Calcium (Ca2+) and the cAMP-dependent protein kinase (PKA) are pleiotropic cellular regulators and both exert powerful, diverse effects on cytoskeletal dynamics, cell adhesion and cell migration. Localization, by A-kinase anchoring proteins (AKAPs), of PKA activity to the protrusive leading edge, integrins, and other regulators of cytoskeletal dynamics has emerged as an important facet of its role in cell migration. Additional recent work has firmly established the importance of Ca2+ influx through mechanosensitive transient receptor potential (TRP) channels and through store-operated Ca2+ entry (SOCE) in cell migration. Finally, there is considerable evidence showing that these mechanisms of Ca2+ influx and PKA regulation intersect – and often interact – and thus may work in concert to translate complex extracellular cues into the intracellular biochemical anisotropy required for directional cell migration.
Introduction
Successful cell migration requires cells to interpret their extracellular environment through diverse receptors in order to regulate and rearrange distinct cytoskeletal and adhesive events in subcellular space. Thus, the cell migration machinery must be regulated by signaling intermediates that can be activated by diverse stimuli and can exert control over a large number of downstream targets – all with temporal and spatial specificity. In this regard, two systems – Ca2+ influx and signaling through the cAMP-dependent protein kinase (PKA) – are perfectly suited [1-3]. However, despite intense study of Ca2+ and PKA, and despite – or perhaps because of – their myriad possible connections to cell migration, our understanding of how these signals control cell motility is far from complete.
Some of the first formal descriptions of Ca2+ patterns in migrating cells established the enduring tenet that [Ca2+]i during migration is highly variable in both time and subcellular space (e.g.[4,5]). Since then, the mechanisms controlling this variability have been a major research focus. Recent work has highlighted the importance of Ca2+ influx from two distinct sources – namely, mechanosensitive channels and store-operated Ca2+ entry (SOCE) – in the regulation of migration.
Over a similar period, the role of PKA as both a positive and negative regulator of cell motility was established in a number of systems [3]. A critical advance in understanding this regulation came from incorporating the importance of AKAP-mediated localization in specifying the effects of PKA [6] and demonstrating that PKA is spatially regulated in motile cells [7••], providing a mechanism through which its promiscuous activity can be brought to bear specifically on processes in migration. Recent work has confirmed and expanded this observation, bringing specific migration- or cytoskeleton-associated AKAPs to the fore and providing mechanisms for focusing PKA activity even more tightly on discrete cytoskeletal events.
Finally, a wealth of literature demonstrates the substantial cross-talk and coincidence between Ca2+ and cAMP/PKA [8•-11••], and recent observations further support the hypothesis that these pathways may act in concert to achieve the specificity and diversity of controls required for the regulation of cell migration.
Ca2+ in migration I – It's a stretch, but there are flickers of hope
Recent work investigating Ca2+ in migration has capitalized on our growing understanding of the importance of mechanical forces in cell migration [12] by demonstrating important roles for mechanosensitive stretch-activated Ca2+ channels (SACCs) during migration. Motile cells generate a number of cellular forces [12] which inevitably impinge on the shape and tension of the plasma membrane. Given that nearly every type of cell expresses some form of mechanosensitive or stretch-activated ion channel [13,14•], one would hypothesize that such channels might regulate and/or be regulated by cell migration. Indeed, the importance of SACCs in migration was definitively demonstrated by Lee and co-workers who reported that tension-dependent, SACC-mediated Ca2+ transients are required for trailing edge release and retraction [15••]. Conversely, a role for SACCs in the leading edge was established later by studies in which local application of Gd3+, an effective (but not exclusive) inhibitor of SACC-mediated Ca2+ influx, to the leading (but not trailing) edge resulted in global inhibition of traction forces and migration [16•]. In neither case, however, was the identity of the SACC known.
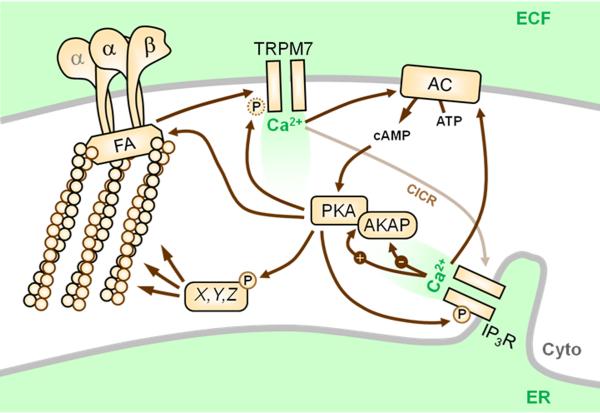
A wealth of recent evidence [17-27] suggests that excellent candidates for such SACCs include members of the transient receptor potential (TRP) family [28]. A prominent, recent example is a report describing that TRPM7 ([23••]; Figure 1), a unique SACC which also contains an active α-kinase domain [28], mediates generation of transient microdomains of elevated cytosolic Ca2+ - coined ‘Ca2+ flickers’ – that occur within leading edge lamellae in an actomyosin contractility-dependent manner. The importance of TRPM7 in migration is further underscored by work showing that TRPM7 interacts with F-actin and myosin IIA, and phosphorylates the latter, in an agonist-dependent manner [17], and that TRPM7-mediated Ca2+ entry regulates cell-substrate adhesion through localized modulation of m-calpain activity [18].
Figure 1.
Connections between Ca2+ flickers and PKA. During cell migration, highly localized Ca2+ transients – ‘flickers’ – are mediated by TRPM7 through a mechanism that requires cellular tension through integrins (αβ), focal adhesions (FA), and actomyosin contractility. Ca2+ entering through TRPM7 stimulates Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER) through the IP3 receptor (IP3R), which increases flicker amplitude. Local Ca2+ may directly activate nearby Ca2+-sensitive adenylyl cyclases (AC) or indirectly activate others, e.g. via protein kinase C. Ca2+ can also exert both positive and negative effects on AKAP function. PKA is known to enhance or activate TRPM7 currents and our unpublished observations suggest that PKA can directly phosphorylate the channel (see Figure 3). PKA can also phosphorylate and enhance the activity of the IP3R, which contributes to flicker amplitude. Finally, many focal adhesion proteins and cytoskeletal regulatory proteins (X,Y,Z) are regulated by PKA, and this may also represent a route for cross-talk between PKA and TRPM7 activity. ECF, extracellular fluid; Cyto, cytoplasm.
Other TRP channels also have connections to the cytoskeleton and cell migration [29]. TRPC1 is recruited to the leading edge of glioma cells migrating in response to EGF, and inhibition or silencing of TRPC function inhibits EGF-induced chemotaxis [24]. At least one function of TRPC1 appears to be the generation of a gradient of Ca2+ in leading edge lamellipodia (in contrast to the punctuate leading edge flickers generated by TRPM7) required for chemotactic cell polarity [21]. Expression of TRPV1 enhances motility in some systems [20] but exerts a negative role in others [27], while the closely-related TRPV4 is required for cytoskeletal remodeling and reorientation in endothelial cells in response to cyclic stretch [22] – an effect quite likely dependent on TRPV4's functional and physical association with β1 integrins [22,26] and F-actin [19]. Even in these detailed reports, however, the exact molecular mechanisms coupling TRP and other mechanosensitive channels to cellular tension are not well understood [14•], and thus represent an important avenue for future study.
Ca2+ in migration II – What's in store?
As mentioned above, TRPM7-mediated flickers do not act alone, but rather occur on the backdrop of a diffuse gradient of Ca2+ that increases from the front to the rear of the cell [23••]. Also, while flicker activity was completely eliminated by RNAi against TRPM7, flicker amplitude (but not probability) was also partially blunted by inhibition of the function or expression of the inositol (3,4,5) trisphosphate receptor (IP3R) [23••]. This suggests that Ca2+ flickers are locally triggered by tension-dependent activation of TRPM7, but are amplified by local IP3R-mediated store release ([23••]; Figure 1). In broad support of this, Ca2+ influx through TRPM7 was shown to promote Ca2+-induced Ca2+ release through the ryanodine receptor during migration [25].
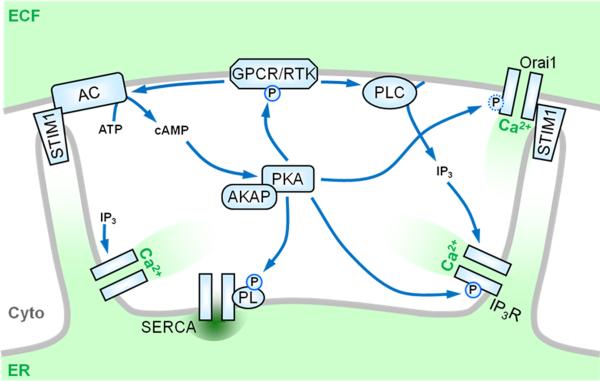
The connection between Ca2+ stores and cell migration is further highlighted by recent work focusing on store-operated Ca2+ entry (SOCE) mediated by STIM1 and Orai1. In SOCE, receptor-mediated activation of phospholipases C leads to IP3-mediated depletion of Ca2+ from the ER, which in turn stimulates Ca2+ influx through the plasma membrane ([30] and Figure 2). Groundbreaking work (reviewed in [30]) identified STIM1 as the ER-localized Ca2+ sensor which, upon store depletion, interacts with and activates Orai1 at the plasma membrane to allow influx of extracellular Ca2+. Recent work elegantly demonstrates that STIM1, Orai1, and SOCE play critical roles in the migration of a number of cell types. Specifically, genetic inhibition of Orai1 or STIM1 or selective pharmacological inhibition of SOCE inhibits the in vitro migration of breast cancer cells [31••], smooth muscle cells [32•], neutrophils [33•], and endothelial cells [34•], and also inhibits breast tumor metastasis [31••] and angiogenesis [34•] in vivo. Of note, there is strong evidence demonstrating that, in addition to Orai1, STIM1 can activate TRPC1 and possibly other TRPC isoforms in response to store-depletion [30], and it may be this axis of SOCE that regulates some programs of chemotaxis (see above and [24]).
Figure 2.
Connection between store-operated Ca2+ entry (SOCE) and PKA. SOCE, and its principal mediators STIM1 and Orai1, play increasingly important roles in the migration of a number of cell types. During SOCE, signals generated by receptors (e.g. G-protein coupled receptors (GPCR) or receptor tyrosine kinases (RTK)) at the plasma membrane activate phospholipases C (PLC) to generate IP3, which causes the release of Ca2+- from intracellular stores via the IP3R. Store depletion activates STIM1, which re-localizes within the ER membrane to allow interaction with and activation of the plasma membrane Ca2+ channel Orai1. Store-depletion has also been shown to stimulate adenylyl cyclase (AC) and activate PKA. Reciprocally, PKA may regulate the SOCE pathway at a number of levels, e.g. through phosphorylation and modulation of many PLC-coupled receptors, the IP3R, phospholamban associated with the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA), and possibly Orai1 itself (see Figure 3).
Whither goest the Ca2+ in migration?
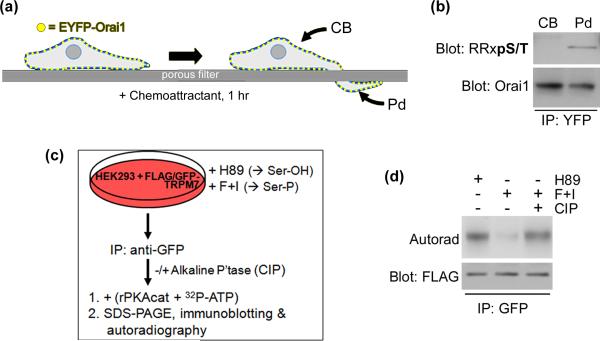
Currently, it is unknown whether Stim1/Orai1/SOCE exert a global or localized effect in migrating cells. In that regard, an intriguing hypothesis is that, in a cell undergoing chemotaxis, the higher relative concentration of extracellular stimulus at the leading edge would produce a localized or graded concentration of intracellular IP3, which would in turn elicit localized store depletion and SOCE within the leading edge. This would result in the generation of leading edge Ca2+ signals with kinetics, localization, and perhaps targets that would be distinct from Ca2+ flickers. The idea of differential regulation of SOCE or its components at the leading edge is supported by preliminary evidence from our laboratory that phosphorylation of Orai1 is differentially regulated in cell bodies versus chemotactic pseudopodia (Figure 3).
Figure 3.
New observations of PKA phosphorylation of migration-associated Ca2+ channels. (a) Schematic depicting separation and isolation of cell bodies (CB) from chemotactic pseudopodia (PD) (see [7••] and references therein). COS7 cells were transfected with pEYFP-Orai1 then cultured as depicted in to form pseudopodia in response to a gradient of EGF. (b) YFP-tagged Orai1 was immunoprecipitated from isolated cell bodies and pseudopodia and analyzed by SDS-PAGE and immunoblotting against Orai1 and the phosphorylated PKA consensus site (RRXpS/T). (c) Schematic for back-phosphorylation of TRPM7 by PKA. HEK293 cells were transfected with pcDNA4TO-FLAG-GFP-TRPM7 then treated with either (to inhibit PKA and promote accessibility of PKA sites) or forskolin and IBMX (F+I, to increase cAMP, activate PKA, & drive full phosphorylation of PKA sites). FLAG-GFP-TRPM7 was then immunoprecipitated and subject to in vitro phosphorylation with recombinant PKA + 32P-γ-ATP with or without prior dephosphorylation with calf intestinal phosphatase (CIP). (d) Reaction products were analyzed by SDS-PAGE, immunoblotting, and autoradiography.
This begs the question – what specific purposes are served by distinct Ca2+ signals such as SOCE or flickers during cell migration? Flickers appear to be critical for cells to reorient towards an oblique gradient of growth factor [23••] and thus resemble the filopodial Ca2+ transients that steer growth cones during axon guidance [35]. And while SOCE has been recognized for decades, its specific physiological effects are largely unknown [36]. One possibility is that SOCE may regulate migration through highly localized control of focal adhesions. Nanometer-scale targeting of microtubules to focal adhesions promotes focal adhesion disassembly, putatively through localized delivery of one or more “relaxing signals” [37,38]. Importantly, localized Ca2+ waves can trigger disassembly of focal adhesions [39] and STIM1 has been shown to track to microtubule plus ends in an EB1-dependent manner [40]. These observations support a hypothesis in which microtubules target STIM1 to focal adhesions and thus promote localized SOCE and focal adhesion turnover. The question of what functions are served by distinct types of Ca2+ signals during cell migration will ultimately be answered by a better understanding of the distinct effectors regulated by these signals.
PKA in cell migration – Still in the lead
PKA has numerous cytoskeleton- and migration-associated targets [3] – indeed, various subunits of PKA have recently been identified as part of a myosin II-responsive focal adhesion proteome [41], placing PKA in immediate proximity to many known and potential targets. Given this, and the fact that PKA can exert both negative and positive effects on cell migration [7••], it is not surprising that the role of PKA in migration is critically dependent on its localization through AKAPs. In all systems in which it has been directly examined, PKA activity is enriched in the leading edge [7••,42,43] and AKAP-mediated localization is required for leading edge formation and/or maintenance and for efficient cell migration [7••,42-45]. The question of which AKAP(s) are responsible for localizing PKA during cell migration is not resolved. The most direct investigation so far demonstrated that leading edge gradients of PKA activity in breast and colon carcinoma cells are mediated by AKAP-Lbc, a PKA scaffold and Rho-specific guanine nucleotide exchange factor [43]. However, several other AKAPs are also physically or functionally associated with cytoskeletal or adhesive elements [6]. The Wiskott-Aldrich Syndrome protein family verprolin-homologous protein 1 (WAVE1) was one of the first and most direct examples of a cytoskeleton-associated AKAP [46] and recent work suggests that WAVE2 may serve a similar function [45]. Similarly, an early report of the interaction between IQGAP1 and AKAP79 [47] is complemented by recent work demonstrating a ternary complex between IQGAP2, AKAP220, and PKA, in which PKA-mediated phosphorylation of IQGAP2 enhances its interaction with active Rac [48]. Finally, non-canonical mechanisms of PKA anchoring, e.g. via α4 integrins [44••] or actin filaments [49], may also play an important role during migration.
As mentioned above, the role for PKA in migration is certainly not always a positive one. A recent, elegant example of this is the ability of PKA to act as a critical switch during vascular sprouting by suppressing endothelial cell polarity and migration while up-regulating cadherin-mediated cell-cell adhesion [50•]. This occurs through indirect inhibition of Src activity via PKA-mediated phosphorylation and activation of the C-terminal Src kinase (Csk). This inhibition of Src is in contrast to a parallel ability of PKA to directly phosphorylate and activate Src and related kinases [51,52]. The differential regulation of Src by PKA under different cellular circumstances highlights both the breadth of PKA's effects and the fundamental requirement for mechanisms that specify these effects.
Ca2+ and cAMP/PKA: Connections
The collaboration between Ca2+ and cAMP/PKA signaling in regulating cell function has been appreciated for decades [8•] and has since been expertly reviewed [9-11••]. Indeed, there are numerous, elegant examples of how closely connected the two signals can be in both time and space (e.g. [53]; reviewed in [11••]). The number of levels at which Ca2+ and PKA can regulate one another, combined with the strong influence each signal has over migration-associated processes, makes it quite likely that cross-talk between Ca2+ and PKA will play an important role in regulating cell migration. This notion is underscored by recent, compelling reports of Ca2+/PKA cross-talk – including unpublished observations from our laboratory – related to both TRPM7 and SOCE.
The most direct route connecting Ca2+ to PKA is through Ca2+-mediated regulation of AC activity and thus cAMP production. This effect is governed by the isoform of AC expressed; ACs 1, 3, 5, 6, 8, and 9 are Ca2+-regulated, while the remaining isoforms are insensitive ([54]; see also [53] and references therein). Specifically, AC1 and AC8 are activated by Ca2+ via calmodulin, while the other isoforms are inhibited by Ca2+, either directly or through phospho-regulation. It should be noted, however, that even the classically ‘insensitive’ isoforms (i.e. ACs2, 4, and 7) may still be indirectly regulated by Ca2+ via phosphorylation by PKC [54]. Ca2+ may also regulate PKA signaling by affecting AKAP function. Ca2+/CaM binding inhibits the interaction of two AKAPs (AKAP79 and gravin/SSeCKS) with membrane phospholipids [55,56]. Conversely, Ca2+ promotes the binding of IQGAP2 to AKAP220, thereby facilitating PKA-mediated phosphorylation of IQGAP2 [48]. These observations support a mechanism whereby local increases of Ca2+ may exclude PKA or, alternatively, displace specific AKAP-containing complexes while retaining or enhancing others – an efficient way for Ca2+ to regulate the specificity of PKA signaling. In addition, the anchoring of PKA to IQGAPs [47,48], coupled with the ability of IQGAPs to serve as Ca2+/calmodulin-dependent regulators of cytoskeletal and microtubule dynamics [57], bring IQGAPs to the fore as examples of cytoskeletal effectors at the nexus of PKA and Ca2+ signals.
A recent and elegant study showed that depletion of ER Ca2+ can elicit cAMP synthesis and PKA activation in a STIM1-dependent manner ([58••]; Figure 2). Also, SOCE efficiently activates AC8 [53,59] and induces PKA, STIM1, and AC8 to co-localize at the plasma membrane and cofractionate in lipid rafts [59]. Conversely, PKA can potentiate SOCE in a variety of cells [10], although the mechanism for this is unclear. Given the hypothesis outlined earlier, suggesting a mechanism of focal adhesion turnover through microtubule-mediated targeting of Stim1, it is intriguing to note that PKA has also been shown to associate with microtubule plus ends [60] and may phosphorylate Orai1 (Figure 3). These considerations, combined with the growing recognition of the importance of STIM1, Orai1, and SOCE in cell migration [31•• ,32•,33•, 34•], make this an area rife with potential future studies.
PKA is implicated in the regulation of a large number of mechanosensitive TRP channels, including those implicated in migration, e.g. V1 [61], V4 [62], and, notably, M7 [63]. For TRPV1, this regulation is critically dependent on AKAP-mediated anchoring of PKA to the channel [64] and there is evidence to suggest a similar requirement for other TRP channels [62]. Furthermore, in all cases, PKA signaling has a positive or sensitizing effect on channel function, and for at least TRPV1 and V4, this requires direct phosphorylation of the channel [61,62]. Takezawa et al [63] showed that the inhibition and stimulation of TRPM7 by muscarinic and beta-adrenergic receptors, respectively, required modulation of cAMP levels and that βAR-mediated stimulation also required PKA activity. While clearly demonstrating that PKA activity positively regulates TRPM7, that report did not demonstrate direct phosphorylation of the channel. However, TRPM7 contains at least four strong consensus sites for PKA phosphorylation and preliminary evidence from our laboratory strongly suggests that TRPM7 can be directly phosphorylated by PKA (Figure 3). Given this, and the ability of mechanical tension to elevate cAMP and activate PKA [65], it is tempting to hypothesize a role for PKA in the mechanosensitive activation of some TRP channels associated with cell migration.
Finally, in addition to increasing [Ca2+]i, PKA can also contribute to clearance of cytosolic Ca2+ – and thus termination of Ca2+ signaling – through regulation of the activity of plasma membrane Ca2+-ATPases (PMCAs) via direct phosphorylation and, in some cells, the sarco-/ endoplasmic reticulum Ca2+-ATPase (SERCA), via phosphorylation of phospholamban [10].
CONCLUSIONS
Beyond the Ca2+ events discussed above, it should be noted that PKA interacts with numerous other factors that control or interpret Ca2+ flux and that have been implicated in cell migration [10,66,67]. The hypothesis – or perhaps ‘prediction’ - being proffered here is that, based on the strong influence of Ca2+ and PKA on cell migration and the staggering complexity and ubiquity of cross-talk between these signals, collaboration between Ca2+ and PKA will play a fundamentally important role in the spatial and temporal regulation of cell migration. This hypothesis prompts two important questions. First, is the regulation of Ca2+ influx – an activity for which PKA is to the manner born – a major target for localized PKA in migrating cells? Conversely, is the spatial regulation of PKA activity – with its numerous targets within the cell migration machinery – a major target for specific Ca2+ signals? While much more experimentation is needed to address these questions, the investigation of the interplay between Ca2+ and PKA during cell migration will inevitably provide important mechanistic insights into the regulation of this complex, fundamental cellular process.
Acknowledgements
The author would like to thank Drs. Richard Lewis (Stanford University) and David Clapham (Harvard University) for Orai1 and TRPM7 plasmids, respectively and Andrew McKenzie for helpful discussions. Work in the author's laboratory is supported by grants from the NIH (GM074204) and the Lake Champlain Cancer Research Organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Pidoux G, Tasken K. Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J Mol Endocrinol. 2010;44:271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- 3.Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Marks PW, Maxfield FR. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J Cell Biol. 1990;110:43–52. doi: 10.1083/jcb.110.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brundage RA, Fogarty KE, Tuft RA, Fay FS. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254:703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 6.Skroblin P, Grossmann S, Schafer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase a anchoring. Int Rev Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- 7••.Howe AK, Baldor LC, Hogan BP. Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc Natl Acad Sci U S A. 2005;102:14320–14325. doi: 10.1073/pnas.0507072102. [This work was the first to establish that it is not just the activity but also the subcellular localization of PKA, mediated by AKAPs, that is important for chemotaxis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Berridge MJ. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [An amazingly thorough and prescient article on the possible mechanisms and consequences of second messenger cross-talk.] [PubMed] [Google Scholar]
- 9.Bugrim AE. Regulation of Ca2+ release by cAMP-dependent protein kinase. A mechanism for agonist-specific calcium signaling? Cell Calcium. 1999;25:219–226. doi: 10.1054/ceca.1999.0027. [DOI] [PubMed] [Google Scholar]
- 10.Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 11••.Borodinsky LN, Spitzer NC. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE. 2006;2006:pe22. doi: 10.1126/stke.3362006pe22. [This is an insightful and extremely well-written review that does a superb job in synthesizing the literature on the cross-talk between Ca2+ and cAMP/PKA signaling, with special emphasis on the closely-coordinated Ca2+/cAMP oscillations observed in many systems. A must-read, to fully appreciate the current article.] [DOI] [PubMed] [Google Scholar]
- 12.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 14•.Kobayashi T, Sokabe M. Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Curr Opin Cell Biol. 2010;22:669–676. doi: 10.1016/j.ceb.2010.08.023. [An excellent and thorough review that focuses, intently and expertly, on a topic only touched upon in the current article - namely, the interface between the cytoskeleton and mechanosensitive ion channels.] [DOI] [PubMed] [Google Scholar]
- 15••.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [This work is the first formal description of the role of SACCs in cell migration, implicating them specifically in release and retraction of the trailing edge.] [DOI] [PubMed] [Google Scholar]
- 16•.Munevar S, Wang YL, Dembo M. Regulation of mechanical interactions between fibroblasts and the substratum by stretch-activated Ca2+ entry. J Cell Sci. 2004;117:85–92. doi: 10.1242/jcs.00795. [One of the first and best papers to ‘break’ the dogma that the role of Ca2+ in migration is relegated to the trailing edge.] [DOI] [PubMed] [Google Scholar]
- 17.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramadass R, Becker D, Jendrach M, Bereiter-Hahn J. Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch Biochem Biophys. 2007;463:27–36. doi: 10.1016/j.abb.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A, Frippiat C, Nilius B, Schwab A. A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium. 2007;42:17–25. doi: 10.1016/j.ceca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Fabian A, Fortmann T, Dieterich P, Riethmuller C, Schon P, Mally S, Nilius B, Schwab A. TRPC1 channels regulate directionality of migrating cells. Pflugers Arch. 2008;457:475–484. doi: 10.1007/s00424-008-0515-4. [DOI] [PubMed] [Google Scholar]
- 22.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrinto-integrin signaling. Circ Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [This study identified a new type of localized Ca2+ transient in the leading edge of cells that is mediated principally by the stretch-activated Ca2+ channel TRPM7. These flickers are generated through an actomyosin contractility-dependent mechanism and are required for the chemotactic response to directional growth factor gradients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bomben VC, Turner KL, Barclay TT, Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J Cell Physiol. 2010 doi: 10.1002/jcp.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JP, Luan Y, You CX, Chen XH, Luo RC, Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca(2+) influx. Cell Calcium. 2010 doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb) 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caprodossi S, Amantini C, Nabissi M, Morelli MB, Farfariello V, Santoni M, Gismondi A, Santoni G. Capsaicin (CPS) promotes a more aggressive gene expression phenotype and invasiveness in null-TRPV1 urothelial cancer cells. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr025. [DOI] [PubMed] [Google Scholar]
- 28.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark K, Middelbeek J, van Leeuwen FN. Interplay between TRP channels and the cytoskeleton in health and disease. Eur J Cell Biol. 2008;87:631–640. doi: 10.1016/j.ejcb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Varnai P, Hunyady L, Balla T. STIM and Orai: the long-awaited constituents of store-operated calcium entry. Trends Pharmacol Sci. 2009;30:118–128. doi: 10.1016/j.tips.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [A beautifully executed study that deftly integrates a range of techniques - from analysis of focal adhesion turnover through single cell migration assays to analysis of metastasis in vivo - to demonstrate the critical role for store-operated Ca2+ entry, and its principal mediators STIM1 and Orai1, for the migration and metastasis of breast cancer cells.] [DOI] [PubMed] [Google Scholar]
- 32•.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol. 2010;298:C993–1005. doi: 10.1152/ajpcell.00325.2009. [Another thorough and well-executed demonstration of the importance of SOCE mediated by STIM1 and Orai1 in cell migration, expanding on earlier work from the Trebak lab. This paper, along with [33] and [34], combine with [31••] to unequivocally establish SOCE and its mediators as central regulators in the migration of a variety of cell types.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Schaff UY, Dixit N, Procyk E, Yamayoshi I, Tse T, Simon SI. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [See [32].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VA, Taylor H, et al. Orai1 and CRAC Channel Dependence of VEGF-Activated Ca2+ Entry and Endothelial Tube Formation. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.243352. [See [32].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 36.Putney JW. The Physiological Function of Store-operated Calcium Entry. Neurochem Res. 2011 doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krylyshkina O, Anderson KI, Kaverina I, Upmann I, Manstein DJ, Small JV, Toomre DK. Nanometer targeting of microtubules to focal adhesions. J Cell Biol. 2003;161:853–859. doi: 10.1083/jcb.200301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannone G, Ronde P, Gaire M, Beaudouin J, Haiech J, Ellenberg J, Takeda K. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem. 2004;279:28715–28723. doi: 10.1074/jbc.M404054200. [DOI] [PubMed] [Google Scholar]
- 40.Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr., Hoogenraad CC, et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo JC, Han X, Hsiao CT, Yates Iii JR, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim CJ, Kain KH, Tkachenko E, Goldfinger LE, Gutierrez E, Allen MD, Groisman A, Zhang J, Ginsberg MH. Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol Biol Cell. 2008;19:4930–4941. doi: 10.1091/mbc.E08-06-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulucci-Holthauzen AA, Vergara LA, Bellot LJ, Canton D, Scott JD, O'Connor KL. Spatial distribution of protein kinase A activity during cell migration is mediated by A-kinase anchoring protein AKAP Lbc. J Biol Chem. 2009;284:5956–5967. doi: 10.1074/jbc.M805606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Lim CJ, Han J, Yousefi N, Ma Y, Amieux PS, McKnight GS, Taylor SS, Ginsberg MH. Alpha4 integrins are type I cAMP-dependent protein kinase-anchoring proteins. Nat Cell Biol. 2007;9:415–421. doi: 10.1038/ncb1561. [This elegant and thoughtful report not only establishes that ⍰4 integrins anchor PKA but also shows that they do so in a unique way, by interacting only with the tetrameric holoenzyme rather than only through the R subunits, as is typical for most AKAPs. This has far-reaching implications for the future study of PKA anchoring as well as for investigation of PKA regulation during adhesion and migration.] [DOI] [PubMed] [Google Scholar]
- 45.Yamashita H, Ueda K, Kioka N. WAVE2 forms a complex with PKA and is involved in PKA enhancement of membrane protrusions. J Biol Chem. 2011;286:3907–3914. doi: 10.1074/jbc.M110.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westphal RS, Soderling SH, Alto NM, Langeberg LK, Scott JD. Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J. 2000;19:4589–4600. doi: 10.1093/emboj/19.17.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nauert JB, Rigas JD, Lester LB. Identification of an IQGAP1/AKAP79 complex in beta-cells. J Cell Biochem. 2003;90:97–108. doi: 10.1002/jcb.10604. [DOI] [PubMed] [Google Scholar]
- 48.Logue JS, Whiting JL, Tunquist B, Langeberg LK, Scott JD. Anchored PKA recruitment of active RAC. J Biol Chem. 2011 doi: 10.1074/jbc.M111.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivard RL, Birger M, Gaston KJ, Howe AK. AKAP-independent localization of type-II protein kinase A to dynamic actin microspikes. Cell Motil Cytoskeleton. 2009;66:693–709. doi: 10.1002/cm.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Jin H, Garmy-Susini B, Avraamides CJ, Stoletov K, Klemke RL, Varner JA. A PKA-Csk-pp60Src signaling pathway regulates the switch between endothelial cell invasion and cell-cell adhesion during vascular sprouting. Blood. 2010;116:5773–5783. doi: 10.1182/blood-2010-07-296210. [A well-executed and thorough study that demonstrates PKA as a potent negative regulator of endothelial cell migration during vasculogenesis, and thus gives importance balance to the concept of PKA as a promoter of leading edge dynamics and cell migration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obara Y, Labudda K, Dillon TJ, Stork PJ. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci. 2004;117:6085–6094. doi: 10.1242/jcs.01527. [DOI] [PubMed] [Google Scholar]
- 52.Yeo MG, Oh HJ, Cho HS, Chun JS, Marcantonio EE, Song WK. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J Cell Physiol. 2011;226:236–247. doi: 10.1002/jcp.22335. [DOI] [PubMed] [Google Scholar]
- 53.Willoughby D, Wachten S, Masada N, Cooper DM. Direct demonstration of discrete Ca2+ microdomains associated with different isoforms of adenylyl cyclase. J Cell Sci. 2010;123:107–117. doi: 10.1242/jcs.062067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 55.Gorski JA, Gomez LL, Scott JD, Dell'Acqua ML. Association of an A-kinase-anchoring protein signaling scaffold with cadherin adhesion molecules in neurons and epithelial cells. Mol Biol Cell. 2005;16:3574–3590. doi: 10.1091/mbc.E05-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao J, Shumay E, McLaughlin S, Wang HY, Malbon CC. Regulation of AKAP-membrane interactions by calcium. J Biol Chem. 2006;281:23932–23944. doi: 10.1074/jbc.M601813200. [DOI] [PubMed] [Google Scholar]
- 57.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [A sophisticated and elegant study that establishes that, upon sensing the depletion of ER Ca2+ stores, STIM1 not only couples to Orai1 to effect Ca2+ entry but also activates adenylyl cyclase to increase cAMP and activate PKA. While the mechanism for STIM1-mediated AC activation is not yet known, this study opens up the possibility of a novel and perhaps universal mechanism for coupling Ca2+ flux to cAMP and PKA signaling.] [DOI] [PubMed] [Google Scholar]
- 59.Martin AC, Willoughby D, Ciruela A, Ayling LJ, Pagano M, Wachten S, Tengholm A, Cooper DM. Capacitative Ca2+ entry via Orai1 and stromal interacting molecule 1 (STIM1) regulates adenylyl cyclase type 8. Mol Pharmacol. 2009;75:830–842. doi: 10.1124/mol.108.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005;169:151–165. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takezawa R, Schmitz C, Demeuse P, Scharenberg AM, Penner R, Fleig A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc Natl Acad Sci U S A. 2004;101:6009–6014. doi: 10.1073/pnas.0307565101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 65.Alenghat FJ, Tytell JD, Thodeti CK, Derrien A, Ingber DE. Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J Cell Biochem. 2009;106:529–538. doi: 10.1002/jcb.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao JT, Gui P, Zamponi GW, Davis GE, Davis MJ. Spatial association of the Cav1.2 calcium channel with alpha5beta1-integrin. Am J Physiol Cell Physiol. 2011;300:C477–489. doi: 10.1152/ajpcell.00171.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]