Abstract
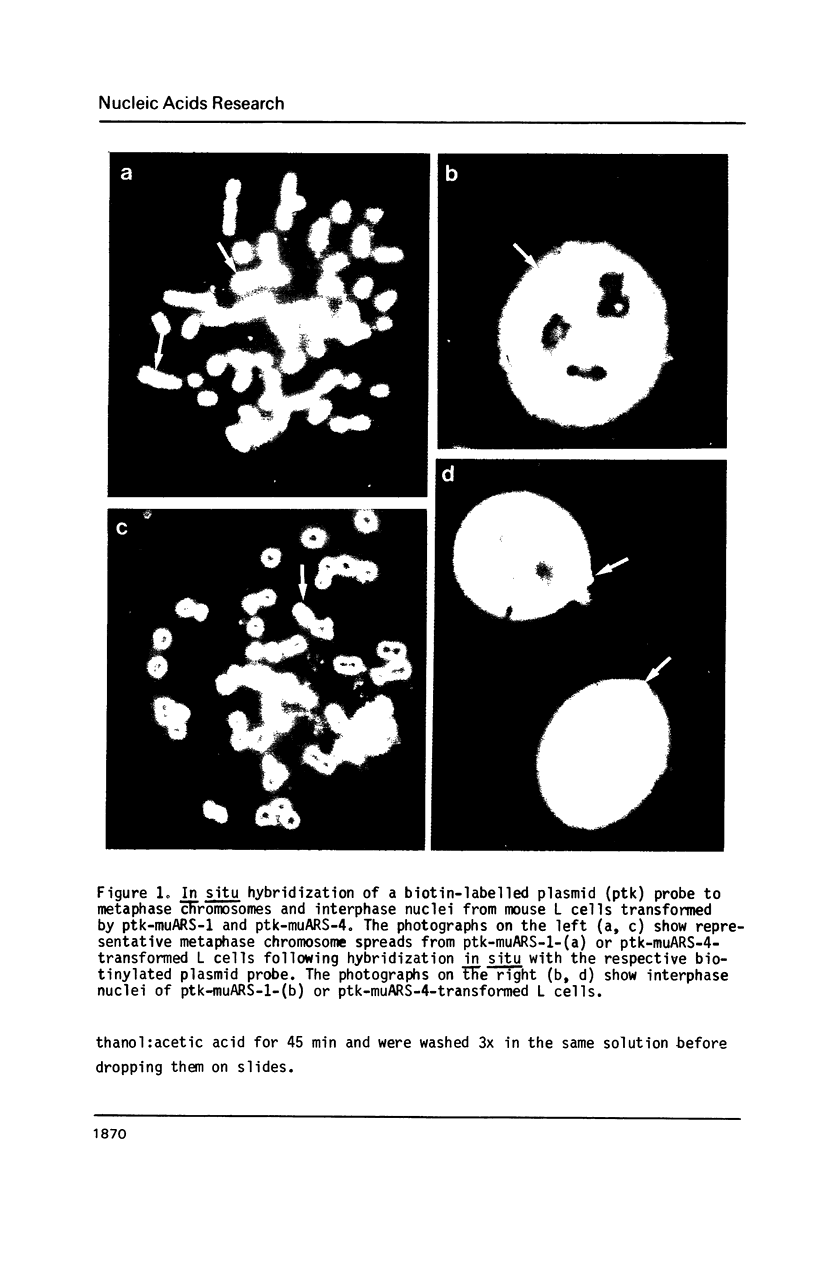
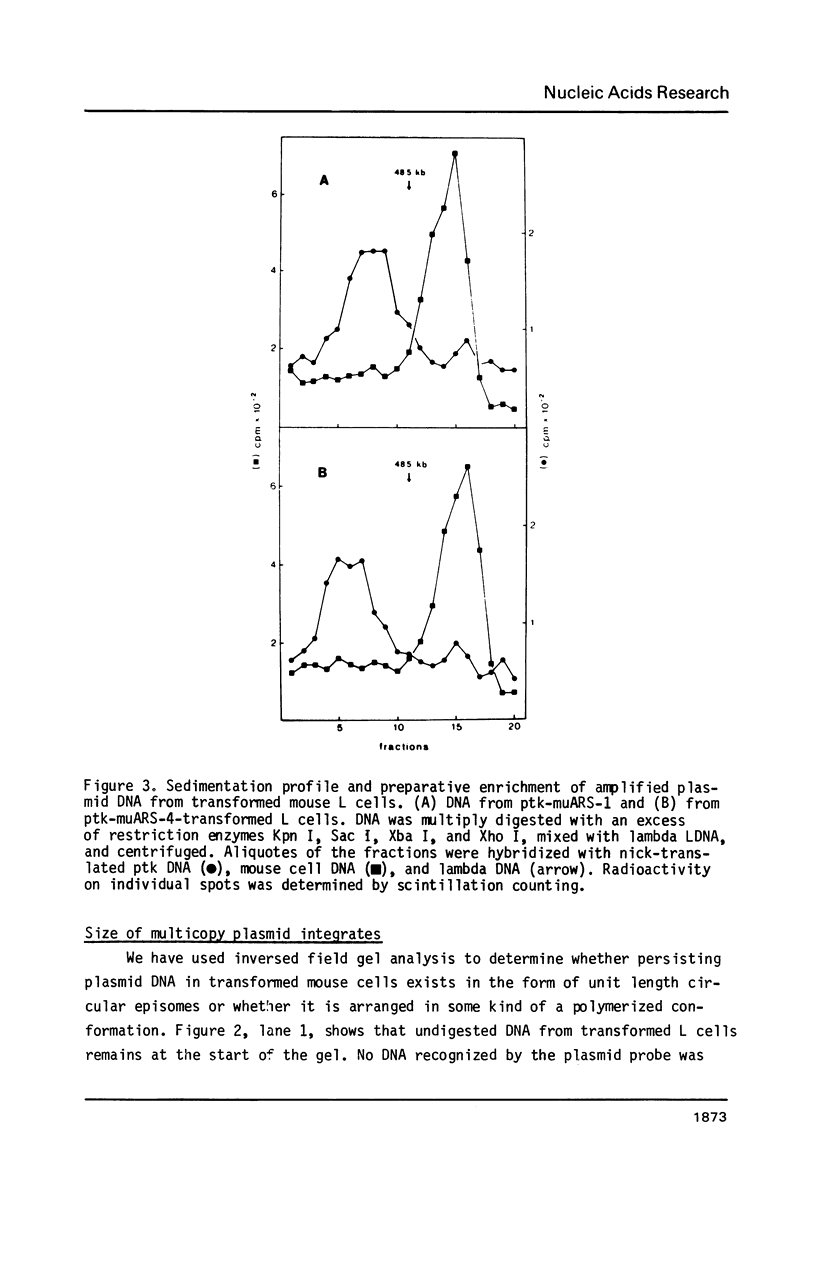
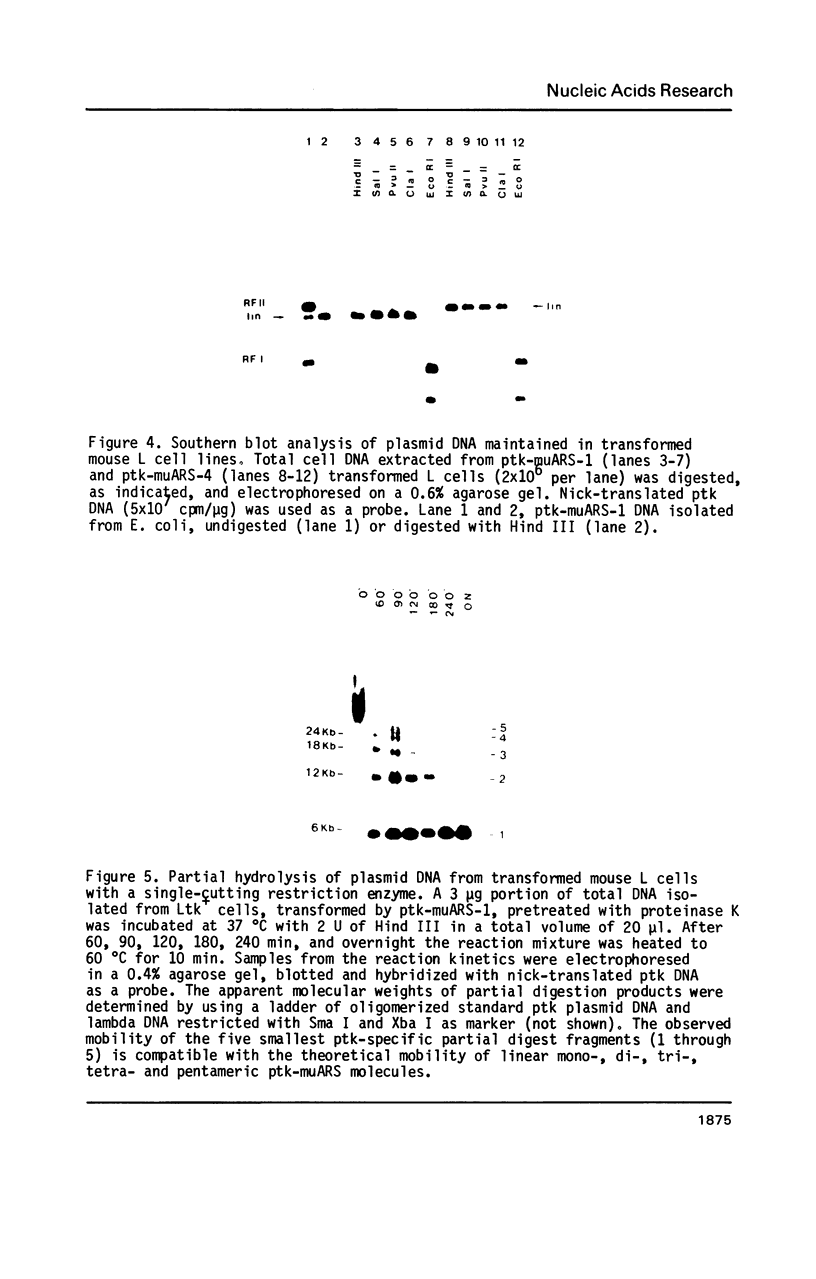
Distinct elements isolated from mouse genomic DNA confer on plasmid DNA the ability to persist at high copy numbers in mouse L fibroblasts (1). Field inversion gel electrophoresis demonstrated that - in contrast to our previous assumption - the persisting plasmid DNA does not exist extrachromosomally but as clusters of tandem repeats integrated into genomic DNA. Digestion with restriction endonucleases that do not cut within the plasmid DNA results in fragments of 50-300 kb in length indicating reiteration of 10-50 plasmid DNA molecules. Restriction with several enzymes that cut once or twice within the plasmid sequences lead to fragment(s) indicative for head-to-tail tandem repeats. In situ hybridization revealed signals for a long homogeneously staining region (HSR) in one or two chromosomes per cell nucleus. Possibilities how these elements could act in the establishment and/or maintenance of the head-to-tail polymers of plasmid DNA in mouse cells are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros P. F., Matzke A. J. M., Matzke M. A. Localization of Agrobacterium rhizogenes T-DNA in plant chromosomes by in situ hybridization. EMBO J. 1986 Sep;5(9):2073–2077. doi: 10.1002/j.1460-2075.1986.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G., Barlow D. P., Lehrach H. A large inverted duplication allows homologous recombination between chromosomes heterozygous for the proximal t complex inversion. Cell. 1987 Mar 13;48(5):813–825. doi: 10.1016/0092-8674(87)90078-x. [DOI] [PubMed] [Google Scholar]
- Hiller S., Hengstler M., Kunze M., Knippers R. Insertional activation of a promoterless thymidine kinase gene. Mol Cell Biol. 1988 Aug;8(8):3298–3302. doi: 10.1128/mcb.8.8.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst A., Müller F., Zastrow G., Zentgraf H., Schwender S., Dinkl E., Grummt F. Murine genomic DNA sequences replicating autonomously in mouse L cells. Cell. 1988 Feb 12;52(3):355–365. doi: 10.1016/s0092-8674(88)80028-x. [DOI] [PubMed] [Google Scholar]
- Landegent J. E., Jansen in de Wal N., van Ommen G. J., Baas F., de Vijlder J. J., van Duijn P., Van der Ploeg M. Chromosomal localization of a unique gene by non-autoradiographic in situ hybridization. Nature. 1985 Sep 12;317(6033):175–177. doi: 10.1038/317175a0. [DOI] [PubMed] [Google Scholar]
- Lötscher E., Siwka W., Zimmer F. J., Grummt F., Zachau H. G. Transposed human immunoglobulin V kappa gene regions carry clusters of conserved sequence elements. Gene. 1988 Sep 30;69(2):225–236. doi: 10.1016/0378-1119(88)90433-7. [DOI] [PubMed] [Google Scholar]
- Mantei N., Boll W., Weissmann C. Rabbit beta-globin mRNA production in mouse L cells transformed with cloned rabbit beta-globin chromosomal DNA. Nature. 1979 Sep 6;281(5726):40–46. doi: 10.1038/281040a0. [DOI] [PubMed] [Google Scholar]
- Matz B. Herpes simplex virus infection generates large tandemly reiterated simian virus 40 DNA molecules in a transformed hamster cell line. J Virol. 1987 May;61(5):1427–1434. doi: 10.1128/jvi.61.5.1427-1434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. C., Spence A., Smith M. The distal transcription signals of the herpesvirus tk gene share a common hexanucleotide control sequence. Cell. 1984 May;37(1):253–262. doi: 10.1016/0092-8674(84)90321-0. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., Dailey L., Basilico C. Amplification and excision of integrated polyoma DNA sequences require a functional origin of replication. Cell. 1984 Apr;36(4):943–949. doi: 10.1016/0092-8674(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Pülm W., Knippers R. Transfection of mouse fibroblast cells with a promoterless herpes simplex virus thymidine kinase gene: number of integrated gene copies and structure of single and amplified gene sequences. Mol Cell Biol. 1985 Feb;5(2):295–304. doi: 10.1128/mcb.5.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Axel R. Gene amplification and gene correction in somatic cells. Cell. 1982 May;29(1):109–119. doi: 10.1016/0092-8674(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- SZYBALSKA E. H., SZYBALSKI W. Genetics of human cess line. IV. DNA-mediated heritable transformation of a biochemical trait. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2026–2034. doi: 10.1073/pnas.48.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci U S A. 1985 Feb;82(3):694–698. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschueren H. Interference reflection microscopy in cell biology: methodology and applications. J Cell Sci. 1985 Apr;75:279–301. doi: 10.1242/jcs.75.1.279. [DOI] [PubMed] [Google Scholar]
- Weidle U. H., Buckel P., Grummt F. A new expression system for mammalian cells based on putative replicator sequences of the mouse and a truncated thymidine kinase gene. Gene. 1988 Dec 20;73(2):427–437. doi: 10.1016/0378-1119(88)90507-0. [DOI] [PubMed] [Google Scholar]
- Weidle U. H., Buckel P., Wienberg J. Amplified expression constructs for human tissue-type plasminogen activator in Chinese hamster ovary cells: instability in the absence of selective pressure. Gene. 1988 Jun 30;66(2):193–203. doi: 10.1016/0378-1119(88)90356-3. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]