was highly consistent across all individual subjects.
Letter Identification
Because orientation discrimination relies on the fine discrimination of a single feature, it is likely related more with the early visual cortex. It is possible that the observed meridian effect is specific to this stimulus and task and may not be a general property of crowding. Here, we examined the VM effect on crowding with more complex visual stimuli and a more difficult task, i.e., identification of letters. The experimental design was essentially the same as the orientation discrimination experiment except that the target and distractor were letters. Similar to the orientation discrimination task, there was a significant main effect for these test conditions [F(2,14) = 39.7, p < 0.001] (Figure 2C). The target alone condition had the best performance (84.0% ± 2.3%), and both ipsilateral and contralateral distractors induced significant crowding effects (67.4% ± 3.0% for ipsilateral condition and 74.4% ± 1.7% for contralateral condition; p < 0.005 for both conditions). Importantly, the difference between the ipsilateral and contralateral conditions was also significant (p < 0.03). Distractors presented in the same hemifield as the target induced a more severe crowding effect than when presented in the opposite hemifield. Consistent with the orientation discrimination experiment, results from the letter identification task further demonstrated that hemispheric projection can modulate the crowding effect and that the VM effect is likely a general property of crowding. The findings from these experiments constrain the cortical locus of crowding to visual areas where left and right visual fields are represented distinctly but are not completely independent from each other. More implications of these results will be considered in the Concluding Remarks.
Crowding Is Insensitive to the Horizontal Meridian
In visual areas V2 and V3, the upper and lower visual quadrants are represented separately in the ventral and dorsal projections. A spatially adjacent target and distractor presented near the HM can be mapped to either close or distant sites in V2 and V3 depending upon whether the two stimuli are on the same or opposite sides of the HM (Figures 1C–1D). It should be noted that, in either case (whether they are on the opposite or the same side of the HM), the cortical representations of the two items near the HM would be continuous in V1 and other visual areas (e.g., V4) that contain more-than-quarter or hemifield representation.
The experimental design of the HM experiments was essentially the same as the VM experiments except that the spatial locations of the target and distractor were arranged relative to the HM. The HM effect was tested on all three tasks: target detection, orientation discrimination, and letter identification (see Experimental Procedures for details). For the target detection experiment, contrast threshold was not significantly different when the distractor was added on either side (15.6% ± 1.3% for ipsilateral distractor and 16.1% ± 1.4% for contralateral distractor) compared with the target alone condition (14.0% ± 1.3%, p > 0.1 for both conditions) (Figure 3A). There was also no significant difference between the contralateral and ipsilateral conditions (p > 0.9). In both the orientation discrimination (Figure 3B) and letter identification (Figure 3C) experiments, compared to the target alone (uncrowded) condition (93.3% ± 1.0% for orientation discrimination and 92.0% ± 1.4% for letter identification), both the ipsilateral distractor (86.8% ± 2.2%, p < 0.03 for orientation discrimination and 78.0% ± 3.3%, p < 0.01 for letter identification) and the contralateral distractor (87. 5% ± 2.0%, p < 0.04 for orientation discrimination and 80.5% ± 2.8%, p < 0.01 for letter identification) induced significant crowding effects. However, there was no significant difference between the two crowded conditions (ipsilateral versus contralateral) for either the orientation discrimination or letter identification experiments (p > 0.9).
Figure 3. Target-Distractor Interaction across the Horizontal Meridian.
(A) Contrast threshold for target detection under three conditions (target alone, with a contralateral distractor, with an ipsilateral distractor). There was no significant difference across conditions on target detection.
(B and C) Results from orientation discrimination (B) and letter identification (C) are similar. Both ipsilateral and contralateral distractors induced significant crowding effects compared with the target alone condition, but there was no significant difference between the ipsilateral and contralateral conditions.
Error bars represent 6 SEM. *p < 0.05, **p < 0.01.
An additional analysis showed that the interaction between the crowding effect of distractor location (ipsilateral versus contralateral) and the meridian position (HM versus VM) was significant for both tasks [orientation discrimination: F(1,7) = 12.2, p < 0.01; letter identification: F(1,7) = 5.66, p < 0.05]. Thus, results from the experiments with a target placed near the HM provide a clear contrast to the pattern of results when a target is placed near the VM. There was no difference, whether the distractor was on the same or different side as the target relative to the HM. In other words, whether the distractor was mapped to the adjacent or distant sites relative to the target in V2 and V3 did not modulate the crowding effect.
Concluding Remarks
With the distractor placed at an equal distance from the target but on the same or opposite side of the major meridians (VM and HM), crowding was observed for both ipsilateral and contralateral distractors, but it was stronger for ipsilateral than contralateral distractor relative to the VM and indifferent to the HM. A significant interaction between the distractor location (ipsilateral versus contralateral) and meridian position (VM versus HM) for both the orientation and letter tasks further supports the difference between the VM and HM in their modulation of the crowding effect. These results, combined with the distinct cortical representations of two stimuli across either the VM or HM as confirmed by the supporting imaging experiment (see Supplemental Experimental Procedures), may constrain the neural site(s) of the crowding effect to a cortical area(s) where the left and right as well as the upper and lower visual spaces are brought together, with the left and right hemifield representations maintaining some degree of discontinuity but with the upper and lower visual fields represented continuously. Similar logic has been used in several studies to infer the underlying neuronal mechanisms of certain visual phenomena. For example, perceptual completion is much poorer when illusory contours cross the VM than when they reside entirely within the left or right visual hemifield [13]. Twice as many targets can be successfully tracked by attention when they are divided and presented across the left and right hemifields compared with all presented within the same hemifield [14]. Because this hemifield-level effect on attentional selection can also be extended to a quadrant-level effect, the underlying mechanism was presumably linked to extrastriate areas V2 and V3 [15]. Although it is difficult to quantify the cortical distance across the VM versus across the HM, the key point relevant to our study is that, in both cases, in some brain areas, the cortical distance between the target and contralateral distractors (across the meridians) is orders of magnitude larger than that between the target and ipsilateral distractors (on the same side of the meridians).
Results obtained from the current study suggest that crowding occurs where the left and right visual hemifields are represented distinctly (but not independently of each other) and the upper and lower visual fields are represented continuously. All of the early visual areas meet the criterion of a split representation of the left and right visual hemifields. However, V2 and V3, with split representation of the upper and lower visual quadrants, do not meet the criterion of continuous upper-lower field representation. This leaves the primary visual cortex (V1) and/or visual areas beyond V2 and V3 to be candidates for the neural substrate of crowding. However, both existing evidence and results from the current study suggest that crowding likely occurs beyond V1. For example, orientation-specific adaptation is largely preserved under crowding, implying that the influence of crowding on spatial resolution may take place beyond the primary visual cortex [8]. Although crowding does affect the strength of orientation adaptation [16], it is not clear how much of the effect could be attributed to attention. Additionally, several functional properties of crowding, such as the large extent and substantial anisotropy of crowding, are also inconsistent with V1 as the cortical locus of crowding [1]. Indeed, a substantial crowding effect was seen in the current study with the contralateral distractor across the VM, further supporting that V1 is unlikely to be the primary site for crowding.
Beyond V2 and V3, which have split quadrant representations, the likely candidate for the neural substrate of crowding could be V4 and possibly even the LOC. Although the retinotopic representation of V4 in humans is still under debate, V4 (or hV4) is likely to be the candidate place where crowding takes place. For primates, V4 is divided into dorsal and ventral parts, with the ventral part representing most of the hemifield, including the HM [17]. For humans, the corresponding visual area hV4 in each hemisphere is suggested to represent more than a quarterfield of visual space [18, 19]. Furthermore, it was suggested that, in addition to hV4 representing a larger range of angular span than ventral V4 in the macaque, a portion of lateral occipital cortex should be grouped with hV4, completing the representation of the whole hemifield [20]. For both scenarios, crowding could be modulated by the VM, but not the HM, which leaves hV4 as a reasonable candidate for the cortical locus of crowding. Indeed, there is additional evidence that makes hV4 an appealing locus of crowding as well, including the correlation between the V4 receptive field and the spatial extent and the marked anisotropy of crowding [21]. Crowding between first- and second-order targets and distractors [22] is consistent with V4 being the neural site as well [23, 24]. Even though hV4 seems to be the favorable candidate for the cortical locus of crowding, our results are also compatible with crowding occurring at other or multiple visual areas that meet the split left-right but continuous upper-lower representations of visual space.
Although we observed that the crowding effect was stronger for the ipsilateral distractor than for the contralateral distractor relative to the VM, it also needs to be emphasized that the contralateral distractor did induce severe crowding. Is this crosshemispheric interaction occurring through callosal connections? A study in a patient with a posterior callosectomy [25] showed that adding distractors to the contralateral visual field impaired the performance of both the patient and normal controls, suggesting that interhemispheric crowding occurs despite the lack of direct posterior callosal connections. Thus, the observed interhemispheric interaction is more likely to occur at higher visual areas. Indeed, recent studies showing the influence of configuration on crowding [26] and the holistic face crowding effects [27] also point to the involvement of higher-level visual areas. Our observation that contralateral distractors generate a strong crowding effect further supports this view.
In summary, taking advantage of the distinct properties of cortical representation of the visual space, the current study shows that crowding occurs with distractors projecting to either the same or opposite hemisphere as the target and that crowding also occurs with distractors projecting to either the same or different quadrant representations as the target. In addition, a significant hemispheric modulation of the crowding effect could be seen across multiple tasks, but there was no modulation of the crowding effect due to quadrant projection. These results provide strong constraints on the cortical sites where visual crowding could take place. They suggest that crowding occurs beyond V2 and V3 and further point to area hV4 as a likely candidate for the neural correlate of crowding, with potential involvement of higher-level visual areas as well.
Experimental Procedures
Participants
Eight subjects (three females) were recruited in the psychophysical experiments. All participants had normal or corrected-to-normal vision. Participants gave written informed consent in accordance with procedures and protocols approved by the human subjects review committee of the University of Minnesota.
Stimuli and Procedure
Stimuli were generated by using software MATLAB (Mathworks, Inc.) together with the Psychophysics Toolbox extensions [28, 29]. For psychophysical experiments, the visual stimuli were presented on a 22 inch, gamma-corrected Gateway monitor (1280 × 1024 at 85 Hz). The target stimulus that was used for both the detection and discrimination tasks was a 3 cycle/degree Gabor patch, spanning a visual angle of 1°. Target contrast was adjusted and fixed for each subject to avoid both ceiling and floor effects in the orientation discrimination experiment. A full-contrast plaid made of two orthogonally aligned Gabor patches of the same spatial frequency and space constant as the target was used as distractor. For the letter identification task, sloan letters were used as the stimuli (http://psych.nyu.edu/pelli/software.html). The target and distractor were randomly sampled from the same alphabet, excluding the possibility of identical target and distractor. Each letter was 1° in both height and width. The target letters were dark gray, and the distractors were black, presented on a mean gray background (34.6 cd/m2). The contrast of the distractors was always kept at 100%, and the contrast of the target was adjusted for each subject to avoid both ceiling and floor effects. A small red cross was always presented as a fixation point. The target and distractor stimuli in all three tasks (target detection, orientation discrimination, and letter identification) were located along an imaginary isoeccentricity circle 15° from fixation. For the VM experiments, the target was either on the right or left side of the VM, with its center being 1° away from the meridian in the lower visual field. For the HM experiments, the target was either above or below the HM, with its center being 1° away from the meridian in the right visual field. The distractor was either on the same or opposite side of the meridian (VM or HM) as the target, with center-to-center distance between the target and distractor being 2°. In other words, the distractor could be presented in the same or different hemifield as the target in the VM experiments or in the same or different quadrant as the target in the HM experiments (schematically depicted in Figure 1).
Observers sat 50 cm away from the monitor and viewed the display binocularly. A chin rest was used to stabilize head position. The VM and HM effects were measured separately, each with two target positions (right or left relative to the VM and upper or lower relative to the HM). There were three conditions (target alone, distractor ipsilateral, and distractor contralateral to target) for each target location in all experiments. The contrast threshold for target (Gabor) detection was measured by the Quest staircase procedure implemented in the Psychophysics Toolbox by using a performance criterion of 82% with β = 3.5. Each run had 50 trials, and each experimental condition was measured four times on two separate days. For each trial, after a 1000 ms fixation presentation, there were two temporal intervals indicated by two auditory cues. Each interval lasted 200 ms with a 500 ms gap between them in which only the fixation was displayed. The target was presented in either the first or second interval, and subjects were asked to press one of two buttons to indicate in which interval they thought the target was displayed. The target could be tilted 5° from the horizontal or vertical direction either clockwise or counterclockwise in each trial, exactly the same as in the orientation discrimination experiment. Each subject performed 2400 trials for this task (1200 trials each for the VM experiment and HM experiment).
For the orientation discrimination task, the contrast level of the target was adjusted and fixed for each subject. In each trial, the central fixation was displayed for 1000 ms, followed by test stimuli for 200 ms, and the subjects were asked to discriminate which direction the target was tilted (±5° from the horizontal direction for the VM experiment and ±5° from the vertical direction for the HM experiment). Two possible target locations (left or right relative to the VM and upper or lower relative to the HM) were examined in separate sessions. Each session was measured twice on different days. Each subject completed 1920 trials for this task (960 trials for the VM experiment and 960 trials for the HM experiment). The same procedure applied to the letter identification experiment except that the target and distractor stimuli were letters. After the presentation of the test stimuli, a uniform gray screen was displayed, with all possible letters at full contrast aligned horizontally in the center of the screen. Subjects were asked to identify which letter they thought was the target by pointing and clicking the specific letter with a mouse-controlled cursor. In order to ensure that the subject’s gaze came back to the fixation point before the beginning of the next trial, the duration of fixation display following the response was slightly increased to 1200 ms. Each subject performed 960 trials for this task (480 trials each for the VM and the HM experiment).
Observers were required to maintain fixation on the fixation point throughout all experiments. For the letter identification task, observers had to maintain fixation during the fixation period as well as during the stimulus presentation period but were free to move their eyes during the response period. An auditory feedback was given if the response was incorrect. The order of the conditions within each session and for different sessions was counterbalanced both within and across subjects.
Data Analysis
We combined the data from the two target locations (left and right of the VM and upper and lower of the HM) according to whether the distractor was on the same or different side of the meridians as the target. For target detection, the contrast threshold was the dependent variable. Data calculated by the Quest procedure to exceed 95% confidence interval from the mean standard deviation of each individual observer were excluded. The contrast threshold was then averaged for each condition. For orientation discrimination and letter identification tasks, percentage of correct responses was the dependent variable. Repeated measures analyses of variance (ANOVAs) were carried out with test condition (target alone, distractor ipsilateral, and distractor contralateral to target) as a within-subject factor, and Bonferroni correction for multiple comparisons was used for all post hoc analyses. Furthermore, another repeated measures ANOVA was carried out to test the interaction between the crowding effect of distractor location (ipsilateral versus contralateral, with target alone condition as the baseline) and the meridian position (HM versus VM).
Supplementary Material
Acknowledgments
This research was supported by the James S. McDonnell Foundation, the National Institutes of Health Grant R01 EY02934, the National Science Foundation, the National Basic Research Program of China 2007CB512204, and the National Natural Science Foundation of China 30571996. The 3T scanner at the University of Minnesota is supported by Biotechnology Research Resource (BTRR) grant P41 008079 and by the Mental Illness and Neuroscience Discovery (MIND) Institute.
Footnotes
Supplemental Data
Supplemental Data include a Supplemental Experiment, Supplemental Experimental Procedures, and two figures and can be found with this article online at http://www.currentbiology.com/supplemental/S0960-9822(08)01625-4.
References
- 1.Levi DM. Crowding–an essential bottleneck for object recognition: A mini-review. Vision Res. 2008;48:635–654. doi: 10.1016/j.visres.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelli DG, Tillman KA. The uncrowded window of object recognition. Nat. Neurosci. 2008;11:1129–1135. doi: 10.1038/nn.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flom MC, Heath GG, Takahashi E. Contour interaction and visual resolution: Contralateral effects. Science. 1963;142:979–980. doi: 10.1126/science.142.3594.979. [DOI] [PubMed] [Google Scholar]
- 4.Kooi FL, Toet A, Tripathy SP, Levi DM. The effect of similarity and duration on spatial interaction in peripheral vision. Spat. Vis. 1994;8:255–279. doi: 10.1163/156856894x00350. [DOI] [PubMed] [Google Scholar]
- 5.Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Res. 1985;25:963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- 6.Westheimer G, Hauske G. Temporal and spatial interference with vernier acuity. Vision Res. 1975;15:1137–1141. doi: 10.1016/0042-6989(75)90012-7. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy SP, Levi DM. Long-range dichoptic interactions in the human visual cortex in the region corresponding to the blind spot. Vision Res. 1994;34:1127–1138. doi: 10.1016/0042-6989(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 8.He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- 9.Levi DM, Waugh SJ. Spatial scale shifts in peripheral vernier acuity. Vision Res. 1994;34:2215–2238. doi: 10.1016/0042-6989(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 10.Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: Distinguishing feature integration from detection. J. Vis. 2004;4:1136–1169. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- 11.Intriligator J, Cavanagh P. The spatial resolution of visual attention. Cognit. Psychol. 2001;43:171–216. doi: 10.1006/cogp.2001.0755. [DOI] [PubMed] [Google Scholar]
- 12.Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 13.Pillow J, Rubin N. Perceptual completion across the vertical meridian and the role of early visual cortex. Neuron. 2002;33:805–813. doi: 10.1016/s0896-6273(02)00605-0. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol. Sci. 2005;16:637–643. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 15.Carlson TA, Alvarez GA, Cavanagh P. Quadrantic deficit reveals anatomical constraints on selection. Proc. Natl. Acad. Sci. USA. 2007;104:13496–13500. doi: 10.1073/pnas.0702685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proc. Natl. Acad. Sci. USA. 2006;103:4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattass R, Sousa AP, Gross CG. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wade AR, Brewer AA, Rieger JW, Wandell BA. Functional measurements of human ventral occipital cortex: Retinotopy and colour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:963–973. doi: 10.1098/rstb.2002.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer AA, Liu J, Wade AR, Wandell BA. Visual field maps and stimulus selectivity in human ventral occipital cortex. Nat. Neurosci. 2005;8:1102–1109. doi: 10.1038/nn1507. [DOI] [PubMed] [Google Scholar]
- 20.Hansen KA, Kay KN, Gallant JL. Topographic organization in and near human visual area V4. J. Neurosci. 2007;27:11896–11911. doi: 10.1523/JNEUROSCI.2991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinon MC, Gattass R, Sousa AP. Area V4 in Cebus monkey: Extent and visuotopic organization. Cereb. Cortex. 1998;8:685–701. doi: 10.1093/cercor/8.8.685. [DOI] [PubMed] [Google Scholar]
- 22.Chung ST, Li RW, Levi DM. Crowding between first- and second-order letter stimuli in normal foveal and peripheral vision. J. Vis. 2007;7:10.1–10.13. doi: 10.1167/7.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrera VP, Nealey TA, Maunsell JH. Mixed parvocellular and magnocellular geniculate signals in visual area V4. Nature. 1992;358:756–761. doi: 10.1038/358756a0. [DOI] [PubMed] [Google Scholar]
- 24.Ferrera VP, Nealey TA, Maunsell JH. Responses in macaque visual area V4 following inactivation of the parvocellular and magnocellular LGN pathways. J. Neurosci. 1994;14:2080–2088. doi: 10.1523/JNEUROSCI.14-04-02080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afraz SR, Montaser-Kouhsari L, Vaziri-Pashkam M, Moradi F. Interhemispheric visual interaction in a patient with posterior callosectomy. Neuropsychologia. 2003;41:597–604. doi: 10.1016/s0028-3932(02)00201-4. [DOI] [PubMed] [Google Scholar]
- 26.Livne T, Sagi D. Configuration influence on crowding. J. Vis. 2007;7:4.1–4.12. doi: 10.1167/7.2.4. [DOI] [PubMed] [Google Scholar]
- 27.Louie EG, Bressler DW, Whitney D. Holistic crowding: Selective interference between configural representations of faces in crowded scenes. J. Vis. 2007;7:24.1–24.11. doi: 10.1167/7.2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brainard DH. The psychophysics toolbox. Spat. Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 29.Pelli DG. The Video Toolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997;10:437–442. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.