Abstract
Some of the most prevalent human degenerative diseases appear as a result of the misfolding and aggregation of proteins. Compelling evidence suggest that misfolded protein aggregates play an important role in cell dysfunction and tissue damage, leading to the disease. Prion protein (Prion diseases), amyloid-beta (Alzheimer’s disease), alpha-synuclein (Parkinson’s disease), Huntingtin (Huntington’s disease), serum amyloid A (AA amyloidosis) and islet amyloid polypeptide (Type 2 Diabetes) are some of the proteins that trigger disease when they get misfolded. The recent understanding of the crucial role of misfolded proteins as well as the structural requirements and mechanism of protein misfolding have raised the possibility that these diseases may be transmissible by self-propagation of the protein misfolding process in a similar way as the infamous prions transmit prion diseases. Future research in this field should aim to clarify this possibility and translate the knowledge of the basic disease mechanisms into development of novel strategies for early diagnosis and efficient treatment.
Keywords: prion, amyloid, protein misfolding, transmission, prion-like propagation, infectious proteins
1. Introduction
It is well established that protein misfolding diseases (PMDs), also known as “conformational diseases”, are caused by the misfolding of proteins into intermolecular β-sheet aggregated structures. This conformation is stabilized by intermolecular interactions, leading to the formation of oligomers, proto-fibrils and fibrils, which then accumulate as amyloid deposits in affected tissues [1,2]. Aggregates of prion protein (PrPSc) in prion diseases (also known as transmissible spongiform encephalopathies or TSEs), amyloid-beta (Aβ) in Alzheimer’s disease (AD), islet amyloid polypeptide (IAPP) in type 2 diabetes (T2D) or serum amyloid A (SAA) in secondary amyloidosis accumulate extracellularly. Other misfolded aggregates accumulate intracellularly, such as alpha-synuclein (α-syn) in Parkinson disease (PD), superoxide dismutase (SOD) in amyotrophic lateral sclerosis (ALS), tau in Tauopathies or AD, and huntingtin (Htt) in Huntington disease (HD) [1].
2. The role of Misfolded Proteins in disease
Although the presence of misfolded protein aggregates in affected tissue was recognized long ago, their role in the disease etiology remained controversial. Only in the past 10 years misfolded proteins have been widely considered to be the triggering factors in the disease. Perhaps the most compelling pieces of evidence in favor of this view came from genetic studies. Most PMDs mainly arise sporadically, without detectable genetic origins; however a portion (usually small) of the cases can be inherited. Interestingly, mutations in the genes encoding the protein component of the misfolded aggregates have been shown to be genetically associated with inherited forms of the disease [3–6]. The familial forms usually have an earlier onset and higher severity than sporadic cases. Mutations in the respective misfolded proteins have been associated with familial forms of many diseases, including AD, TSEs, HD, PD, T2D, ALS and various rarer amyloid-related diseases such as familial amyloid polyneuropathy, cardiac amyloidosis, visceral amyloidosis, cerebral haemorrhage with amyloidosis of the Dutch and Icelandic type, cerebral amyloidosis of the British and Danish type [3–6]. The fact that mutations in the gene encoding the misfolded proteins produce inheritable disease is by itself a very strong argument for a crucial role of protein misfolding in the disease.
Other evidence for the important role of protein misfolding came from studies aiming to generate transgenic animal models for PMDs. Insertion of human genes encoding mutant proteins with a high propensity to misfold and aggregate leads to emergence of several pathological and clinical hallmarks of the different diseases. Over-expression of human prion protein transgene in mice generates spontaneous neurodegeneration accompanied by brain vacuolization as happens in natural TSEs [7]. In the AD field, the most common transgenic models over-express the amyloid precursor protein (APP) and/or the presenilin 1 (PS1), both genes associated to familial forms of AD [3]. Transgenic mice expressing human mutated APP show amyloid plaques, cognitive impairment, cell death and related inflammatory processes [8]. Although PS1 transgenic animals do not develop amyloid plaques or related alterations, the presence of this gene accelerates disease alterations in the presence of the APP gene through increasing the concentration or amyloidogenicity of Aβ [8]. Over-expression of human IAPP transgene in rodent models leads to beta-cell death and diabetes because of accumulation of oligomeric IAPP, which is toxic for beta-cells [9]. Transgenic mice of HD, expressing human Htt gene with expanded poly-glu repeats show intracellular deposits of Htt, neuronal impairment and motor dysfunction [10]. Human α-syn gene expression in transgenic mice induces some of the hallmarks of PD, as dopaminergic cell loss, Lewy bodies accumulation and motor dysfunction [11]. All these findings suggest that misfolding and aggregation of amyloid proteins play a critical role in the pathology and could be the main cause of conformational diseases.
3. The mechanisms and intermediates of Protein Misfolding
Misfolding is produced by an incorrect folding process that results in the formation of a protein with a different conformation from its native fold. Protein misfolding can occur by several reasons [6,12]: (i) somatic mutations in the gene sequence leading to the production of a protein unable to adopt the native folding; (ii) errors on the processes of transcription or translation leading to the production of modified proteins unable to properly fold; (iii) failure of the folding and chaperone machinery; (iv) mistakes on the post-translational modifications or trafficking of proteins; (v) structural modification produced by environmental changes or (vi) induction of protein misfolding by seeding and cross-seeding mechanisms.
The most frequent destiny for misfolded proteins is self-aggregation, because the mistaken exposure of fragments to the solvent that are normally buried inside the protein, lead to a high degree of stickiness. The β-sheet structural motif offers the most favorable organization for these intermolecular aggregates and can accommodate an almost unlimited number of polypeptide chains [12,13]. As a result misfolded proteins exist as a large and heterogenous continuum of polymeric sizes, which are usually classified in broad and not very well defined categories, such as oligomers, protofibrils and fibrils [14–16]. Soluble oligomers are small assemblies of misfolded proteins that are present in the buffer soluble fraction of tissue extracts and usually include structures ranging in size from dimers to 24-mers [15,16]. Recent compelling evidence coming from several independent studies of different proteins indicates that oligomers might be the most toxic species in the misfolding and aggregation pathway [14–16]. Indeed, both synthetic and natural oligomers have been shown to induce apoptosis in cell cultures at very low concentrations [17–19], block long term potentiation in brain slice cultures [20] and impair synaptic plasticity and memory in animals [21,22]. Protofibrils are larger aggregates that can be seen using electron microscopy as curvilinear structures of 4–11 nm diameter and <200 nm long [14,23]. Protofibrils increase in size with increased time and protein concentration and are elongated by growth on their ends [24]. Annular protofibrils are pore-like assemblies that form in the cell membrane and may contribute to cell death [25,26]. Protofibrils and annular protofibrils have also been shown to be highly toxic in various in vitro studies [26,27]. Amyloid fibrils are long, straight and unbranched structures of around 10 nm diameter and usually several μm lengths [13]. They bind the dies Congo red and thioflavin and show a typical “cross-β” X-ray diffraction pattern consisting of two major reflections at 4.7 Å and 10 Å found on orthogonal axes [13]. Fibrils can also elicit toxicity in cultured cells, but usually at much higher concentrations than oligomers and protofibrils [14].
The mechanism of protein misfolding and aggregation follows the so-called “seeding-nucleation” model [28,29]. In this process, the initial steps of misfolding are thermodynamically unfavorable and progress slowly, until the minimum stable oligomeric unit is formed that then grows exponentially at a fast speed. There is two kinetic phases in the seeding-nucleation model of polymerization. Firstly, during the lag phase, a low amount of misfolded and oligomeric structures are produced in a slow process, generating seeds for the next step. Once nuclei are formed, the elongation phase takes place and results in fast growing of the polymers. The addition of pre-formed seeds can reduce the length of the lag phase, accelerating the exponential phase. Oligomers are perhaps the best seeds to propagate the misfolding process in an exponential manner. However, larger structures as fibrils could be as well important to propagate this event in vivo, due to their higher resistance to biological clearance than smaller aggregates.
4. When Misfolded Proteins behave as Pathogens: The case of Prion diseases
Among PMDs prion diseases are unique because the pathology can be transmitted by an infectious process. The widely accepted prion hypothesis proposes that PrPSc is the sole component of the infectious agent, which propagates by auto-catalytically converting the native protein (PrPC) into more of the misfolded form [30,31]. Compelling evidence has accumulated over the years to support this hypothesis. An important proof for this theory is the fact that inoculation of animals with ultrapure PrPSc causes prion disease and that infectivity titer is directly proportional to the PrPSc concentration. As mentioned above, transgenic animals expressing human misfolded protein transgene develop some clinical and neuropathological features of the human disease, whereas PrP knock-out mice are resistant to prion infection [32], showing that misfolded proteins are intimately implicated in the malady. Indeed, all inherited cases of prion disease are linked with mutations in the prion protein gene, which usually have an earlier onset and more severe phenotype than the sporadic forms [30]. Moreover, low concentrations of PrPSc produce neurotoxicity and induce apoptosis, which demonstrate their neurodegenerative capability [33]. Furthermore, PrPSc is able to induce misfolding of PrPC in vitro by cyclic amplification of protein misfolding (PMCA), resulting in generation of prion infectious material in the test tube in the absence of living cells [34]. In addition, prions themselves encode many phenotypic TSE variants, termed prion strains. Prion strains, after inoculation into distinct hosts, cause infection with typical features, such as incubation period, clinical signs, characteristic pattern of neuropathological lesions and specific PrPSc biochemical features [35]. These prion strains, as well as the associated phenomena of species barrier and strain adaptation are exclusive features of the misfoded prion protein and can be reproduced during in vitro replication by PMCA [31].
The molecular basis for prion infectivity is the ability of PrPSc to efficiently induce the transformation of PrPC into PrPSc. This conversion process follows the seeding-nucleation model with infectious PrPSc acting as a seed to capture PrPC into the polymer [28]. New molecules of PrPC added to the edges of PrPSc aggregates acquire the same conformation of the PrPSc template to be able to incorporate stably into the polymer. In this way PrPSc aggregates grow, and at certain stage polymers breakdown into smaller pieces multiplying the number of oligomeric seeds to further propagate prion replication. We still do not understand how this key fragmentation process occurs in vivo, but is likely to be mostly a mechanical disruption when polymers become too large.
5. The Prion phenomenon in other PMDs
As discussed before, protein misfolding and aggregation in other PMDs also follows a seeding-nucleation model involving the formation of similar intermediates and end-products as in TSEs [28,29]. Indeed, acceleration of protein aggregation by addition of seeds has been convincingly reported in vitro for several proteins implicated in diverse diseases [36,37]. These findings suggest that protein misfolding processes have the inherent ability to be transmissible [28]. Strikingly, a series of recent and exciting reports, using cellular and/or animal models, have provided evidence suggesting that the transmission of protein misfolding by a prion-like mechanism might be at the heart of the most common PMDs, including Alzheimer’s, Parkinson’s, Huntington’s diseases and various systemic amyloidosis [38–42]. A possible transmissible origin by infectious proteins could clarify the unknown etiology of many of these spontaneous age-related degenerative diseases.
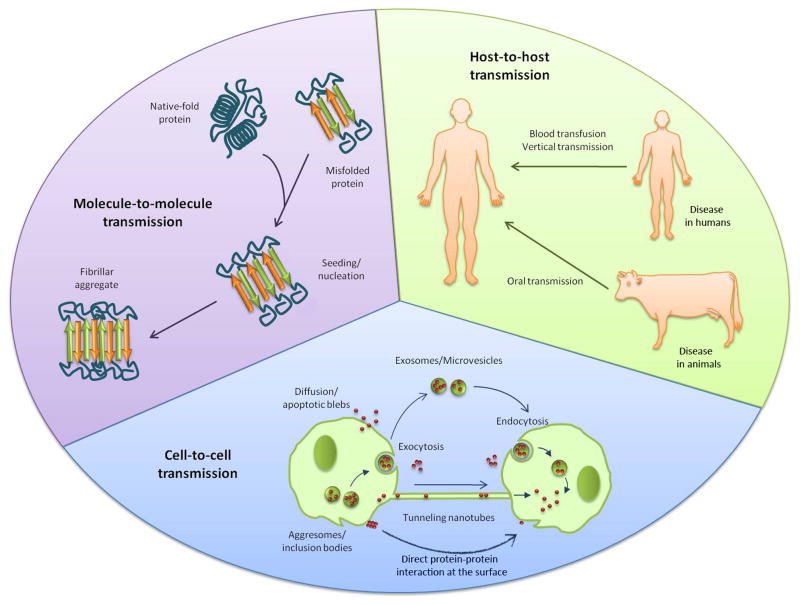
The prion-like phenomenon could contribute to the transmission/spread/propagation of the pathological misfolded conformation between proteins, within cells and tissues and among individuals (Fig. 1). Thus, transmission by infectious proteins can be regarded at different stages. At molecular level, misfolded proteins can propagate the conformational changes into the native proteins, modifying their function and inducing cellular stress and damage. Protein misfolding can be transmitted also from one cell to another to propagate the pathology throughout the affected tissue. Several subcelullar pathways and cellular connections may participate in the spreading process, allowing that misfolded aggregates get in contact with the native protein in the neighboring cells. Misfolded protein aggregates can accumulate intra- or extracellularly [1]. Cytoplasmic aggregates are usually encapsulated in so-called aggresomes, which are microtubule-dependent cytoplasmatic inclusion bodies that are produced in the cell when its capacity to degrade misfolded proteins is overstepped [43,44]. Aggresomes formation could help in the recruitment of protein aggregates which are then targeted to elimination by macroautophagy and degradation into lysosomes. In most PMDs, the autophagy-lysosome pathway is dysregulated or inhibited, as occurs in HD, AD, PD and ALS [45,46]. Both autophagosomal and lysosomal vesicles can spread aggregates within the cell and act as a reservoir for misfolded proteins. Moreover, lysosomal proteolysis could be an important source of fragmentation of large polymers leading to multiplication of seeds. In neurodegenerative PMDs, axonal transport of misfolded aggregates, both anterograde and retrograde, could contribute to their propagation along neuronal processes, as it has been demonstrated for prion proteins [47]. Cell-to-cell transmission of intracellular aggregates requires their release to the extracellular space, the recruitment of the aggregates by other cells and, finally, the nucleation process in the host cell (Fig. 1). Exit of misfolded protein aggregates from a cell can occur upon cell death or be mediated by exocytosis. It has been reported that exosomes are used by prion protein as vehicles for their dissemination [48–50] and a small fraction of Aβ peptides can be secreted from the cells associated with exosomes [51]. Exosomes are membranous vesicles mainly implicated in sorting and release of membrane proteins but can also harbor cytosolic content. Therefore, exosomes and microvesicles plasma membrane evaginations-, by exocytosis/endocytosis can be exchanged to deliver their content, contributing to the spreading of protein misfolding. Exosomes have been found in plasma and other body fluids [52] and could contribute to systemic spread of PMDs. Microvesicles can also participate in horizontal transfer of material among cells. For example, PrPSc is released from infected neurons in association with microvesicles that are infectious both in vitro and in vivo [53]. Another possible mechanism for cellular spreading of misfolded aggregates is the use of tunneling nanotubes. These structures are membrane bridges between cells for intercellular long distance communication to transfer organelles, vesicles, plasma membrane and cytoplasmic molecules [54]. Tunneling nanotubes have been implicated in propagation of endogenous PrP between dendritic cells and uninfected neurons [55, 56] and could be a spreading pathway for other misfolded proteins, such as Aβ and α-syn [56]. Once misfolded aggregates are in the extracellular space, they are internalized by other cells through diffusion, endosomes, receptor-mediated transport or fusion of the transporting vesicles with the plasma membrane. The amyloidogenic proteins Aβ, tau and α-syn have been shown to be internalized by cells through receptor-mediated transport and packed by endocytosis [42,57–59]. Once internalized, misfolded protein aggregates can act as seeds to propagate protein misfolding in the new host cell. The cellular spreading and propagation of protein misfolding have been already reported for various proteins, such as α-syn, Tau, Htt and PrP [41,42,60]. In the case of proteins sitting on the cell surface, such as PrP, cell-to-cell spreading could simply occur by direct contact between the misfolded protein in one cell with the natively folded protein in the neighboring cells.
Figure 1. Transmission of Protein Misfolding between molecules, cells and individuals.
Prion-like transmission of protein misfolding may operate at various levels, including molecule-to-molecule, cell-to-cell and host-to-host. Propagation of the pathological conformational changes and downstream effects to cells, tissues and the entire individual appears to be a universal property of misfolded protein aggregates.
Most neurodegenerative PMDs spreads progressively from a rather small initial affected region to other distant areas of the brain in an organized, stepwise and specific manner. This has been described in AD, PD, HD and frontotemporal dementia [40, 61]. It is likely that prion-like transmission of protein misfolding in these diseases play an important role on the tissue spreading of the pathology. Several studies shown that α-syn aggregates can be transmitted from host cells to grafted neuronal cells in PD patients [62,63]. In the same way, soluble Aβ in host transgenic AD animals can be transferred to grafted cells and induce amyloid plaque formation [64]. Furthermore, misfolded aggregates can be disseminated beyond the tissue where they are produced and spread through the entire organism in a peripheral and systemic transmission thanks to immune system cells, peripheral nerves and bloodstream. It has been reported that PrPSc-loaded dendritic cells and peripheral nerve fibers can initiate and spread prion neuroinvasion [65]. Most of the disease-associated misfolded proteins are found circulating in the CSF and plasma, which could facilitate their spread through the body [66–70].
The recent developments in the field have demonstrated that misfolded proteins associated with various PMDs can initiate the conversion of the normal form of the protein into the misfolded form and propagate these changes to neighboring cells in experimental models. However, prions not only do that, but also can transfer the disease from individual to individual acting as bona fide infectious agents [35,52]. A series of recent studies have shown experimental evidences for prion-like mechanisms of pathological transmission among individuals under defined experimental settings. Transgenic mice expressing the human amyloid precursor protein were injected intra-cerebrally with brain homogenates derived from AD patients [38,71]. Inoculated animals exhibited accelerated Aβ-deposition in the brain. Injection of material in which Aβ aggregates were priorly immunodepleted did not produce acceleration of the pathology, indicating that pre-formed Aβ aggregates were required to induce amyloid deposition in vivo [38]. In a more recent study from the same group, it was shown that intra-peritoneal inoculation with AD brain homogenates accelerates AD pathology in animal models, indicating that the seeds can be administered peripherally and be able to reach the brain to induce the pathology in the target organ [72]. Similarly, intra-cerebral injection of brain extract containing Tau aggregates into mice without aggregates induced the assembly of native Tau into misfolded aggregate filaments in recipient mice [39]. Interestingly, the pathology spread beyond the site of injection to neighboring brain regions. Even more striking results have been reported in two systemic amyloid diseases. In secondary reactive amyloidosis and mouse senile amyloidosis, associated with deposition of amyloid-A and apolipoprotein AII, respectively, even tiny quantities of misfolded aggregates can be transmitted between individuals and cause disease by diverse routes, including blood transfusion and oral administration [73,74]. In the case of amyloid-A amyloidosis, evidence also exists for natural transmission in captive cheetah populations [75].
Individual to individual transmission can occur through vertical (mother to offspring) or horizontal routes (Fig. 1). Horizontal transmission may involve exposure through contaminated surgical tools, organ transplant, use of drugs from human origin or blood transfusion. All these routes have been demonstrated to occur in the transmission of prion diseases in humans [76]. Again in analogy to TSEs, seeds composed of the misfolded proteins could be acquired from domestic or consumption animals. Although the majority of PMDs have not been described in animals, little or no studies have been done to assess whether misfolded proteins accumulate in animals, even without obvious signs of the disease. It is well established that BSE can be transmitted to humans leading to the induction of the fatal variant Creutzfeldt-Jakob disease [77]. Since prions can be detected in skeletal muscle, saliva, blood, urine and feces [78–82], prion diseases could be acquire through exposure or intake of these fluids and consumption of infected foodstuff derived from afflicted animals. Likewise, AApoAII amyloidosis can be transmitted by oral route both by intake of feces or milk [74,75]. Oral transmission route could have an important role in host-to-host infection if any of the animals that we usually consume develop any of the PMDs typically seen in humans, as happens with BSE in cattle. Other route for host-to-host transmission to bear in mind is the vertical infection, from parents to their offspring [84]. In addition, a medically relevant possible route for transmission of misfolded proteins might be blood transfusion. Transmission of BSE, variant CJD, and scrapie by blood has been proved in several animal models [85]. Epidemiological data indicates that human vCJD blood is infectious during both incubation period and clinical phase and can be transmitted through transfusion route [86]. To our knowledge, besides of prion diseases and systemic amyloidosis, no others PMDs has been reported to be transmissible host-to-host by oral, vertical, blood transfusion or any other natural route, till now.
6. Concluding remarks
Formation and accumulation of misfolded protein aggregates is a central feature of several human diseases, most of which are chronic, progressive and have no cure. Substantial progress has been produced in understanding the molecular basis of these diseases and the crucial role of protein misfolding. However, we still do not understand the origin of these diseases and the mechanism by which misfolded aggregates lead to cell dysfunction and tissue damage. Most of the misfolded protein aggregates that causes PMDs share the same misfolding mechanisms, structures and transmission pathways with infectious prions, which provide them the potential for disease transmission. These similarities may underlie the emergent concept that all misfolded protein aggregates could act as infectious agents using the same transmission mechanisms as prion diseases. So, is transmissibility a common feature in all PMDs? Despite exciting recent reports supporting this possibility, more studies need to be carried out to provide a definitive answer to this important question. Understanding the precise pathways implicated in the transmission process, from molecular to host level, will provide new diagnostic targets and powerful tools for therapeutic approaches to fight against these devastating diseases.
Acknowledgments
This work was supported in part by NIH grant P01 AI077774 to CS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soto C. Unfolding the role of Protein Misfolding in Neurodegenerative Diseases. Nature Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–9. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–8. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 4.Buxbaum JN, Tagoe CE. The genetics of the amyloidoses. Annu Rev Med. 2000;51:543–69. doi: 10.1146/annurev.med.51.1.543. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J. Genetic dissection of primary neurodegenerative diseases. Biochem Soc Symp. 2001:51–7. doi: 10.1042/bss0670051. [DOI] [PubMed] [Google Scholar]
- 6.Soto C. Protein misfolding and disease; protein refolding and therapy. FEBS Lett. 2001;498:204–7. doi: 10.1016/s0014-5793(01)02486-3. [DOI] [PubMed] [Google Scholar]
- 7.DeArmond SJ, Prusiner SB. Prion protein transgenes and the neuropathology in prion diseases. Brain Pathol. 1995;5:77–89. doi: 10.1111/j.1750-3639.1995.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 8.Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–33. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 10.Menalled LB, Chesselet MF. Mouse models of Huntington’s disease. Trends Pharmacol Sci. 2002;23:32–9. doi: 10.1016/s0165-6147(00)01884-8. [DOI] [PubMed] [Google Scholar]
- 11.Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson’s disease progression. Acta Neuropathol. 2008;115:385–98. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly JW. Alternative conformations of amyloidogenic proteins govern their behavior. Curr Opin Struct Biol. 1996;6:11–7. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 13.Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435:773–8. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;31:267–98. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 15.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–5. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 17.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280(17):17294–300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 18.Bucciantini M, Calloni G, Chiti F, Formigli L, Nosi D, Dobson CM, Stefani M. Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J Biol Chem. 2004;279:31374–82. doi: 10.1074/jbc.M400348200. [DOI] [PubMed] [Google Scholar]
- 19.Simoneau S, Rezaei H, Sales N, Kaiser-Schulz G, Lefebvre-Roque M, Vidal C, Fournier JG, et al. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 2007;3:e125. doi: 10.1371/journal.ppat.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, et al. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–40. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 21.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2004;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 22.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, et al. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–52. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 24.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Assembly of A beta amyloid protofibrils: an in vitro model for a possible early event in Alzheimer’s disease. Biochem. 1999;38:8972–80. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan R, Marchant RE, Zagorski MG. ABri peptide associated with familial British dementia forms annular and ring-like protofibrillar structures. Amyloid. 2004;11:10–3. doi: 10.1080/13506120410001667872. [DOI] [PubMed] [Google Scholar]
- 26.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418(6895):291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 27.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, et al. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–5. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Jarrett JT, Lansbury PT. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–8. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 30.Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–52. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Espinoza R, Soto C. Generation of prions in vitro and the protein-only hypothesis. Prion. 2010;4:1–7. doi: 10.4161/pri.4.2.11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–47. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 33.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–45. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–61. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 36.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 37.Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV, Dobson CM. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci. 2004;13:1933–8. doi: 10.1110/ps.04707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–4. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 39.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–25. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–30. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 44.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–98. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menzies FM, Moreau K, Rubinsztein DC. Protein misfolding disorders and macroautophagy. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metcalf DJ, García-Arencibia M, Hochfeld WE, Rubinsztein DC. Autophagy and misfolded proteins in neurodegeneration. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.11.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs GG, Preusser M, Strohschneider M, Budka H. Subcellular localization of disease-associated prion protein in the human brain. Am J Pathol. 2005;166:287–94. doi: 10.1016/S0002-9440(10)62252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;101:9683–8. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fevrier B, Vilette D, Laude H, Raposo G. Exosomes: a bubble ride for prions? Traffic. 2005;6:10–7. doi: 10.1111/j.1600-0854.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Zhou X, Bai Y, Zhang Z, Zhao D. Cellular prion protein released on exosomes from macrophages binds to Hsp70. Acta Biochim Biophys Sin. 2010;42:345–50. doi: 10.1093/abbs/gmq028. [DOI] [PubMed] [Google Scholar]
- 51.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA. 2006;103:11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, andprionoids. Neuron. 2009;64:783–90. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Mattei V, Barenco MG, Tasciotti V, Garofalo T, Longo A, Boller K, et al. Paracrine diffusion of PrP(C) and propagation of prion infectivity by plasma membrane-derived microvesicles. PLoS One. 2009;4:e5057. doi: 10.1371/journal.pone.0005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rustom A. Hen or egg?: some thoughts on tunneling nanotubes. Ann NY Acad Sci. 2009;1178:129–136. doi: 10.1111/j.1749-6632.2009.04997.x. [DOI] [PubMed] [Google Scholar]
- 55.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–36. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 56.Gousset K, Zurzolo C. Tunnelling nanotubes: a highway for prion spreading? Prion. 2009;3:94–8. doi: 10.4161/pri.3.2.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burdick D, Kosmoski J, Knauer MF, Glabe CG. Preferential adsorption, internalization and resistance to degradation of the major isoform of the Alzheimer’s amyloid peptide, A beta 1–42, in differentiated PC12 cells. Brain Res. 1997;746:275–84. doi: 10.1016/s0006-8993(96)01262-0. [DOI] [PubMed] [Google Scholar]
- 58.Nagele RG, D’Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of beta-amyloid (1–42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 59.Sung JY, Kim J, Paik SR, Park JH, Ahn YS, Chung KC. Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J Biol Chem. 2001;276:27441–8. doi: 10.1074/jbc.M101318200. [DOI] [PubMed] [Google Scholar]
- 60.Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem. 2009;111:192–203. doi: 10.1111/j.1471-4159.2009.06324.x. [DOI] [PubMed] [Google Scholar]
- 61.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 63.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 64.Meyer-Luehmann M, Stalder M, Herzig MC, Kaeser SA, Kohler E, Pfeifer M, et al. Extracellular amyloid formation and associated pathology in neural grafts. Nat Neurosci. 2003;6:370–7. doi: 10.1038/nn1022. [DOI] [PubMed] [Google Scholar]
- 65.Dorban G, Defaweux V, Heinen E, Antoine N. Spreading of prions from the immune to the peripheral nervous system: a potential implication of dendritic cells. Histochem Cell Biol. 2010;133:493–504. doi: 10.1007/s00418-010-0687-9. [DOI] [PubMed] [Google Scholar]
- 66.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 67.Tokuda T, Qureshi MM, Ardah MT, Varghese S, Shehab SA, Kasai T, et al. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766–72. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 68.El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–7. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 69.Weiss A, Abramowski D, Bibel M, Bodner R, Chopra V, DiFiglia M, et al. Single-step detection of mutant huntingtin in animal and human tissues: a bioassay for Huntington’s disease. Anal Biochem. 2009;395:8–15. doi: 10.1016/j.ab.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Tasaki M, Ueda M, Ochiai S, Tanabe Y, Murata S, Misumi Y, et al. Transmission of circulating cell-free AA amyloid oligomers in exosomes vectors via a prion-like mechanism. Biochem Biophys Res Commun. 2010;400:559–62. doi: 10.1016/j.bbrc.2010.08.101. [DOI] [PubMed] [Google Scholar]
- 71.Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci. 2000;20:3606–11. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally Applied Abeta-Containing Inoculates Induce Cerebral beta-Amyloidosis. Science. 2010;330:980–2. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundmark K, Westermark GT, Nyström S, Murphy CL, Solomon A, Westermark P. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci USA. 2002;99:6979–84. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, Li F, et al. Transmission of mouse senile amyloidosis. Lab Invest. 2001;81:493–9. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]
- 75.Zhang B, Une Y, Fu X, Yan J, Ge F, Yao J, et al. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc Nat Acad Sci USA. 2008;105:7263–8. doi: 10.1073/pnas.0800367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown P, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075–81. doi: 10.1212/wnl.55.8.1075. [DOI] [PubMed] [Google Scholar]
- 77.Scott MR, Will R, Ironside J, Nguyen HO, Tremblay P, DeArmond SJ, Prusiner SB. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA. 1999;96:15137–42. doi: 10.1073/pnas.96.26.15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–5. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 79.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008;582:3161–6. doi: 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Safar JG, Lessard P, Tamgüney G, Freyman Y, Deering C, Letessier F, et al. Transmission and detection of prions in feces. J Infect Dis. 2008;198:81–9. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korenaga T, Yan J, Sawashita J, Matsushita T, Naiki H, Hosokawa M, et al. Transmission of amyloidosis in offspring of mice with AApoAII amyloidosis. Am J Pathol. 2006;168:898–906. doi: 10.2353/ajpath.2006.050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. Transmission of prion diseases by blood transfusion. J Gen Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 86.Ironside JW, Head MW. Variant Creutzfeldt-Jakob disease and its transmission by blood. J Thromb Haemost. 2003;1:1479–86. doi: 10.1046/j.1538-7836.2003.00268.x. [DOI] [PubMed] [Google Scholar]