Abstract
Background
Integrins mediate invasion and angiogenesis in prostate cancer bone metastases. We conducted a phase II study of Cilengitide, a selective antagonist of αvβ3 and αvβ5 integrins, in non-metastatic castration resistant prostate cancer with rising PSA.
Methods
Patients were observed for 4 weeks with PSA monitoring, and then treated with 2,000 mg IV of cilengitide twice weekly until toxicity/progression. PSA, circulating tumor cells (CTCs) and circulating endothelial cells (CECs) were monitored each cycle with imaging performed every 3 cycles. Primary end point was PSA decline by ≥ 50%. Secondary endpoints were safety, PSA slope, time to progression (TTP), overall survival (OS), CTCs, CECs and gene expression.
Results
16 pts were enrolled; 13 were eligible with median age 65.5 years, baseline PSA 8.4 ng/mL and median Gleason sum 7. Median of 3 cycles was administered. Treatment was well tolerated with 2 grade 3 toxicities and no grade 4 toxicities. There were no PSA responses; 11 patients progressed by PSA after 3 cycles. Median TTP was 1.8 months and median OS has not been reached. Median pre- and on-treatment PSA slopes were 1.1 and 1.8 ng/mL/month. Baseline CTCs were detected in 1/9 patients. CTC increased (0 to 1; 2 pts), remained at 0 (2 pts) or decreased (23 to 0; 1 patient) at progression. Baseline median CEC was 26 (0–61) and at progression, 47 (15–148). Low cell counts precluded gene expression studies.
Conclusions
Cilengitide was well tolerated but had no detectable clinical activity. CTCs are of questionable utility in non-metastatic prostate cancer.
Keywords: EMD 121974, cilengitide, non-metastatic prostate cancer
INTRODUCTION
Non-metastatic castration resistant prostate cancer (CRPC) is a distinct disease state that is characterized by rising PSA despite androgen deprivation therapy without evidence of distant metastases. This clinical state could last a few years and presents an opportunity to intervene with therapy designed to delay progression to metastatic disease [1]. Delay/prevention of clinical systemic metastasis is a clinically meaningful objective.
Formation of bone metastasis is a multi-step process that involves invasion of the vasculature by tumor cells, cell migration to and adhesion at distant bone sites, angiogenesis and tumor growth [2,3]. Interactions between tumor and endothelial cells on one hand and the extracellular matrix (ECM) components (such as vitronectin, fibronectin and osteopontin) on the other mediate several of these steps. Interactions of the ECM with tumor cells and endothelial cells are dependent on a class of transmembrane cell surface receptors called integrins.
The role of integrins in prostate cancer metastases
Integrins transduce signals between the ECM and the intracellular cell signaling pathways of endothelial or tumor cells in both directions[4]. Structurally, they are heterodimers consisting of an alpha and a beta subunit. At least 18 alpha and 8 beta subunits have been identified with more than 24 unique integrin heterodimers recognized so far[5].
Integrins play important roles in cell migration, adhesion, invasion, proliferation, survival and angiogenesis of epithelial neoplasms [4,6,7]. αvβ3 is expressed in prostate cancer cells but not in normal prostate cells[8]. Prostate cancer cell lines derived from bone metastases uniformly express αvβ3[9]. Preclinical studies show that αvβ3 integrin mediates the adhesion of prostate cancer cells to ECM components of the bone such as osteopontin [10,11]. αv integrins also promote survival of prostate cancer cells in bone[12] and siRNAs directed against αv integrins induce apoptosis of PC3 prostate cancer cells in bone[13]. αvβ3 also mediates osteopontin (ECM component) triggered proliferation of castration resistant prostate cancer cells in bone[14]. Bone turnover by osteoblasts and osteoclasts involves interaction of αvβ3 and αvβ5 with osteopontin and bone sialoprotein [15,16]. Blockade of αvβ3 reduces osteoclast recruitment and bone lysis initiated by metastatic cancer cells[17]. Thus, integrins αvβ3 and αvβ5 promote metastasis of prostate cancer cells to bone in each step of the metastatic process [5,4,18].
Endothelial cells when activated by tumor secreted cytokines express αvβ3[19]. A crucial role of αvβ3 in activated endothelial cells is to inhibit apoptosis by up-regulating NF-kB activity [20,21]. Antagonists of αvβ3 and αvβ5 block endothelial cell proliferation and differentiation induced by fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF) in cell lines, chicken chorioallantoic membrane (CAM) and severe combined immunodeficient (SCID) mice [22]. Novel agents that target integrins have shown promising clinical activity in glioblastoma multiforme [23].
Cilengitide
Cyclo-l-Arg-Gly-l-Asp-d-Phe-N (Me) l-Val; (Merck KGaA, Darmstadt, Germany) is a cyclic pentapeptide and RGD mimetic that selectively and competitively antagonizes ligand binding to αvβ3 and αvβ5 in vitro. Cilengitide or EMD121974 inhibited proliferation and increased apoptosis in cell lines and caused tumor regression in cell culture [24,25]. It blocks angiogenesis stimulated by VEGF and FGF in a 3-D gel of bovine endothelial cells [26]. Cilengitide also inhibited αvβ3 and αvβ5 in CAM and in orthotopic models of human melanoma, medulloblastoma and glioblastoma (GBM) in nude and SCID mice [27,28].
In a phase I clinical trial of cilengitide in advanced solid tumors, twice-weekly infusions of cilengitide were administered to 37 patients continuously at doses from 30 mg/m2 up to 1600 mg/m2 in 4 week cycles [29]. In another phase I trial, 20 patients were treated at 2 doses (600 and 1200 mg/m2 on the same schedule as the above study) [30]. In both studies, no dose limiting toxicity was observed. The terminal half-life at all doses in both studies was around 4 hours. Cmax concentrations achieved in plasma at 120 mg/m2 were comparable to tumor inhibitory plasma levels in mice. No hematological or grade 3/4 non-hematologic toxicity were reported. In the phase I component of two NCI sponsored studies (NCT00022113 and NABTT-9911/ NCT00006093) of cilengitide given intravenously twice weekly, no dose limiting toxicity was observed at doses as high as 2400 mg/m2.
Given the critical role of integrins αvβ3 and αvβ5 in promoting angiogenesis and bone metastasis in prostate cancer and the preclinical and clinical safety profile of cilengitide we conducted a single-arm multi-center NCI sponsored phase II study of single agent cilengitide (NCI-6735) in non-metastatic rising PSA-only castration-resistant prostate cancer . The dosing and schedule were based on earlier phase I trials of cilengitide and phase II trials in advanced melanoma (00082875 MDACC 2004) and recurrent GBM (NCT00093964).
PATIENTS AND METHODS
Patients were eligible if they had a histologic or cytologic diagnosis of prostate cancer with no evidence of metastatic disease or local progression on radiologic imaging (Bone scan, CT/MRI of abdomen and pelvis) and had 3 consecutive rising levels of prostate specific antigen (PSA) at a minimum of one week intervals with the last of those values ≥ 2 ng/mL. Patients had to have PSA progression despite androgen deprivation therapy and anti-androgen withdrawal (≥ 4 weeks off flutamide; ≥ 6 weeks off bicalutamide or nilutamide). ECOG performance status of 0–2 and adequate organ and hematologic function were required (ANC ≥1500/uL, platelets ≥100,000/uL, serum creatinine ≤1.5 × upper limits of normal, normal bilirubin and LFTs ≤ 2.5 × upper limits of normal). Patients who had not had orchiectomy were required to continue on LHRH agonist therapy with a castrate range testosterone level. Patients on stable doses of steroids or megace for longer than 1 month continued on the same doses. Patients had to be > 4 weeks from major surgery and prior systemic anti-cancer therapy. No previous treatment with cilengitide was allowed. Continuing bisphosphonate use was permitted if on stable doses for > 6 weeks prior to registration on protocol but was not allowed to be initiated while on the study. No concurrent herbal or food supplements (such as PC-SPES or saw palmetto) other than a daily multivitamin were allowed during the study. Patients with a second active malignancy (less than 2 years from completion of therapy or with current evidence of disease) were excluded except for superficial bladder cancer or non-melanomatous skin cancer. Men of reproductive potential had to agree to use effective contraception. All patients on the study signed an informed consent approved by the institutional review board at the respective participating institution prior to study entry. This Cancer Therapy Evaluation Program (CTEP) sponsored trial was conducted by the Department of Defense-Prostate Cancer Clinical Trials Consortium.
Treatment plan
Patients had a lead-in observation period of 4 weeks with PSA measured at 2 weeks and 4 weeks (figure 1). Treatment with cilengitide began after the 4 weeks lead-in period. Cilengitide was administered at a starting dose of 2000 mg intravenously over one hour twice weekly each week in 4 week cycles without any planned breaks between cycles. Grade 4 hematologic or grade 3 or 4 non-hematologic toxicities by NCI CTCAE version 3.0 necessitated holding the drug until resolution of toxicities to grade ≤1 and re-starting treatment at −1 dose level (1600 mg/dose). Recurrent serious toxicity triggered reduction to −2 dose level (1200 mg/dose) after resolution to ≤ 1 grade. Therapy was stopped for a third occurrence of toxicity of that grade. Treatment could be interrupted for a maximum of 2 consecutive doses or 4 doses in a 12-week period. Based on phase I studies of cilengitide that demonstrated no dose-toxicity relationship and no DLT at doses up to 2000 mg, dosing was not based on body weight or surface area [23,29]. Cilengitide was provided by DCTD, NCI.
Figure 1.
Treatment Schema
Duration of therapy and monitoring
In the absence of toxicity, patients were treated on protocol for a minimum of 3 cycles (12 weeks) prior to response assessment in order to permit an adequate evaluation of the effect of the investigational agent. Patients were evaluated for toxicity and had PSA measured each cycle. Imaging with bone scan and CT or MRI abdomen/pelvis was performed every 3 cycles. Beyond the first 3 cycles, treatment was stopped when any one of the following occurred: clinical or PSA progression, after 3 additional cycles beyond complete response, recurrence of serious toxicity in spite of dose reduction to −2 dose level and maximally allowed dose interruptions, patient preference or worsening of the patient's general medical condition that precluded further treatment in the judgment of the investigator. The PSA value at the end of the 4 week lead-in period prior to the first dose was considered the baseline PSA.
End points and statistical design
Complete response was defined as PSA <0.2 ng/mL, partial response as decline in PSA by 50% from baseline and progression as ≥ 25% rise in PSA over baseline or nadir whichever was lower[31]. PSA responses and progression needed confirmation by a successive PSA at least 4 weeks later. Patients not meeting criteria for either response or progression were considered to have stable disease. Patients with partial response or stable disease by PSA criteria with no evidence of objective disease progression continued treatment with cilengitide until criteria for halting therapy were met.
The primary end-point of the study was PSA response rate (complete and partial response) in patients treated with single-agent cilengitide in non-metastatic castration-resistant prostate cancer. Secondary endpoints were safety of cilengitide, changes in PSA slope with treatment, response duration, time to progression and survival. For calculation of pre-treatment PSA slope, at least 3 PSA values including the lead-in observation period values on weeks 2 and 4 (baseline) were included. For on-treatment PSA slope, the baseline PSA and all PSA values in the first 6 months of treatment with cilengitide were considered. The study was designed to accrue 32 patients to provide 90% power at the 10% significance level to detect a difference between a 5% versus a 20% response rate. If 4 or more PSA responses were seen in this population, further study would be undertaken. To prevent against excess toxicity, if ≥ 3 of the first 12 patients experienced a high-grade non-hematologic toxicity (grade 3 and/or 4) excluding alopecia, nausea or vomiting, the trial would stop early. All of the eligible patients (with the exception of those who received no study medication) are included in the main analysis of the response rate. Survival and time to progression was determined by Kaplan-Meier analysis.
Correlative biology studies
In the absence of objective disease in non-metastatic CRPC, we planned to evaluate circulating tumor and endothelial cells (CTC and CEC). Correlatives included enumeration of CTC using the CellSearch assay (Veridex, Huntington Valley, PA) and CEC using the CellTracks reagents (Veridex, formerly Immunicon Corp.). All CTC and CEC enumeration was performed at Immunicon Corp. and results were communicated to the study authors. RNA isolation was performed from CTCs and CECs from blood collected at baseline and the beginning of each cycle. Analyses included serial enumeration of CTCs and CECs in study patients, comparison of CTC/CEC numbers between patients, and microarray genotyping of CTCs/CECs.
RESULTS
Baseline Characteristics
Between January 2005 and May 2007, 16 patients were registered to the protocol at 6 centers. The protocol was closed due to lack of any PSA response coupled with slow accrual. 1 patient progressed clinically before any treatment and was not included in the toxicity or efficacy analysis. 2 patients who received study drug were deemed ineligible as they did not meet entry PSA criteria of 3 consecutive rises in PSA but were included in the toxicity analysis. Table 1 describes the baseline demographic and clinical data of the 13 eligible patients. Median age was 65.5 years, median baseline PSA at registration was 8.4 with a range of 2.2 to 77, Gleason sum was 7 in 46.2% and 8–9 in another 38.5%, and median Karnofsky performance score was 90 (range, 80–100). 6 patients had undergone prior radical prostatectomy and 5 had undergone definitive radiation treatment. 3 patients each had received salvage and adjuvant radiation therapy. Median time since hormone initiation for the 13 eligible patients was 4.7 years (range, 1–10.6 years). Median pre-treatment PSA slope was 1.1 ng/mL/month.
Table 1.
Baseline characteristics of eligible patients (n=13)
| Median Age (range) | 65.5 yrs (53.8–78.1) |
|
| |
| Median Karnofsky Performance Score (range) | 90 (80–100) |
|
| |
| Median baseline PSA (range) | 8.4 (2.2–77) |
|
| |
| Gleason sum(%) 6 | 2 (15.4%) |
| 7 | 6 (46.2%) |
| 8 | 2 (15.4%) |
| 9 | 3 (23.1%) |
|
| |
| Prior Radiation to prostate | 11 |
| Definitive | 5 |
| Adjuvant | 3 |
| Salvage | 3 |
|
| |
| Radical Prostatectomy | 6 |
|
| |
| No Local Treatment Modality | 2 |
|
| |
| Median time since ADT initiation (range) | 4.7 yrs (1–10.6) |
Efficacy and Survival
Patients were treated for a median of 3 cycles (range 3–8) with cilengitide. There were no PSA responses; 2 patients had stable disease (SD) at 12 weeks (figure 2) and 11 patients had progressed by PSA criteria (2 by imaging also) at first assessment after 3 cycles. Median on-treatment PSA slope was 1.8 ng/mL/month (not significantly different from pre-treatment slope) (fig. 2). Time to PSA progression was 1.8 months (95% CI: 0.9–2.8). All patients are off protocol therapy. With a median follow-up of 3.1 years (range, 16 months –5 years), median overall survival has not been reached for the cohort; 5 of 13 (38 %) evaluable patients have died.
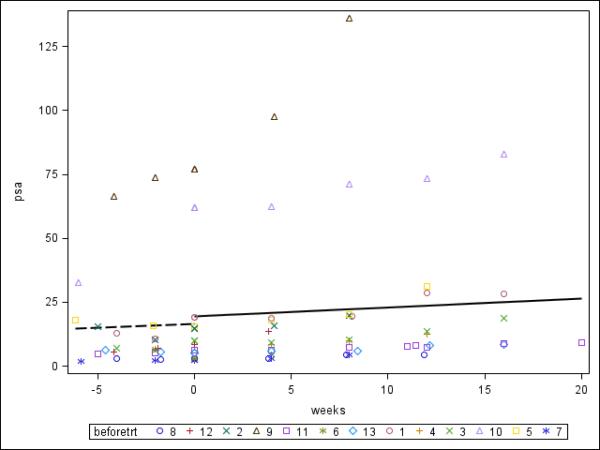
Figure 2.
PSA velocity before and after treatment with Cilengitide in evaluable patients (n=13)
The broken and solid lines represent median pre-treatment and post-treatment PSA velocity respectively. Treatment with Cilengitide started at week 0. Individual PSA values for all 13 eligible patients are shown as a scatter plot.
Treatment related toxicity
Toxicity was evaluated by NCI-CTCAE (ver. 3) criteria in all 15 treated patients including the 2 ineligible patients. Therapy was tolerated very well with no grade 4 or higher adverse events reported (Table 2), There were 2 grade 3 (atrial fibrillation) and 3 grade 2 adverse events (dyspnea, lymphopenia and osteonecrosis). The patient who developed osteonecrosis was not on bisphosphonates when he was diagnosed with avascular necrosis of the femoral head. There were 22 grade 1 adverse events. Dose reduction to −1 dose level was needed in 1 patient (atrial fibrillation).
Table 2.
Treatment related adverse events
| Adverse Event | Grade | Number |
|---|---|---|
| Arthritis | 1 | 2 |
| Increased aspartate aminotransferase | 1 | 1 |
| Constipation | 1 | 1 |
| Diarrhea | 1 | 1 |
| Dry eye syndrome | 1 | 1 |
| Edema | 1 | 1 |
| Fatigue | 1 | 4 |
| Flushing | 1 | 1 |
| Headache | 1 | 1 |
| Decreased hemoglobin | 1 | 2 |
| Hyperglycemia NOS | 1 | 1 |
| Hyperglycemia | 1 | 1 |
| Hyponatremia | 1 | 1 |
| Memory impairment | 1 | 1 |
| Nausea | 1 | 1 |
| Rash (desquamating) | 1 | 1 |
| Toothache | 1 | 1 |
| Dyspnea | 2 | 1 |
| Lymphopenia | 2 | 1 |
| Osteonecrosis | 2 | 1 |
| Atrial fibrillation | 3 | 2 |
Includes all grade 1 and above toxicities considered unknown, possible, likely or probably related to Cilengitide.
Correlative analysis
In patients tested at baseline for CTCs (n=9), only 1 had any CTCs (range 0–23) reflecting the relative paucity of CTCs. For those with CTC data at progression (n=5), CTC increased from 0 to 1 (2 patients), remained at 0 (2 pt) and decreased from 23 to 0 (1 pt). In patients with baseline CEC data (n=10), median CEC number was 26 (range 0–61). 8 patients had serial CEC counts. At progression (n=7), median CEC was 47 (range 15–148). Low cell counts and RNA yield precluded correlative gene expression studies. The trend of CECs on treatment is shown in figure 3. The significance of the transient increase in CECs on treatment is unclear.
Figure 3.
Circulating endothelial cells on treatment (0 weeks indicates start of treatment).
DISCUSSION
Routine PSA measurement after definitive local treatment and use of early androgen deprivation therapy have resulted in non-metastatic castration resistant prostate cancer disease state which is characterized by rising levels of PSA despite castrate levels of testosterone without other evidence of disease[32]. On the control arms of 2 separate randomized phase III trials evaluating atrasentan and zoledronic acid in non-metastatic CRPC patients, the median time to metastases was 25 and 30 months respectively[33,1]. Non-metastatic CRPC offers a potential therapeutic window to decrease morbidity from CRPC by delaying or preventing systemic metastases yet few trials have been conducted in this stage due to the substantial challenges posed by the lack of measurable disease. However, the natural history of non-metastatic CRPC is variable with greater PSA velocity and absolute PSA value predicting a more aggressive clinical course [1]. A risk adapted approach defined by such factors or other biomarkers including CTCs would certainly optimize clinical trial design in this setting.
In the absence of a control arm, a lead-in period of observation was proposed to utilize each patient as his own control by analyzing changes in PSA slope before and on treatment. We hypothesized that CTC and CEC changes could reflect disease activity and also provide a method of performing gene expression studies to verify drug activity on the intended target (the integrin pathway).
In this trial, there was no evidence of activity of Cilengitide as a single agent in this setting. There are several possible explanations for the result. It is possible that integrin mediated cell signaling was not abrogated adequately. Our ability to verify if this indeed was the case and detect drug effect on the intended target was hampered by the paucity of CTCs for the planned correlative analyses. In retrospect, CTCs (as assayed by the Veridex CellSearch test) were not ideal correlates for this trial as they are infrequently detected in the non-metastatic setting [34–36]. Though CTCs have been shown to be prognostic [37] and possibly predictive of a survival benefit with treatment in metastatic CRPC [38,39], CTC number appears to be dependent on the tumor burden [35,40]. CTCs are detected more frequently and at higher numbers per patient in metastatic prostate cancer. In one study, >65% patients had ≥5 CTCs/7.5ml blood [41]. In contrast, only 14% of patients with localized epithelial cancer have ≥2 CTCs/7.5 ml. This difference becomes especially relevant when gene expression studies are planned on CTCs as ≥ 100 CTCs per patient were necessary in one study to perform such studies [42]. CTCs by currently approved assays are of questionable value in non-metastatic prostate cancer due to low sensitivity. Methods of enrichment for CTCs or alternative techniques of detection could prove promising in non-metastatic CRPC[43].
CECs have been investigated as surrogates for angiogenesis and as prognostic and predictive biomarkers [44,45]. However, experience with CECs in prostate cancer is more limited than with CTCs. One study of CECs in metastatic prostate cancer treated with docetaxel found CEC declines after 2–5 weeks of treatment but not baseline CECs to be of prognostic value[39].
It is conceivable that integrin signaling was indeed blocked (suggested by the activity of Cilengitide at similar doses in GBM and modest activity in metastatic CRPC[46]) but was not adequate in and of itself in non-metastatic CRPC. The presence of multiple integrin molecules and other pro-angiogenic pathways provides significant redundancy in intracellular signaling pathways. Compensatory pathways could be triggered by inhibition of specific molecular targets (e.g. treatment with an anti-angiogenic peptide Angiotensin II (1–7) resulted in higher serum levels of pro-angiogenic factors such as placental derived growth factor [47]). A broad acting pan-integrin inhibitor may show greater clinical activity. Combination of an integrin antagonist with other therapies including conventional chemotherapy could enhance activity.
The trial suffered from a familiar problem seen in previous studies of non-metastatic castration resistant prostate cancer: poor accrual. An ECOG study of chemotherapy compared to ketoconazole (ECOG 1899) closed due to poor accrual. Novel trial designs and endpoints to assess potentially cytostatic therapies in non-metastatic CRPC are urgently needed. PSA based endpoints are likely not suitable to assess activity of cytostatic agents in non-metastatic CRPC. Change in PSA slope was designed into the trial as one possible indicator of drug activity but also relies on PSA. It is also unknown how PSA endpoints relate to clinical objectives in non-metastatic CRPC. The PCCTWG has recommended not relying solely on PSA to stop therapy [32]. In a phase II trial in metastatic CRPC, this approach demonstrated evidence of modest activity for single agent Cilengitide[48,46]. Several investigators have pointed out the drawbacks in utilizing conventional endpoints in trials of targeted agents and have recommended time to event or progression free survival at a particular timepoint as more suitable[32,49,50]. A placebo controlled randomized trial with a clinical end point (e.g. metastasis free survival) may be a more optimal trial design to investigate biological agents in non-metastatic CRPC. The low clinical event rate in the context of non-metastatic CRPC presents a problem in utilizing such an approach as well [1].
There was no MTD identified in the phase I trials of Cilengitide. It is unclear if higher doses of Cilengitide would exhibit increased activity in non-metastatic CRPC. In our trial with this agent in metastatic CRPC, there was a modest increase in TTP between the 500 mg and 2000 mg/dose arms which is the dose we used in the current trial.[46].
Cilengitide was well tolerated but did not elicit PSA responses in this trial of non-metastatic CRPC patients. CTCs are of questionable utility in non-metastatic prostate cancer.
Acknowledgement
Cancer Therapy Evaluation Program (CTEP), PC051382, PC051375, Prostate Cancer Foundation (PCF) N008367, Veridex (formerly Immunicon Corp.)
Footnotes
This study was presented in part at the 2010 American Society of Clinical Oncology Genitourinary Symposium.
References
- 1.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, Wynne C, Murray R, Zinner NR, Schulman C, Linnartz R, Zheng M, Goessl C, Hei YJ, Small EJ, Cook R, Higano CS. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–2925. doi: 10.1200/JCO.2005.01.529. doi:23/13/2918 [pii] 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim T, Flamini E, Mercatali L, Sacanna E, Serra P, Amadori D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer. 2010;116(6):1406–1418. doi: 10.1002/cncr.24896. doi:10.1002/cncr.24896. [DOI] [PubMed] [Google Scholar]
- 3.Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: Targeting the lethal phenotype. J Clin Oncol. 2005;23(32):8232–8241. doi: 10.1200/JCO.2005.03.0841. doi:23/32/8232 [pii] 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- 4.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20(3):203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 5.Cooper CR, Chay CH, Pienta KJ. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia. 2002;4(3):191–194. doi: 10.1038/sj.neo.7900224. doi:10.1038/sj/neo/7900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20(3–4):321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 7.Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: Implications for prostate cancer. J Cell Biochem. 2004;91(1):41–46. doi: 10.1002/jcb.10665. doi:10.1002/jcb.10665. [DOI] [PubMed] [Google Scholar]
- 8.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59(7):1655–1664. [PubMed] [Google Scholar]
- 9.Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, Zahn R, Riethmuller G, Pantel K. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: Establishment of working models for human micrometastases. Cancer Res. 1999;59(1):241–248. [PubMed] [Google Scholar]
- 10.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of alpha(v)beta(3) integrin-mediated cell migration and phosphatidylinositol 3-kinase/akt pathway activation. J Biol Chem. 2000;275(32):24565–24574. doi: 10.1074/jbc.M002646200. doi:10.1074/jbc.M002646200 M002646200 [pii] [DOI] [PubMed] [Google Scholar]
- 11.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26(42):6238–6243. doi: 10.1038/sj.onc.1210429. doi:1210429 [pii] 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91(4):718–729. doi: 10.1002/jcb.10662. doi:10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- 13.Bisanz K, Yu J, Edlund M, Spohn B, Hung MC, Chung LW, Hsieh CL. Targeting ecm-integrin interaction with liposome-encapsulated small interfering rnas inhibits the growth of human prostate cancer in a bone xenograft imaging model. Mol Ther. 2005;12(4):634–643. doi: 10.1016/j.ymthe.2005.05.012. doi:S1525-0016(05)00280-7 [pii] 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Thalmann GN, Sikes RA, Devoll RE, Kiefer JA, Markwalder R, Klima I, Farach-Carson CM, Studer UE, Chung LW. Osteopontin: Possible role in prostate cancer progression. Clin Cancer Res. 1999;5(8):2271–2277. [PubMed] [Google Scholar]
- 15.Ross FP, Chappel J, Alvarez JI, Sander D, Butler WT, Farach-Carson MC, Mintz KA, Robey PG, Teitelbaum SL, Cheresh DA. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268(13):9901–9907. [PubMed] [Google Scholar]
- 16.Cheng SL, Lai CF, Fausto A, Chellaiah M, Feng X, McHugh KP, Teitelbaum SL, Civitelli R, Hruska KA, Ross FP, Avioli LV. Regulation of alphavbeta3 and alphavbeta5 integrins by dexamethasone in normal human osteoblastic cells. J Cell Biochem. 2000;77(2):265–276. doi: 10.1002/(sici)1097-4644(20000501)77:2<265::aid-jcb9>3.0.co;2-6. doi:10.1002/(SICI)1097-4644(20000501)77:2<265∷AID-JCB9>3.0.CO;2-6 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Nemeth JA, Cher ML, Zhou Z, Mullins C, Bhagat S, Trikha M. Inhibition of alpha(v)beta3 integrin reduces angiogenesis, bone turnover, and tumor cell proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis. 2003;20(5):413–420. doi: 10.1023/a:1025461507027. [DOI] [PubMed] [Google Scholar]
- 18.Manes T, Zheng DQ, Tognin S, Woodard AS, Marchisio PC, Languino LR. Alpha(v)beta3 integrin expression up-regulates cdc2, which modulates cell migration. J Cell Biol. 2003;161(4):817–826. doi: 10.1083/jcb.200212172. doi:10.1083/jcb.200212172 jcb.200212172 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 20.Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM. Osteoprotegerin is an alpha vbeta 3-induced, nf-kappa b-dependent survival factor for endothelial cells. J Biol Chem. 2000;275(28):20959–20962. doi: 10.1074/jbc.C000290200. doi:10.1074/jbc.C000290200 C000290200 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. Nf-kappab mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141(4):1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L. Inhibition of angiogenesis and tumor growth by sch221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res. 2001;61(5):2232–2238. [PubMed] [Google Scholar]
- 23.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA. Phase i and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25(13):1651–1657. doi: 10.1200/JCO.2006.06.6514. doi:25/13/1651 [pii] 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79(7):1157–1164. doi: 10.1016/0092-8674(94)90007-8. doi:0092-8674(94)90007-8 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Oliveira-Ferrer L, Hauschild J, Fiedler W, Bokemeyer C, Nippgen J, Celik I, Schuch G. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of fak/src/akt pathway. J Exp Clin Cancer Res. 2008;27:86. doi: 10.1186/1756-9966-27-86. doi:1756-9966-27-86 [pii] 10.1186/1756-9966-27-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS. Alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6(2):105–119. doi: 10.1023/B:AGEN.0000011801.98187.f2. doi:10.1023/B:AGEN.0000011801.98187.f2 5255607 [pii] [DOI] [PubMed] [Google Scholar]
- 27.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, Laug WE. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48(1):151–157. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Taga T, Suzuki A, Gonzalez-Gomez I, Gilles FH, Stins M, Shimada H, Barsky L, Weinberg KI, Laug WE. Alpha v-integrin antagonist emd 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98(5):690–697. doi: 10.1002/ijc.10265. doi:10.1002/ijc.10265 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Eskens FA, Dumez H, Hoekstra R, Perschl A, Brindley C, Bottcher S, Wynendaele W, Drevs J, Verweij J, van Oosterom AT. Phase i and pharmacokinetic study of continuous twice weekly intravenous administration of cilengitide (emd 121974), a novel inhibitor of the integrins alphavbeta3 and alphavbeta5 in patients with advanced solid tumours. Eur J Cancer. 2003;39(7):917–926. doi: 10.1016/s0959-8049(03)00057-1. doi:S0959804903000571 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O'Bryant CL, Baron A, Gallemann D, Colevas D, Eckhardt SG. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (emd 121974), in patients with advanced solid tumors. Ann Oncol. 2007;18(8):1400–1407. doi: 10.1093/annonc/mdm140. doi:18/8/1400 [pii] 10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- 31.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G, et al. Eligibility and response guidelines for phase ii clinical trials in androgen-independent prostate cancer: Recommendations from the prostate-specific antigen working group. J Clin Oncol. 1999;17(11):3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 32.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. doi:26/7/1148 [pii] 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, Qian J, Steinberg J, Carducci M. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113(9):2478–2487. doi: 10.1002/cncr.23864. doi:10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis JW, Nakanishi H, Kumar VS, Bhadkamkar VA, McCormack R, Fritsche HA, Handy B, Gornet T, Babaian RJ. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: Initial results in early prostate cancer. J Urol. 2008;179(6):2187–2191. doi: 10.1016/j.juro.2008.01.102. discussion 2191. doi:S0022-5347(08)00249-8 [pii] 10.1016/j.juro.2008.01.102. [DOI] [PubMed] [Google Scholar]
- 35.Helo P, Cronin AM, Danila DC, Wenske S, Gonzalez-Espinoza R, Anand A, Koscuiszka M, Vaananen RM, Pettersson K, Chun FK, Steuber T, Huland H, Guillonneau BD, Eastham JA, Scardino PT, Fleisher M, Scher HI, Lilja H. Circulating prostate tumor cells detected by reverse transcription-pcr in men with localized or castration-refractory prostate cancer: Concordance with cellsearch assay and association with bone metastases and with survival. Clin Chem. 2009;55(4):765–773. doi: 10.1373/clinchem.2008.117952. doi:clinchem.2008.117952 [pii] 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maestro LM, Sastre J, Rafael SB, Veganzones SB, Vidaurreta M, Martin M, Olivier C, VB DELO, Garcia-Saenz JA, Alfonso R, Arroyo M, Diaz-Rubio E. Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res. 2009;29(11):4839–4843. doi:29/11/4839 [pii] [PubMed] [Google Scholar]
- 37.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13(23):7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. doi:13/23/7053 [pii] 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 38.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. doi:14/19/6302 [pii] 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 39.Strijbos MH, Gratama JW, Schmitz PI, Rao C, Onstenk W, Doyle GV, Miller MC, de Wit R, Terstappen LW, Sleijfer S. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur J Cancer. doi: 10.1016/j.ejca.2010.03.030. doi:S0959-8049(10)00261-3 [pii] 10.1016/j.ejca.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Moreno JG, O'Hara SM, Gross S, Doyle G, Fritsche H, Gomella LG, Terstappen LW. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology. 2001;58(3):386–392. doi: 10.1016/s0090-4295(01)01191-8. doi:S0090-4295(01)01191-8 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV, Terstappen LW, Lilja H, Heller G, Fleisher M, Scher HI. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13(7):2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. doi:13/7/2023 [pii] 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 42.Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, Connelly MC, Terstappen LW, O'Hara SM. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65(12):4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. doi:65/12/4993 [pii] 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 43.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2(25):25ra23. doi: 10.1126/scitranslmed.3000403. doi:2/25/25ra23 [pii] 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgiou HD, Namdarian B, Corcoran NM, Costello AJ, Hovens CM. Circulating endothelial cells as biomarkers of prostate cancer. Nat Clin Pract Urol. 2008;5(8):445–454. doi: 10.1038/ncpuro1188. doi:ncpuro1188 [pii] 10.1038/ncpuro1188. [DOI] [PubMed] [Google Scholar]
- 45.Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15(1):139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 46.Bradley DA, Daignault S, Ryan CJ, Dipaola RS, Smith DC, Small E, Gross ME, Stein MN, Chen A, Hussain M. Cilengitide (emd 121974, nsc 707544) in asymptomatic metastatic castration resistant prostate cancer patients: A randomized phase ii trial by the prostate cancer clinical trials consortium. Invest New Drugs. doi: 10.1007/s10637-010-9420-8. doi:10.1007/s10637-010-9420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petty WJ, Miller AA, McCoy TP, Gallagher PE, Tallant EA, Torti FM. Phase i and pharmacokinetic study of angiotensin-(1–7), an endogenous antiangiogenic hormone. Clin Cancer Res. 2009;15(23):7398–7404. doi: 10.1158/1078-0432.CCR-09-1957. doi:1078-0432.CCR-09-1957 [pii] 10.1158/1078-0432.CCR-09-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley DA, Daignault S, Ryan CJ, Dipaola RS, Smith DC, Small E, Gross ME, Stein MN, Chen A, Hussain M. Cilengitide (emd 121974, nsc 707544) in asymptomatic metastatic castration resistant prostate cancer patients: A randomized phase ii trial by the prostate cancer clinical trials consortium. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9420-8. doi:10.1007/s10637-010-9420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adjei AA, Christian M, Ivy P. Novel designs and end points for phase ii clinical trials. Clin Cancer Res. 2009;15(6):1866–1872. doi: 10.1158/1078-0432.CCR-08-2035. doi:1078-0432.CCR-08-2035 [pii] 10.1158/1078-0432.CCR-08-2035. [DOI] [PubMed] [Google Scholar]
- 50.Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002;7(5):401–409. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]