Abstract
Objective
To assess the diagnostic accuracy of the urine lipoarabinomannan (LAM) test among ambulatory HIV-infected persons.
Design
Cross-sectional
Methods
HIV-infected persons consecutively presenting to the HIV Clinic at Tembisa Main Clinic in Ekhuruleni, South Africa were screened for symptoms of tuberculosis (TB), and asked to provide sputum and blood samples for smears for acid-fast bacilli (AFB) and mycobacterial culture, and a urine specimen for a LAM enzyme-linked immunosorbent assay (ELISA). Fine needle aspirates were obtained from participants with enlarged lymph nodes, and sent for histopathology. Non-pregnant participants underwent chest x-ray.
Results
422 HIV-infected participants were enrolled with median age 37 years [interquartile range (IQR) 31-44 years], median CD4+ T-cell count 215 cells/μL (IQR 107-347 cells/μL), and 212 (50%) receiving antiretroviral therapy (ART). 30 (7%) had active TB: 18 with only pulmonary TB (PTB), 5 with only extrapulmonary TB (ETB), and 7 with both PTB and ETB. 27% (95% CI 12-48%) of TB cases were sputum AFB-positive. The sensitivity and specificity of the urine LAM compared to the gold standard of positive bacteriology or histopathology were 32% (95% confidence interval [CI] 16-52%) and 98% (95% CI 96-99%) respectively. Urine LAM had higher sensitivity in TB cases with higher bacillary burdens, though these differences were not statistically significant.
Conclusions
The sensitivity of urine LAM testing is inadequate to replace mycobacterial culture. In contrast to prior research on the urine LAM, this study was conducted among less sick, ambulatory HIV-infected patients presenting for routine care.
Keywords: tuberculosis, HIV, lipoarabinomannan, sensitivity, specificity, predictive value, screening
Introduction
Tuberculosis (TB) in HIV-infected persons is predominantly smear negative, necessitating a multi-step diagnostic algorithm to confirm or exclude TB in smear-negative TB suspects. Completing this algorithm is onerous and costly for patients and providers alike.1 Moreover, considerable uncertainty remains even when the full diagnostic algorithm is followed.2 A rapid, sensitive, point-of-care test for TB, allowing TB disease status to be accurately assigned on the day of initial presentation could transform TB diagnosis and screening, allowing for prompt initiation of TB treatment and for intensified case-finding (ICF), isoniazid preventive therapy (IPT) and antiretroviral therapy (ART) to be readily integrated into the first days of HIV care.
The ideal TB diagnostic test would be accurate, rapid, point-of-care, safe, robust, widely applicable to different populations and forms of TB; affordable and acceptable to clinicians, laboratory technicians and patients; and should either replace sputum smear microscopy, or increase microbiologic confirmation of TB among sputum smear-negative cases.3
Lipoarabinomannan (LAM) is a cell wall lipopolysaccharide only found in mycobacteria,4 and can be detected in urine of patients with mycobacterial infections.5 Enzyme-linked immunosorbent assays (ELISAs) have been developed to detect LAM in urine.6
The aims of this study were to assess the sensitivity and positive predictive value of urine LAM to screen HIV-infected persons for TB, and the specificity and negative predictive value of the urine LAM to exclude TB among HIV-infected persons prior to initiation of ART and/or IPT.
Methods
Study site and population
This study was conducted at the Tembisa Main Clinic in Ekurhuleni, South Africa. Consecutive patients presenting to the clinic between October 2009 and May 2010 for HIV-related care were asked to participate in the study. Inclusion criteria were age ≥18 years, HIV infection (testing was provided by routine clinical services, not the study), ability to communicate in English, Zulu or Sepedi, and written informed consent. Patients who were already on treament for TB or who had discontinued TB treatment in the past 3 months, patients on dialysis, and prisoners were excluded.
Study procedures
Demographic information and clinical history were obtained through interview and medical record review. All study participants provided two sputum, one blood and one urine specimen regardless of symptoms. Participants unable to expectorate spontaneously underwent percussion, and if necessary, sputum induction with 3% hypertonic saline. All participants were examined for lymphadenopathy and a fine needle aspiration (FNA) was done on all superficial, easily accessible lymph nodes ≥1 cm in size.
Additional diagnostic work up and all treatment was provided at the discretion of the treating clinicians, who were given the results of all study-related diagnostic testing except the urine LAM test.
Urine specimens were refrigerated after collection, transported to the laboratory in cooler boxes at 2-8°C on the day of collection, heat inactivated, spun down, and stored at −20°C until testing was performed. All other specimens were transported to the laboratory at ambient temperature and processed on the day of collection.
Sputum smears were decontaminated with N-acetyl-L-cysteine-sodium hydroxide. A Ziehl-Neelsen-stained smear was prepared, examined under 100x magnification using a light microscope, and graded according to the World Health Organization (WHO) grading system. Processed sputum sediment from both specimens was cultured using the BACTEC MGIT 960 system (BD Diagnostics, Sparks, USA).
Blood was sent for complete blood count and CD4+ T-cell counts, and inoculated directly into BACTEC Myco-F/Lytic bottles at the time of collection. Smears of lymph node aspirates were prepared at the time of collection and sent for histopathology.
Mycobacterial species identification was performed using the Capilia® MPB64 monoclonal antibody test (TAUNS, Numazu, Japan).
Urine was tested for LAM using the Clearview® TB ELISA, Inverness Medical Innovations, Waltham, USA, according to the manufacturer’s instructions in batches.6 The laboratory technicians reading the urine LAM ELISA optical densities were blinded to the participants’ histories and other diagnostic test results.
A rapid urine pregnancy test was also performed on all female participants not already known to be pregnant. All participants who were not pregnant underwent a chest x-ray. The chest x-ray was read by a single reader for active TB.
Case definitions
Participants were classified as having pulmonary TB (PTB) if they had at least one sputum culture that was positive for Mycobacterium tuberculosis, or at least one sputum smear that was acid fast bacilli (AFB)-positive and culture was not performed or contaminated. Participants were classified as having extrapulmonary TB (ETB) if they had at least one blood culture that was positive for M. tuberculosis, or at least one FNA from an enlarged lymph node that was AFB-positive and/or histologically consistent with TB.
Data management and statistical analyses
We assumed that the urine LAM test would have a sensitivity of 50-80%,7, 8 and thus that we would be able to estimate the sensitivity of the test with 95% confidence intervals (CIs) of ±15% if we enrolled at least 400 participants with a TB prevalence of 10-15%. We assumed that the urine LAM test would have a specificity of 85-100%, and thus that we would be able to estimate the specificity of the test with 95% CIs of ±5% if we enrolled at least 400 participants with a TB prevalence of 10-15%.
The statistical software package SAS 9.2 (SAS Institute Inc., Cary, USA) was used for data analysis. The performance characteristics of the urine LAM as compared to the gold-standard case definitions and the prevalence of TB was calculated along with 95% CIs using the exact binomial method.
Ethics
The study was approved by the Gauteng Province Department of Health, the Ekhuruleni Metropolitan Municipality Executive Director for Health, the Ekhuruleni Metropolitan Municipality Ethics Committee, the University of the Witwatersrand Human Research Ethics Committee, the Johns Hopkins University School of Medicine Institutional Review Board, and the London School of Hygiene and Tropical Medicine Ethics Committee.
Results
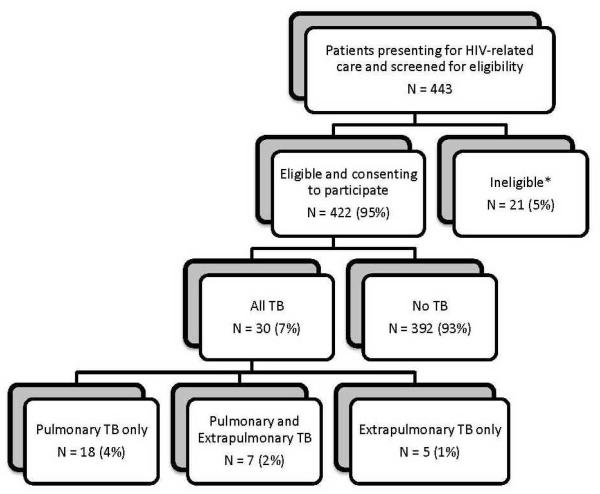
Between October 2009 and May 2010, 443 persons presenting to the study clinic for routine HIV-related care were invited and consented to participate and were screened for eligibility; 21 (5%) were ineligible [HIV-uninfected (8), currently being treated for TB (12), and completion of TB treatment <3 months prior to eligibility screening (1)]. 144 (34%) of participants were male, the median age was 37 years [interquartile range (IQR) 31-44 years], median CD4+ T-cell count was 215 cells/μL (IQR 107-347 cells/μL), and 212 (50%) were receiving ART. The median CD4+ T-cell count among those not receiving ART was 160 cells/μL (IQR 65-315 cells/μL). Participants receiving ART had a median CD4+ T-cell count of 258 cells/μL (IQR 165-357 cells/μL), and had been receiving ART for a median duration of 247 days (IQR 99-848 days) excluding those who has just been initiated on ART the day of enrollment in the study.
361 (86%) of participants reported having any duration of cough, fever, night sweats or weight loss. A total of 30 TB cases (7%, 95% CI 5-10%) was identified, 18 (60%) had only PTB, 7 (23%) had PTB and ETB, and 5 (17%) had only ETB. Seven PTB cases were diagnosed on the basis of a positive sputum smear, and 25 on the basis of a positive sputum culture. Of the 12 cases with ETB, 7 had a positive blood culture, 3 had lymph node histopathology consistent with TB, and 2 had both. Twenty-seven percent (95% CI 12-48%) of TB cases were sputum AFB-positive.
The performance characteristics of symptoms, body mass index (BMI), routine labs and urine LAM are shown in Table 1. The sensitivity, specificity, positive predictive value and negative predictive value of the urine LAM test were 32% (95% CI 16-52%), 98% (95% CI 96-99%), 53% (95% CI 28-77%), and 95% (95% CI 93-97%) respectively. The urine LAM test had sensitivities of 40% (5-85%) and 20% (95% CI 6-44%) among sputum AFB-positive and -negative TB cases. The urine LAM test was more sensitive in TB cases with night sweats (42%, 95% CI 20-67%), weight loss (36%, 95% CI 18-57%), BMI <18.5 kg/m2 (67%, 95% CI 30-93%), anemia (43%, 95% CI 22-66%), CD4+ T-cell counts ≤50 cells/μL (56%, 95% CI 21-86%), moderate/advanced disease on chest x-ray (38%, 95% CI 14-68%), or ETB (64%, 95% CI 31-89%), but none of these differences achieved statistical significance.
Table 1.
Performance characteristics of body mass index (BMI), routine labs and urine LAM for diagnosis of TB
| Test | Sensitivity [x/n] = % (95% CI) |
Specificity [x/n] = % (95% CI) |
Positive predictive value [x/n] = % (95% CI) |
Negative predictive value [x/n] = % (95% CI) |
|---|---|---|---|---|
| BMI <18.5 kg/m2 | [9/30] = 30 (15, 49) |
[364/387] = 94 (91, 96) |
[9/32] = 28 (14, 47) |
[364/385]= 95 (92, 97) |
| Anemia (1) | [22/30] = 73 (54, 88) |
[196/388] = 51 (45, 56) |
[22/214] = 10 (7, 15) |
[196/204] = 96 (92, 98) |
| Sputum smear microscopy |
[7/26] = 27 (12, 48) |
[228/228] = 100 |
[7/7] = 100 |
[228/247] = 92 (88, 95) |
| Chest x-ray (CXR) (2) | [21/22] = 95 (77, 100) |
[58/123] = 47 (38, 56) |
[21/86] = 24 (16, 35) |
[58/59] = 98 (91, 100) |
| Urine LAM | [9/28] = 32 (16, 52) |
[378/386] = 98 (96, 99) |
[9/17] = 53 (28, 77) |
[378/397] = 95 (93, 97) |
| Site of TB | ||||
| PTB (3) | [5/23] = 22 (7, 44) |
[378/386] = 98 (96, 99) |
[5/13] = 38 (14, 68) |
[378/396] = 95 (93, 97) |
| ETB (4) | [7/11] = 64 (31, 89) |
[378/386] = 98 (96, 99) |
[7/15] = 47 (21, 73) |
[378/382] = 99 (97, 100) |
| Smear positivity | ||||
| AFB+ | [3/6] = 50 (12, 88) |
[0/0] = – |
[3/3] = 100 |
[0/3] = 0 |
| AFB− | [3/19] = 16 (4, 40) |
[222/227] = 98 (95, 99) |
[3/8] = 38 (9, 76) |
[222/238] = 93 (89, 96) |
| Absolute CD4+ T-cell count (cells/μL) | ||||
| ≤50 | [5/9] = 56 (21, 86) |
[36/40] = 90 (76, 97) |
[5/9] = 56 (21, 86) |
[36/40] = 90 (76, 97) |
| ≤200 | [8/23] = 35 (16, 57) |
[164/170] = 96 (92, 99) |
[8/14] = 57 (29, 82) |
[164/179] = 92 (87, 95) |
| 201-350 | [1/5] = 20 (1, 72) |
[113/114] = 99 (95, 100) |
[1/2] = 50 (1, 99) |
[113/117] = 97 (91, 99) |
| 351-500 | [0/0] = – |
[55/56] = 98 (90, 100) |
[0/1] = 0 |
[55/55] = 100 |
| >500 | [0/0] = – |
[46/46] = 100 |
[0/0] = – |
[46/46] = 100 |
| Currently receiving antiretroviral therapy | ||||
| No | [6/19] = 32 (13, 57) |
[178/183] = 97 (94, 99) |
[6/11] = 55 (23, 83) |
[178/191] = 93 (89, 96) |
| Yes | [3/9] = 33 (7, 70) |
[199/202] = 99 (96, 100) |
[3/6] = 50 (12, 88) |
[199/205] = 97 (94, 99) |
| Urine LAM plus BMI <18.5 kg/m2 (5) |
[12/28] = 43 (24, 63) |
[354/384] = 92 (89, 95) |
[12/42] = 29 (16, 45) |
[354/370] = 96 (93, 98) |
| Urine LAM plus anemia (1, 5) |
[21/28] = 75 (55, 89) |
[192/385] = 50 (45, 55) |
[21/214] = 10 (6, 15) |
[192/199] = 96 (93, 99) |
| Urine LAM plus sputum smear microscopy (5) |
[9/25] = 36 (18, 57) |
[222/227] = 98 (95, 99) |
[9/14] = 64 (35, 87) |
[222/238] = 93 (89, 96) |
| Urine LAM plus CXR (2, 5) |
[20/20] = 100 |
[55/123]= 45 (36, 54) |
[20/88] = 23 (14, 33) |
[55/55] = 100 |
Anemia: hemoglobin <12 g/dL in women, <13 g/dL in men
Evidence of active TB on chest x-ray.
The performance of the urine LAM test was calculated after excluding the 5 participants who had only ETB.
The performance of the urine LAM test was calculated after excluding the 18 participants who had only PTB.
Excluding participants for whom one of the test results was missing.
Having either a positive urine LAM test or sputum smear was 36% sensitive (95% CI 18-57%), 98% specific (95% CI 95-99%), and had positive and negative predictive values of 64% (95% CI 35-87%) and 93% (95% CI 89-96%).
Four of 7 participants who grew NTMs and did not grow TB from sputum and/or blood had positive urine LAM tests.
Discussion
The sensitivity of the urine LAM among HIV-infected ambulatory patients in this study was only 50% among sputum AFB-positive and 16% among AFB-negative TB cases respectively, making it inadequate to replace sputum smear microscopy in AFB-positive TB cases or mycobacterial culture in AFB-negative TB cases, even if the urine LAM is formatted into a rapid lateral flow assay.
Our study differed from prior studies in that we studied the urine LAM test to screen a high-risk population for TB rather than to confirm a diagnosis of TB among symptomatic persons; our participants were ambulatory patients presenting for routine HIV-related care, who were less immunosuppressed than populations in which this test has previously been studied; and a highly sensitive standard diagnostic work up for TB was performed in all study participants regardless of symptoms reported.
The prevalence of active TB among ambulatory HIV-infected adults in our study was 7%, which was lower than previously found by Bassett et al. and Houlihan et al. in KwaZulu Natal and by Lawn et al. in the Western Cape, South Africa, where the prevalence of TB was 20-30% among HIV-infected ambulatory patients presenting for initiation of ART; however the HIV-infected patients in these prior studies had a lower median CD4+ T-cell counts (100-125 cells/μL) than the median CD4+ T-cell count of participants in our study (215 cells/μL), and were not receiving ART in contrast to the 50% in our study who were on ART.9-11
The sensitivity of the urine LAM in our study (32%, 95% CI 16-52%) was comparable to or lower than that reported in previous studies (20-80%),7, 8, 12-16 likely because our study participants were ambulatory, relatively less sick persons with lower bacillary burdens of TB. Prior studies have demonstrated that the urine LAM has greater sensitivity for detecting TB among patients with a higher bacillary burden: sputum AFB-positive versus AFB-negative TB patients;7, 8, 13, 15-19 patients with definite rather than probable or possible TB;7, 8, 15, 16 HIV-infected versus -uninfected TB patients;7, 14-16 HIV-infected patients with lower CD4+ T-cell counts;7, 12, 14, 17 PTB versus ETB patients;17 and TB patients with versus without mycobacteremia.7, 17
The sensitivity of the urine LAM alone did not exceed 64% in any subgroup, and thus the urine LAM was not a useful test to rule out TB in any subgroup. The specificity of the urine LAM exceeded 90% in all subgroups and may be a useful test for ruling in TB among sick, hospitalized HIV-infected persons with a high burden of TB, but in this out-patient population the positive predictive value reached only 57%, even among those with CD4+ T-cell counts ≤200 cells/μL.
In the past year there has been much excitement about the Cepheid GeneXpert MTB/RIF assay, a molecular test for TB that is run within two hours, could be used at the point of care, and which has >98% and >72% sensitivity for sputum smear-positive and -negative TB respectively and >98% specificity, significantly better performance characteristics than that of the urine LAM.20
One of the main benefits of active TB case-finding is to detect patients early in the natural history of disease, and such programs will have limited impact if they rely on TB diagnostics with poor sensitivity in this group. Development of TB diagnostics should focus on rapid, point-of-care technologies that have significantly higher sensitivity, specificity and predictive value among patients with both early as well as advanced TB disease.
Figure 1. Flow diagram.
* Reasons for exclusion due to ineligibility included: HIV-uninfected (8), currently being treated for TB (12), and completion of TB treatment <3 months prior to eligibility screening (1).
Acknowledgments
We thank the staff and patients of Tembisa Main Clinic, and the staff of the Aurum Institute for Health Research and the Bioanalytical Research Corporation who made this study possible. Dr. Celine Gounder was funded by NIH grant T32AI007291. This study was funded in part by the United States Agency for International Development (USAID). The contents of this report are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Sources of support: Dr. Celine Gounder was funded by NIH grant T32AI007291. This study was funded in part by a grant from the United States Agency for International Development (USAID).
Footnotes
Contributors: All authors contributed to the conception and design of the study, interpretation of the data, and reviewing drafts of the manuscript. TK was responsible for managing the field site. CRG and NIW analyzed the data. CRG drafted and revised the manuscript.
Conflict of interest statement: We declare that we have no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kemp JR, Mann G, Simwaka BN, et al. Can Malawi’s poor afford free tuberculosis services? Patient and household costs associated with a tuberculosis diagnosis in Lilongwe. Bull World Health Organ. 2007;85:580–5. doi: 10.2471/BLT.06.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson D, Nachega J, Morroni C, et al. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10:31–8. [PubMed] [Google Scholar]
- 3.Foulds J, O’Brien R. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int J Tuberc Lung Dis. 1998;2:778–83. [PubMed] [Google Scholar]
- 4.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 5.Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–51. [PubMed] [Google Scholar]
- 6.Inverness Medical . Package insert. Scarborough; Maine, USA: 2008. Clearview TB ELISA: LAM specific direct urinary antigen test. [Google Scholar]
- 7.Shah M, Variava E, Holmes CB, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J Acquir Immune Defic Syndr. 2009;52:145–51. doi: 10.1097/QAI.0b013e3181b98430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg. 2005;99:893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Bassett IV, Wang B, Chetty S, et al. Intensive Tuberculosis Screening for HIV-Infected Patients Starting Antiretroviral Therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–29. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Kranzer K, Edwards DJ, et al. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houlihan CF, Mutevedzi PC, Lessells RJ, et al. The tuberculosis challenge in a rural South African HIV programme. BMC Infect Dis. 2010;10:23. doi: 10.1186/1471-2334-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Edwards DJ, Kranzer K, et al. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–80. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- 13.Daley P, Michael JS, Hmar P, et al. Blinded evaluation of commercial urinary lipoarabinomannan for active tuberculosis: a pilot study. Int J Tuberc Lung Dis. 2009;13:989–95. [PMC free article] [PubMed] [Google Scholar]
- 14.Dheda K, Davids V, Lenders L, et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One. 2010;5:e9848. doi: 10.1371/journal.pone.0009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutetwa R, Boehme C, Dimairo M, et al. Diagnostic accuracy of commercial urinary lipoarabinomannan detection in African tuberculosis suspects and patients. Int J Tuberc Lung Dis. 2009;13:1253–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Reither K, Saathoff E, Jung J, et al. Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC Infect Dis. 2009;9:141. doi: 10.1186/1471-2334-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah M, Martinson NA, Chaisson RE, et al. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J Clin Microbiol. 2010;48:2972–4. doi: 10.1128/JCM.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessema TA, Hamasur B, Bjun G, et al. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand J Infect Dis. 2001;33:279–84. doi: 10.1080/003655401300077306. [DOI] [PubMed] [Google Scholar]
- 19.Tessema TA, Bjune G, Hamasur B, et al. Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis. Scand J Infect Dis. 2002;34:97–103. doi: 10.1080/00365540110077263. [DOI] [PubMed] [Google Scholar]
- 20.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Rie A, Menezes C, Scott L, et al. High Yield, Sensitivity, and Specificity of Xpert MTB/RIF for M. tuberculosis Detection in Fine Needle Aspirates from HIV-Infected TB Suspects; 18th Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2011; Mar 1, Session 174. Abstract 879. [Google Scholar]
- 22.Hillemann D, Rusch-Gerdes S, Boehme C, et al. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49:1202–5. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Causse M, Ruiz P, Juan Bautista GA, et al. Comparison of Two Molecular Methods for the Rapid Diagnosis of Extrapulmonary Tuberculosis. J Clin Microbiol. 2011 doi: 10.1128/JCM.00491-11. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadwai V, Boehme C, Nabeta P, et al. Xpert MTB/RIF, a new pillar in the diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011 doi: 10.1128/JCM.02319-10. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peter J, Green C, Hoelscher M, et al. Urine for the diagnosis of tuberculosis: current approaches, clinical applicability, and new developments. Curr Opin Pulm Med. 2010;16:262–70. doi: 10.1097/MCP.0b013e328337f23a. [DOI] [PMC free article] [PubMed] [Google Scholar]