Abstract
How does motivation interact with cognitive control during challenging behavioral conditions? Here, we investigated the interactions between motivation and cognition during a response conflict task and tested a specific model of the effect of reward on cognitive processing. Behaviorally, participants exhibited reduced conflict during the reward vs. no-reward condition. Brain imaging results revealed that a group of subcortical and fronto-parietal regions was robustly influenced by reward at cue processing and, importantly, that cue-related responses in fronto-parietal attentional regions were predictive of reduced conflict-related signals in the medial prefrontal cortex (PFC)/anterior cingulate cortex during the upcoming target phase. Path analysis revealed that the relationship between cue responses in the right intraparietal sulcus (IPS) and interference-related responses in the medial PFC during the subsequent target phase was mediated via signals in the left fusiform gyrus, which we linked to distractor-related processing. Finally, reward increased functional connectivity between the right IPS and both bilateral putamen and bilateral nucleus accumbens during the cue phase, a relationship that covaried with across-individual sensitivity to reward in the case of the right nucleus accumbens. Taken together, our findings are consistent with a model in which motivationally salient cues are employed to upregulate top-down control processes that bias the selection of visual information, thereby leading to more efficient stimulus processing during conflict conditions.
Introduction
Cognition interacts with motivation in important ways, and a significant amount of research in the past decade has attempted to elucidate the neural bases of these interactions (Watanabe, 2002; Braver et al., 2007; Pessoa, 2009). Whereas it is clear that motivation affects cognitive function, the mechanisms by which cognition is altered remain poorly understood. One possibility is that the effect is relatively unspecific, such as an “energizing” function that speeds up performance. Contrariwise, motivation may have more specific effects on cognition, for instance, by enhancing executive function. In the latter scenario, motivation would be expected, for instance, to influence the selection of information pertinent to the task at hand.
To probe this question, we investigated the effects of reward during a response-conflict task (Fig. 1A). Based on recent findings (Engelmann et al., 2009; Pessoa and Engelmann, 2010), we anticipated that motivation would enhance processing in attentional regions in fronto-parietal cortex. We reasoned that these regions would then be better positioned to exert top-down control that favored the processing of task-relevant information in visual cortex during the target phase – for instance, by amplifying task-relevant information (Egner and Hirsch, 2005) – and/or by improving filtering of task-irrelevant information (Polk et al., 2008). Thus, behavioral conflict would be reduced during the reward vs. no-reward condition; likewise conflict-related brain responses in medial prefrontal cortex (MPFC) (Carter et al., 1998) would be expected to be reduced. A central goal of the present study was to understand the link between the processing of cues signaling potential reward and interference-related activity during the subsequent target phase (see Fig. 1A). Critically, we sought to advance our understanding of the effects of motivation on cognition by evaluating potential network interactions that might have subserved these effects. Specifically, we employed both mediation analysis and functional connectivity analysis to test the model outlined in Fig. 2 that summarizes the interactions hypothesized in the present study.
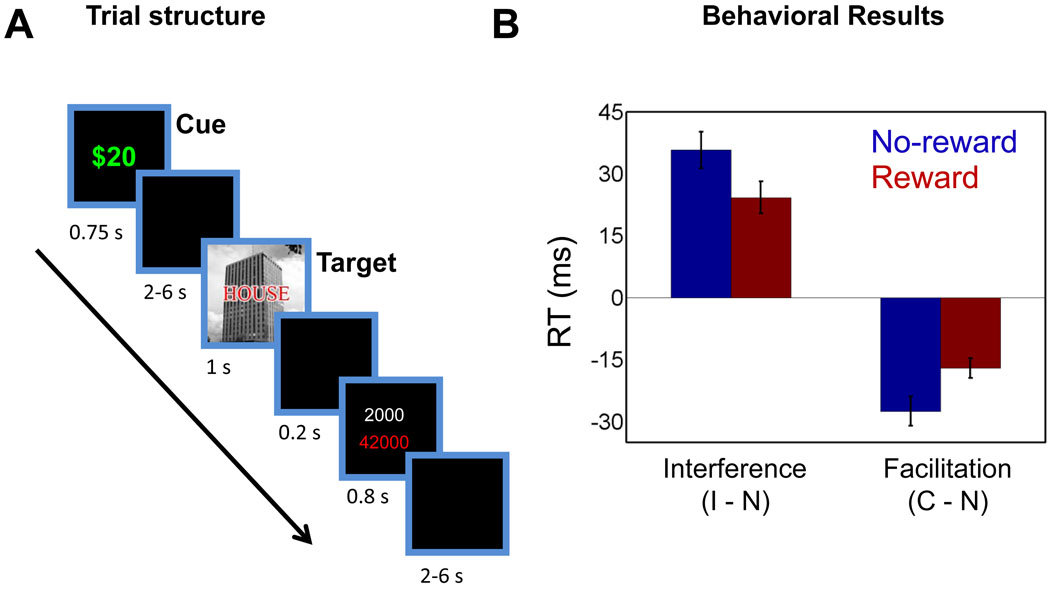
Figure 1.
Response-conflict paradigm and behavioral data. Subjects performed a response conflict task under two motivational contexts: (A) during the reward condition (shown here), a cue stimulus (“$20”) signaled that participants would be rewarded for fast and correct performance; during the control condition (not shown here), a cue stimulus (“$00”) signaled that no reward was involved. Following a variable-length delay, a target stimulus containing a picture of a house or building was shown together with a task-irrelevant word (an incongruent condition is illustrated here). After the target stimulus, subjects were informed about the potential reward and about the total number of points accrued. Finally, a variable-length delay ended the trial. (B) Plot of interference (incongruent vs. neutral) and facilitation (congruent vs. neutral) reaction time scores illustrating the interaction patterns. C, congruent trials; I, incongruent trials; N, neutral trials. Error bars represent the standard error of the mean.
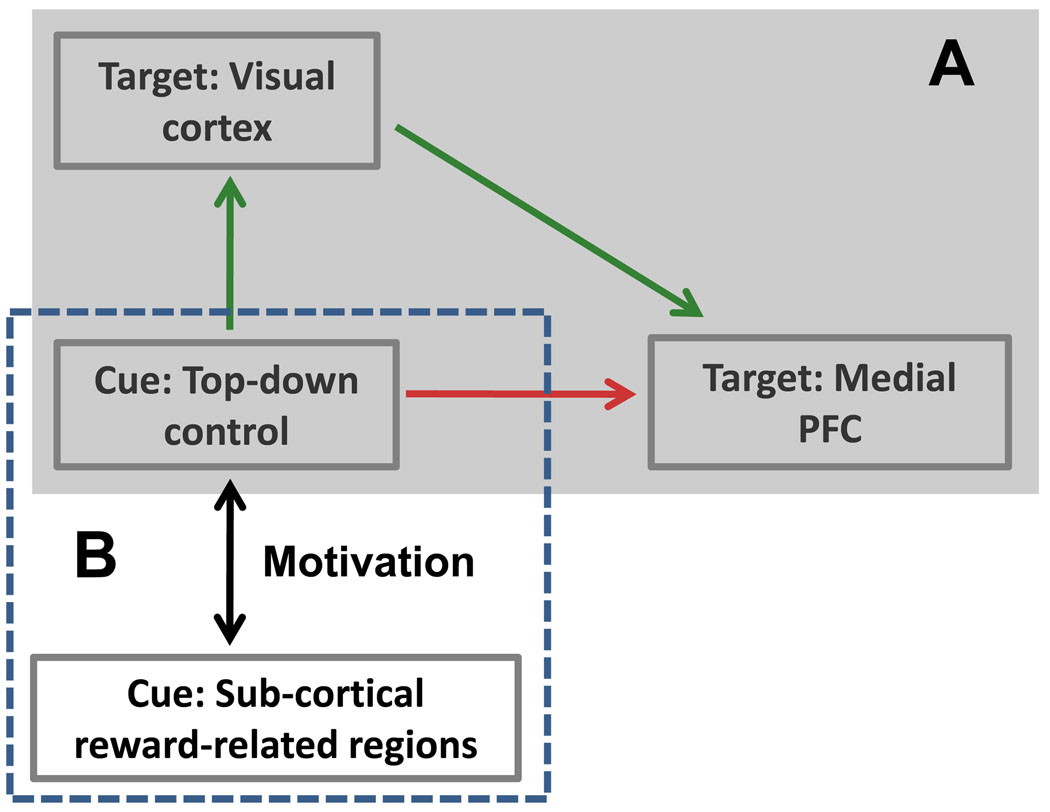
Figure 2.
Hypothesized network interactions. (A) The relationship between attentional control implemented in fronto-parietal cortex during the cue phase, and conflict-related activity in medial PFC during the subsequent target phase was hypothesized to be mediated via the amount of target/distractor processing in visual cortex. (B) We also hypothesized that the functional coupling between fronto-parietal cortex and subcortical regions involved in reward processing would be affected by motivational context. PFC, prefrontal cortex.
Methods
Subjects
Fifty-four volunteers (22 ± 5 years old; 28 females) participated in the study, which was approved by the Institutional Review Board of Indiana University, Bloomington. All subjects were in good health with no past history of psychiatric or neurological disease. All participants had normal or corrected-to-normal vision. All participants gave informed written consent. One male participant’s data were excluded from the analysis because of excessive head motion (greater than one voxel size) and data from three other participants (one male, two females) were removed because of poor performance (less than 60% correct; two of the participants reported being unable to concentrate due to fatigue; the other responded only during reward trials).
Personality questionnaire
Before the fMRI experiment, participants completed the Behavioral Activation System (BAS) scale, which assesses multiple personality characteristics related to sensitivity to reward (Carver and White, 1994). As in our recent study (Engelmann et al., 2009), we employed the BAS-drive subscale, which has the highest internal reliability (Carver and White, 1994; Jorm et al., 1998), has been suggested to be the strongest predictor of positive affective responses to reward (Beaver et al., 2006), and has been proposed to provide a clearer measure of appetitive motivation and approach behavior (Dawe et al., 2004).
Stimuli and behavioral paradigm
Compound scene-plus-word stimuli were employed (Fig. 1A). These categories were chosen because they are associated with category-related responses in visual cortex. Specifically, scenes strongly recruit the parahippocampal gyrus, bilaterally (Epstein et al., 1999), whereas words robustly recruit the left fusiform gyrus (Polk and Farah, 2002; McCandliss et al., 2003). Accordingly, images of houses and buildings overlaid with the words “HOUSE”, “BLDNG” were used to create congruent and incongruent trials; the letter string “XXXXX” was used to create a neutral trial type. Each trial begun with a cue indicating the motivational condition (“$00” or “$20”) shown for 750 ms. Following a 2–6 s jittered inter-stimulus-interval, a composite scene-plus-word target image was shown for 1000 ms. Participants were instructed to press the index finger button to indicate that they saw a house image and the middle finger button to indicate that they saw a building image irrespective of the overlaid word (responses were always made with the right hand; the response button mapping was counterbalanced across participants). Thus, image stimuli were task relevant and word stimuli were task irrelevant. After 200 ms from the offset of the target, participants received visual feedback for 800 ms consisting of the total number of points won on the trial, as well as their cumulative earnings (in points) until that moment in time (so as to minimize the “cognitive load” related to keeping track of earnings). During reward trials, participants won 2000 points per trial if they were fast and accurate in their response and they won zero points during error or slow trials. The reaction time (RT) threshold to determine “fast” responses was set at 800 ms based on behavioral pilot data. During no-reward trials, participants won zero points irrespective of their performance. During correct trials, points won were displayed in white color and during error trials points were displayed in blue color, thus providing performance feedback for both motivational conditions. Finally, a 2–6 s inter-trial-interval containing a blank screen terminated the trial.
Both inter-stimulus and inter-trial intervals were selected from an exponential distribution favoring shorter intervals and helped in the estimation of separate cue- and target-related responses (see below). Before the start of the experiment, participants were informed that they could earn 2000 points during each reward trial if performance was fast and accurate and that at the end of the experiment the points would be converted to cash, such that they could earn an additional $20 based on their performance. At the end of the experiment, each participant’s base pay ($25) was potentially increased such that for each point they earned .01 cents (average reward-based earning: $18).
We used Presentation software (Neurobehavioral Systems, Albany, CA, USA) for the presentation of visual stimuli and responses were recorded using an MRI-compatible response box inside the scanner room.
Each participant performed 6 runs of the conflict task. Each run consisted of 36 trials, resulting in a total of 216 trials and 36 trials per condition. Trial order was balanced such that each trial type was preceded by the other trial types with the same frequency. For each condition, trials were equally divided between those containing house and building scenes, and different images of houses and buildings were used during the control and reward conditions (counterbalanced across participants). Within condition, each image was used only once during congruent, incongruent, or neutral trial types. No scene images appeared in consecutive trials to minimize potential priming effects (Mayr et al., 2003). Finally, each run ended with a 10-s fixation cross allowing us to record the hemodynamic response for the final trial of the run.
Functional localizer
At the end of the main experimental runs, subjects participated in an additional functional “localizer” run during which they performed a simple one-back working memory task administered in a alternating blocked fashion and containing novel word and scene stimuli. Five blocks were performed per condition, each with 12 trials during which a neutral word or scene (house/building) stimulus was presented for 1000 ms and followed by a 250-ms blank screen. Blocks lasted 15 s and were separated by a 15-s rest block during which participants passively viewed a white fixation cross on the screen.
MR data acquisition
MR data were collected using a 3 Tesla Siemens TRIO scanner (Siemens Medical Systems, Erlangen, Germany) with a 32-channel head coil (without parallel imaging). Each scanning session began with a high-resolution MPRAGE anatomical scan (TR = 1900 ms, TE = 4.15 ms, TI = 1100 ms, 1 mm isotropic voxels, 256 mm field of view). Subsequently, in each functional run of the main experiment, 165 EPI volumes were acquired with a TR of 2500 and TE of 25 ms. Each volume consisted of 44 oblique slices with a thickness of 3 mm and an in-plane resolution of 3 × 3 mm (192 mm field of view). Slices were positioned approximately 30 degrees relative to the plane defined by the line connecting the anterior and posterior commissures. For the final functional localizer run, 123 EPI volumes were collected with the same scanning parameters.
Behavioral data analysis
Mean RT data (excluding condition-specific extreme points that were situated more than 3 standard deviations from the mean; 3.4% on average) and mean accuracy data were initially analyzed using a 2 motivation (reward, no-reward) x 3 congruency (neutral, congruent, incongruent) repeated-measures ANOVA. Additional 2 × 2 repeated-measures ANOVAs were evaluated to further characterize the results.
General fMRI data analysis
Pre-processing of the data was done using tools from the AFNI software package (Cox, 1996) (http://afni.nimh.nih.gov/afni). The first 3 volumes of each functional run were discarded to account for equilibration effects. The remaining volumes were slice-time corrected using Fourier interpolation such that all slices were realigned to the first slice to account for the timing offset between slices. Six-parameter rigid-body motion correction within and across runs was performed using Fourier interpolation (Cox and Jesmanowicz, 1999) such that all volumes were spatially registered to the volume acquired closest in time to a particular subject’s high-resolution anatomy. To normalize the functional data to Talairach space (Talairach and Tournoux, 1988), initially each subject’s high-resolution MPRAGE anatomical volume was spatially registered to the so-called TT_N27 template (in Talairach space) using a 12-parameter affine transformation; the same transformation was then applied to the functional data. All volumes were spatially smoothed using a Gaussian filter with a full-width at half maximum of 6 mm (i.e., two times the voxel dimension). Finally, the signal intensity of each voxel was scaled to a mean of 100 (on a per run basis), which allowed the interpretation of the estimated regression coefficients in terms of percent signal change.
Voxelwise analysis
Each participant’s fMRI data were analyzed using a general linear model framework in AFNI. There were a total of 8 experimental event types in the design matrix: no-reward and reward events during the cue phase, and neutral, congruent, and incongruent events during the target phase, separately for the no-reward and reward conditions. In addition, both error and slow trials (in both cases pooled across reward and no-reward conditions) were treated separately from correct trials, and involved two additional regressors, one accounting for cue-related activity and another accounting for target-related activity. No assumptions were made about the shape of the hemodynamic response function as responses were estimated via deconvolution. The lack of shape assumption was especially pertinent given that cue-related responses could be more or less sustained depending on brain region (and experimental condition; see below). Responses were estimated starting from event onset to 15 s post onset using cubic spline basis functions. This method is closely related to the use of finite impulses (“stick functions”), the commonly employed technique that can be considered the simplest form of basis expansion. Cubic splines allow for a smoother approximation of the underlying responses, instead of the discrete approximation obtained by finite impulses. Constant, linear, and quadratic terms were included for each run separately (as covariates of no interest) to model baseline and drifts of the MR signal. As an index of activation, for each event type, we averaged the estimated responses at 5 and 7.5 s after stimulus onset (as determined via the spline-based estimates). Exploratory analyses at earlier stages of data collection suggested that a similar pattern of results was obtained when the peak response was used as response index. Finally, note that no separate estimate of the reward outcome phase was determined, given its close temporal proximity to the target phase. In this manner, the estimates of target-related activity included contributions from the outcome phase (see supplementary text). Note, however, that given our interest in evaluating interaction terms, the effect of the outcome phase was largely canceled out. Specifically, because both incongruent and neutral trials during the reward condition had a reward-outcome phase, in our analyses we focused on difference scores, that is (incongruent – neutral)reward, and contrasted this difference to (incongruent – neutral)no-reward, (i.e., we evaluated the motivation by congruency interaction). However, as our design did not allow the separation of outcome-from target-related activity, we could not rule out the possibility that some difference existed in outcome-related activity as a function of cognitive trial type (incongruent, neutral), particularly in the reward condition.
Estimation of cue and target responses
Event-related designs allow the estimation of different event types when they occur in a randomized fashion. However, the present study, by design, required a fixed order between the cue and target phases. In comparable situations, at times, a partial-trial design is employed (Ollinger et al., 2001). Here, instead, we randomized the delay between cue and target phases and the inter-trial interval. Because cue-related activation may have been sustained (at least in some brain regions), sequential dependencies may not have been fully eliminated (Ruge et al., 2009). We evaluated our design in a set of computer simulations described in the supplementary text. As described, the contamination in the estimation procedure produced by the sequential nature of our design was negligible. In other words, target-related activity at 5 and 7.5 sec after stimulus onset was not contaminated by cue-related responses. These results are also consistent with the fact that the correlations between different regressors in our model were only modest, and did not exceed .37. Critically, our main objective was to investigate a potential motivation by congruency interaction; accordingly, any spillover of motivation-related cue signals would lead to a main-effect type of contamination, and not one exhibiting an interaction pattern (note that information about congruency was not provided at the time of the cue).
Group analysis
Whole-brain voxelwise random-effects analyses were conducted separately for the cue and target phases and were restricted to grey-matter voxels based on the FSL automated segmentation tool (http://www.fmrib.ox.ac.uk/fsl/). For the cue phase, a paired t test was run to compare the activations between reward and no-reward conditions. For the target phase, a separate 2 × 2 repeated-measures ANOVA was run to probe the interaction between motivation (reward, no-reward) and interference (incongruent vs. neutral). Given the working hypotheses of this study, when reporting interactions (see Table 2), we focused on those regions that also exhibited a main effect of interference (incongruent vs. neutral pooled over reward and no-reward conditions). Note that no bias was associated with this approach as main effects and interactions are orthogonal to each other. Finally, all voxelwise statistical tests were corrected for multiple comparisons using the false discovery rate approach (Genovese et al., 2002) at p < .05. Given that this method corrects only at the voxel level and not in terms of topological features such as peaks or regions of activation, we report here activations with a minimum cluster extent of 10 contiguous voxels. As illustrated by Chumbley and Friston (2009), this approach reduces the likelihood that false discovery rate correction will reveal spurious activations.
Table 2.
Voxelwise analysis at target phase (peak Talairach coordinates, and F values)
| REWARD x INTERFERENCE | INTERFERENCE | REWARD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Location | x | y | z | F(1,49)* | x | y | z | F(1,49)* | x | y | z | F(1,49)* | |
| Occipital | |||||||||||||
| Middle Occipital gyrus | R | 41 | −70 | 17 | 18.96 | ||||||||
| Inferior Occipital gyrus | L | −37 | −76 | −11 | 20.75 | ||||||||
| Parietal | |||||||||||||
| Intraparietal sulcus | R | 35 | −57 | 35 | 16.89 | 35 | −53 | 34 | 18.35 | 38 | −61 | 41 | 25.03 |
| L | −32 | −59 | 34 | 12.2 | −31 | −55 | 38 | 15.64 | |||||
| Inferior Parietal lobe | R | 41 | −43 | 38 | 30.81 | ||||||||
| Frontal | |||||||||||||
| rostral ACC | R | 8 | 26 | 20 | 14.11 | 5 | 32 | 14 | 23.82 | ||||
| dorsal ACC | R | 8 | 8 | 38 | 23.35 | ||||||||
| Medial PFC | R/L | 0 | 19 | 45 | 15.05 | 0 | 19 | 45 | 14.82 | ||||
| SMA/pre-SMA | R/L | 0 | 12 | 50 | 7.52 | 0 | 12 | 50 | 23.86 | ||||
| Frontal Eye Field | R | 35 | −7 | 47 | 18.34 | ||||||||
| L | −35 | −6 | 51 | 16.24 | |||||||||
| Precentral gyrus | R | 42 | 7 | 31 | 11.39 | 40 | 2 | 29 | 16.98 | ||||
| L | −41 | 2 | 31 | 12.2 | −42 | 5 | 29 | 29.39 | |||||
| Superior Frontal gyrus | R | 31 | 46 | 14 | 8.97 | 21 | 45 | 24 | 17.32 | ||||
| Middle Frontal gyrus | R | 46 | 14 | 32 | 11.98 | 44 | 27 | 30 | 14.84 | 39 | 53 | 3 | 18.49 |
| L | −48 | 24 | 21 | 17.59 | |||||||||
| Anterior Insula | R | 31 | 19 | 8 | 17.45 | 31 | 19 | 8 | 15.7 | 35 | 17 | −4 | 31.96 |
| L | −31 | 19 | 4 | 14.09 | |||||||||
| Subcortical | |||||||||||||
| Caudate | R | 9 | 6 | 11 | 17.57 | 12 | 6 | 14 | 10.72 | ||||
p < .05 (False Discovery Rate corrected; minimum cluster extent: 10 voxels)
Relationship between cue- and target-related activity
An important goal of the present study was to understand the link between cue-related responses, which were anticipated to vary as a function of reward, and target-related responses, which were anticipated to vary as a function of both congruency and reward. Accordingly, we correlated cue-and target-related responses. For the cue phase, we considered differential reward vs. no-reward responses. For the target phase, we employed an interaction index that contrasted the differential response incongruent vs. neutral during the reward and no-reward conditions: [(incongruent – neutral)reward – (incongruent – neutral)no-reward]. As outlined in the next paragraph, this interaction index was investigated in the medial prefrontal cortex (PFC)/anterior cingulate cortex (ACC) given this region’s purported role in conflict monitoring (Botvinick et al., 2001). In the paper, we refer to this site as “medial PFC”.
Given our focused hypotheses, we performed Pearson correlation analysis at the region of interest (ROI) level. Cue-related ROIs were selected among fronto-parietal regions involved in attentional processing, namely the intraparietal sulcus (IPS), frontal eye field (FEF), middle frontal gyrus (MFG), and pre-supplementary motor area/supplementary motor area (pre-SMA/SMA; see Table 1 for locations). A 5-mm radius sphere was utilized and centered on the peak voxel of the reward vs. no-reward contrast at the group level. In a similar fashion, target-related activity was obtained from a 5-mm radius sphere centered on the peak voxel of the interaction in the medial PFC, as revealed by the 2 motivation (reward, no-reward) x 2 congruency (neutral, incongruent) repeated-measures ANOVA at the group level. Note that because this interaction-based selection criterion is orthogonal to a main effect of motivation, which was used to define the cue-related regions, no selection bias was incurred in the creation of our ROIs (as stated, no congruency information was available at the time of the cue).
Table 1.
Voxelwise analysis at cue phase (peak Talairach coordinates and t values)
| Reward vs. No-reward | |||||
|---|---|---|---|---|---|
| Peak Location | x | y | z | t(49)* | |
| Occipital | |||||
| Calcarine sulcus | R/L | 0 | −80 | 3 | 4.39 |
| Middle Occipital gyrus | R | 26 | −88 | 3 | 7.27 |
| L | −22 | −88 | 3 | 6.09 | |
| InferiorOccipital gyrus | R | 34 | −80 | −5 | 4.19 |
| L | −34 | −78 | −4 | 4.23 | |
| Precuneus | R | 25 | −69 | 24 | 3.4 |
| L | −23 | −71 | 30 | 2.85 | |
| Parietal | |||||
| Intraparietal sulcus | R | 24 | −54 | 40 | 2.56 |
| L | −27 | −52 | 41 | 3.54 | |
| Inferior Parietal lobe | L | −28 | −42 | 41 | 2.92 |
| Frontal | |||||
| rostral ACC | R | 13 | 39 | 8 | 2.83 |
| ACC | R | 6 | 8 | 39 | 6.38 |
| L | −8 | 7 | 39 | 5.03 | |
| SMA/pre-SMA | R/L | 0 | −6 | 57 | 5.72 |
| Frontal Eye Field | R | 34 | −11 | 48 | 5.86 |
| L | −31 | −12 | 50 | 4.6 | |
| Precentral gyrus | L | −48 | −4 | 37 | 5.03 |
| Middle Frontal gyrus | R | 26 | 46 | 25 | 3.74 |
| L | −28 | 35 | 29 | 4.41 | |
| Anterior Insula | R | 31 | 17 | 11 | 4.03 |
| L | −35 | 26 | 5 | 3.7 | |
| Subcortical | |||||
| Midbrain | R | 7 | −15 | −8 | 4.63 |
| L | −10 | −18 | −8 | 5.3 | |
| Putamen | R | 17 | 9 | −2 | 5.34 |
| L | −19 | 9 | 2 | 5.7 | |
| Caudate | R | 10 | 9 | 2 | 5.66 |
| L | −10 | 9 | 2 | 5.77 | |
| Nucleus Accumbens | R | 13 | 6 | −7 | 5.03 |
| L | −13 | 6 | −7 | 4.77 | |
p < .05 (False Discovery Rate corrected; minimum cluster extent: 10 voxels)
Network analysis
To probe potential network interactions, we performed mediation analysis (Baron and Kenny, 1986), with the goal of assessing the model shown in Fig. 2A (for recent applications to neuroimaging data, see (Wager et al., 2008; Lim et al., 2009; Atlas et al., 2010)). This model formalizes the hypothesis that attentional control during the cue phase is linked to the amount of target/distractor processing in visual cortex during the target phase, thereby determining the extent of conflict-related responses during the target phase. A standard statistical approach was adopted, which involved evaluating the following components (Baron and Kenny, 1986): (1) total effect c (initial variable → outcome, which can also be written in terms of the indirect effect ab plus the direct effect c'), (2) indirect path a (initial variable → intervening variable), (3) indirect path b (intervening variable → outcome, after controlling for the initial variable), and (4) direct effect c' (initial variable → outcome, after controlling for the intervening variable). The final mediation effect was tested by assessing the significance of the product of paths a and b (see Fig. 6) by using Sobel’s test (Sobel, 1982).
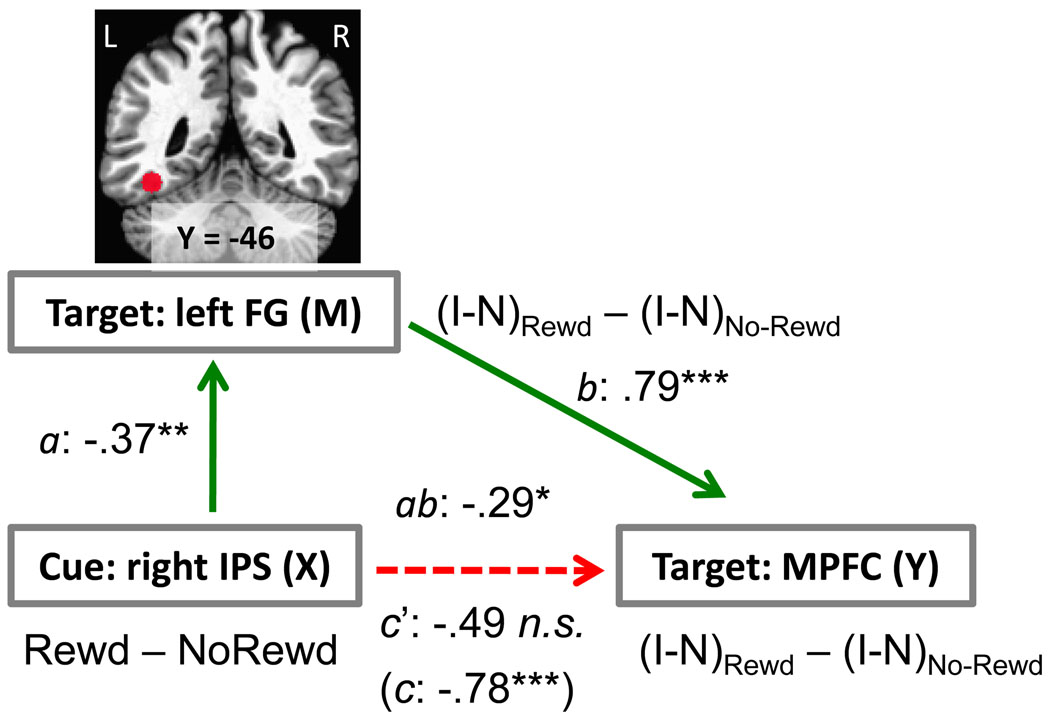
Figure 6.
Network analysis. The relationship between cue-related responses in the right IPS (X: initial variable) and target-related responses in the medial prefrontal cortex (Y: outcome variable) was mediated via the left fusiform gyrus (M: mediator/intervening variable). The X, Y, and M variables employed in the analysis involved contrast or interaction terms, as indicated in red font. The letters a, b, c, c’, refer to estimated path coefficients (see SI Text). FG, fusiform gyrus; IPS, intraparietal sulcus; MPFC, medial prefrontal cortex; Rewd, reward condition; No-Rewd, no-reward condition; I, incongruent trial; N, neutral trial. *P < .05, **P < .01, ***P < .005, two-tailed. The dotted line indicates a path that was not statistically significant after the mediator was taken into account.
Cue-related responses from the right IPS that were modulated by reward and correlated with conflict-related activity in the medial PFC at the target phase were employed as the initial predictor in the mediation analysis (see Results section below). For the mediator variable, responses evoked in the left fusiform gyrus during the target phase were employed, and medial PFC target-related activity comprised the outcome variable. Because we were interested in evaluating how reward affected these interactions, the analyses employed indices involving differential responses, as indicated in Fig. 6. In particular, an interaction-type index was employed during the target phase. In the left fusiform gyrus, we reasoned that this provided a measure of word-related processing (incongruent vs. neutral; note that “XXXX” was employed in the neutral condition) and how this was affected by reward. For the medial PFC, we assumed that the interaction term measured how conflict-related processing (incongruent vs. neutral) varied with reward.
For the network analysis, the fronto-parietal ROIs were the same as the ones in the previous correlation analysis between cue and target activity. As stated, the interaction-based selection criterion used to select the MPFC ROI is orthogonal to a main effect of motivation, which was used to define the fronto-parietal ROIs. Accordingly, no selection bias was incurred in the creation of our ROIs. In addition, the ROI for the left fusiform gyrus was obtained from separate localizer data, as described in the next section.
In addition to the right IPS ROI, cue-related responses from the pre-SMA/SMA and right FEF ROIs also exhibited significant correlations with conflict-related activity in the medial PFC ROI at the target phase (see Results section below). Therefore, for completeness, we ran two additional mediation analyses: one employing pre-SMA/SMA cue-related responses as the initial variable, another employing right FEF cue-related responses as the initial variable.
Definition of regions of interest (ROIs) in visual cortex
ROIs in visual cortex, namely bilateral parahippocampal gyrus and left fusiform gyrus, were defined based on data from the localizer run. These ROIs were defined at the individual level via the contrast of word vs. scene blocks. Specifically, for the left fusiform gyrus, voxels were considered that exhibited stronger response for word relative to scene blocks (p < .005, uncorrected); for the parahippocampal gyrus, the reverse contrast was employed. In both cases, a 5-mm radius sphere centered on the peak voxel was used. Mean individual peak coordinates were as follows: left fusiform gyrus: X = −39, Y=−46, Z = −14; left parahippocampal gyrus: X = −24, Y=−44, Z = −6; right parahippocampal gyrus: X = 25, Y=−44, Z = −7.
For the left fusiform gyrus ROI, we probed interference and facilitation effects. Accordingly, for target-related responses, two separate 2 × 2 repeated-measures ANOVAs were run to probe the interactions between motivation (reward, no-reward) and interference (incongruent, neutral) and between motivation (reward, no-reward) and facilitation (congruent, neutral). In the parahippocampal gyrus, surprisingly, we observed reduced responses during the reward relative to the no-reward condition irrespective of congruency, potentially due to a contamination of the responses linked to reward outcome (see supplementary text). Accordingly, we did not perform ROI analyses on parahippocampal gyrus data.
Functional connectivity analysis at cue phase
We investigated potential interactions between the right IPS ROI and subcortical reward-related ROIs by performing a functional connectivity analysis based on the trial-by-trial methods proposed by D’Esposito and colleagues (Rissman et al., 2004). Application of these methods to connectivity analysis has been reported in several previous studies (Buchsbaum et al., 2005; Daselaar et al., 2006; Nee et al., 2007; Camara et al., 2008). Here, for each participant, trial-based responses were estimated during both the cue and target phases (as a function of trial type). Responses to individual trials were estimated simultaneously. Specifically, for each participant, a design matrix was set up such that each trial’s cue and target phase was coded as a separate event. To provide response estimates for the cue and target phases of each trial, a hemodynamic response was assumed (Cohen, 1997). Although in so doing we assumed that evoked responses were transient (which may not have been the optimal assumption for some brain regions, overall, trial-based estimates provided an excellent fit to the data as illustrated in Fig. S2 (for an evaluation of this method in the context of functional connectivity analysis, see (Zhou et al., 2009)). Note, however, that without assuming a fixed shape, the estimation of single-trial responses during relatively fast-paced event-related designs is poor, and possibly unfeasible.
The right IPS ROI and subcortical reward-related ROIs were based on 5-mm radius spheres centered on the peak voxel of the contrast reward vs. no-reward defined at the group level. Trial-based responses were averaged across all voxels within the seed ROI as well as within reward-related ROIs to create representative estimates. We then calculated the trial-by-trial Pearson correlations between right IPS responses and subcortical reward-related ROIs, separately for the reward and no-reward conditions. To contrast the correlations at the group level, they were initially transformed (via Fisher’s Z-transform) and then compared via a paired t test. Because correlation values are orthogonal from differential activation scores, our procedure did not incur circularities in the selection process.
Results
Behavioral results
A 2 motivation (reward, no-reward) x 3 congruency (neutral, congruent, incongruent) repeated-measures ANOVA on mean RT data (Fig. 1B) revealed main effects of motivation (F(1,49) = 99.24, p < .001) and congruency (F(2,98) = 128.18, p < .001), as well as a motivation x congruency interaction (F(2,98) = 12.11, p < .001). Further 2 × 2 ANOVAs revealed that reward decreased both interference (incongruent vs. neutral) and facilitation effects (congruent vs. neutral), consistent with the notion that motivation enhanced attentional filtering, thereby reducing the influence of the task-irrelevant word item (see supplementary text for additional behavioral results; see also Fig. S1-B for error data).
Functional MRI results
Cue and target phase
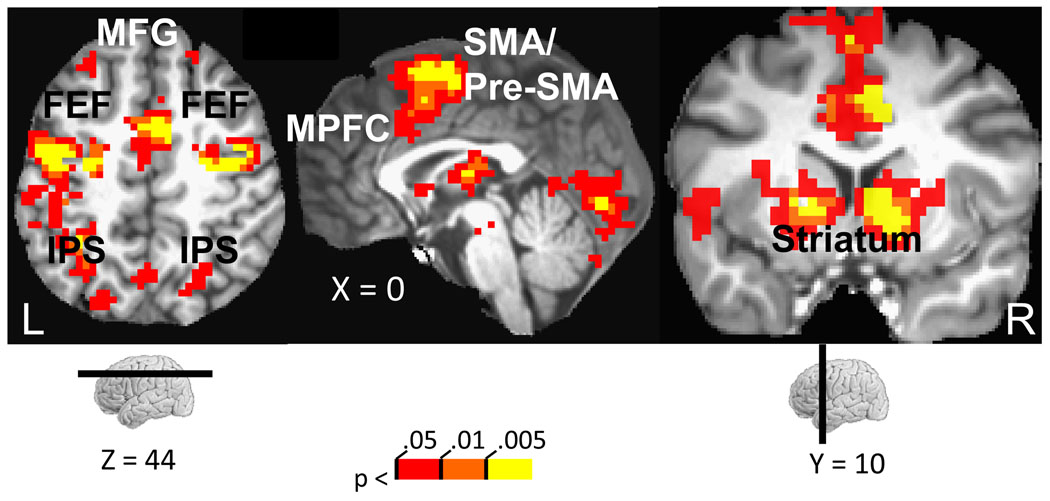
Cue-related responses were probed by contrasting the reward vs. no-reward conditions. Increased evoked responses during the reward condition were observed in several brain regions, including dorsal and ventral striatum, subcortically, and fronto-parietal regions, cortically (Fig. 3; Table 1). The target phase involved several task components, including attentional selection, conflict-related processing (during incongruent trials), and reward outcome processing (during rewarded trials). Our main goal was to evaluate interference-related responses in MPFC and how these were influenced by reward. Accordingly, as with behavioral data (see supplementary text), we performed a 2 motivation (reward, no-reward) x 2 congruency (neutral, incongruent) repeated-measures ANOVA, which revealed a significant main effect and, critically, a significant interaction in MPFC (Fig. 4A; Table 2), such that interference-related activity (incongruent – neutral) was reduced during the reward compared to no-reward condition (Fig. 4B). No significant effect of motivation was observed on facilitation (congruent – neutral) related activity in the MPFC. Based on these voxelwise analyses at the cue and target periods, we defined a series of ROIs that allowed us to evaluate the model shown in Fig. 2 in a focused manner, as described in the subsequent sections (note also that all ROI analyses avoided problems of “circularity” (Kriegeskorte et al., 2009); see Methods).
Figure 3.
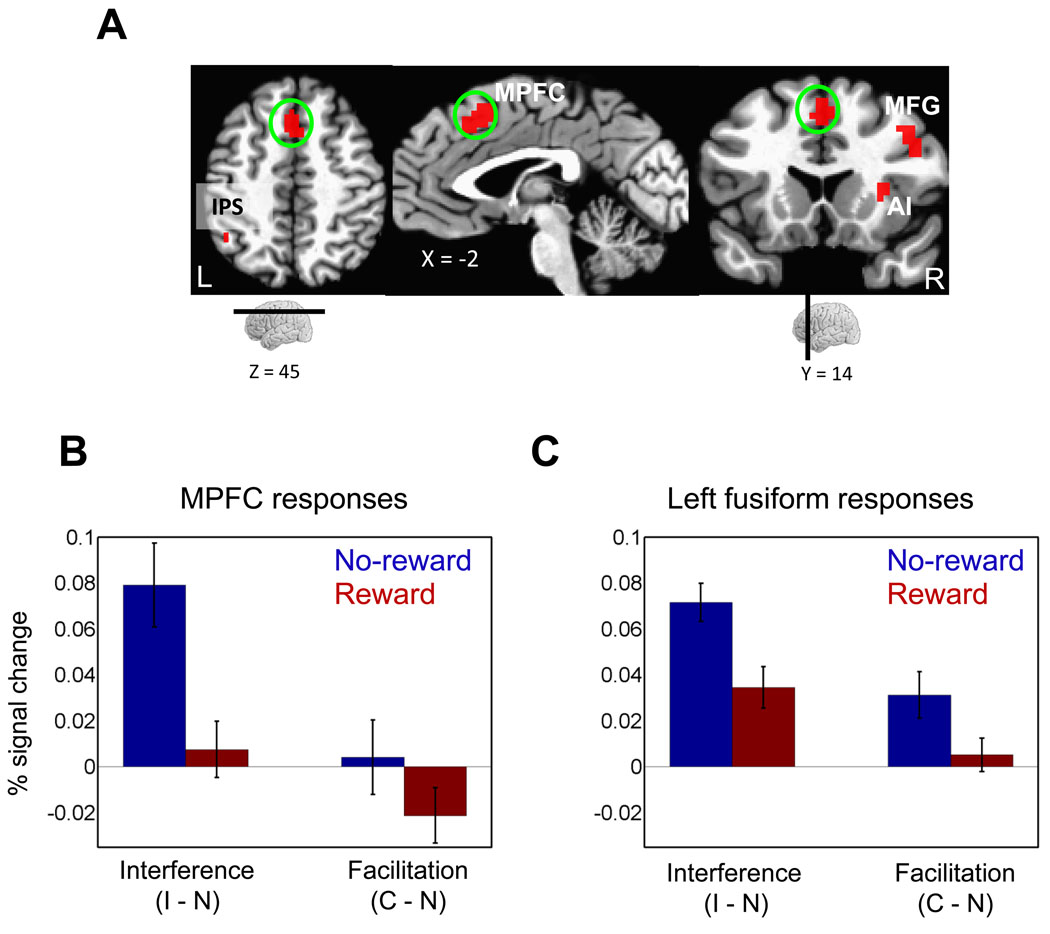
Cue-related responses. Increased evoked responses to reward relative to no-reward were observed across fronto-parietal cortex and subcortical regions. MPFC, medial prefrontal cortex; FEF, frontal eye field; IPS, intraparietal sulcus; MFG, middle frontal gyrus; SMA/pre-SMA, supplementary motor area/ pre-supplementary motor area. The color scale represents p-values corrected for multiple comparisons via false discovery rate.
Figure 4.
Target-related responses. (A) Regions that showed significant motivation (reward, no-reward) x interference (incongruent, neutral) interactions. MPFC, medial prefrontal cortex; IPS, intraparietal sulcus; MFG, middle frontal gyrus; AI, anterior insula. (B) Plot of interference (incongruent vs. neutral) and facilitation (congruent vs. neutral) effects in the medial prefrontal cortex ROI illustrating the interaction patterns. (C) Plot of interference (incongruent vs. neutral) and facilitation (congruent vs. neutral) effects in the left fusiform gyrus ROI illustrating the interaction patterns. C, congruent trials; I, incongruent trials; N, neutral trials. Error bars in panels B and C represent the standard error of the mean.
Relationship between cue- and target-related activity
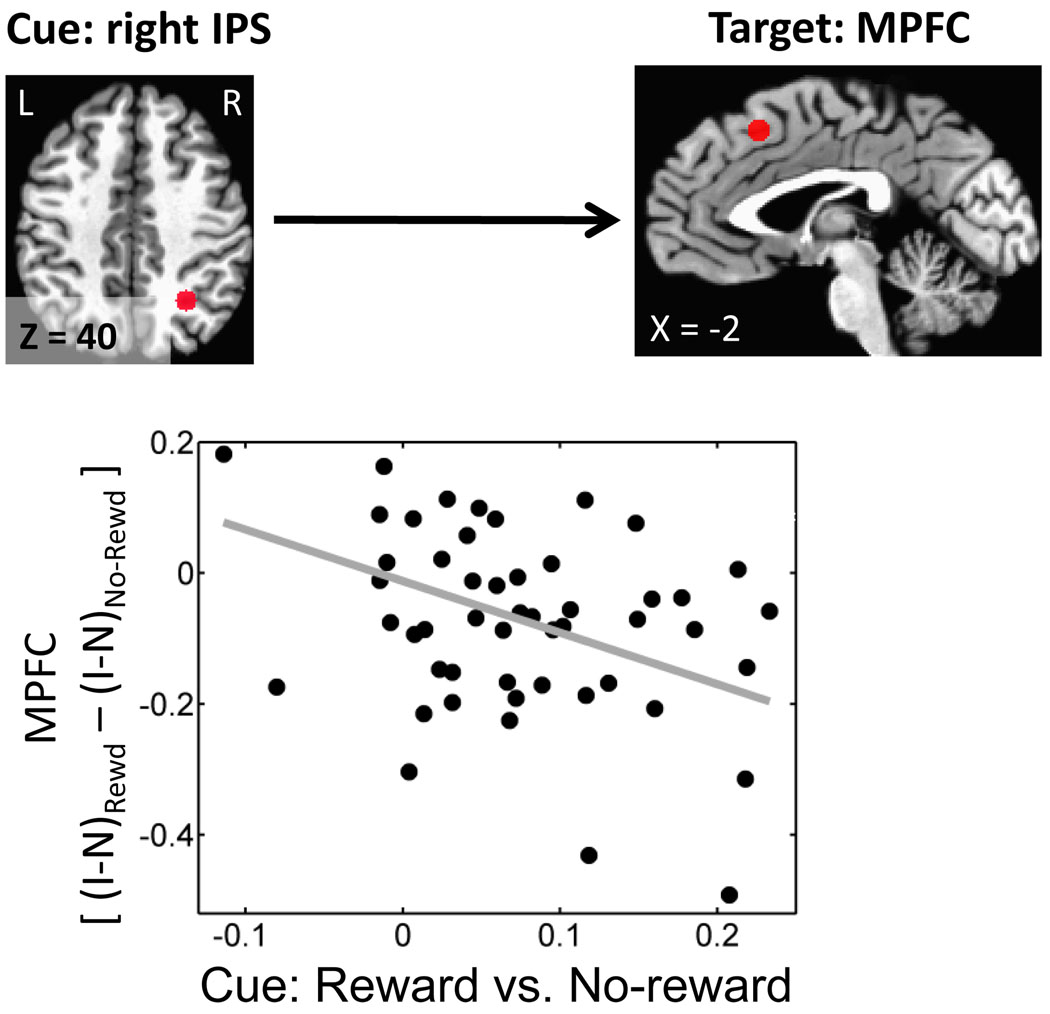
To probe the link between cue processing and interference-related activity during the target phase, and how these were influenced by reward, we performed correlation analyses. Cue-related ROIs were selected among fronto-parietal regions involved in attentional processing, namely the IPS, FEF, MFG, and pre-SMA/SMA (see Table 1 for locations). The target-related ROI focused on the MPFC, which was defined based on the motivation by congruency interaction determined in the voxelwise analysis (see Table 2 for location). Significant Pearson correlations were obtained with the right IPS (r(49) = −.39, P < .005; Fig. 5), pre-SMA/SMA (r(49) = −.34, P < .05) and right FEF (r(49) = −.39, P < .05), such that increased cue-related responses in fronto-parietal regions during the reward condition were correlated with reduced interference in MPFC during the subsequent target phase.
Figure 5.
Relationship between cue- and target-phase responses. The scatter plot illustrates the relationship between cue-related responses in the right IPS ROI and conflict-related responses in the medial prefrontal cortex ROI. Increased differential responses during the cue phase were linked to decreased conflict-related responses during the subsequent target phase. The linear fit is shown for illustration purposes. IPS, intraparietal sulcus; MPFC, medial prefrontal cortex; Rewd, reward condition; No-Rewd, no-reward condition.
Visual responses during the target phase in category-responsive regions
We investigated category-related responses in inferior temporal cortex, including those in the left fusiform gyrus, which responds to word stimuli, and in the parahippocampal gyrus, bilaterally, which responds to scene stimuli. We probed target-related responses in these ROIs (defined based on separate localizer runs), according to 2 × 2 repeated-measures ANOVAs. In the left fusiform gyrus, significant motivation x congruency interactions were detected both in the case of interference (incongruent vs. neutral : F(1,49) = 11.39, P < .001) and facilitation (congruent vs. neutral : F(1,49) = 4.44, P < .05), such that both incongruent vs. neutral and congruent vs. neutral differential activity was reduced during the reward relative to the no-reward condition (Fig. 4C). Given that parallel behavioral results were observed, namely, decreased interference and facilitation RT effects, visual responses were consistent with the notion that word items underwent reduced processing during the reward condition, possibly due to increased attentional filtering.
Mediation analysis
As described above, differential reward vs. no-reward responses in fronto-parietal regions to the cue were inversely correlated with interference-related activity during the target phase in the MPFC (see Fig. 5). Based on connectivity analysis, Wang and colleagues recently suggested that the IPS plays an important role in coordinating sites in medial and lateral PFC during conflict resolution (Wang et al., 2009). The IPS is also important for top-down attentional control and can bias the selection of visual information (Kastner et al., 1999; Hopfinger et al., 2000; Greenberg et al., 2010). We thus reasoned that, in our task, the relationship between the IPS and medial PFC might be mediated via visual cortical regions involved in processing target and distractor stimuli (see Fig. 2A). We tested this hypothesis by carrying out a mediation analysis by focusing on the left fusiform gyrus, the region identified in the previous section. A statistically significant mediation was observed, such that the total effect between the right IPS and the MPFC (see Fig. 5) was significantly reduced once the contribution of the left fusiform gyrus was taken into account – a relationship evaluated by assessing the significance of the product of path weights a and b (ab = −.29, SE = .14, t(48) = 2.02, P < .05; Fig. 6).
We interpreted the relationship between responses in the right IPS and left fusiform gyrus in terms of a filtering mechanism. If filtering was indeed involved, responses in the right IPS should be correlated with reduced responses in the left fusiform gyrus not only during conflict processing as established, but also during facilitation (behaviorally, facilitation decreased, too). Accordingly, across participants, we correlated differential responses in the right IPS during the cue phase (reward vs. no-reward) and facilitation-related responses in the left fusiform gyrus at the target phase [(congruent – neutral)reward – (congruent – neutral)no-reward]. Stronger responses in the right IPS were associated with reduced scores in the left fusiform gyrus (r(50) = −.47, P < .005), consistent with the filtering proposal.
Functional connectivity analysis at cue phase and individual differences
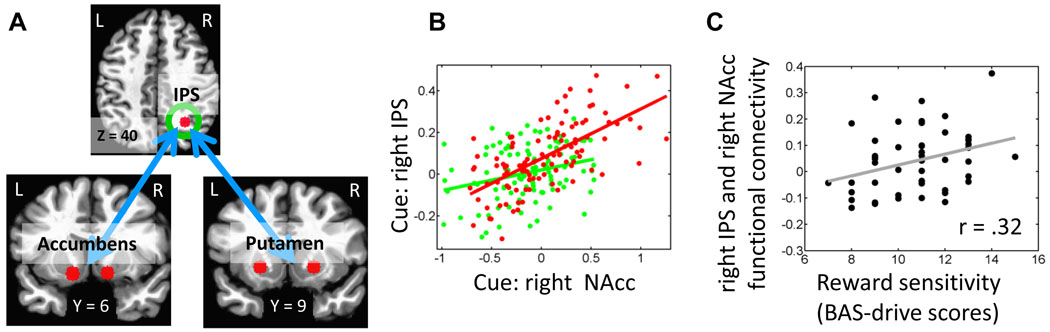
We reasoned that, if motivationally salient cues engage fronto-parietal regions more robustly during the cue phase, they would exhibit increased “functional coupling” with reward-related brain regions that are capable of evaluating the motivational significance of the cues (see Fig. 2B). Accordingly, we investigated the functional connectivity between the right IPS ROI and subcortical regions that were sensitive to the reward manipulation. Increased correlation with the right IPS during reward (vs. no-reward) was observed in the following subcortical ROIs: bilateral putamen (right: t(49) = 2.63, P <.05; left: t(49) = 2.28, P < .05) and bilateral nucleus accumbens (right: t(49) = 2.37, P < .05; left: t(49) = 2.67, P < .05; Fig. 7A and B).
Figure 7.
Functional connectivity. (A) Regions exhibiting stronger functional connectivity with the right IPS ROI during the cue-phase (reward vs. no-reward). (B) The scatter plot illustrates the trial-by-trial relationship between right IPS and right nucleus accumbens signals during the reward (red dots and line) and no-reward (green dots and line) conditions. Data are illustrated for one individual. The lines are included for illustration purposes and are linear fits to the data. IPS, intraparietal sulcus; NAcc, nucleus accumbens. (C) The scatter plot illustrates the relationship between BAS-drive scores and functional connectivity between right IPS and right nucleus accumbens. Participants with higher BAS-drive scores exhibited increased functional coupling. The linear fit to the data is included for illustration purposes.
Do individual differences in reward sensitivity influence the strength of coupling between the right IPS and these subcortical ROIs? To evaluate this possibility, BAS-drive scores, which have been proposed to be a good measure of appetitive motivation and approach behavior (Dawe et al., 2004), were correlated with functional connectivity scores. Positive correlations were observed between right IPS-right accumbens connectivity and BAS-drives scores (r(50) = .32, P < .05; Fig. 7C).
Discussion
How does motivation interact with cognitive control during challenging behavioral conditions? We hypothesized that a motivationally salient cue stimulus would allow participants to upregulate control and thereby handle a response-conflict inducing stimulus in an improved manner. Our goal was not only to probe how evoked responses were influenced by cognitive condition and reward, but to advance our understanding of the network interactions subserving the impact of reward on cognitive control and visual processing. Behaviorally, participants exhibited reduced conflict during the reward vs. no-reward condition. Brain imaging results are discussed next.
Stronger cue-related responses during the reward condition were observed in a network of fronto-parietal regions involved in attention, several of which have been shown to generate preparatory cue-related activity during attentional tasks (Kastner and Ungerleider, 2000; Corbetta and Shulman, 2002). Previously, we observed that responses in fronto-parietal attentional regions increased as a function of the absolute magnitude of an incentive (Engelmann et al., 2009). In the present study, several sub-cortical sites also exhibited stronger reward-related signals at the cue phase, including the nucleus accumbens, caudate, putamen, and a site in the midbrain that appeared to include the ventral tegmental area, and to some extent the substantia nigra. All of these sub-cortical structures are involved in reward processing (Schultz, 2000; Delgado, 2007; Camara et al., 2009; Haber and Knutson, 2010).
During the target phase, paralleling the behavioral data, we were interested in probing conflict-related responses (i.e., incongruent vs. neutral). In particular, we targeted responses in the MPFC, as this region has been implicated in conflict processing (Botvinick et al., 2001) and other executive functions (Brown and Braver, 2005; Weissman et al., 2005; Aarts et al., 2008). Regardless of the precise functions carried out by the MPFC, in specific paradigms it appears to provide an index of the amount of conflict (MacDonald et al., 2000; van Veen et al., 2001; Kerns et al., 2004; Yeung and Nieuwenhuis, 2009). Here, conflict-related responses in the MPFC decreased during the reward vs. no-reward condition (i.e., an interference by reward interaction was observed).
To investigate the relationship between cue- and target-related responses, we correlated differential (i.e., reward vs. no-reward) responses at the cue phase with interference-related responses (i.e., (incongruent – neutral)reward – (incongruent – neutral)no-reward) at the target phase. We observed an inverse linear relationship between cue and target responses, such that as differential cue-related responses increased in fronto-parietal cortical regions involved in attentional control, conflict-related responses in MPFC decreased at the subsequent target phase. We interpret these findings as suggesting that motivationally salient cues enhanced top-down control, thereby reducing conflict-related responses (as well as reducing behavioral conflict) – and thus increasing the likelihood of reward.
In this study, we tested a model of how increased top-down control may affect conflict processing, namely by biasing information processing in visual cortex in favor of task-relevant information (Egner and Hirsch, 2005) and against task-irrelevant information (only the latter was tested because our design did not allow us to isolate scene-related processing; see supplementary text). To test this model, we performed a path analysis, which revealed that the relationship between the right IPS (during cue) and the medial PFC (during target) was mediated via responses in the left fusiform gyrus (during target). For the latter region, we reasoned that the contrast of incongruent and neutral trials provided an index of word-related processing, as only the former included a word stimulus (but note that this approach does not assume that the left fusiform gyrus is strictly dedicated to word processing; see (Xue and Poldrack, 2007)). According to the path analysis, stronger differential responses in the right IPS were observed in conjunction with decreased word-related responses in the left fusiform gyrus in the reward vs. no-reward condition (path a), consistent with the idea that enhanced attentional control biased processing in a way that decreased the influence of word stimuli. Put another way, reward improved the filtering of task-irrelevant stimuli. At the same time, decreased word-related processing in the left fusiform gyrus was observed in conjunction with reduced conflict-related responses in the MPFC after controlling for right IPS cue responses (both during the target phase; path b), strengthening the notion that the filtering of word stimuli reduced the amount of conflict registered in MPFC. This interpretation is consonant with the behavioral data where both interference and facilitation RT effects were reduced with reward. In particular, the fact that facilitation also decreased speaks in favor of the filtering notion, as the potentially advantageous effect of a congruent word stimulus was reduced. It is worth pointing out that we did not find evidence for enhancement effects in visual cortex because, as stated above, our design did not allow us to investigate this scenario. It is quite possible, however, that task-relevant information (i.e., scenes) represented in the parahippocampal gyrus was also amplified (Egner and Hirsch, 2005; Polk et al., 2008) in the present task.
The role of the parietal cortex in general, and the IPS in particular, in controlling attention is well documented (Corbetta and Shulman, 2002; Bisley and Goldberg, 2003; Serences and Yantis, 2006; Shomstein and Behrmann, 2006; Knudsen, 2007). Furthermore, the parietal cortex is important for the filtering of distractor information (Friedman-Hill et al., 2003). Although great emphasis is often put on the role of prefrontal cortex in conflict processing, interestingly, the parietal cortex also appears to be involved (Egner et al., 2007). Recently, it was proposed that conflict resolution relies on attentional processes carried out by the IPS via interactions with the medial and lateral PFC (Wang et al., 2009). Our findings are consistent with these reports and showed that the IPS is involved in attentional control during conflict processing.
We also observed effects of reward during the cue phase on lateral prefrontal cortex (for example bilateral middle frontal gyrus; MFG). This effect is noteworthy given the importance of the lateral PFC in the implementation of attentional control (Kerns et al., 2004; Banich et al., 2009). However, the network interactions that were observed involving the IPS were not detected in the case of the MFG. It is possible that, in the present case, the IPS was involved more specifically given its role in attentional filtering (Friedman-Hill et al., 2003), and that the MFG would be involved in other types of control settings (but see Egner et al, 2005).
A final goal of our study was to advance the understanding of the mechanisms by which motivation influences top-down control. In particular, we anticipated that the coupling between subcortical regions important in evaluating the motivational significance of the cue stimulus and fronto-parietal attentional regions important for attention would be enhanced during rewarded trials. Indeed, increased functional coupling with the right IPS was observed with the bilateral nucleus accumbens and bilateral putamen (note that directionality cannot be inferred from the analysis performed).
Personality traits related to appetitive motivation and approach behavior influence both how participants react to incentives behaviorally (van Steenbergen et al., 2009; Savine et al., 2010) and, importantly, the strength of the modulatory effects of reward on brain activity (Locke and Braver, 2008; Engelmann et al., 2009). Here, the functional connectivity between right IPS and right nucleus accumbens was also correlated with BAS-drive scores, which suggests that reward sensitivity influences how regions interact with each other during motivated conditions.
Conflict-related tasks have generated a healthy database of behavioral and neuroimaging findings in the past couple of decades. Our goal in the present study was to capitalize upon this rich paradigm to probe interactions between motivation and executive function. Our findings suggest that participants are able to employ motivationally salient cues to upregulate top-down control processes that bias the selection of visual information in a way that reduces both behavioral conflict and conflict-related brain responses. It is noteworthy that, in the case of congruent trials, this strategy entailed less use of the task-irrelevant information and, accordingly, reduced facilitation scores. It should be pointed out, however, that in most trials this strategy did not compromise the likelihood of obtaining a reward given that RTs were already fast in the congruent condition. Overall, given that participants were not provided information about the upcoming cognitive trial type (congruent, neutral, or incongruent) at the time of the cue, the overall best strategy might have been to attempt to reduce the impact of the task-irrelevant words. Finally, it appears that the ability to upregulate control is associated with how reward-related brain regions process motivationally salient cues and interact with fronto-parietal regions important for the control of attention.
Supplementary Material
Acknowledgements
Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589) and the Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc. We would like to thank Morta Lapkus for assistance with summarizing personality scores, Andrew Bauer for assistance with data collection, and Jong-Moon Choi for discussions.
Contributor Information
Srikanth Padmala, Department of Psychological and Brain Sciences Indiana University, Bloomington, IN spadmala@indiana.edu.
Luiz Pessoa, Department of Psychology University of Maryland, College Park, MD pessoa@umd.edu.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J Neurosci. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in working memory. Oxford: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Munte TF. Functional connectivity of reward processing in the brain. Front Hum Neurosci. 2008;2:19. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Ye Z, Munte TF. Reward networks in the brain as captured by connectivity measures. Front Neurosci. 2009;3:350–362. doi: 10.3389/neuro.01.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chumbley JS, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox R, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addict Behav. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju EC, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: Transient and sustained motivational effects. Frontiers in Human Neuroscience. 2009;3 doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill S, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proceedings of the National Academy of Sciences USA. 2003;2003:4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of spatial and feature-based attention in frontoparietal cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B. Using the BIS/BAS scales to measure behavioral inhibition and behavioral activation: factor structure, validity and norms in a large community sample. Personality and Individual Differences. 1998;26:49–58. [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior Cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci U S A. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cogn Affect Behav Neurosci. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. Neuroimage. 2007;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive function? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010:4. doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. Functional MRI evidence for an abstract, not perceptual, word-form area. J Exp Psychol Gen. 2002;131:65–72. doi: 10.1037//0096-3445.131.1.65. [DOI] [PubMed] [Google Scholar]
- Polk TA, Drake RM, Jonides JJ, Smith MR, Smith EE. Attention enhances the neural processing of relevant features and suppresses the processing of irrelevant features in humans: a functional magnetic resonance imaging study of the stroop task. J Neurosci. 2008;28:13786–13792. doi: 10.1523/JNEUROSCI.1026-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Ruge H, Goschke T, Braver TS. Separating event-related BOLD components within trials: the partial-trial design revisited. Neuroimage. 2009;47:501–513. doi: 10.1016/j.neuroimage.2009.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Beck SM, Edwards BG, Chiew KS, Braver TS. Enhancement of cognitive control by approach and avoidance motivational states. Cognition & Emotion. 2010;24:338–356. doi: 10.1080/02699930903381564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Behrmann M. Cortical systems mediating visual attention to both objects and spatial locations. Proc Natl Acad Sci U S A. 2006;103:11387–11392. doi: 10.1073/pnas.0601813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. 1982. pp. 290–312. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- van Steenbergen H, Band GP, Hommel B. Reward Counteracts Conflict Adaptation: Evidence for a Role of Affect in Executive Control. Psychol Sci. 2009 doi: 10.1111/j.1467-9280.2009.02470.x. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective connectivity of the fronto-parietal network during attentional control. J Cogn Neurosci. 2009;22:543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Integration across multiple cognitive and motivational domains in monkey prefrontal cortex. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 326–337. [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Xue G, Poldrack RA. The neural substrates of visual perceptual learning of words: implications for the visual word form area hypothesis. J Cogn Neurosci. 2007;19:1643–1655. doi: 10.1162/jocn.2007.19.10.1643. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nieuwenhuis S. Dissociating response conflict and error likelihood in anterior cingulate cortex. J Neurosci. 2009;29:14506–14510. doi: 10.1523/JNEUROSCI.3615-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Thompson WK, Siegle G. MATLAB toolbox for functional connectivity. Neuroimage. 2009;47:1590–1607. doi: 10.1016/j.neuroimage.2009.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.