Abstract
Oligothymidylates involving alternating alkyl phosphotriester-phosphodiester or methylphosphonate-phosphodiester backbones and covalently linked to an acridine derivative have been studied using circular dichroism. Two isomers with the same diastereoisomeric configuration for all the phosphotriesters (ethyl triester and neopentyl triester) or the methylphosphonate linkages were studied. These six compounds were compared to the parent oligonucleotide with unmodified phosphodiester bonds. Intramolecular interactions between the acridine and the bases of the oligonucleotides were revealed by the induced circular dichroism of the acridine dye. Binding to poly(rA) and poly(dA) induced large changes in the circular dichroism signal. All oligothymidylates formed double-stranded complexes with poly(rA). Substitution of phosphotriesters and methylphosphonates to phosphates allowed both double- and triple-stranded structures to be formed with with poly(dA). The double-stranded structures formed with poly(rA) and poly(dA) were characterized by different environments of the acridine dye. The circular dichroism spectra of the complexes with poly(dA) and the thermal stabilities of the complexes formed with both poly(rA) and poly(dA) were drastically dependent of the diastereoisomeric configuration of the phosphate modification. For the complexes formed with the pseudoequatorial stereoisomer the modification of the phosphate groups increased the stability of the complexes as compared with the oligothymidylate containing only phosphodiester linkages whereas it decreased it for pseudoaxial modifications.
Full text
PDF




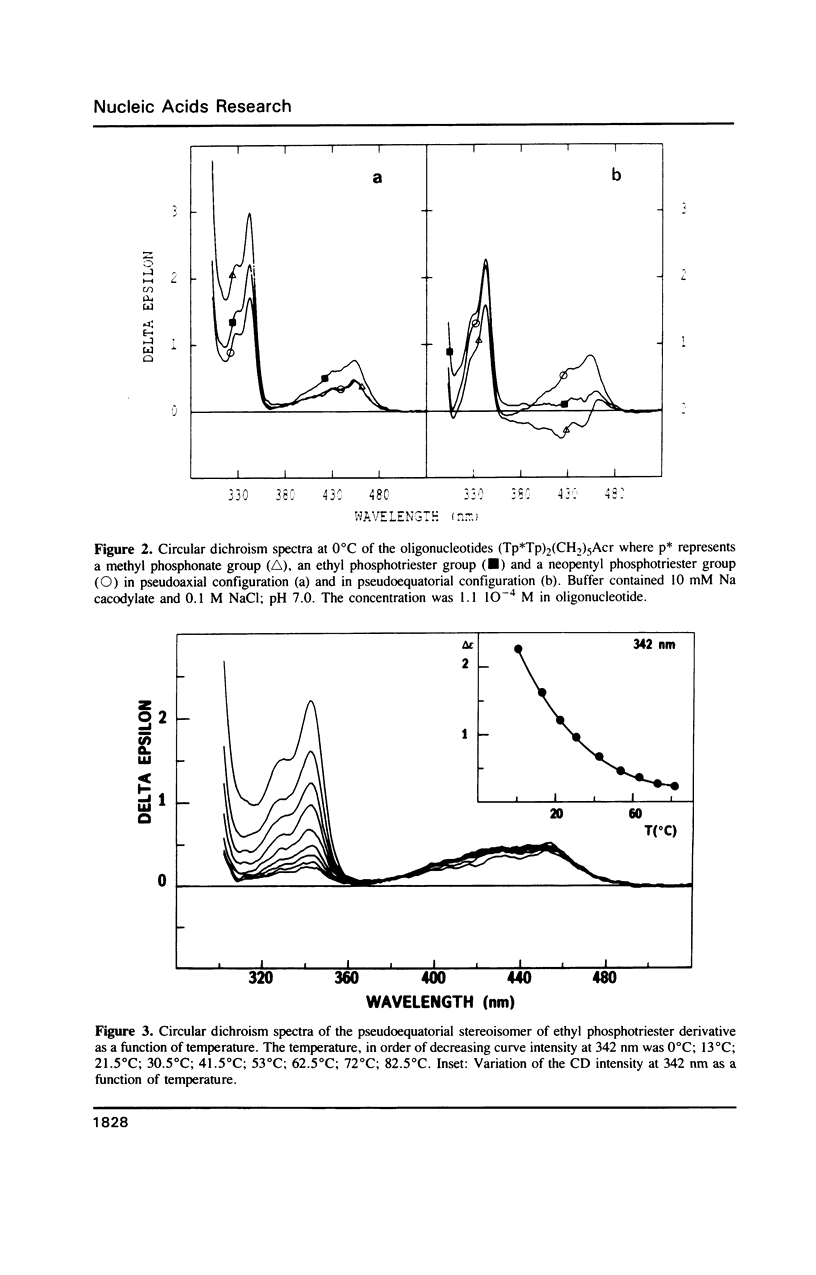
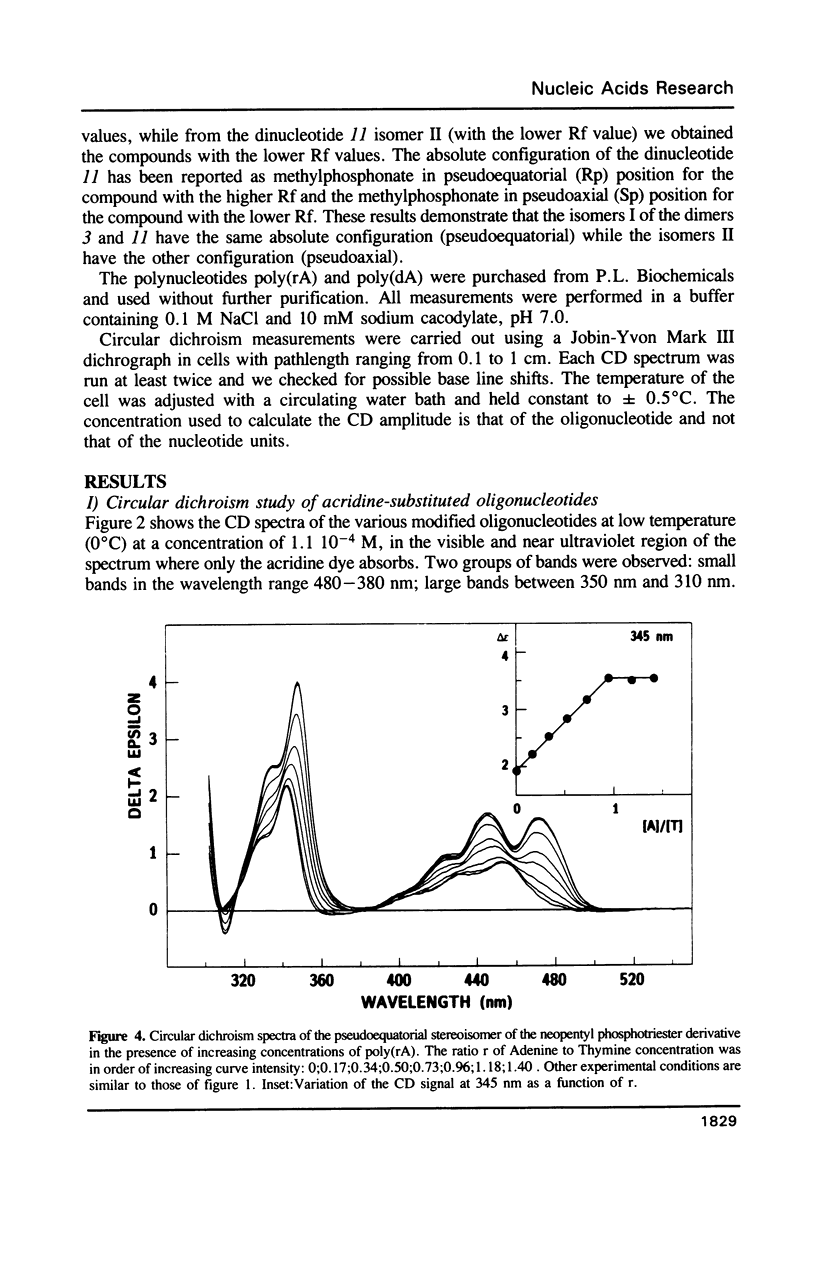
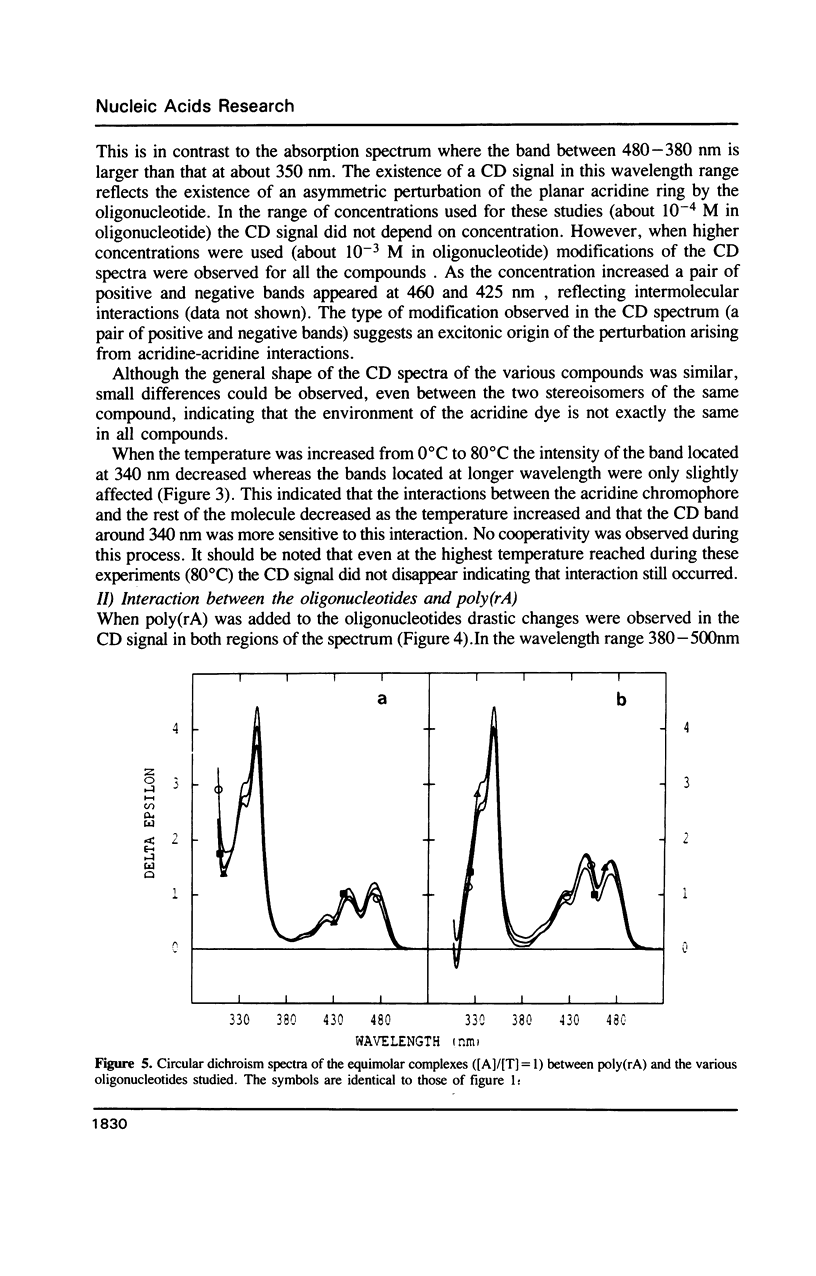
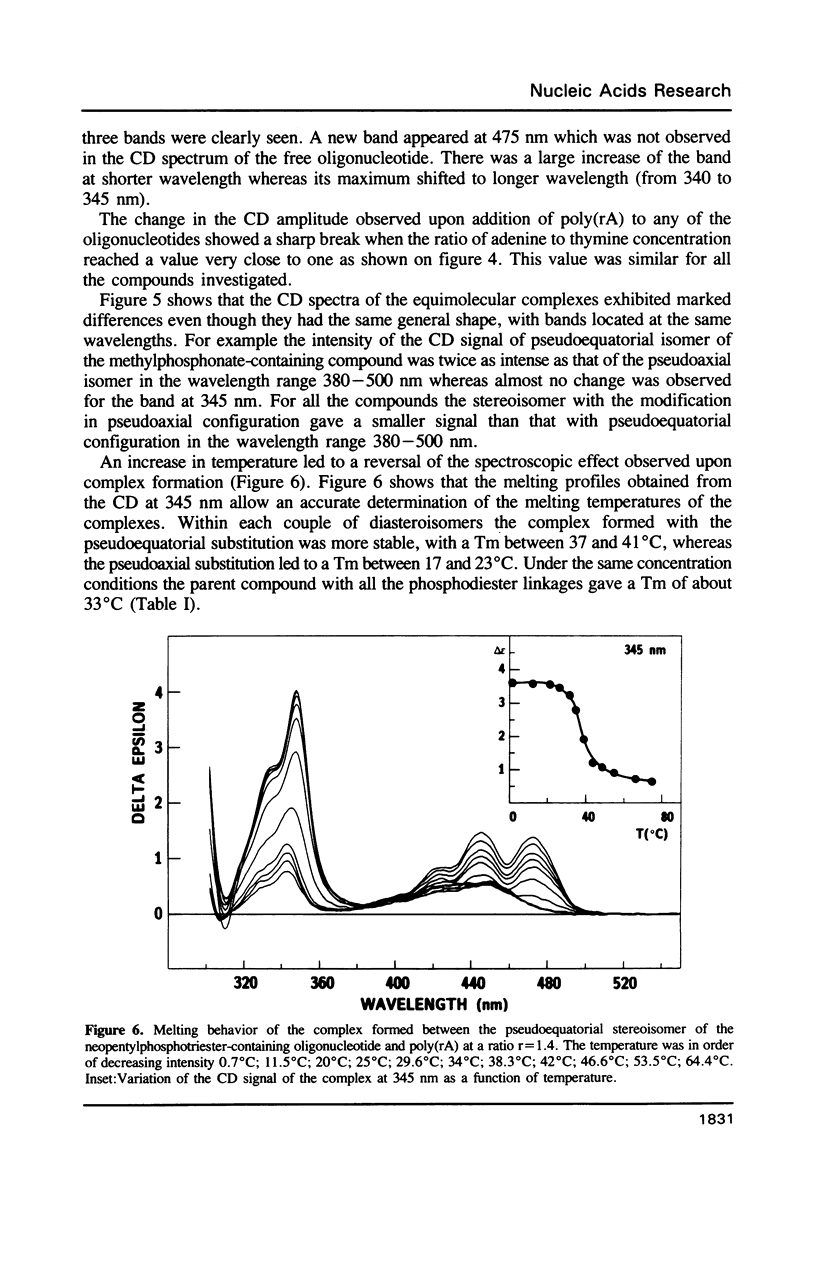





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexeev D. G., Lipanov A. A., Skuratovskii IYa Poly(dA).poly(dT) is a B-type double helix with a distinctively narrow minor groove. 1987 Feb 26-Mar 4Nature. 325(6107):821–823. doi: 10.1038/325821a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseline U., Toulme F., Thuong N. T., Delarue M., Montenay-Garestier T., Hélène C. Oligodeoxynucleotides covalently linked to intercalating dyes as base sequence-specific ligands. Influence of dye attachment site. EMBO J. 1984 Apr;3(4):795–800. doi: 10.1002/j.1460-2075.1984.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behling R. W., Kearns D. R. Determination of NOE "silent" dipolar interactions between magnetically equivalent nuclei: application to poly(dA).poly(dT). Biopolymers. 1985 Jul;24(7):1157–1167. doi: 10.1002/bip.360240705. [DOI] [PubMed] [Google Scholar]
- Bower M., Summers M. F., Powell C., Shinozuka K., Regan J. B., Zon G., Wilson W. D. Oligodeoxyribonucleoside methylphosphonates. NMR and UV spectroscopic studies of Rp-Rp and Sp-Sp methylphosphonate (Me) modified duplexes of (d[GGAATTCC])2. Nucleic Acids Res. 1987 Jun 25;15(12):4915–4930. doi: 10.1093/nar/15.12.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani G. R., Bollum F. J. Oligodeoxythymidylate: polydeoxyadenylate and oligodeoxyadenylate: polydeoxythymidylate interactions. Biochemistry. 1969 Oct;8(10):3928–3936. doi: 10.1021/bi00838a008. [DOI] [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Jollès B., Laigle A., Chinsky L., Turpin P. Y. The poly dA strand of poly dA.poly dT adopts an A-form in solution: a UV resonance Raman study. Nucleic Acids Res. 1985 Mar 25;13(6):2075–2085. doi: 10.1093/nar/13.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelot G., Asseline U., Thuong N. T., Hélène C. Proton and phosphorus nuclear magnetic resonance studies of an oligothymidylate covalently linked to an acridine derivative and of its binding to complementary sequences. Biochemistry. 1985 May 7;24(10):2521–2529. doi: 10.1021/bi00331a019. [DOI] [PubMed] [Google Scholar]
- Lancelot G., Guesnet J. L., Asseline U., Thuong N. T. NMR studies of complex formation between the modified oligonucleotide d(T*TCTGT) covalently linked to an acridine derivative and its complementary sequence d(GCACAGAA). Biochemistry. 1988 Feb 23;27(4):1265–1273. doi: 10.1021/bi00404a029. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Aurelian L., Blake K. R., Murakami A., Reddy M. P., Spitz S. A., Ts'o P. O. Control of ribonucleic acid function by oligonucleoside methylphosphonates. Biochimie. 1985 Jul-Aug;67(7-8):769–776. doi: 10.1016/s0300-9084(85)80166-8. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Dreon N., Pulford S. M., McParland K. B. Oligothymidylate analogues having stereoregular, alternating methylphosphonate/phosphodiester backbones. Synthesis and physical studies. J Biol Chem. 1980 Oct 25;255(20):9659–9665. [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Yano J., Yano E., Carroll C., Jayaraman K., Ts'o P. O. Nonionic nucleic acid analogues. Synthesis and characterization of dideoxyribonucleoside methylphosphonates. Biochemistry. 1979 Nov 13;18(23):5134–5143. doi: 10.1021/bi00590a017. [DOI] [PubMed] [Google Scholar]
- Murakami A., Blake K. R., Miller P. S. Characterization of sequence-specific oligodeoxyribonucleoside methylphosphonates and their interaction with rabbit globin mRNA. Biochemistry. 1985 Jul 16;24(15):4041–4046. doi: 10.1021/bi00336a036. [DOI] [PubMed] [Google Scholar]
- Pless R. C., Ts'o P. O. Duplex formation of a nonionic oligo(deoxythymidylate) analogue (heptadeoxythymidylyl-(3'-5')-deoxythymidine heptaethyl ester (d-(Tp(Et))7T)) with poly(deoxyadenylate). Evaluation of the electrostatic interaction. Biochemistry. 1977 Mar 22;16(6):1239–1250. doi: 10.1021/bi00625a033. [DOI] [PubMed] [Google Scholar]
- Roy S., Borah B., Zon G., Cohen J. S. Conformation of the oligonucleotide d(AAAAAATTTTTT)2 by two-dimensional nuclear Overhauser effect spectroscopy and its relevance to poly(dA).poly(dT). Biopolymers. 1987 Apr;26(4):525–536. doi: 10.1002/bip.360260406. [DOI] [PubMed] [Google Scholar]
- Sarma M. H., Gupta G., Sarma R. H. Untenability of the heteronomous DNA model for poly(dA).poly(dT) in solution. This DNA adopts a right-handed B-DNA duplex in which the two strands are conformationally equivalent. A 500 MHz NMR study using one dimensional NOE. J Biomol Struct Dyn. 1985 Jun;2(6):1057–1084. doi: 10.1080/07391102.1985.10507624. [DOI] [PubMed] [Google Scholar]
- Summers M. F., Powell C., Egan W., Byrd R. A., Wilson W. D., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. VI. NMR and UV spectroscopic studies of ethyl phosphotriester (Et) modified Rp-Rp and Sp-Sp duplexes, (d[GGAA(Et)TTCC])2. Nucleic Acids Res. 1986 Sep 25;14(18):7421–7436. doi: 10.1093/nar/14.18.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuong N. T., Chassignol M., Lancelot G., Mayer R., Hartmann B., Leng M., Hélène C. Synthesis and structural studies of a self-complementary decadeoxynucleotide d(AATTGCAATT). I.-Synthesis and chemical characterization of the decanucleotide. Biochimie. 1981 Oct;63(10):775–784. doi: 10.1016/s0300-9084(81)80037-5. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Livingston D. C. Synthesis and properties of oligodeoxyribonucleotides containing an ethylated internucleotide phosphate. Biochemistry. 1986 Sep 9;25(18):5083–5091. doi: 10.1021/bi00366a016. [DOI] [PubMed] [Google Scholar]
- Zama M., Ichimura S. Induced circular dichroism of acridine orange bound to double-stranded RNA and transfer RNA. Biopolymers. 1976 Sep;15(9):1693–1699. doi: 10.1002/bip.1976.360150907. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. A RNA.DNA hybrid that can adopt two conformations: an x-ray diffraction study of poly(rA).poly(dT) in concentrated solution or in fibers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):78–82. doi: 10.1073/pnas.78.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]



