Abstract
Herpes simplex virus (HSV) infections are ubiquitous in humans, but the determinants of clinical and virologic severity are not completely understood. Prior research has suggested that psychological distress can be a co-factor in reactivation of latent HSV infection. Personality traits such as extraversion and neuroticism influence stress attributions and may inform the relationship between psychological distress and health outcomes. Earlier studies in this area have primarily focused on subjective reports of HSV lesion recurrence, but such reports may be influenced by both personality traits and distress. We report results from a randomized, double-blind, placebo-controlled, crossover trial of acyclovir in 19 women for whom personality was assessed at baseline and daily assessments of genital lesions, stress, anxiety, and depression levels were collected for 22 weeks. In addition, daily swabs of the genital mucosa were collected to assess HSV-2 viral reactivation. We found that daily stress predicted genital lesion frequency, and that daily stress, anxiety, and depression predicted genital lesion onset approximately 5 days before onset. Anxiety was also associated with genital lesions 3 days after onset. Distress and viral reactivation were not associated; and no personality traits were associated with any of the outcomes. These results support the hypothesis that psychological distress is both a cause and a consequence of genital lesion episodes.
1. Introduction
Herpes simplex virus (HSV) infection has been a principal focus of psychoneuroimmunology research, because HSV infections are ubiquitous in humans and the determinants of clinical and virologic severity are not completely understood. Data from both animal and human research suggest that psychological distress can be a co-factor in reactivating latent HSV infection. For example, Freeman et al. (2007) found that restraint stress in mice could both deplete numbers of HSV-specific CD8+ T cells and impair the release of interferon-gamma— which prevents viral replication—resulting in reactivation of latent HSV infection. Unfortunately, many of the human studies have relied on patient assessment of severity of HSV disease, and such perceptions can be influenced by stress (Rand et al., 1990). Despite this shortcoming, a recent meta-analysis of stress and HSV that focused on prospective studies with clinically relevant outcomes found a small but positive correlation between frequency of recurrence and stress, particularly among women (Chida and Mao, 2009).
Recent research on stress has suggested that measures of stable personality traits that influence stress attributions may provide additional information on the link between health and psychological distress. Traits such as neuroticism predictably affect appraisals of, and responses to, events (Bolger and Zuckerman, 1995; Cohen et al., 1999a; Dickerson and Kemeny, 2004; Kiecolt-Glaser et al., 2002; Segerstrom, 2005, 2006), and may be associated with stress-related immune modulation as well as negative health outcomes such as cardiovascular disease (Friedman, 2008). Findings in humans have been replicated in animal studies that differentiate between controllable and uncontrollable environmental stressors (Bonneau et al., 2007; Dess et al., 1983). Failure to consider individual differences in personality and stress appraisals may account for the inconsistent results observed in some areas of psychoneuroimmunology research (Kemeny, 2003; Segerstrom and Miller, 2004; Taylor et al., 2000).
To address these gaps, we conducted a prospective study that included measures of daily viral reactivation in the genital tract of women both on and off antiviral therapy. Because HSV reactivation of mucosa is intermittent and highly variable, prolonged follow up is required to reliably assess disease severity. In addition, the correlation between lesions and viral detection is imperfect. This reflects frequent asymptomatic reactivation (Tronstein et al., in press) and the presence of HSV-negative lesions from which the virus has been cleared but complete healing has not yet occurred. In the last 2 decades, we have used daily swabs of genital secretions obtained by research participants at home to assess the frequency and predictors of HSV reactivation, as well as the efficacy of antiviral therapy in suppressing HSV shedding (Casper et al., 2008; Corey et al., 2004; Straus et al., 1997; Wald et al., 1997; Wald et al., 1996).
The psychological assessment of the participants included a baseline evaluation of personality traits and daily measures of psychological distress. Inclusion of the antiviral therapy condition allowed us to test whether increased viral shedding—with or without clinical symptoms—predicted increased distress (Maier and Watkins, 1998). We hypothesized that 1) psychological distress and personality factors are associated with viral shedding and genital lesions; 2) increased psychological distress precedes viral shedding and genital lesions; and 3) personality factors influence viral shedding and genital lesions both directly and indirectly by influencing levels of psychological distress.
2. Methods
2.1 Participants
Study data were collected as part of a randomized, placebo-controlled, crossover clinical trial of acyclovir as a treatment for HSV-2 infection. Healthy women with laboratory-documented HSV-2 infection were recruited from a previous study of subclinical HSV-2 shedding (Wald et al., 1996). The time between the initial trial and the present study was approximately 2 years; 19 of the original 26 participants elected to participate in this follow-up.
2.2 Materials
2.2.1 Personality
Neuroticism and extraversion were measured using the revised Eysenck Personality Questionnaire, a scale with well established psychometric properties for those constructs (Eysenck et al., 1985). Each scale consisted of 24 yes/no items. Sample items include “Would you call yourself a nervous person?” and “Do you worry about awful things that might happen?” (Neuroticism) and “Do you like going out a lot?” and “Would you be very unhappy if you could not see lots of people most of the time?” (Extraversion). Items were re-coded before analysis to ensure proper scoring.
2.2.2 Psychological Distress
Psychological distress was measured daily by single-item visual analog scales (VAS) of stress, anxiety, and depression. Participants were instructed to draw a line along the scale that represented their stress, anxiety, or depression levels over the course of the entire day. The lines were 10 cm in length and had anchors of “No stress” and “Most intense stress imaginable,” “No anxiety or worry’ and “Most intense anxiety imaginable,” and “No depression” and “Most intense depression imaginable.” This measurement approach had the advantage of strong face validity and was easy for women to complete on a daily basis. Similar VAS and single-item distress measures have been used in prior published HSV research (Cohen et al., 1999b; Dalkvist et al., 1995; Rand et al., 1990).
2.3 Procedures
Participants were randomized to receive either 1) acyclovir 400 mg twice daily (the standard suppressive dose), or 2) placebo for 10 weeks. Each arm was followed by 2 weeks of washout and then 10 weeks in the other study arm. Participants returned every 2 weeks to the University of Washington Virology Research Clinic in Seattle. All procedures were approved by the University’s Institutional Review Board, and all participants provided informed consent for participation.
At enrollment, all participants completed a standardized questionnaire and interview that included demographic characteristics, clinical history of genital and oral herpes, other medical history, and a personality assessment. Participants were instructed to maintain a daily diary in which they recorded presence or absence of genital herpes lesions in the morning or as symptoms occurred. All clinical and laboratory information was recorded on standardized data collection forms. Clinicians noted the presence or absence of lesions during clinic visits.
For at-home collection, participants were instructed how to swab their genital area to obtain a sample of secretions for HSV DNA polymerase chain reaction (PCR). Women collected cervicovaginal, vulvar, and perianal samples, as described elsewhere (Wald et al., 1996; Wald et al., 1995). The swabs for HSV DNA PCR were placed in vials containing 1 mL of PCR digestion buffer and refrigerated by the participants until transport to the Virology Research Clinic. Refrigerated samples were delivered to the PCR laboratory at regular intervals and stored at −20ºC until processed.
2.4 Laboratory Methods
HSV Western blot was used to identify HSV-2 antibodies at study entry (Ashley et al., 1988). A validated, quantitative, real-time PCR assay with glyocoprotein B primers was used to measure the amount of HSV in genital secretions, as described previously (Jerome et al., 2002). Each specimen was assayed in duplicate. Specimens were considered positive only if the results of both replications exceeded the cutoff value and at least 3 copies of HSV DNA/reaction (150 copies of HSV DNA/mL of transport medium) were detected (Magaret et al., 2007).
2.5 Statistical analysis
Viral shedding was defined as the detection of HSV in the genital mucosa by PCR. A lesion day was defined as a day on which the participant reported genital lesions consistent with genital herpes. The frequency of HSV detection in genital swabs was calculated as the proportion of total genital swabs with HSV detected by PCR. Similarly, the frequency of genital lesions was calculated as the proportion of follow-up days in which lesions were reported. The first day of each treatment arm and all wash-out days were excluded. A lesion episode was defined as a consecutive series of days with genital lesions reported in which there was at least 1 lesion-free day before and after. A shedding episode was defined as a consecutive series of days with HSV detected via PCR with at least 2 HSV- days before and after. Single days with missing diaries or swabs within an episode were considered positive. Lesion and shedding episode onset was defined as the first day a lesion or shedding episode occurred, respectively.
Number of lesion episodes was compared by treatment arm, using the Wilcoxon signed-rank test for matched pairs. Spearman rank coefficients were used to measure correlations between number of lesion episodes and personality traits, as well as correlations among daily stress measures. Duration of lesion episodes was compared by treatment arm and personality traits, using generalized estimating equation regression with a normal distribution and robust standard errors.
Poisson random effects models with random intercepts to account for within-individual variability in detection of HSV and genital lesions were used to identify predictors of shedding and lesions, respectively. Except for treatment (placebo or acyclovir) all covariates (age, years since primary episode, neuroticism, extraversion, and daily stress measures) were entered as continuous measures unless otherwise stated. Multivariate models for rates of lesions and shedding included covariates that were associated with lesion detection or shedding at a p < 0.2 significance level. Poisson random effects models were also used to evaluate the association of daily stress measures and rate of lesion and shedding episode onset. The effect of treatment was not a primary focus of this study; however, all results from the primary analyses were adjusted for treatment effects by including treatment in the multivariate model. Statistical significance was set at p < 0.05, two-sided. STATA (version 11.0, College Station, TX) was used for all statistical analyses.
3. Results
3.1 Clinical and demographic variables
The median age for the 19 women was 29 (range = 24–53) and the median number of years since primary infection was 3 (range = 2–5) (Table 1). The median score was 9 (range = 3–21) for neuroticism and 12 (range = 3–20) for extraversion. Median ratings of stress, anxiety, and depression were all in the lowest quartile (≤ 2.5 cm out of 10 cm) and did not differ by treatment condition. All women were 100% compliant with diaries of lesion episodes. Of the 2,694 possible swabs, 329 (12.2%) were missing; however, 16 (84%) of 19 women collected genital swabs on at least 80% of days. Daily stress was missing for a total of 163 (6.1%) days with 17 (89%) of 19 women responding on at least 80% of days. Results were similar for measures of anxiety and depression and did not dramatically differ by treatment arm.
Table 1.
Study participant demographics, personality trait measures, and levels of stress, anxiety, and depression by treatment arm
| Median (range)
|
||
|---|---|---|
| Age, years | 29 (24–53) | |
| Years since first lesion episode | 3 (2–5) | |
| Extraversion | 12 (3–20) | |
| Neuroticism | 9 (3 – 21)
|
|
| Placebo | Acyclovir | |
|
|
||
| Stress | 2.5 (0–9.5) | 2.5 (0–10) |
| Anxiety | 2.3 (0–10) | 2.4 (0–10) |
| Depression | 1 (0–9.5) | 1 (0–10) |
|
|
||
3.2 Treatment
3.2.1 The effect of treatment on HSV-2 lesions
Overall, 11 women (58%) experienced a total of 35 lesion episodes (median = 2, range = 0 – 5) consisting of 259 (10%) lesion days (Table 2). Of the 35 lesion episodes, 27 had HSV detected on at least one day. Participants had fewer episodes on acyclovir than placebo (9 episodes vs. 26 episodes, p = 0.03); however, episode duration did not differ by treatment arm (p = 0.88). Acyclovir use was associated with a 68% reduction in the frequency of days with genital lesions (rate ratio (RR) = 0.32, 95% CI = 0.24–0.42, p < .001) (Table 3).
Table 2.
Frequency of HSV-2 genital lesions by treatment arm
| All sessions | Placebo Arm | Acyclovir Arm | |
|---|---|---|---|
| Number of days with genital lesions/all days (%) | 259 / 2694 (10) | 197 / 1360 (14) | 62 / 1334 (5)** |
| Number of genital lesion episodes, N (%) | 35 (100) | 26 (74) | 9 (26)* |
| Duration of genital lesion episodes (days), median (range) | 6 (1–19) | 7 (2–19) | 4 (1–17) |
p < 0.05
p < 0.001
Table 3.
Univariate and multivariate rate ratios (RR) and 95% confidence intervals (CI) for rates of HSV-2 genital lesions
| Univariate | Multivariate | |||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Age, years | 0.97 (0.82–1.14) | 0.67 | ||
| Years since first lesion episode | 0.38 (0.06–2.35) | 0.30 | ||
| Acyclovir | 0.32 (0.24–0.42) | < 0.001 | 0.32 (0.23–0.42) | < 0.001 |
| Extraversion | 0.95 (0.71–1.28) | 0.73 | ||
| Neuroticism | 0.81 (0.61–1.08) | 0.15 | 0.81 (0.62–1.08) | 0.15 |
| Stress | 1.08 (0.99–1.17) | 0.08 | 1.10 (1.02–1.20) | 0.02 |
| Anxiety | 1.08 (0.99–1.17) | 0.08 | ||
| Depression | 1.02 (0.92–1.12) | 0.74 | ||
3.2.2 The effect of treatment on HSV-2 shedding
A total of 2,365 genital swabs were collected over a median of 155 days (range = 147–174). Of the swabs, 383 (16%) had HSV (Table 4). There were 122 (5.2%) of 2,365 days with both lesions and HSV detected in genital swabs. These 122 days occurred on 47% of the 259 days with lesions and on 32% of the 383 days with viral shedding. The overall frequency of HSV shedding was lower during acyclovir sessions than placebo sessions (8% vs. 24%). The associated univariate rate ratio (RR) for reduction in shedding during acyclovir use was 0.32 (95% CI = 0.25–0.41, p < 0.001) (Table 5).
Table 4.
Frequency of detection of HSV-2 in genital swabs by treatment arm
| All sessions | Placebo Arm | Acyclovir Arm | |
|---|---|---|---|
| Number of HSV-2 positive genital swabs/all swabs (%) | 383 / 2365 (16) | 291 / 1211 (24) | 92 / 1154 (8)** |
| HSV-2 DNA quantitya (log10 copies/mL), median (range) | 4.7 (2.2–10.1) | 5.2 (2.2 – 8.8) | 3.2 (2.2–10.1) |
p < 0.05
p < 0.001
on days HSV-2 was detected
Table 5.
Univariate and multivariate rate ratios (RR) and 95% confidence intervals (CI) for rates of HSV-2 genital shedding
| Univariate | Multivariate | |||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Age, years | 1.01 (0.97–1.06) | 0.55 | ||
| Years since first lesion episode | 0.82 (0.46–1.44) | 0.48 | ||
| Acyclovir | 0.32 (0.25–0.41) | <0.001 | 0.32 (0.25–0.41) | <0.001 |
| Extraversion | 0.98 (0.89–1.06) | 0.58 | ||
| Neuroticism | 0.94 (0.87–1.02) | 0.12 | 0.94 (0.87–1.02) | 0.12 |
| Stress | 1.03 (0.96–1.10) | 0.46 | ||
| Anxiety | 0.99 (0.92–1.07) | 0.88 | ||
| Depression | 1.00 (0.93–1.09) | 0.91 | ||
3.3 Personality
3.3.1 Associations between personality and lesions
With each 1-unit increase in neuroticism, the rate of lesion days decreased by 19% (RR = 0.81, 95% CI: 0.61–1.08; Table 3); however, this change was not statistically significant (p = 0.15). The number of episodes was not correlated with either extraversion or neuroticism (Spearman’s rho = −0.08 and −0.28, respectively). The duration of episodes did not differ by neuroticism (p = 0.38) or extraversion (p = 0.86).
3.3.2 Associations between personality and shedding
Although we observed a 6% lower rate of genital shedding with each 1-unit increase in level of neuroticism, this trend was not statistically significant (RR = 0.94, 95% CI = 0.87 – 1.02, p = 0.12) (Table 5). The trend remained unchanged after adjusting for treatment. Extraversion was not associated with frequency of genital shedding (p = 0.58).
3.4 Psychological distress
3.4.1 Associations between distress and genital lesions
Average stress over the sampling session was associated with a 10% greater frequency of lesions in the multivariate model (RR = 1.10, 95% CI = 1.02–1.20, p = 0.02; Table 3). Anxiety was highly correlated with stress (Spearman’s rho = 0.67) and, therefore, was not included in the multivariate model. When anxiety was included in a multivariate model instead of stress, the association was very similar to that described for stress (data not shown). To address whether psychological distress preceded, was concurrent with, or followed genital herpes episodes, we compared the proportion of lesion episode onsets as a function of stress, anxiety, and depression levels on the 7 days preceding, the same day as, and the 3 days following the onset of shedding or lesion episodes. To control for the possibility that lesions might induce stress, anxiety, and/or depression, we excluded episodes for which fewer than 10 lesion-free days preceded episode onset. All non-lesion days that fell at least 10 days before the start or at least 10 days after the end of an episode were considered to be at risk for lesion onset. The rate of lesion onset that we calculated by using this method (27 lesion onset days / 1943 days at risk for onset = 1.4%) did not differ from the rate of lesion onset that we would obtain if all non-lesion days were considered to be at risk (35 lesion onset days / 2434 days at risk for onset = 1.4%).
On visual inspection of the data, the proportion of lesion onset days did not differ by stress, anxiety, or depression for scores below 6 cm on the 10 cm VAS (graphs not shown). Because these associations were limited to women with relatively high levels of stress, anxiety, or depression, and because they were non-linear, we dichotomized these exposures (≥ 6 = “high” or < 6 “low”) for all subsequent analyses.
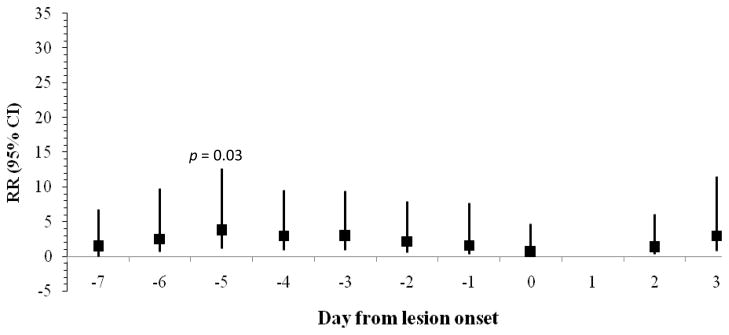
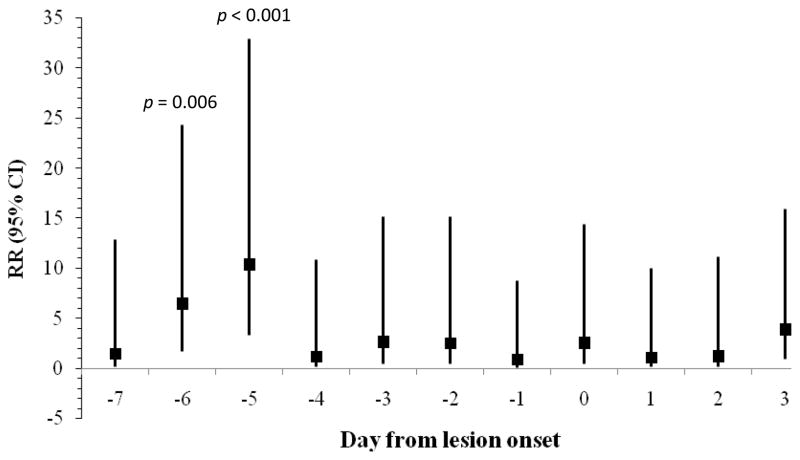
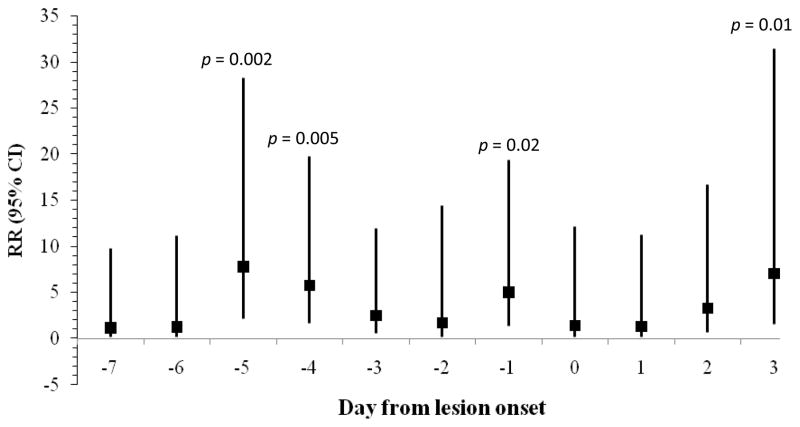
As summarized in Figures 1–3, rates of lesion onset were associated with stress, anxiety, and depression as follows: rates of lesion onset were ≥ 3 times as high following high stress levels 5 days before onset relative to low stress levels at that time (p = 0.03); rates of lesion onset were ≥ 5 times as high following high anxiety levels 1, 4, and 5 days before onset relative to low anxiety on those days (all ps < 0.02); and rates of lesion onset were ≥ 6 times as high following high depression levels 5 and 6 days before onset (ps < 0.006). Women with high anxiety levels 3 days after lesion onset also had higher rates of lesion onset than those with lower anxiety levels (RR > 6, p = 0.01). Other covariates such as age, years since diagnosis, extraversion, and neuroticism were not related to lesion onset.
Figure 1.
Rate Ratio (95% CI) for frequency of genital lesion onset comparing women with high and low stress levels by days from lesion onset (N = 27 lesion onsets).
Figure 3.
Rate Ratio (95% CI) for frequency of genital lesion onset comparing women with high and low depression levels by days from lesion onset (N = 27 lesion onsets).
3.4.2 Associations between distress and shedding
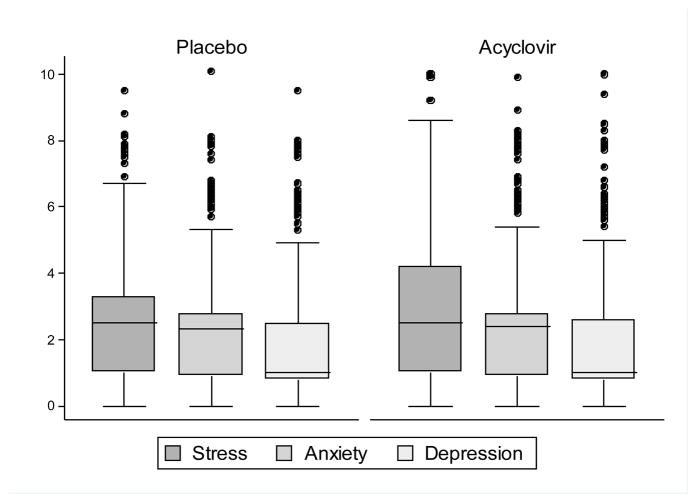
Levels of stress, anxiety, and depression were not associated with the rate of viral shedding (Table 5). The methods described in Section 3.4.1 were used to assess whether psychological distress had an effect on the frequency of shedding episodes. In addition, to test whether shedding might induce stress, anxiety, or depression, we examined whether levels of distress were higher in the placebo (increased shedding) versus treatment (decreased shedding) conditions. We detected no differences in levels of stress, anxiety, or depression by treatment arm (Figure 4) or in rates of shedding episode onset by any psychological distress variables (data not shown).
Figure 4.
Levels of stress, anxiety, and depression by treatment condition
4. Discussion
Our prospective longitudinal study of psychological distress and HSV-2 infection in women found that, consistent with our hypothesis, psychological distress was temporally associated with the onset of HSV-2 lesions. Specifically, lesion onset was associated with high levels of psychological distress—measured as daily levels of stress, anxiety, and depression—approximately 5 days before the episode and with high levels of anxiety 3 days after the episode. Likewise, frequency of lesion episodes was associated with high daily levels of stress in the multivariate model controlling for treatment condition and neuroticism. Contrary to our hypotheses, no effect was found for viral shedding, an important outcome in terms of disease transmission risk. Neither shedding nor lesions were reliably associated with neuroticism or extraversion.
4.1 Psychological distress as cause and effect of HSV-2 reactivation
Despite the small sample size, this study provides evidence that psychological distress may be associated with herpes lesions both before and after lesion onset, and its findings are consistent with causal models of psychological distress and HSV-2 reactivation (Freeman et al., 2007). This study also supports the utility of frequent collection of distress ratings for research in this area. Of the 7 prospective studies of genital herpes lesions (Chida and Mao, 2009), 5 found positive associations with psychological distress. Notably, the 2 largest effects were based on daily distress measurements (Dalkvist et al., 1995; McLarnon and Kaloupek, 1988), and a third was based on weekly measurements (Cohen et al., 1999b). Although we found a small association between stress and lesion episodes across the entire study period, our strongest finding was that daily distress ratings predict lesion onset.
Timing of stress may be critical for another reason. Research has shown that it takes approximately 5 days for HSV to travel from the sacral dorsal root ganglia to the epithelial surface of the genital tract (Perna et al., 1987; Rooney et al., 1992). For example, in a study of ultraviolet radiation-induced reactivation of genital HSV-2, mean time to site-specific lesion recurrence was 5 days (Rooney et al., 1992). These data match ours very closely and allow us to surmise that the physiological effect of psychological distress occurred at the site of latent infection rather than reflecting immune modulation at the site of lesion recurrence. However, similar time frames from stressor to infection have been observed in upper respiratory infections which do not have a similar virus “travel time” component (Pedersen et al., 2010). We cannot therefore rule out a more general immune modulation process.
Post-lesion anxiety was not significantly higher until 3 days after the onset of lesions. One possibility is that lesions were in fact associated with earlier increases in anxiety, but we lacked power to detect the association. Another possibility is that increased anxiety is related to continuation of symptoms rather than mere onset.
4.2 Psychological distress does not predict viral shedding
Contrary to our hypothesis, psychological distress did not predict episodes of viral shedding in the same way that it predicted lesion episodes. Although it may seem somewhat puzzling that distress could cause additional lesions but not additional shedding, mathematical modeling of HSV-2 shedding suggests that HSV-2 infection is dynamic, and that viral particles are slowly but regularly released into the genital area (Schiffer et al., 2009). The virus reactivates frequently, despite the presence of HSV-specific immune cells both in the ganglia and in the mucosa (Verjans et al., 2007; Zhu et al., 2007). Lesions may occur when, in addition to viral shedding, systemic or local immunity is transiently impaired, allowing for sufficient local epithelial replication to result in a discernable lesion.
4.3 Viral shedding does not predict psychological distress
Maier and colleagues (Maier, 2003; Maier and Watkins, 1998) have suggested that the immune system is a “diffuse sensory organ” that engages in bi-directional feedback with the central nervous system. One implication of their theory is that levels of psychological distress can increase when the immune system is actively fighting an infection. Although supportive evidence for this theory exists in some areas of psychoneuroimmunology research (Segerstrom and Miller, 2004) we did not find any such evidence in the context of genital herpes. Although treatment with acyclovir had a clear and clinically significant impact on both lesions and shedding compared to placebo, no differences were observed in stress, anxiety, or depression levels by treatment condition.
4.4 Personality factors did not affect viral shedding or lesion episodes
Prior research on personality and HSV infection has not detected a strong relationship between herpes lesions and traits such as neuroticism and extraversion. Nevertheless, because personality traits are thought to reflect differences in underlying physiology—for example, autonomic nervous system reactivity—we postulated that personality traits, especially high neuroticism, might predict increased shedding. However, we observed a trend in the opposite direction, more consistent with reports that neuroticism occasionally predicts positive health outcomes (Friedman, 2008). These inconsistent findings may be explained in part by the hypothesis that some people who are characterized by high levels of neuroticism engage in unhealthy lifestyles, lose sleep, and lack social support (all of which increase the risk of poor health) while others with similarly high levels enjoy better health because of their health-related vigilance and treatment adherence (Friedman, 2008). Recent research points to conscientiousness (conventionality, reliability, orderliness) as the personality construct that interacts with neuroticism to moderate health outcomes and conscientious individuals may have been more likely to volunteer for and complete the type of demanding study described here. Further research on the joint relationship of neuroticism, conscientiousness, and immunity could be beneficial.
4.5 Strengths and limitations
The strengths of our study include the prospective design and the incorporation of an objective measure of viral reactivation measured daily over the entire 22-week study period. In addition, because the results of the PCR assays were unknown until the study concluded, positive results did not influence subsequent distress in the same way that a lesion might have influenced subsequent distress. Daily diary data provided details on lesion episodes as well as psychological distress, allowing us to examine the temporal relationship between distress and episodes of shedding and lesions. The prospective crossover design allowed us to test whether HSV-2 shedding influences psychological distress. Finally, we assessed personality as a potentially important variable in the distress-disease model.
The study’s primary limitation was its small sample size, which limits both power and generalizability. In addition, the length of the study and the somewhat onerous methods may have led to a self-selection bias. A larger study might have detected associations between personality and genital herpes, and might also have been able to test mediation hypotheses related to personality, distress, and HSV-2 infection. Evaluation of associations at several points in time introduced the potential for spurious significance through repeated testing. However, the consistency of the timing of our associations increases the strength of our findings and reduces the potential for error, because stress, anxiety, and depression noted 5 days before lesion onset were all independently associated with increased rates of lesion onset.
4.5 Summary and future directions
This study supports the hypothesis that psychological distress can be both a cause and a consequence of genital herpes lesions, especially when distress reaches high levels. We emphasize the importance of examining distress on the days surrounding lesion onset individually—rather than averaging across days—to capture the temporal relationship between distress fluctuations and lesion onset. We found no effects for genital shedding, and personality traits did not contribute to the effects of distress on either disease outcome. Future research should use similar methods—along with a broader array of personality traits—to focus on oral herpes infections. In addition, given the apparent effect of psychological distress on lesion episodes, we recommend research on interventions to reduce stress in otherwise healthy individuals.
Figure 2.
Rate Ratio (95% CI) for frequency of genital lesion onset comparing women with high and low anxiety levels by days from lesion onset (N = 27 lesion onsets).
Acknowledgments
This research was supported by National Institutes of Health (NIH) awards P01 AI-30731 (A. Wald) and K24 AI-071113 (A. Wald). Dr. Strachan is also supported in part by NIH R21 AI-81347. An earlier version of this study was presented at the 15th Annual Psychoneuroimmunology Research Meeting, Madison, WI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. J Pers Soc Psychol. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Bonneau RH, Padgett DA, Sheridan JF. Twenty years of psychoneuroimmunology and viral infections in Brain, Behavior, and Immunity. Brain Behav Immun. 2007;21:273–280. doi: 10.1016/j.bbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, Hamilton S, Huang ML, Wald A. Valganciclovir for suppression of human herpesvirus 8 replication: A randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Mao X. Does psychosocial stress predict symtomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studies. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Cohen F, Kearney KA, Zegans LS, Kemeny ME, Neuhaus JM, Stites DP. Differential immune system changes with acute and persistent stress for optimists vs pessimists. Brain Behav Immun. 1999a;13:155–174. doi: 10.1006/brbi.1998.0531. [DOI] [PubMed] [Google Scholar]
- Cohen F, Kemeny ME, Kearney KA, Zegans LS, Neuhaus JM, Conant MA. Persistent stress as a predictor of genital herpes recurrence. Arch Intern Med. 1999b;159:2430–2436. doi: 10.1001/archinte.159.20.2430. [DOI] [PubMed] [Google Scholar]
- Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM, Paavonen J, Morrow RA, Beutner KR, Stratchounsky LS, Mertz G, Keene ON, Watson HA, Tait D, Vargas-Cortes M for the Valacyclovir HSV Transmission Study Group. Once daily valacyclovir to reduce the transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- Dalkvist J, Tarja-Brita RW, Bartsch E, Forsbeck M. Herpes simplex and mood: A prospective study. Psychosom Med. 1995;57:127–137. doi: 10.1097/00006842-199503000-00005. [DOI] [PubMed] [Google Scholar]
- Dess NK, Linwick D, Patterson J, Overmier JB, Levine S. Immediate and proactive effects of controllability and predictability on plasma cortisol responses to shocks in dogs. Behav Neurosci. 1983;97:1005–1016. doi: 10.1037//0735-7044.97.6.1005. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ, Barrett P. A revised version of the Psychotocism scale. Pers Individ Dif. 1985;6:21–29. [Google Scholar]
- Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS. The multiple linkages of personality and disease. Brain Behav Immun. 2008;22:668–675. doi: 10.1016/j.bbi.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME. An interdisciplinary research model to investigate psychosocial cofactors in disease: Application to HIV-1 pathogenesis. Brain Behav Immun. 2003;17:S62–S72. doi: 10.1016/s0889-1591(02)00069-7. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- McLarnon LD, Kaloupek DG. Psychological investigation of genital herpes recurrence: Prospective assessment and cognitive-behavioral intervention for a chronic physical disorder. Health Psychol. 1988;7:231–249. doi: 10.1037//0278-6133.7.3.231. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Zachariae R, Bovbjerg DH. Influence of psychological stress on upper respiratory infecdtion--a meta-analysis of prospective studies. Psychosom Med. 2010;72:823–832. doi: 10.1097/PSY.0b013e3181f1d003. [DOI] [PubMed] [Google Scholar]
- Perna JJ, Mannix ML, Rooney JF, Notkins AL, Straus SE. Reactivation of latent herpes simplex virus infection by ultraviolet light: a human model. J Am Acad Dermatol. 1987;17:473–478. doi: 10.1016/s0190-9622(87)70232-1. [DOI] [PubMed] [Google Scholar]
- Rand KH, Hoon EF, Massey JK, Johnson JH. Daily stress and recurrence of genital herpes simplex. Arch Intern Med. 1990;150:1889–1893. [PubMed] [Google Scholar]
- Rooney JF, Straus SE, Mannix M, Wohlenberg CR, Banks S, Jagannath S, Brauer JE, Notkins AL. UV light-induced reactivation of herpes simplex virus type 2 and prevention by acyclovir. J Infect Dis. 1992;166:500–506. doi: 10.1093/infdis/166.3.500. [DOI] [PubMed] [Google Scholar]
- Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Magaret AS, Wald A, Corey L. Frequent release of low amounts of herpes simplex virus from neurons: Results of a mathematical model. Sci Transl Med. 2009;1:1–9. doi: 10.1126/scitranslmed.3000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism and immunity: Do positive thoughts always lead to positive effects? Brain Behav Immun. 2005;19:195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC. How does optimism suppress immunity? Evaluation of three affective pathways. Health Psychol. 2006;25:653–657. doi: 10.1037/0278-6133.25.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus SE, Wald A, Kost G, McKenzie R, Langenberg AGM, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, Izu A, Dekker C, Corey L. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoprotein D and B: Results of a placebo-controlled vaccine trial. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Kemeny ME, Reed GM, Bower JE, Gruenewald TL. Psychological resources, positive illusions, and health. Am Psychol. 2000;55:99–109. doi: 10.1037//0003-066x.55.1.99. [DOI] [PubMed] [Google Scholar]
- Tronstein E, Johnston C, Huang M-L, Selke S, Magaret A, Warren T, Corey L, Wald A. Gential shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. doi: 10.1001/jama.2011.420. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proceedings of the National Academy of Sciences USA. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital HSV-2 shedding in immunocompetent women. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of sub-clinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. Journal of Experimental Medicine. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]