Abstract
Background
Tenofovir gel, an antiretroviral-based vaginal microbicide, reduced HIV acquisition by 39% in women in a recent randomised controlled clinical trial in South Africa.
Methods
To inform policy we used a dynamical model of HIV transmission, calibrated to the epidemic in South Africa, to determine the population-level impact of this microbicide on HIV incidence, prevalence and deaths and to evaluate its cost-effectiveness.
Results
If women use Tenofovir-gel in 80% or more of sexual encounters (high coverage), it could avert 2.33 (0.12 to 4.63) million new infections and save 1.30 (0.07 to 2.42) million lives and if used in 25% of sexual encounters (low coverage), it could avert 0.50 (0.04 to 0.77) million new infections and save 0.29 (0.02 to 0.44) million deaths, over the next 20 years. At US$0.50 per application, the cost per infection averted at low coverage is US$2,392 (US$562 to US$4,222) and the cost per disability-adjusted life year saved is US$104 (US$27 to US$181); at high coverage the costs are about 30% less.
Conclusion
Over twenty years the use of Tenofovir gel in South Africa could avert up to 2 million new infections and 1 million AIDS deaths. Even with low rates of gel use it is highly cost-effective and compares favourably with other control methods. This female controlled prevention method could have a significant impact on the epidemic of HIV in South Africa. Programmes should aim to achieve gel use in more than 25% of sexual encounters to significantly alter the course of the epidemic.
Keywords: HIV prevention, microbicide gel, Tenofovir, South Africa, cost effectiveness
Introduction
The global HIV epidemic continues unabated, and despite recently observed declines in HIV incidence among young women in some countries in Africa, incidence rates remain unacceptably high.1 Efforts to reduce HIV transmission have yet to show a significant population level effect in many countries in Africa.2 The evidence that treating sexually transmitted infections reduces HIV-incidence remains equivocal;3 behaviour change interventions have not shown a demonstrable impact on HIV-incidence;4 male circumcision reduces the risk of infection by about 60% in men and is now being made available in much of Africa.5 A recent study reports that male circumcision might reduce HIV-infection directly in women but the reduction was not statistically significant in the trials quoted.6 Condoms are effective, if used consistently and correctly, and promoting condoms among sex workers has yielded positive results, but condom use remains limited.7 Where it is available anti-retroviral treatment has reduced mortality rates but there is little evidence that it has significantly reduced community-level transmission because it is provided mainly in the late stages of infection.8 There is therefore an urgent need for new prevention technologies that women can use to reduce their risk of infection.
In the CAPRISA 004 randomized controlled trial a vaginal microbicide containing 1% Tenofovir significantly reduced the transmission of HIV-19 from men to women. The availability of this female controlled method of prevention could have a significant impact on the future epidemic of HIV. In countries like South Africa, where the HIV epidemic is generalised and incidence rates among young women are high,10,11 policies on Tenofovir gel are being developed for post-licensure implementation. To help inform policy development and resource allocation, we estimate the potential long-term impact of Tenofovir gel on HIV prevalence, HIV incidence and AIDS mortality in South Africa as well as its cost-effectiveness.
Methods
A mathematical model,12 previously developed to explore the impact of male circumcision on the HIV epidemic, has been adapted for this study. Briefly, we compartmentalise the population into 5 groups: people that are not infected with HIV and people in four stages of HIV infection. We assume a mean survival time from HIV infection to death, without anti-retroviral treatment, of eleven years and let transmission rates fall as prevalence rises to allow for heterogeneity in sexual risk behaviour as discussed fully in our previous study.12 We fitted the model to trends in the prevalence of HIV among adults as estimated by UNAIDS.2 The model is not structured by age or gender. Assuming that the microbicide reduces male-to-female transmission with a relative risk of infection r, say, but does not reduce female-to-male transmission, then this is equivalent to a reduction in overall transmission with a relative risk of .12 We have previously shown that the long term, population level impact using this model, without stratifying by gender, is not significantly different from the impact calculated using a model that is stratified by gender.12
We consider three coverage scenarios, where coverage is defined as the proportion of all sexual encounters that are protected by the use of the gel. `High' is for women who used the gel in 80% or more of sexual contacts in the CAPRISA 004 trial9 for which the relative risk of infection was rh = 0.46 (0.20–0.96; limits are 95% confidence intervals here and elsewhere). We assume that the average coverage in the `high' scenario was 90%. If the relative risk of infection in one sexual encounter protected by the gel is r and the coverage is c then the average risk, at coverage c, is
| 1 |
Setting c = 0.90 and rc = rh gives r = 0.40 (0.11–0.96). For the `medium' scenario we assume that coverage is 50% so that rm = 0.70 (0.56–0.98) and for the low scenario that coverage is 25% so that rl = 0.85 (0.78–0.99) (Equation 1). In short, based on the CAPRISA 004 trial results, we assume 90% (high) coverage achieves 60% protection from HIV, 50% (medium) coverage achieves 30% protection and 25% (low) coverage achieves 15% protection.
We let the cost effectiveness, C, be the cost of using the microbicide divided by the reduction in the incidence of infection. If the cost per dose is γ, the coverage is c, and susceptible women, who use the microbicide, have η sexual contacts per year, then the annual cost is γηc. We let the incidence of HIV infection among susceptible women be I and the reduction in the risk of infection resulting from the use of the microbicide be . In non-linear epidemiological models, when the epidemic is far from elimination, the proportional reduction in incidence is generally less than the proportional reduction in transmission by an amount ϕ, say, which will depend on the natural history of the epidemic and the structure of the model that is used to fit the data.13 The annual incidence of HIV, per susceptible woman, is then ϕρI so that the cost per infection averted is
| 2 |
If averting one infection saves δ years of disability adjusted life, the cost per disability adjusted life-year (DALY) saved is
| 3 |
Verguet et al.14 suggested that the cost of the microbicide in a developing country is US$0.50. In the CAPRISA 004 Trial,9 the cost of the gel was US$0.01, the Tenofovir was donated free for the trial but is likely to cost about US$0.02, while the cost of the applicator was US$ 0.11 and of the wrapping US$ 0.16, giving a total cost for two applications (one before and one after the sexual encounter) of γ = US$0.60. Large scale production of the gel and cheaper applicators could reduce this cost further. For the purposes of this analysis, the full trial production costs of the product are used. Verguet et al.14 estimate that averting one HIV infection saves δ = 23 DALYs. To decide if the use of the gel is cost effective we compare the cost of preventing one person from being infected with HIV with the life-time cost of keeping one person on ART and we also compare the cost of saving one disability adjusted life year (DALY) with the per capita gross national income per year.
Results
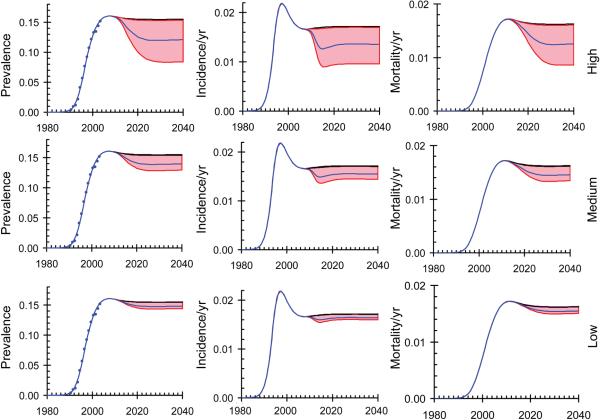
The model provides a good fit to data from sero-prevalence surveys in South Africa and the predicted trends in HIV prevalence, incidence and mortality are shown in Fig. 1. We assume that the microbicide is rolled-out starting in 2012 and is available throughout South Africa by 2015. HIV incidence falls rapidly as gel use increases (Fig. 1, centre) and the highest level of impact is seen by 2015. Prevalence falls less rapidly as transmission declines (Fig. 1, left) and mortality falls more slowly still (Fig. 1, right) because of the average life expectancy of eleven years for people who are infected with HIV and are not on ART; the full impact on mortality is not seen until 2030.
Fig. 1.
Trends in prevalence, incidence and mortality resulting from the roll-out of a vaginal microbicide programme between 2010 and 2015. The model is fitted to the trend in HIV prevalence among adults of age 15 years or more. Black line (immediately above the shaded areas): no intervention. Blue lines: microbicide is used in 90% (High), 50% (Medium) and 25% (Low), of all sex acts. Shaded areas give the 95% confidence limits.
Between 2011 and 2020, low coverage (25%) of the microbicide would avert 0.25 (0.02–0.38) million incident cases and prevent 0.05 (0.01–0.07) million deaths while high coverage (90%) would avert 1.10 (0.06–2.04) million cases and prevent 0.20 (0.01–0.35) million deaths (Table 1). In the following ten years (2021 to 2030) the impact on incidence would be only slightly greater but the impact on mortality would be much greater. Low coverage of the microbicide would still avert 0.25 (0.02–0.39) million incident cases but would prevent 0.24 (0.02–0.37) million deaths while high coverage would avert 1.23 (0.06–2.59) million cases and prevent 1.10 (0.06–2.07) million deaths.
Table 1.
The reduction in prevalence (at the end of each decade) and the cumulative incidence and deaths (over each decade). Data are given in columns assuming that the microbicide is used in 90%, 50% and 25% of sex acts. Numbers are in millions and numbers in brackets give 95% confidence limits.
| Coverage |
||||
|---|---|---|---|---|
| Period | Measure | 90% | 50% | 25% |
| 2011 to 2020 | Prevalence | 0.93 (0.05–1.72) | 0.44 (0.03–0.72) | 0.21 (0.01–0.32) |
| Incidence | 1.10 (0.06–2.04) | 0.53 (0.03–0.85) | 0.25 (0.02–0.38) | |
| Deaths | 0.20 (0.01–0.35) | 0.10 (0.01–0.16) | 0.05 (0.01–0.07) | |
|
| ||||
| 2021 to 2030 | Prevalence | 1.19 (0.06–2.42) | 0.54 (0.03–0.90) | 0.25 (0.02–0.38) |
| Incidence | 1.23 (0.06–2.59) | 0.56 (0.03–0.93) | 0.25 (0.02–0.39) | |
| Deaths | 1.10 (0.06–2.07) | 0.52 (0.03–0.85) | 0.24 (0.02–0.37) | |
|
| ||||
| 2011 to 2030 | Prevalence | 1.19 (0.06–2.42) | 0.54 (0.03–0.90) | 0.25 (0.02–0.38) |
| Incidence | 2.33 (0.12–4.63) | 1.09 (0.06–1.78) | 0.50 (0.04–0.77) | |
| Deaths | 1.30 (0.07–2.42) | 0.62 (0.04–1.01) | 0.29 (0.02–0.44) | |
In the CAPRISA 004 trial9 the annual incidence in the placebo arm was I = 9.1% (6.9%–11.7%). The women in the trial had an average of 5 sexual encounters per month, with the number decreasing from 8.5 to 3.0 per month at the end of the trial, so that η ≈ 60 ±12 per year. Fitting our model to the epidemic in South Africa, the reduction in incidence under the high coverage scenario is 65% of the reduction in transmission while at low coverage it is 53% of the reduction in transmission so that ϕ = 0.65 and 0.53 in the two scenarios. Substituting these values in Equation 2 the cost per infection averted at 90% coverage is US$1,701 (±US$1281) while at 25% coverage it is US$2,392 (±US$1,830) and with Equation 3 the cost per DALY averted at 90% coverage is US$74 (±US$56) and at 25% coverage, is US$104 (±US$77) (Table 2).
Table 2.
The cost effectiveness of the use of a vaginal microbicide at low and high coverage. Numbers in brackets are 95% confidence limits. The cost per infection not-averted is the estimated cost of keeping a person on ART for 23 years.14
| Coverage | US$/infection averted | US$/infection not-averted | US$/DALY saved | US$ Gross National Income |
|---|---|---|---|---|
| 25% | 2,392 (1,830) | 8,396 | 104 (77) | 5,770 |
| 90% | 1,701 (1,281) | 74 (56) |
Discussion
Widespread use of the vaginal microbicide, Tenofovir gel, as part of prevention programs, could lead to significant reductions in the rate of new HIV infections and AIDS related mortality. In South Africa, high coverage (Tenofovir gel used in 90% of sex encounters) could avert 2.33 (0.12 to 4.63) million new infections and save 1.30 (0.07 to 2.42) million lives over the next 20 years in South Africa. In a low coverage scenario (Tenofovir gel used in 25% of sex encounters), it could avert 0.50 (0.04 to 0.77) million new infections and save 0.29 (0.02 to 0.44) million deaths over 20 years in South Africa.
At low coverage the cost of preventing one person from being infected with HIV is 28% of the life-time cost of providing ART to one person which Verguet et al. estimate to be US$8,39614 while the cost of saving one DALY is only 2% of the per capita gross national income per year15 which in South Africa is $5,770.16 Condom social marketing, the use of sterile needles, female condom use by sex workers, ART for the prevention of mother-to-child transmission, blood safety, STI treatment, peer education and male circumcision were investigated by Verguet et al.14 and their cost effectiveness lay between US$5/DALY for condom social marketing in Chad, and US$194/DALY for the use of sterile needles in South Africa.17 The cost-effectiveness of using Tenofovir-gel microbicides at about US$100 per DALY saved (Table 2), compares favourably with these other interventions. In this analysis the cost of the microbicide is US$0.60 per sexual encounter but it is likely that this could be significantly reduced especially if the cost of the applicator and the wrapping can be reduced. Providing women with access to this microbicide should be cost-saving and cost effective. None of the estimates allow for the savings in treatment and hospitalization costs which will be incurred by HIV-positive people before they die or start ART and which will further improve the cost-benefit ratio.
In countries where the incidence of HIV-infection is lower than it is in southern Africa the cost-effectiveness will be less favourable since the cost per infection averted rises as the incidence falls (Equation 1). In countries such as Senegal, where the prevalence and the incidence are about one tenth of that in South Africa, Tenofovir gel would be less cost effective if used in the general population. However, if gel-use can be targeted at women at highest risk, such as commercial sex-workers, the impact of the gel on HIV-incidence would be increased. Now that the effectiveness of Tenofovir gel to prevent HIV infection has been demonstrated, we hope that further research into new drugs and new delivery methods will lead to lower costs with even greater efficacy.
The CAPRISA 004 trial included information, counselling, condom promotion and treatment of sexually transmitted infections for all study participants. Hence, the trial's effectiveness level estimates, which we have used in this analysis, provide an incremental measure of adding Tenofovir gel to an existing prevention program. This highlights the need for Tenofovir gel to be implemented as part of a combination prevention approach.
The model we used is not stratified on age and sex but captures the most important features of the impact of vaginal microbicides on the transmission of HIV. A model structured on age and sex would provide further insights into the way in which microbicide use can best be targeted at those women at highest risk. For example, the incidence of HIV in women in South Africa peaks at the age of about 25 years and close to 60% of all new infections in women are among those aged between 20 and 30 years18 so that targeting women in this age group, at least initially, could yield substantial benefits.
The model did not include Tenofovir resistance because the CAPRISA 004 trial showed no evidence of Tenofovir resistance in women who became infected with HIV in the CAPRISA 004 trial. Resistance may, however, become an important consideration over time as Tenofovir use for AIDS treatment becomes widespread. Further, the model did not include any impact of Tenofovir-gel on female-to-male transmission as there is currently no evidence for this.
While the factor ϕ, which relates the reduction in population level incidence to the reduction in the individual risk of infection, depends on the details of the epidemic and the structure of the model, it is unlikely to differ greatly for countries in East and southern Africa where the epidemic pattern is similar to that in South Africa. If epidemics can be brought close to elimination, formally with R0 close to 1, a small reduction in individual risk can lead to a much greater proportional reduction in population level incidence so that the cost effectiveness would be correspondingly greater.
The concept of combination prevention draws upon the idea that multiple prevention interventions can achieve synergy and thereby reduce HIV incidence to a greater extent than possible with each singly. The synergy goal of combination prevention is critical for the eventual reversal in the current upward trend in the HIV epidemic. Since young women have the highest HIV incidence rates in the southern African epidemic, high coverage of Tenofovir gel in this group in combination with high coverage of circumcision in men plus education, HIV testing and condom promotion could achieve the synergy necessary to turn the tide of the epidemic in this region.
The most important limitation of this analysis lies in the uncertainty in the estimates of the extent to which gel use reduces transmission and further studies to confirm the results of the CAPRISA 004 trial, which should also provide better estimates of the impact, are urgently needed.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gouws E. The International Group on Analysis of Trends in HIV Prevalence and Behaviours in Young People in Countries most Affected by HIV. Trends in HIV prevalence and sexual behaviour among young people aged 15–24 years in countries most affected by HIV. Sex. Transm. Infect. 2010;86(Suppl 2):ii72–ii83. doi: 10.1136/sti.2010.044933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS . Report on the Global AIDS Epidemic. United Nations Joint Programme on AIDS; Geneva: 2008. [Google Scholar]
- 3.White RG, Orroth KK, Glynn JR, et al. Treating curable sexually transmitted infections to prevent HIV in Africa: still an effective control strategy? J. Acquir. Immune Defic. Syndr. 2008;47(3):346–353. doi: 10.1097/QAI.0b013e318160d56a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross DA, Changalucha J, Obasi AI, et al. Biological and behavioural impact of an adolescent sexual health intervention in Tanzania: a community-randomized trial. AIDS. 2007;21(14):1943–1955. doi: 10.1097/QAD.0b013e3282ed3cf5. [DOI] [PubMed] [Google Scholar]
- 5.Lissouba P, Taljaard D, Rech D, et al. A model for the roll-out of comprehensive adult male circumcision services in African low-income settings of high HIV incidence: the ANRS 12126 Bophelo Pele Project. PLoS Med. 2010;7(7):e1000309. doi: 10.1371/journal.pmed.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallett TB, Alsallaq RA, Baeten JM, et al. Will circumcision provide even more protection from HIV to women and men? New estimates of the population impact of circumcision interventions. Sex. Transm. Infect. 2011;87(2):88–93. doi: 10.1136/sti.2010.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foss AM, Watts CH, Vickerman P, Heise L. Condoms and prevention of HIV. Br. Med. J. 2004;329(7459):185–186. doi: 10.1136/bmj.329.7459.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egger M. Outcomes of ART in resource-limited and industrialized countries. 14th Conference on Retriviruses and Opportunistic Infections; San Francisco. 25–28 Februray 2007; 2007. [Google Scholar]
- 9.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2011;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. The Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouws E, Stanecki KA, Lyerla R, Ghys PD. The epidemiology of HIV infection among young people aged 15–24 years in southern Africa. AIDS. 2008;22(Suppl 4):S5–16. doi: 10.1097/01.aids.0000341773.86500.9d. [DOI] [PubMed] [Google Scholar]
- 12.Williams BG, Lloyd-Smith JO, Gouws E, et al. The potential impact of male circumcision on HIV in sub-Saharan Africa. PLoS Med. 2006;3(7) doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford University Press; Oxford: 1991. [Google Scholar]
- 14.Verguet S, Walsh JA. Vaginal microbicides save money: a model of cost-effectiveness in South Africa and the USA. Sex. Transm. Infect. 2010;86(3):212–216. doi: 10.1136/sti.2009.037176. [DOI] [PubMed] [Google Scholar]
- 15.Sachs J. Report of the Commission on Macroeconomics and Health. World Health Organization; Geneva: Dec, 2001. 2001. [Google Scholar]
- 16.World Development Indicators database, Revised 9 July 2010. World Bank; Washington: 2010. [Accessed 13 September, 2010]. http://siteresources.worldbank.org/DATASTATISTICS/Resources/GNIPC.pdf. [Google Scholar]
- 17.Bertozzi S, Padian NS, Wegbreit J, et al. HIV/AIDS prevention and treatment. In: Jamison DT, editor. Disease control priorities in developing countries. Washington, DC: 2006. [Google Scholar]
- 18.Gouws E, Williams BG, Sheppard HW, Enge B, Karim SA. High incidence of HIV-1 in South Africa using a standardized algorithm for recent HIV seroconversion. J. Acquir. Immune Defic. Syndr. 2002;29(5):531–535. doi: 10.1097/00126334-200204150-00015. [DOI] [PubMed] [Google Scholar]