Abstract
Objective
To quantify fetal cardiovascular parameters with Spatio-Temporal Image Correlation (STIC) and Virtual Organ Computed-aided AnaLysis (VOCAL™) utilizing the sub-feature: “Contour Finder: Trace”.
Study Design
A cross-sectional study was designed consisting of patients with normal pregnancies between 19 and 40 weeks of gestation. After STIC datasets were acquired, analysis was performed offline (4DView) and the following cardiovascular parameters were evaluated: ventricular volume in end systole and end diastole, stroke volume, cardiac output, and ejection fraction. To account for fetal size, cardiac output was also expressed as a function of head circumference, abdominal circumference, or femoral diaphysis length. Regression models were fitted for each cardiovascular parameter to assess the effect of gestational age and paired comparisons were made between the left and right ventricles.
Results
1) Two hundred and seventeen patients were retrospectively identified, of whom 184 had adequate STIC datasets (85% acceptance); 2) ventricular volume, stroke volume, cardiac output, and adjusted cardiac output increased with gestational age; whereas, the ejection fraction decreased as gestation advanced; 3) the right ventricle was larger than the left in both systole (Right: 0.50 ml, IQR: 0.2 – 0.9; vs. Left: 0.27 ml, IQR: 0.1 – 0.5; p<0.001) and diastole (Right: 1.20 ml, IQR: 0.7 – 2.2; vs. Left: 1.03 ml, IQR: 0.5 – 1.7; p<0.001); 4) there were no differences between the left and right ventricle with respect to stroke volume, cardiac output, or adjusted cardiac output; and 5) the left ventricular ejection fraction was greater than the right (Left: 72.2%, IQR: 64 – 78; vs. Right: 62.4%, IQR: 56 – 69; p<0.001).
Conclusion
Fetal echocardiography, utilizing STIC and VOCAL™ with the sub-feature: “Contour Finder: Trace”, allows assessment of fetal cardiovascular parameters. Normal fetal cardiovascular physiology is characterized by ventricular volumes that are larger on the right and ejection fractions that are greater for the left ventricle resulting in similar left and right ventricular stroke volume and cardiac output.
Keywords: Four-dimensional ultrasonography, three-dimensional ultrasonography, fetal echocardiography, fetus, Contour-Finder, ventricular volume, stroke volume, cardiac output, ejection fraction, ultrasound, 3D, 4D, prenatal diagnosis
Introduction
The physiology of the fetal heart in normal and pathologic states has been the subject of intense investigation.1–20 Examining fetal cardiac output may provide insight into the fetal response to pathologic conditions. While direct measurement in-utero is not possible, indirect two-dimensional sonographic calculation of cardiac output has been described utilizing differing methodologies. These include: 1) interrogation of Doppler velocities across either the atrioventricular2;4;9 or the semilunar valves5–7 in conjunction with the valvular diameters; and 2) cross-sectional measurement of the left and right ventricle with subsequent estimation of both end systolic and end diastolic ventricular volumes using either orthogonal ventricular measurements12;14;21 or a biplane multiple disc method, Simpson’s rule.22
Each of these techniques has limitations. Minor measurement variation in the atrioventricular and semilunar valves can lead to large differences in the estimated cardiac output.1;23 Additionally, the use of two-dimensional measurements to estimate a volume requires making assumptions about the three dimensional geometry of the fetal heart which may not be valid and could lead to inaccuracy in the estimation of cardiac output.1;23 Furthermore, measurements of left ventricular end diastolic volume and stroke volume are difficult to reproduce and have coefficients of variation greater than 10%.23 As a result, clinical implementation of these methods has not occurred.1
Three- and four-dimensional ultrasonography has the potential to minimize the limitations inherent in two-dimensional estimations of cardiac output because: 1) no geometric assumptions are made since it is possible to assess ventricular volume directly; 2) with a single cardiac dataset acquired with Spatio-Temporal Image Correlation (STIC), all parameters required for calculation (ventricular volumes for left and right) are acquired in the same volume reducing the risk inherent in measurements of the two chambers at different times with two-dimensional ultrasound; 3) neither small outflow diameters nor angle dependent Doppler measurements are required for calculation. We have previously described a repeatable and reproducible approach to quantify ventricular volume calculations utilizing STIC.24;25 The purpose of this study was to use this methodology to quantify cardiovascular parameters including ventricular volume, stroke volume, cardiac output, and ejection fraction in a group of normal fetuses over a range of gestational ages.
Materials and Methods
Study population
A cross-sectional study was designed consisting of patients with normal pregnancies between 19 and 42 weeks of gestation by searching our database of patients enrolled into protocols that included examination of the fetal heart with three-dimensional and four-dimensional ultrasound. Women were considered to have normal pregnancies if they were without medical or obstetrical complication, carried a singleton pregnancy without chromosomal or congenital anomaly, were dated by either a first or second trimester ultrasound scan, and delivered an appropriate for gestational age neonate at term (≥37 weeks) who experienced an uncomplicated neonatal course. All others were excluded. All women provided written informed consent prior to sonographic examination. Participation was approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Examination technique
Ultrasound examinations were performed using a system with STIC capability (Voluson 730 Expert and E8, General Electric Medical systems, Kretztechnik, Zipt, Austria) utilizing a motorized curved array transabdominal transducer (2–5 or 4–8 MHz) by six experienced sonographers. A transverse view of the fetal chest at the level of the four chamber view was obtained from which STIC datasets were acquired. The acquisition time was 10 seconds with a sweep angle that was sufficient to encompass the fetal cardiac structures (25 to 35 degrees). Color Doppler ultrasonography was not utilized during the acquisition process. Adequate cardiac datasets were accepted for postprocessing if acoustic shadowing (signal loss in the sound path secondary to echogenic structures), dropout (signal loss in the sound path without intervening structures), and motion artifact were absent.
Cardiac datasets were acquired to investigate the following fetal cardiovascular parameters: ventricular volume, stroke volume (end diastolic volume – end systolic volume), cardiac output (stroke volume x fetal heart rate), and ejection fraction (stroke volume / end diastolic volume x 100). Fetal biometric measurements of the femoral diaphysis length (FL), abdominal circumference (AC), head circumference (HC), and biparietal diameter (BPD) were obtained utilizing two-dimensional sonography at the time of cardiac dataset collection. Cardiac output was expressed as both a function of estimated fetal weight26 and as a function of biometric components (FL, AC, HC).
Analysis was performed offline (4D View versions 5.0 – 7.0, GE Healthcare, Milwaukee, WI) in a standardized manner. In the “A plane” of the multi-planar display the fetal heart was re-oriented such that the left ventricle was located on the left side of the screen with the apex of the heart directed up. The interventricular septum was then rotated to 90 degrees in both the “A plane” and the “C plane”. The atrioventricular valves were located by scrolling from front to back in the “A plane”. The image was then optimized by selecting “chroma color 1” (Sepia) and “SRI 5”. After the image brightness and contrast settings were optimized, end systole and end diastole were identified by scrolling through each frame and identifying the image preceding atrioventricular (AV) valve opening (systole) and following AV valve closure (diastole). [supplemental video clip 1]
Cardiac ventricular volumes were calculated in a semi-automated fashion utilizing Virtual Organ Computed-aided AnaLysis (VOCAL™). “VOCAL II” was selected and the “Contour Finder: Trace” option was utilized with 15 degrees of rotation and a “sensitivity” of “1” (default = 5). The image was enlarged and the reference dot repositioned into the ventricle of interest. Because of the complex geometry of the ventricles, the location of the reference dot within the ventricle was selected to meet the software requirement that the contour only cross the rotation line twice. With these selections, 12 rotational steps were made and a volume was computed. Datasets were accepted for analysis if the ventricular septum, ventricular walls, and AV valves were visible throughout each rotational step. [supplemental video clip 2]
Statistical analysis
Data were first assessed using numerical and graphical techniques, including scatter plots of each response versus gestational age, to determine if they met the assumptions of the statistical tests being used to analyze them. All but two scatter plots revealed the presence of curvilinear relationships and heteroscedasticity; hence, natural logarithmic transformations (from the Box-Cox family of transformations) of each response and gestational age were performed to linearize the data and correct for heteroscedasticity.
Weighted regression analysis was performed using this transformed data for each of the models to address heteroscedasticity. The weights were calculated as 1/(predicted value of response x 1.253)2 as they are the best linear unbiased estimates (BLUE) of each of the true responses.27–30 The weights were multiplied by √(π/2) = 1.253, using a half standard normal distribution.27–30 Since all of the responses are a function of gestational age, the residuals should have a normal distribution at each value of gestational age and the absolute values of the residuals should have a half normal distribution. It follows that the mean of the absolute residuals multiplied by √(π/2) is an estimate of the standard deviation (SD) of the residuals. If the SD is not fairly constant for each parameter, their predicted values from regressing absolute residuals against gestational age multiplied by √(π/2) will provide age-specific estimates of the SD of the signed residuals, and hence of the response. For each model, studentized residuals from the weighted regression analysis were assessed for normality using a normal probability plot. Main effects polynomial weighted regression models were fitted to the data to capture the curvature in the data. As the two scatter plots of ejection fraction for the left and right ventricles did not indicate curvilinear relationships or the presence of heteroscedasticity, simple linear regression analysis was used to evaluate the mean change in ejection fraction in each ventricle due to gestational age. Linear and/or quadratic terms for gestational age in the model typically provided a good fit to the data as evidenced by random patterns in the residual analyses. The quadratic term was not dropped from the model whenever it was found to be non significant, as it provided a better fit to the data than its corresponding simplified model with only the linear term in it. Model p-values and corresponding adjusted R-squares were reported. The 5th, 50th, and 95th percentiles were computed on the back-transformed data and weighted regression equations.
For bivariate analysis, the Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution. Since the data was not normally distributed, non-parametric statistics were employed. The Wilcoxon Signed-Rank test was used to determine the difference between paired variables and Spearman’s rank correlation coefficient (rs) was utilized to assess correlations. A p-value of <0.05 was considered statistically significant for all comparisons. Statistical analyses were performed with SPSS package version 14 (SPSS Inc, Chicago IL, USA) as well as the SAS System for Windows version 9.2 (SAS Institute Inc, Cary NC, USA).
Results
Two hundred and seventeen patients met the inclusion criteria, of whom 184 had an adequate dataset acquired, yielding an 85% (184/217) rate of acceptance. Because multiple datasets were available, a random number generator was employed such that each patient was represented by a single STIC. These 184 fetuses were evaluated at a median gestational age of 27.7 weeks (range: 19.1 – 38.9). Each fetus was delivered at term with a median gestational age at delivery of 39.6 weeks (range: 37.0 – 41.9) and a median birth weight of 3217 grams (range: 2545 – 4070).
Ventricular volumes increase as gestation advances
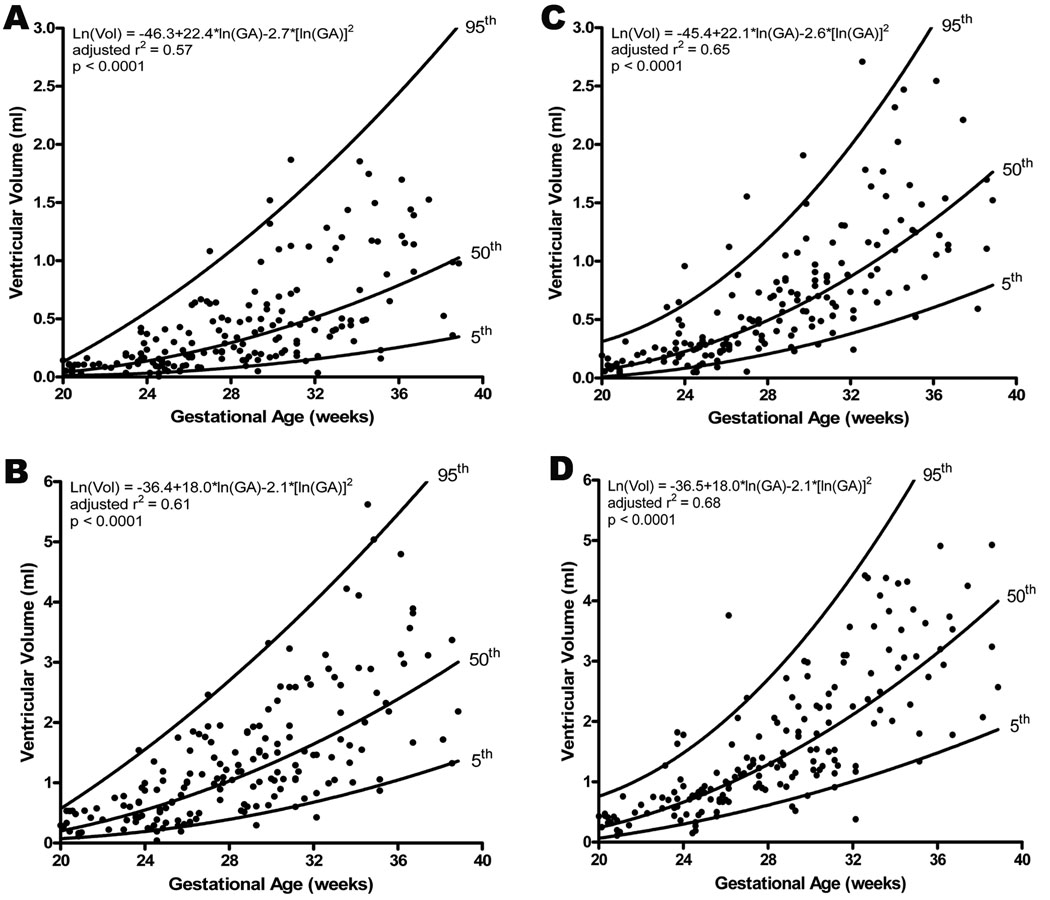
Both left ventricular (Systole: adjusted r2 = 0.567, p<0.0001; Diastole: adjusted r2 = 0.612, p<0.0001; Figure 1) and right ventricular (Systole: adjusted r2 = 0.654, p<0.0001; Diastole: adjusted r2 = 0.675, p<0.0001; Figure 1) volumes (ml) increased as gestation advanced. Pairwise comparisons of the median ventricular volumes demonstrated significantly greater volume for the right side in both systole (Right: 0.50 ml, IQR: 0.2 – 0.9; Left: 0.27 ml, IQR: 0.1 – 0.5; p<0.001) and diastole (Right: 1.20 ml, IQR: 0.7 – 2.2; Left: 1.03 ml, IQR: 0.5 – 1.7; p<0.001). Furthermore, when a ratio of right to left was constructed the right ventricle was found to be volumetrically greater in both systole (mean Right/Left ratio: 2.0, 95% CI: 1.8 – 2.2) and diastole (mean Right/Left ratio: 1.4, 95% CI: 1.3 – 1.5), an effect which was independent of gestational age.
Figure 1.
Volume measurements taken in end systole and end diastole for the left and right ventricles increased significantly with advancing gestational age (A: left ventricle in end systole, B: left ventricle in end diastole, C: right ventricle in end systole, D: right ventricle in end diastole). The regression line with the 5% and 95% confidence intervals is plotted with the regression equation, adjusted r2, and p value for each. Additionally, the median ventricular volumes were significantly greater for the right side in both systole (Right: 0.50 ml, IQR: 0.2 – 0.9; Left: 0.27 ml, IQR: 0.1 – 0.5; p<0.001) and diastole (Right: 1.20 ml, IQR: 0.7 – 2.2; Left: 1.03 ml, IQR: 0.5 – 1.7; p<0.001).
Stroke volume is similar for the left and right ventricles
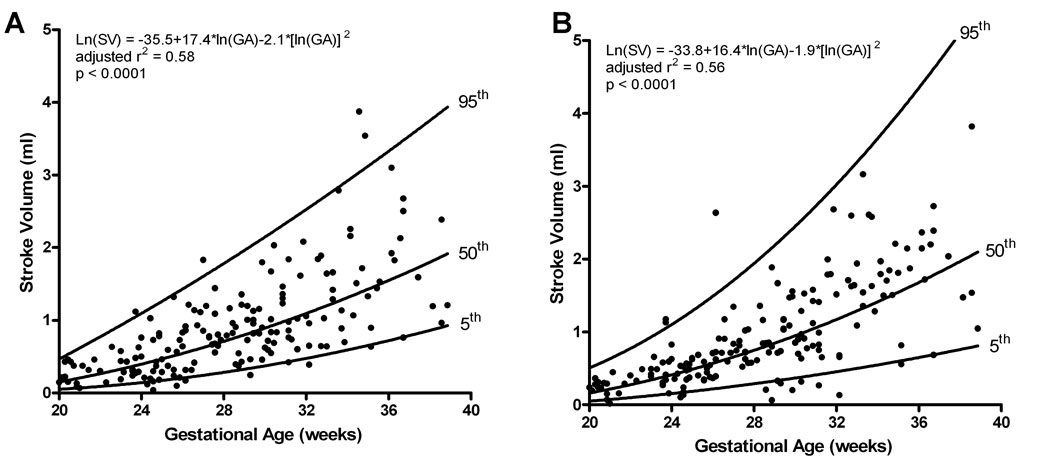
Left and right ventricular stroke volume (ml) increased as gestation advanced (Left: adjusted r2 = 0.585, p<0.0001; Right: adjusted r2 = 0.561, p<0.0001; Figure 2). When compared pairwise there was no significant difference between the left and right stroke volume (z=−1.3, p=0.21).
Figure 2.
Stroke volume (SV; end diastolic volume – end systolic volume) obtained for left (A) and right (B) ventricles increased significantly as gestation advanced; however, there was not a significant difference between the two ventricles. The regression line with the 5% and 95% CI’s is plotted with the regression equation, adjusted r2, and p value for each.
Cardiac output does not differ between the left and right ventricles
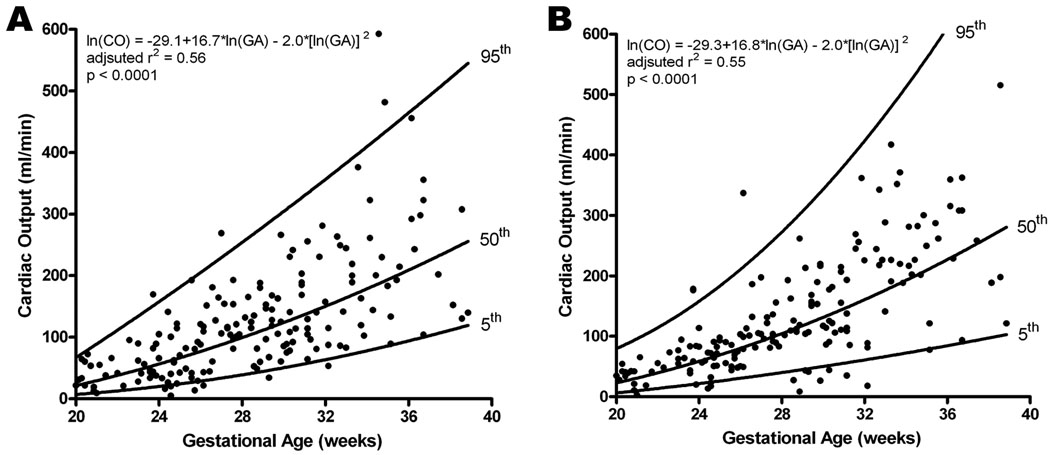
Fetal cardiac output (ml/min) was determined by multiplying the stroke volume by the fetal heart rate (median 140 bpm, range: 116 – 170) recorded during cardiac dataset acquisition. The left and right ventricular cardiac output increased with gestational age (Left: adjusted r2 = 0.561, p<0.0001; Right: adjusted r2 = 0.555, p<0.0001; Figure 3). Comparison in a paired manner yielded no significant difference between left and right sided cardiac output (z=−1.3, p=0.19).
Figure 3.
Cardiac output (CO; stroke volume x fetal heart rate) obtained for left (A) and right (B) ventricles increased with advancing gestational age; however, there was no significant difference found between the two ventricles. The regression line with the 5% and 95% CI’s is plotted with the regression equation, adjusted r2, and p value for each.
Cardiac output per unit of estimated fetal weight does not change with gestational age or differ between left and right ventricles
Fetal cardiac output (CO) was adjusted by the estimated fetal weight26 (EFW) which was calculated using two-dimensional sonographic parameters (BPD, HC, AC, FL) obtained at the time of cardiac dataset acquisition. Adjustment for the estimated fetal weight (CO / EFW; CO(EFW)) demonstrated that the left and right ventricular cardiac output (ml/min/kg) did not change as gestation advanced (Left CO(EFW): rs = 0.002, p=0.98; Right CO(EFW): rs = 0.037, p=0.62); additionally, when comparing the left and the right sides no significant difference was noted (z=−1.1, p=0.25; Figure 4).
Figure 4.
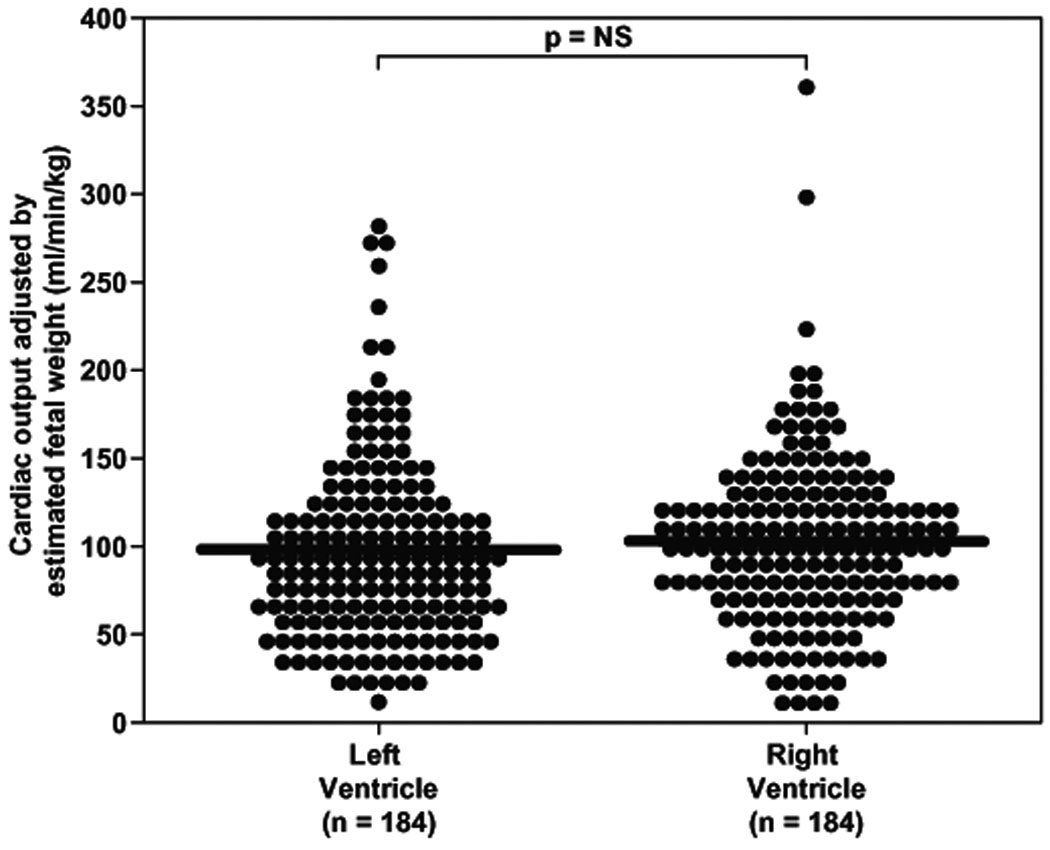
Cardiac output (CO; stroke volume x fetal heart rate) obtained for left and right ventricles adjusted by the estimated fetal weight (EFF; CO / EFW) was not correlated with gestational age and there was no significant difference in the median cardiac output between the two ventricles (LV CO(EFW): 90.0 ml/min/kg, IQR: 56.0 – 179.8 vs. RV CO(EFW): 99.9 ml/min/kg, IQR: 72.2 – 179.2; p=ns).
Cardiac output adjusted by fetal biometric parameter increases with gestational age with no difference between left and right ventricles
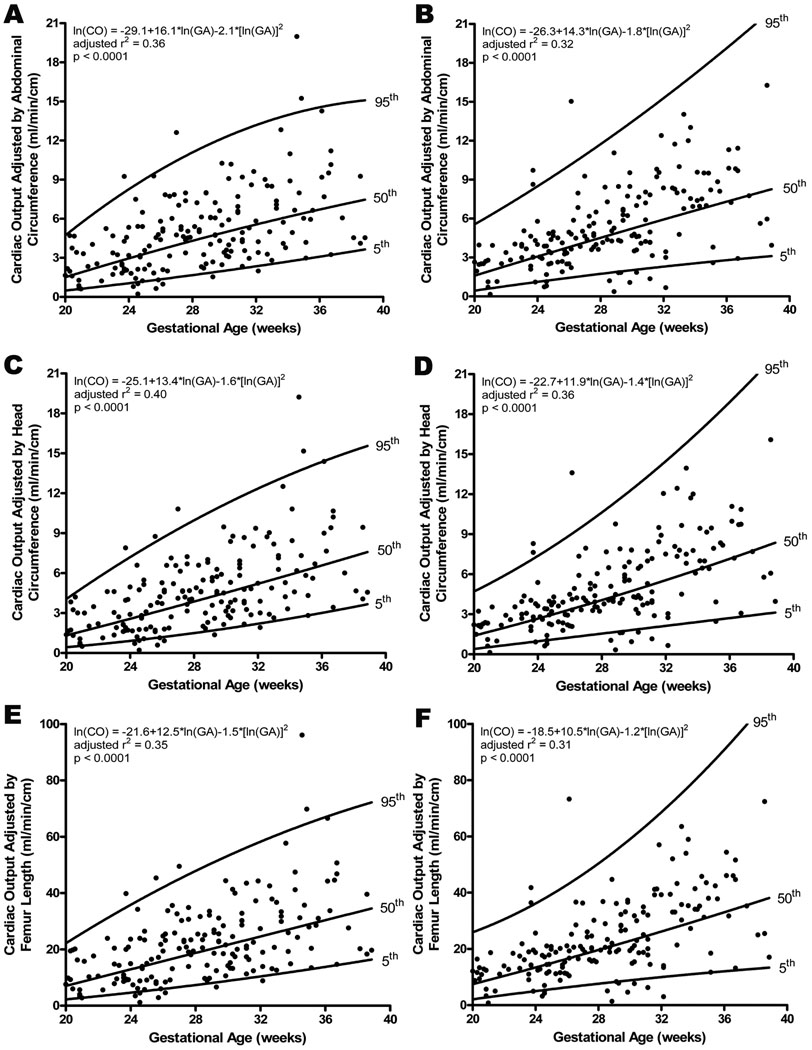
Fetal cardiac output was also adjusted for fetal size by dividing the cardiac output (ml/min) by the abdominal circumference (AC, cm; CO(AC)), head circumference (HC, cm; CO(HC)), and femoral diaphysis length (FL, cm; CO(FL)). Adjustment by the abdominal circumference demonstrated that the left and right ventricular cardiac output increased with gestational age (Left CO(AC): adjusted r2 = 0.356, p<0.0001; Right CO(AC): adjusted r2 = 0.321, p<0.0001; Figure 5a and 5b). Similarly, significant correlations were noted when cardiac output was adjusted by the head circumference (Left CO(HC): adjusted r2 = 0.400, p<0.0001; Right CO(HC): adjusted r2 = 0.363, p<0.0001; Figure 5c and 5d) and by the femoral diaphysis length (Left CO(FL): adjusted r2 = 0.351, p<0.0001; Right CO(FL): adjusted r2 = 0.313, p<0.0001; Figure 5e and 5f). Comparing the left and right sided adjusted cardiac outputs did not yield a significant difference for any of the comparisons (AC: z=−1.3, p=0.20; HC: z=−1.3, p=0.19; FL: z=− 1.3, p=0.18).
Figure 5.
Cardiac output (CO; stroke volume x fetal heart rate) obtained for left (A, C, E) and right (B, D, F) ventricles, adjusted by fetal biometric measurement, increased significantly with advancing gestational age. There was no significant difference when left and right were compared. The regression line with the 5% and 95% CI’s is plotted with the regression equation, adjusted r2, and p value for each.
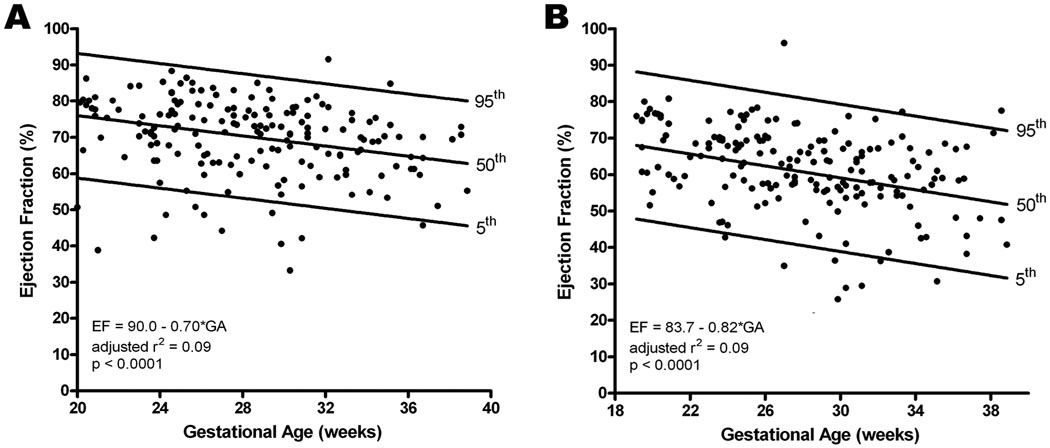
Fetal ejection fraction decreases as gestation advances and is greater for the left ventricle
Ejection fraction was also determined for the left and right ventricles. Both left and right ventricular ejection fractions decreased significantly with advancing gestational age (Left: adjusted r2 = 0.094, p<0.0001; Right: adjusted r2 = 0.094, p<0.0001; Figure 6). In addition, the left ventricular ejection fraction was significantly greater than the right (Left: 72.2%, IQR: 64 – 78; Right: 62.4%, IQR: 56 – 69; p<0.001).
Figure 6.
Ejection fraction (stroke volume / end diastolic volume x 100) for the left (A) and right (B) ventricles decreased with increasing gestational age. The regression line with the 5% and 95% CI’s is plotted with the regression equation, adjusted r2, and p value for each. Additionally, the left side was significantly greater than the right (Left: 72.2%, IQR: 64 – 78; Right: 62.4%, IQR: 56 – 69; p<0.001).
Comment
Principal findings of the study
1) Ventricular volume, stroke volume, and cardiac output increase with gestational age; whereas, the ejection fraction decreases as gestation advances; 2) right ventricular volumes are greater than left; 3) there is no difference between the left and right ventricles for stroke volume, cardiac output, or adjusted cardiac output; and 4) the left ventricular ejection fraction is greater than the right.
Fetal cardiovascular parameters change as gestation advances
Right and left ventricular volumes in both systole and diastole increased with gestational age, consistent with previous reports utilizing two-dimensional22 or three-dimensional imaging.31–34 When fetal heart function was evaluated, by computing the parameters stroke volume and cardiac output, the same correlation was found. These findings have been well supported in the literature by utilizing both two-dimensional2;4;5;7;9;14;21;22;35 and three-dimensional31–34;36;37 ultrasonography.
Since fetal size increases dramatically as gestation advances,38 adjusting cardiac output for fetal size may allow a better estimation of the changes that occur over time. First, an adjustment based on the estimated fetal weight26 was chosen because of its routine use in the obstetrical community as well as in animal studies.39–41 When adjusting cardiac output by the estimated fetal weight (CO / EFW), no significant change with gestational age was observed. However, unlike animal studies where it is possible to weigh the animal, clinicians have only estimates of fetal weight in humans which can carry significant variation and could introduce errors in the calculations.42 In order to minimize variability introduced into these calculations cardiac output was also expressed as a function of three biometric parameters (HC, AC, FL), each of which has a reliability coefficient that approaches 143 and are measured directly without mathematical treatment or modeling of the observed results. Each adjusted cardiac output (CO(HC), CO(AC), CO(FL)) increased with gestational age, findings similar to those obtained from chronically instrumented fetal baboons.39
The results on ejection fraction reported in this study are consistent with a previous report from Wladimiroff et al14 who used M-mode. Left ventricular volumes in systole and diastole were calculated and a decrease in fetal ejection fraction with advancing gestation was observed. Additionally, three-dimensional31;33;34 imaging has also been utilized to calculate the fetal ejection fraction; specifically, Esh-Broder et al utilized a free-hand technique to compute ejection fraction and reported lower values than those described herein.31 Furthermore, Uittenbogaard et al calculated fetal ejection fraction by manually tracing the ventricle in multiple slices and summing the volume of each slice in a method that employs Simpson’s rule.34 The authors did not find a significant correlation with gestational age and reported lower ejection fractions than those reported in this study. Lastly, Messing et al, utilizing STIC with the post-processing features “Inversion Mode” and VOCAL™, computed ejection fractions that were comparable to those obtained in this study but did not find a significant decrease with advancing gestational age.33 The apparent discrepancies between these studies and the results herein may be attributed to differences in method repeatability or smaller sample sizes. Esh-Broder et al and Uittenbogaard et al employed techniques with a coefficient of variation of 16% and 13.7%, respectively, and the sample size utilized by Esh-Broder et al and Messing et al was 25 and 100, respectively.31;33;34
The finding that ejection fraction decreases with advancing gestational age is plausible, and could be a reflection of the physiologic changes that occur in the myocardium, the fetus, and the developing placenta. Supporting evidence for this can be found in the early filling wave (E-wave) of the mitral and tricuspid valves. The peak velocity of the E-wave increases with gestational age indicating increased ventricular compliance and enhanced active relaxation.44 Afterload, as reflected by the umbilical artery pulsatility or resistance indices, also decreases with advancing gestational age.45 Each of these parameters could explain the finding of the current study that the fraction of blood ejected from either ventricle decreases as gestational age advances.
Systolic and diastolic volumes are greater in the right ventricle
Comparable results were obtained when a ratio of right to left end diastolic ventricular volumes in the human fetus were calculated using either two-dimensional22 or three-dimensional33 ultrasonography; specifically, the right ventricular volume was greater with results ranging from 1.122 to 1.4.33 When ventricular volumes in end systole were compared the right ventricle remained greater than the left. This finding is consistent with results reported by Uittenbogaard et al; although, the ratio reported herein of 2 was greater than that of Uittenbogaard et al (1.12).34 Furthermore, ventricular volumes reported in this study are consistent with those of Messing et al.33 However, since the measurement of volume does not serve as an effective indicator of function we sought to further explore these relationships by evaluating several fetal cardiovascular parameters.
Left and right ventricular stroke volume, cardiac output, and ejection fraction
When comparing the stroke volume and cardiac output of the left and right sides of the fetal heart no significant differences were noted. Moreover, after the cardiac output was adjusted for parameters of fetal size (EFW, AC, HC, FL), the non-significant difference between the left and right ventricles held. These findings are supported by recent work from Molina et al36 who found that the ratio of right to left stroke volume remained close to 1 across gestation, increasing slowly from 0.97 at 12 weeks to 1.13 at 34 weeks. These results are plausible when one considers that: 1) the pulmonary artery annulus is larger than the aortic annulus across gestation;2 and 2) the mean and maximum flow velocity waveform (FVW) of the ascending aorta is greater than that of the main pulmonary artery.9 Taken together, a slightly smaller aorta with a greater FVW could produce the same stroke volume and cardiac output as a slightly larger pulmonary artery with a lower FVW.
Fetal assessment studies utilizing two-dimensional techniques have reported greater cardiac output for the right ventricle than the left, leading investigators to conclude that the fetal heart is right dominant.2;4;7;9 This finding could relate to the potential sources of bias inherent in the measurement technique which can include the following: 1) a change in the fetal status during an examination requiring up to 30 minutes;2 2) inherent variation when computing an area (A = π x r2) utilizing outflow diameters due to a magnification by a power of 2;9;23 and 3) assuming that the semilunar valves are circular.4 None of these factors affect the computation of a ventricular volume or the calculation of stroke volume or cardiac output when STIC and VOCAL™ are utilized for the following reasons: 1) a cardiac dataset is acquired in 12.5 seconds or less making a change in the fetal status unlikely; 2) neither small outflow diameters nor angle dependent Doppler measurements are required; and 3) no geometric anatomical assumptions are made. Therefore, if the heart is volumetrically right dominant and there is no difference between the left and right ventricle in terms of stroke volume and cardiac output, then the only mechanism that could account for this finding is a functional difference between the left and right ventricular ejection fraction.
A novel finding is that the left ventricular ejection fraction was significantly greater than the right ventricular ejection fraction. Structurally, the human brain is relatively large compared to that of other species46;47 and a greater proportion of the cardiac output is allocated to this developing organ in the human.47 This mechanism, in combination with the high metabolic demand47 of the human fetal brain, may explain this finding.
Limitations
There are several important limitations to the present work. First, there is no gold standard at present to serve as a means of comparison for the calculation of fetal cardiovascular parameters. Recent work from Rizzo et al suggests that the mean bias between two-dimensional and three-dimensional estimates of stroke volume may be small; however, the reported range is greater than 40% for both left and right ventricular stroke volume estimates.37 Therefore, using two-dimensional ultrasonographic estimation of volume as a standard for comparison to STIC datasets should be interpreted with caution. Second, it is important to note that STIC produces a single, computer generated, cardiac cycle which is an assemblage of between 20 and 30 real cardiac cycles and a smoothing or averaging of the ventricular borders could occur, introducing error into the calculations.48 We have previously demonstrated that using STIC and VOCAL™ is both repeatable and reproducible for calculating fetal ventricular volumes.24;25 Furthermore, Bhat et al addressed the validity of STIC using a balloon model with volumes ranging between 2.5 ml and 10 ml.49 The authors concluded that STIC was acceptably accurate over this volume range. In the present study, nearly all volume calculations in end systole and half of those in end diastole were less than 2.5 ml; therefore, it remains unclear if STIC compilation affects volume calculation since the absolute error of STIC has not been described in this range of volumes. Third, adequate volume datasets are limited by fetal position, movement, or respirations, as well as acoustic shadowing and drop out which contributed to an overall rate of rejection of 15%, a rate similar to results reported by Uittenbogaard et al (11.6%) for experienced sonographers.50 Lastly, while the acquisition time of a STIC is at most 12.5 seconds, there is a significant learning curve and time commitment required to orient and analyze the data.
Conclusions
In conclusion, this study demonstrates that ventricular volumes, stroke volume, and cardiac output all increase with advancing gestational age. Furthermore, the fetal heart is volumetrically right dominant; however, there is not a significant difference between the left and right sides with respect to stroke volume or cardiac output. Finally, the ejection fraction decreases with advancing gestation, with the left ventricle ejecting significantly more blood than the right. These findings enhance our knowledge of normal fetal physiology and may allow an assessment of cardiovascular function, utilizing familiar variables, in pathologic conditions.
Supplementary Material
This video clip illustrates how to orient a cardiac STIC dataset prior to calculating ventricular volumes.
This video clip illustrates how to calculate cardiac ventricular volumes in a semi-automated fashion utilizing VOCAL™ and Contour Finder: Trace with 15° of rotation.
Acknowledgements
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services.
Footnotes
Presentation information: The findings of this submission were presented at the annual meeting of the International Society of Ultrasound in Obstetrics and Gynecology in Chicago IL on August 24, 2008 (abstract number OC004).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Simpson J. Echocardiographic evaluation of cardiac function in the fetus. Prenat.Diagn. 2004;24:1081–1091. doi: 10.1002/pd.1065. [DOI] [PubMed] [Google Scholar]
- 2.Allan LD, Chita SK, Al-Ghazali W, Crawford DC, Tynan M. Doppler echocardiographic evaluation of the normal human fetal heart. Br.Heart J. 1987;57:528–533. doi: 10.1136/hrt.57.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baschat AA. Pathophysiology of fetal growth restriction: implications for diagnosis and surveillance. Obstet.Gynecol.Surv. 2004;59:617–627. doi: 10.1097/01.ogx.0000133943.54530.76. [DOI] [PubMed] [Google Scholar]
- 4.De Smedt MC, Visser GH, Meijboom EJ. Fetal cardiac output estimated by Doppler echocardiography during mid- and late gestation. Am.J.Cardiol. 1987;60:338–342. doi: 10.1016/0002-9149(87)90238-4. [DOI] [PubMed] [Google Scholar]
- 5.Kenny JF, Plappert T, Doubilet P, Saltzman DH, Cartier M, Zollars L, et al. Changes in intracardiac blood flow velocities and right and left ventricular stroke volumes with gestational age in the normal human fetus: a prospective Doppler echocardiographic study. Circulation. 1986;74:1208–1216. doi: 10.1161/01.cir.74.6.1208. [DOI] [PubMed] [Google Scholar]
- 6.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet.Gynecol. 2006;28:126–136. doi: 10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- 7.Mielke G, Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation. 2001;103:1662–1668. doi: 10.1161/01.cir.103.12.1662. [DOI] [PubMed] [Google Scholar]
- 8.Rasanen J, Debbs RH, Huhta JC. Echocardiography in intrauterine growth restriction. Clin.Obstet.Gynecol. 1997;40:796–803. doi: 10.1097/00003081-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Reed KL, Meijboom EJ, Sahn DJ, Scagnelli SA, Valdes-Cruz LM, Shenker L. Cardiac Doppler flow velocities in human fetuses. Circulation. 1986;73:41–46. doi: 10.1161/01.cir.73.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Severi FM, Rizzo G, Bocchi C, D'Antona D, Verzuri MS, Arduini D. Intrauterine growth retardation and fetal cardiac function. Fetal Diagn.Ther. 2000;15:8–19. doi: 10.1159/000020969. [DOI] [PubMed] [Google Scholar]
- 11.Turan OM, Turan S, Gungor S, Berg C, Moyano D, Gembruch U, et al. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet.Gynecol. 2008;32:160–167. doi: 10.1002/uog.5386. [DOI] [PubMed] [Google Scholar]
- 12.Veille JC, Sivakoff M, Nemeth M. Evaluation of the human fetal cardiac size and function. Am.J.Perinatol. 1990;7:54–59. doi: 10.1055/s-2007-999447. [DOI] [PubMed] [Google Scholar]
- 13.Verburg BO, Jaddoe VW, Wladimiroff JW, Hofman A, Witteman JC, Steegers EA. Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. Circulation. 2008;117:649–659. doi: 10.1161/CIRCULATIONAHA.107.709717. [DOI] [PubMed] [Google Scholar]
- 14.Wladimiroff JW, Vosters R, McGhie JS. Normal cardiac ventricular geometry and function during the last trimester of pregnancy and early neonatal period. Br.J.Obstet.Gynaecol. 1982;89:839–844. doi: 10.1111/j.1471-0528.1982.tb05037.x. [DOI] [PubMed] [Google Scholar]
- 15.DeVore GR. Assessing fetal cardiac ventricular function. Semin.Fetal Neonatal Med. 2005;10:515–541. doi: 10.1016/j.siny.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Alfred Z Abuhamad, Rabih Chaoui. A Practical Guide to Fetal Echocardiography: Normal and Abnormal Hearts. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 17.Nelson TR, Pretorius DH, Sklansky M, Hagen-Ansert S. Three-dimensional echocardiographic evaluation of fetal heart anatomy and function: acquisition, analysis, and display. J.Ultrasound Med. 1996;15:1–9. [PubMed] [Google Scholar]
- 18.Terry J Dubose. Fetal Sonography. Philadelphia: W.B. Saunders Company; 1996. [Google Scholar]
- 19.Timor-Tritsch IE, Bashiri A, Monteagudo A, Arslan AA. Qualified and trained sonographers in the US can perform early fetal anatomy scans between 11 and 14 weeks. Am.J.Obstet.Gynecol. 2004;191:1247–1252. doi: 10.1016/j.ajog.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Zimmer EZ, Chao CR, Sharma S, Timor-Tritsch IE. Interfetal heart rate and size variation in first-trimester multifetal pregnancies and heart rate of surviving fetuses after fetal reduction. Ultrasound Obstet.Gynecol. 1997;9:253–256. doi: 10.1046/j.1469-0705.1997.09040253.x. [DOI] [PubMed] [Google Scholar]
- 21.Wladimiroff JW, McGhie J. Ultrasonic assessment of cardiovascular geometry and function in the human fetus. Br.J.Obstet.Gynaecol. 1981;88:870–875. [PubMed] [Google Scholar]
- 22.Schmidt KG, Silverman NH, Hoffman JI. Determination of ventricular volumes in human fetal hearts by two-dimensional echocardiography. Am.J.Cardiol. 1995;76:1313–1316. doi: 10.1016/s0002-9149(99)80365-8. [DOI] [PubMed] [Google Scholar]
- 23.Simpson JM, Cook A. Repeatability of echocardiographic measurements in the human fetus. Ultrasound Obstet.Gynecol. 2002;20:332–339. doi: 10.1046/j.1469-0705.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- 24.Hamill N, Romero R, Myers SA, et al. Fetal cardiac output determination by four-dimensional fetal echocardiography using spatiotemporal image correlation (STIC) and VOCAL. Ultrasound Obstet.Gynecol. 32(3):244. 8-1-2008. Ref Type: Abstract. [Google Scholar]
- 25.Hamill N, Romero R, Hassan S, et al. Repeatability and Reproducibility of Fetal Cardiac Ventricular Volume Calculations Using Spatiotemporal Image Correlation and Virtual Organ Computer Aided Analysis. J.Ultrasound Med. 2009;28:1301–1311. doi: 10.7863/jum.2009.28.10.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–540. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG. Construction of age-related reference centiles using absolute residuals. Stat.Med. 1993;12:917–924. doi: 10.1002/sim.4780121003. [DOI] [PubMed] [Google Scholar]
- 28.Altman DG, Chitty LS. Charts of fetal size: 1. Methodology. Br.J.Obstet.Gynaecol. 1994;101:29–34. doi: 10.1111/j.1471-0528.1994.tb13006.x. [DOI] [PubMed] [Google Scholar]
- 29.Altman DG, Chitty LS. Design and analysis of studies to derive charts of fetal size. Ultrasound Obstet.Gynecol. 1993;3:378–384. doi: 10.1046/j.1469-0705.1993.03060378.x. [DOI] [PubMed] [Google Scholar]
- 30.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Regression Models. New York: McGraw-Hill; 1996. [Google Scholar]
- 31.Esh-Broder E, Ushakov FB, Imbar T, Yagel S. Application of free-hand three-dimensional echocardiography in the evaluation of fetal cardiac ejection fraction: a preliminary study. Ultrasound Obstet.Gynecol. 2004;23:546–551. doi: 10.1002/uog.1059. [DOI] [PubMed] [Google Scholar]
- 32.Meyer-Wittkopf M, Cole A, Cooper SG, Schmidt S, Sholler GF. Three-dimensional quantitative echocardiographic assessment of ventricular volume in healthy human fetuses and in fetuses with congenital heart disease. J.Ultrasound Med. 2001;20:317–327. doi: 10.7863/jum.2001.20.4.317. [DOI] [PubMed] [Google Scholar]
- 33.Messing B, Cohen SM, Valsky DV, Rosenak D, Hochner-Celnikier D, Savchev S, et al. Fetal cardiac ventricle volumetry in the second half of gestation assessed by 4D ultrasound using STIC combined with inversion mode. Ultrasound Obstet.Gynecol. 2007;30:142–151. doi: 10.1002/uog.4036. [DOI] [PubMed] [Google Scholar]
- 34.Uittenbogaard LB, Haak MC, Spreeuwenberg MD, Van Vugt JM. Fetal cardiac function assessed with four-dimensional ultrasound imaging using spatiotemporal image correlation. Ultrasound Obstet.Gynecol. 2009;33:272–281. doi: 10.1002/uog.6287. [DOI] [PubMed] [Google Scholar]
- 35.Sutton MS, Gill T, Plappert T, Saltzman DH, Doubilet P. Assessment of right and left ventricular function in terms of force development with gestational age in the normal human fetus. Br.Heart J. 1991;66:285–289. doi: 10.1136/hrt.66.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina FS, Faro C, Sotiriadis A, Dagklis T, Nicolaides KH. Heart stroke volume and cardiac output by four-dimensional ultrasound in normal fetuses. Ultrasound Obstet.Gynecol. 2008;32:181–187. doi: 10.1002/uog.5374. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo G, Capponi A, Cavicchioni O, Vendola M, Arduini D. Fetal cardiac stroke volume determination by four-dimensional ultrasound with spatio-temporal image correlation compared with two-dimensional and Doppler ultrasonography. Prenat.Diagn. 2007;27:1147–1150. doi: 10.1002/pd.1870. [DOI] [PubMed] [Google Scholar]
- 38.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 39.Paton JB, Fisher DE. Organ blood flows of fetal and infant baboons. Early Hum.Dev. 1984;10:137–147. doi: 10.1016/0378-3782(84)90120-8. [DOI] [PubMed] [Google Scholar]
- 40.Rudolph AM, Heymann MA. Circulatory changes during growth in the fetal lamb. Circ.Res. 1970;26:289–299. doi: 10.1161/01.res.26.3.289. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ.Res. 1985;57:811–821. doi: 10.1161/01.res.57.6.811. [DOI] [PubMed] [Google Scholar]
- 42.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am.J.Obstet.Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 43.Perni SC, Chervenak FA, Kalish RB, Magherini-Rothe S, Predanic M, Streltzoff J, et al. Intraobserver and interobserver reproducibility of fetal biometry. Ultrasound Obstet.Gynecol. 2004;24:654–658. doi: 10.1002/uog.1717. [DOI] [PubMed] [Google Scholar]
- 44.Hecher K, Campbell S, Snijders R, Nicolaides K. Reference ranges for fetal venous and atrioventricular blood flow parameters. Ultrasound Obstet.Gynecol. 1994;4:381–390. doi: 10.1046/j.1469-0705.1994.04050381.x. [DOI] [PubMed] [Google Scholar]
- 45.Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of blood velocity and pulsatility index at the intra-abdominal portion, and fetal and placental ends of the umbilical artery. Ultrasound Obstet.Gynecol. 2005;26:162–169. doi: 10.1002/uog.1902. [DOI] [PubMed] [Google Scholar]
- 46.Abitbol MM. Evolution of the ischial spine and of the pelvic floor in the Hominoidea. Am.J.Phys.Anthropol. 1988;75:53–67. doi: 10.1002/ajpa.1330750107. [DOI] [PubMed] [Google Scholar]
- 47.Foley RA, Lee PC. Ecology and energetics of encephalization in hominid evolution. Philos.Trans.R.Soc.Lond B Biol.Sci. 1991;334:223–231. doi: 10.1098/rstb.1991.0111. [DOI] [PubMed] [Google Scholar]
- 48.DeVore GR, Falkensammer P, Sklansky MS, Platt LD. Spatio-temporal image correlation (STIC): new technology for evaluation of the fetal heart. Ultrasound Obstet.Gynecol. 2003;22:380–387. doi: 10.1002/uog.217. [DOI] [PubMed] [Google Scholar]
- 49.Bhat AH, Corbett VN, Liu R, Carpenter ND, Liu NW, Wu AM, et al. Validation of volume and mass assessments for human fetal heart imaging by 4-dimensional spatiotemporal image correlation echocardiography: in vitro balloon model experiments. J.Ultrasound Med. 2004;23:1151–1159. doi: 10.7863/jum.2004.23.9.1151. [DOI] [PubMed] [Google Scholar]
- 50.Uittenbogaard LB, Haak MC, Spreeuwenberg MD, Van Vugt JM. A systematic analysis of the feasibility of four-dimensional ultrasound imaging using spatiotemporal image correlation in routine fetal echocardiography. Ultrasound Obstet.Gynecol. 2008;31:625–632. doi: 10.1002/uog.5351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video clip illustrates how to orient a cardiac STIC dataset prior to calculating ventricular volumes.
This video clip illustrates how to calculate cardiac ventricular volumes in a semi-automated fashion utilizing VOCAL™ and Contour Finder: Trace with 15° of rotation.