Abstract
It is proposed that socioeconomic conditions in early childhood effect immune programming, with poorer conditions resulting in adult phenotypes that are prone to inflammation. Recent evidence supports this possibility, showing an inverse association of childhood SES with adult markers of systemic inflammation. In this study, we further investigate this association, extending prior studies to include an examination of multiple indices of SES across distinct periods of childhood. Subjects were 112 men and women, 40–60 years of age (88.6% Caucasian). Childhood SES was indexed by a composite of three indicators of parental wealth (parental home and vehicle ownership, and number of bedrooms per child in the family home) averaged across 2-year periods of childhood between 1 and 18 years old. Higher adult serum concentrations of interleukin (IL)-6 were associated with lower SES in early childhood (years 1–2) (β = −.05, p < .05), associations that were independent of adult age, personal income, educational attainment, gender, race, body mass index, and physical activity. These associations support recent suggestions that the early environment may program immune phenotypes that contribute to disease risk.
Keywords: childhood socioeconomic status, inflammation, interleukin-6, early biological programming, immune phenotype, SC Childhood Interview
INTRODUCTION
Growing evidence suggests that poorer socioeconomic conditions during childhood confer increased risk for a range of chronic inflammatory diseases and premature mortality among adults; effects that are independent of socioeconomic circumstances during adulthood (Cohen et al., 2010; Galobardes et al., 2004; Galobardes et al., 2006; Gliksman et al., 1995). In this regard, it has been proposed that social inequalities impact psychobiologic programming (Barker, 1998; Hertzman, 1999), resulting in adult phenotypes that are prone to central activation of brain regions involved in processing emotions, such as the amygdala (Gianaros et al., 2008), and increased peripheral activation of the sympatho-adrenal-medullary and hypothalamic-pituitary-adrenal (HPA) systems (Chen & Matthews, 2001; Chen et al., 2004; Chen et al., 2010). Possibly as a result of increased activation of the HPA axis and heightened systemic levels of cortisol, lower childhood SES has also been associated with decreased responsiveness of adult immune cells to glucocorticoid signaling that down-regulates inflammation (Miller et al., 2009; Miller & Chen, 2007), and an associated increase in circulating markers of inflammation, such as C-reactive protein (CRP) and interleukin (IL)-6 (Danese et al., 2007; Phillips et al., 2009; Pollitt et al., 2007; Taylor et al., 2006), although some report more modest effects that are attenuated when controlled for adult SES (Gimeno et al., 2008; Pollitt et al., 2007). Recent evidence also suggests that lifestyle factors, such as smoking, diet and physical activity, may also contribute to associations of childhood SES with adult inflammation (Danese et al., 2007; Pollitt et al., 2007). Further understanding of factors related to higher systemic levels of inflammatory markers in adulthood is warranted given their association with increased risk for a range of chronic diseases, including cardiovascular and metabolic disease, cancer, osteoarthritis, and dementia (Chung et al., 2009).
Animal evidence also supports an association of early adversity with programming of physiologic systems in adulthood (Coe & Lubach, 2003; Shanks & Lightman, 2001; Stevens et al., 2009; Zhang et al., 2006). For example, maternal separation, maternal stress, and pre- and postnatal exposure to corticotrophin-releasing hormone (CRH) have been associated with epigenetic changes to DNA that result in altered neuroplasticity of the amygdala and hippocampus, programming of hippocampal glucocorticoid receptors, and increases in neuroendocrine responses to stress (Gunnar & Quevedo, 2007; Liu et al., 1997; Weaver et al., 2004; Zhang & Meaney, 2010). Growing animal evidence also suggests that the pre- and early postnatal environment are critical for shaping immune phenotypes, with early adversity being associated with increased risk for inflammation in adulthood (Coe & Lubach, 2003; Coe et al., 1992; Pincus-Knackstedt et al., 2006; Shanks & Lightman, 2001; Worlein & Laudenslager, 2001). Although this programming may serve an adaptive role in the short term, preparing the organism to handle threats (e.g. pathogens) in their specific environment, it may be detrimental to long term survival, being associated with increased risk for chronic inflammatory disease (i.e. cardiovascular disease, atopy, asthma), and premature mortality in later life (Shanks & Lightman, 2001; Zhang et al., 2006).
To date, evidence showing an association of childhood SES with systemic inflammation in adults is limited by the use of single markers of SES, typically father’s occupation or education, averaged across childhood. Thus, it remains unclear whether there are critical periods during childhood when socioeconomic conditions have a stronger impact on developing immune function and thus the adult phenotype. One of the only studies to systematically examine socioeconomic factors across different periods of childhood shows that parental home ownership during early childhood (largest effect predicted by the first 2 years of life) is more strongly associated with decreased adult resistance to upper respiratory infection than home ownership later in childhood (Cohen et al., 2004). These results are consistent with the animal evidence and suggest that socioeconomic factors during the early years of life may be more closely related to adult health than those in later childhood. However, it remains to be determined whether the same pattern holds for associations of childhood socioeconomic conditions with adult levels of systemic inflammation. Accordingly, the purpose of the present study was to examine the association of multiple markers of childhood SES, including parental home and vehicle ownership, number of bedrooms per child in the family home, and parental occupational class, as assessed for each year of childhood (1–18 years), with circulating levels of IL-6 among a sample of relatively healthy mid-life volunteers. Based on current theories of early physiologic programming, animal evidence, and existing work showing a role of childhood SES in host resistance, it was hypothesized that indicators of SES in early childhood would be more strongly associated with adult levels of systemic inflammation than those in middle or late childhood.
METHODS
Subjects were recruited through mass mail solicitation in Allegheny County, Pennsylvania to take part in the Vaccination Immunity Project (VIP), a project examining psychosocial, behavioral, and physiological factors that predict antibody response to Hepatitis B vaccination. Participants were excluded from the study if they were taking medications known to affect the endocrine, immune, or nervous systems, were smokers, or reported a history or symptoms of hypertension, coronary heart disease, cardiovascular disease, chronic kidney or liver disease, diabetes, cancer, bleeding problems, asthma, severe allergies, autoimmune disease, chronic infections, hormone disorders, clinical depression, or psychotic disorders. Informed consent was obtained from all individuals in accordance with the University of Pittsburgh Institutional Review Board. From the original sample of 153 community volunteers (58.2% female, 88.2% Caucasian) aged 40–60 years, analyses were conducted on the sample of 112 participants (59.8% female, 88.6% Caucasian) on whom we had circulating serum IL-6 data collected at a laboratory visit prior to administration of the hepatitis B vaccination.
Procedures
Participants were instructed to abstain from strenuous physical activity and non-prescription medications for 24 hours, from alcohol for 48 hours, and from food and all drinks except water for 12 hours before coming into the laboratory between 7 and 9 AM. On arrival, participants completed questionnaires and measurements of height and weight were taken before a 4ml blood sample was drawn.
Measures
Serum IL-6
IL-6 levels were determined using a high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems) according to manufacturer’s directions. The assay standard range is from 0.156 to 10 pg/mL. IL-6 levels were extrapolated from a standard curve with linear regression from a log-linear curve. All samples were run in duplicate and the average coefficient of variation (CV) between samples was 5%, with inter-plate average CV of 7%. Natural log transformation was applied to normalize raw score distributions of the IL-6 values.
Childhood SES Index
Participants completed the SC Childhood Interview, a retrospective measure that assesses multiple indicators of childhood socioeconomic status (Available at www.psy.cmu.edu/~scohen; Cohen, 2010). For each year of childhood (years 1 through 18), participants were asked to report whether their parents owned their home (0 = no, 1 = yes), the number of bedrooms in the home and the number of siblings and adults living in the home (used to calculate number of bedrooms/child, 0 = <1, 1 = 1+), and the number of vehicles owned by the family (0 = 0–1, 1 = 2+). In each case, participants were given the option of responding that they did not know or could not recall. Selection of this option was most frequent in early childhood, with inability to recall the number of bedrooms across distinct periods of childhood ranging from 0%–9.8%, vehicle ownership 0%–6.3%, and home ownership 0%–3.6%. Markers of childhood SES were summed across 2 year intervals (years 1+2, 3+4, etc.). A childhood SES index was calculated for each 2 year interval by summing scores across the 3 indicators (score range = 0–6). Participants also reported the occupation of their father (coded as 0 = blue collar or 1 = white collar), which remained relatively stable across childhood. Incomplete data for any one of the childhood SES indicators resulted in missing values for the sum score, reflected in variations in the final sample size for each 2-year interval (Table 1). Primary missing data was from recall of number of bedrooms in early childhood (n = 12) and knowledge of father’s occupation in adolescence (n = 7).
Table 1.
Descriptive statistics.
| Mean or % | SD | N | |
|---|---|---|---|
| Race (% White) | 88.4% | 112 | |
| Female | 59.8% | 112 | |
| Age | 50.5 | 5.2 | 112 |
| BMI kg/m2 | 26.4 | 3.8 | 112 |
| Physical Activity (kcal/week) | 2,318 | 2,653 | 111 |
| Annual Family Income | $63,642 | $30,371 | 109 |
| Adult Educational Attainment (Years) | 14.9 | 1.6 | 109 |
| Adult SES Index | 0 | 1.75 | 109 |
| Serum IL-6 (pg/mL) | 1.22 | .6 | 112 |
| Childhood SES Index (Age 1–2) | 2.43 | 1.8 | 92 |
| Childhood SES Index (Age 3–4) | 2.48 | 1.7 | 93 |
| Childhood SES Index (Age 5–6) | 2.84 | 1.7 | 100 |
| Childhood SES Index (Age 7–8) | 3.05 | 1.8 | 102 |
| Childhood SES Index (Age 9–10) | 3.17 | 1.8 | 102 |
| Childhood SES Index (Age 11–12) | 3.35 | 1.8 | 104 |
| Childhood SES Index (Age 13–14) | 3.52 | 1.8 | 105 |
| Childhood SES Index (Age 15–16) | 3.75 | 1.7 | 104 |
| Childhood SES Index (Age 17–18) | 3.86 | 1.7 | 103 |
Adult SES
Participants were asked to report their years of education and current annual (pre-tax) family income (selected from following: Less than $5,000; $5,000–$11,999; $12,000–$15,999; $16,000–$24,999; $25,000=$34,999; $35,000–$49,999; $50,000–$74,999; $75,000–$99,999; $100,000 & greater). For analysis, we used the mid-point of the indicated income range (Janicki-Deverts et al., 2007). For example, $42,500 was assigned to those indicating they fell within the range of $35,000–$49,999. An index of individual SES was calculated by standardizing the distributions of years of education and the mid-point value of the family income category, then summing the standardized values for each individual. For 4 subjects lacking education data and 16 subjects missing family income data, individual SES was determined using regression estimation methods. All analyses were run with and without these 20 individuals and in no case did inclusion of these subjects alter findings. Three subjects were missing both education and family income data and were dropped from analyses that included adult SES.
Additional Variables
A number of variables were assessed that might explain associations between childhood SES and inflammation. These variables included age, gender, race, body mass index (BMI: kg/m2), and physical activity (kcal/week), as assessed with the Paffenbarger physical activity questionnaire (PPAQ; Paffenbarger et al., 1993).
Data Analysis
Initial Pearson product-moment correlation analyses were performed to determine bivariate associations between demographics, BMI, physical activity (log transformed), childhood SES, adult SES, and IL-6. Next, linear regression analyses were conducted examining the association of each 2-year measure of childhood SES with adult IL-6. In these models, age, gender, race, and BMI were entered in the first step of the equation, followed by the childhood SES 2-year interval of interest. Our primary hypothesis predicted that SES during the first 2 years of life would be inversely associated with adult IL-6. In secondary analyses we examined whether childhood SES accounted for variability in IL-6 above that explained by adult SES and lifestyle factors by entering demographic characteristics, physical activity, and BMI in step 1, adult SES in step 2, and childhood SES in step 3. Finally, we examined whether any observed associations were moderated by gender. Here, age, race, and BMI were entered in step 1, gender and childhood SES (centered) in step 2, followed by the interaction term in step 3.
Results
Demographic characteristics of the sample are displayed in Table 1. IL-6 was positively associated with BMI (r = .45, p <.001); however, there were no significant associations of IL-6 with age, gender, race, or physical activity. As expected, SES across childhood was inter-correlated (r’s = .43–.96), and SES increased gradually over time (See Table 1) reflecting a movement from renting to owning a home, increases in the size of the home, and the purchasing of more cars.
As shown in Table 2, SES across childhood was inversely associated with adult age. In addition, and consistent with existing literature (Pollitt et al., 2005; Phillips et al., 2009; Taylor et al., 2006), there was a tendency for higher adult BMI to be associated with lower SES in middle childhood. As expected, adult SES was positively related to childhood SES, however, these associations were only significant after age 5 years.
Table 2.
Bivariate correlations of the Childhood SES Index for every two years of childhood with demographics, physical activity, adult SES, and IL-6.
| Childhood SES Index | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–2 years | 3–4 years | 5–6 years | 7–8 years | 9–10 years | 11–12 years | 13–14 years | 15–16 years | 17–18 years | |
| Race (White = 1) | .02 | .04 | .09 | .12 | .14 | .15 | .19 | .16 | .19 |
| Gender (Female =1) | .02 | −.04 | −.02 | .02 | .01 | .04 | .05 | .02 | .02 |
| Age | −.28** | −.24* | −.22* | −.25* | −.25* | −.28** | −.28** | −.30** | −.28** |
| BMI | −.11 | −.12 | −.22* | −.29** | −.30** | −.28** | −.28** | −.29** | −.27** |
| Physical Activity | −.15 | −.09 | .01 | −.04 | −.00 | .04 | .08 | .14 | .12 |
| Adult SES Index | −.04 | .04 | .24* | .29** | .35** | .35** | .36** | .39** | .46** |
| Serum IL-6 (log) | −.25* | −.25* | −.24* | −.17 | −.16 | −.16 | −.17 | −.27** | −.28** |
p < .05,
p < .01.
Values determined using Pearson product-moment correlations. Spearman rank correlations were also tested for gender and race with similar effects.
SES and Adult Serum IL-6
First, to replicate previous reports (Miller et al., 2009; Pollitt et al., 2007), we tested for associations between father’s occupation and adult serum IL-6. Here we found that participants who reported (throughout childhood) a father with a blue collar job (n = 61) showed elevated IL-6 compared to those whose father’s had a white collar job (n = 33) throughout childhood, β(SE) = −.23 (.11), p < .05. Given that there was little variability in father’s occupational status across years of childhood (stable across childhood and adolescence for 94 of 101 participants) we did not include this marker in the childhood SES index we used to evaluate the role of SES at different ages (see earlier description of the index).
Using our index of childhood SES, we tested for an association of childhood SES with adult serum IL-6 across discrete years of childhood. Initial bivariate analyses revealed inverse associations of IL-6 with childhood SES throughout early, middle, and late childhood, although effects were stronger in early and later childhood (See Table 2). Consistent with our primary hypothesis, regression analyses controlling for age, gender, race, physical activity, and BMI showed that childhood SES continued to predict IL-6, but only in early childhood (years 1–2) (see Table 3; Model 1).1 Thus, our findings suggest that socioeconomic factors in the first two years of life are associated with adult levels of IL-6, with study participants who recalled living in less advantaged early socioeconomic situations showing higher serum concentrations of IL-6 than did those who recalled more affluent early life circumstances.
Table 3.
Hierarchical linear regressions showing the contributions of race, gender, age, BMI, and adult and childhood SES to the prediction of log transformed IL-6.
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
| Unstandarized Coefficient | SE | p | Unstandardized Coefficient | SE | p | |
| Step 1 | ||||||
| (Constant) | −1.96 | 0.65 | 0.004 | −1.97 | 0.66 | 0.004 |
| Race | −0.15 | 0.15 | 0.31 | −0.15 | 0.15 | 0.34 |
| Gender | 0.21 | 0.10 | 0.04 | 0.22 | 0.10 | 0.03 |
| Age | 0.01 | 0.01 | 0.40 | 0.01 | 0.01 | 0.40 |
| BMI | 0.07 | 0.01 | 0.00 | 0.07 | 0.01 | 0.00 |
| Physical Activity | −0.01 | 0.04 | .79 | −0.03 | 0.04 | 0.53 |
| Adult SES Index | 0.01 | 0.03 | 0.87 | |||
| Step 2 of separate models | ||||||
| Childhood SES Index (1–2 yrs old) | −0.05 | 0.03 | 0.044 | −0.05 | 0.03 | 0.046 |
| Childhood SES Index (3–4 yrs old) | −0.05 | 0.03 | 0.06 | −0.05 | 0.03 | 0.07 |
| Childhood SES Index (5–6 yrs old) | −0.03 | 0.03 | 0.24 | −0.03 | 0.03 | 0.23 |
| Childhood SES Index (7–8 yrs old) | −0.001 | 0.03 | 0.96 | −0.002 | 0.03 | 0.95 |
| Childhood SES Index (9–10 yrs old) | −0.01 | 0.03 | 0.80 | 0.01 | 0.03 | 0.81 |
| Childhood SES Index (11–12 yrs old) | −0.004 | 0.03 | 0.88 | 0.00 | 0.03 | 0.88 |
| Childhood SES Index (13–14 yrs old) | −0.01 | 0.03 | 0.78 | −0.01 | 0.03 | 0.82 |
| Childhood SES Index (15–16 yrs old) | −0.04 | 0.03 | 0.17 | −0.04 | 0.03 | 0.17 |
| Childhood SES Index (17–18 yrs old) | −0.05 | 0.03 | 0.10 | −0.06 | 0.03 | 0.09 |
Model 1: Regression analyses controlling for race, gender, age, BMI, and physical activity.
Model 2: Regression analyses controlling for race, gender, age, BMI, physical activity and adult SES.
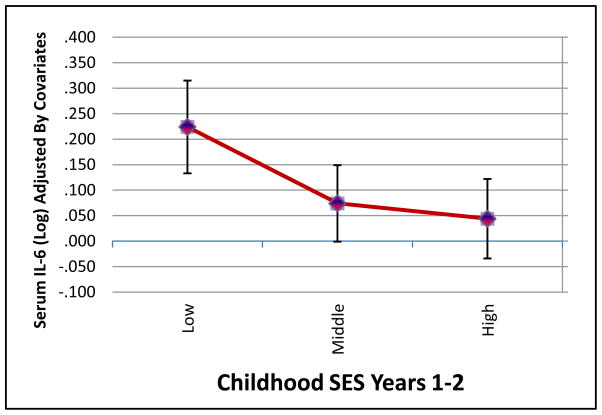
Next, we examined whether the observed association of early childhood socioeconomic factors with IL-6 was independent of adult SES. Consistent with prior literature, IL-6 increased with decreasing adult SES (r = −.22, p < .05). Further adjustments by age, gender, and race did not influence this effect, R2 = .05, p < .05; however, additional adjustments by BMI reduced this effect of adult SES on IL-6 to non-significant (p = .33). To examine whether early childhood SES predicted IL-6 independently of adult SES, we entered demographic characteristics and BMI into step 1, adult SES into step 2, and each 2-year childhood SES index into step 3 of separate regression models predicting IL-6 (Table 3; Model 2). In these full models, childhood SES across years 1–2 remained a predictor of adult IL-6 independently of adult SES (See Figure 1). In addition, there was no significant interaction of gender with childhood SES in the prediction of serum IL-6.2
Figure 1.
Mean Circulating Serum IL-6 (pg/mL; log transformed and adjusted by age, gender, race, BMI, physical activity and adult SES) at low (0–1), middle (2–3), and high (4–6) scores on the Childhood SES Index for years 1–2.
Discussion
The present study provides initial evidence for an association between socioeconomic conditions in early childhood (years 1–2) and a circulating marker of inflammation among a relatively healthy, mid-life sample of adults. Consistent with current theories of early physiologic programming (Coe & Lubach, 2003; Hertzman, 1999; McDade, 2005; Surtees et al., 2003), our findings show that, when compared with adults who recall more affluent early life circumstances, individuals who endorse lower socioeconomic conditions during the first 2 years of life have higher adult levels of circulating IL-6. This association is independent of demographic factors (age, gender, and race), BMI, physical activity, and adult SES. Although these effects are modest, they are consistent with the hypothesis that early social inequalities impact psychobiologic programming (Barker, 1998; Hertzman, 1999), resulting in adult phenotypes that are prone to inflammation (Miller et al., 2009). In this regard, growing evidence shows that socioeconomic conditions during childhood confer risk for inflammatory disease in later life (e.g. atherosclerosis; Libby, 2002; Libby & Ridker, 2004; Ward et al., 2009), with circulating levels of inflammatory markers in mid-life adults predicting risk for a range of chronic diseases, including cardiovascular and metabolic disease, cancer, osteoarthritis, and dementia (Chung et al., 2009). Thus, our findings raise the possibility that socioeconomic conditions during early childhood have a lasting impact on levels of systemic inflammation, which may contribute to the increase in inflammatory disease risk known to accompany a history of poorer childhood socioeconomic circumstances.
Our results were consistent with recently published evidence that adult levels of circulating markers of inflammation, including IL-6 and CRP, covary inversely with socioeconomic indicators that are generally stable across childhood, in this case father’s occupation (Danese et al., 2007; Phillips et al., 2009; Pollitt et al., 2007; Taylor et al., 2006). However, by using a measure of childhood SES that was sensitive to variation across the years of childhood, we extended these findings to suggest that there may be a critical or optimal period in early childhood when socioeconomic conditions have a greater impact on future inflammation.
The mechanisms through which early life experiences may affect systemic inflammation are unclear. However, growing evidence suggests that early experience plays a critical role in the development of brain structure and function (Gianaros et al., 2008; McEwen & Gianaros, 2010; Penza et al., 2003; Taylor et al., 2006), with socioeconomic adversity programming biobehavioral response patterns that are biased toward interpreting ambiguous circumstances with mistrust and anticipation of threat/harm, which, in turn, drives higher levels of physiologic arousal in anticipation of threat (vigilance) and in response to psychological stress (Chen & Matthews, 1999; Chen & Matthews, 2001; Taylor et al., 2004). Peripherally, this heightened central vigilance is proposed to activate physiologic pathways that modulate the magnitude of the inflammatory response, including the autonomic nervous system and the hypothalamic-pituitary-adrenal axis (Cohen et al., 2006; Li et al., 2007; Slavich et al., 2010; Steptoe et al., 2007).
In regard to the inflammatory response, recent evidence suggests that early activation of these peripheral pathways may program the sensitivity of immune cells throughout life. For example, exposure to early stress may decrease the sensitivity of immune cells to the anti-inflammatory action of glucocorticoids (Miller & Chen, 2010; Miller et al., 2009). Recent evidence also shows that early life adversity can program stable changes in the expression of genes through epigenetic processes (Mathews & Janusek, 2010; Zhang & Meaney, 2010). Theoretically, it is proposed that these adaptations serve to fine tune the organism’s physiological phenotype to handle the demands of its environment, which optimizes survival during the reproductive years (Gluckman & Hanson, 2004). Thus, it is possible that prenatal and early childhood adversity may program the nervous, endocrine, and immune systems (Coe & Lubach, 2003; Gluckman & Hanson, 2004; Gunnar & Quevedo, 2007; McEwen & Gianaros, 2010; McGowan & Szyf, 2010; Sandman & Davis, 2010). With regards to the immune system, these adaptations may initially bolster immune responses that increase the probability of survival in the early environment, but have longer term consequences such as increasing risk for inflammatory disease in later life (Miller & Chen, 2010; Miller et al., 2009; Miller & Chen, 2007; Shanks & Lightman, 2001).
It is also possible that socioeconomic attributes of early childhood affect later systemic inflammation via behavioral or environmental pathways. Here, evidence shows that childhood socioeconomic conditions exert a sustained influence on health behaviors, with lower parental education predicting poorer dietary habits, increased likelihood of smoking, decreased physical activity, and higher BMI among adults (e.g. Lynch, Kaplan, & Salonen, 1997; Pollitt et al., 2005). These behaviors, in turn, have been associated with increased systemic inflammation (e.g. Bruunsgaard, 2005; Fröhlich et al., 2003), risk for inflammatory disease (Rosamond et al., 2007), and have, in prior work, partially contributed to associations between childhood SES (averaged across entire childhood) and adult inflammation (Danese et al., 2007; Pollitt et al., 2007). In the current study we observed an association of higher adult BMI, but not physical activity, with childhood SES. However, the association we observed between lower socioeconomic conditions during the first 2 years of life and higher adult levels of circulating IL-6 was independent of BMI and physical activity.
Also of note, the present findings report an association between age and childhood SES, such that older adults report lower childhood SES. These associations likely reflect the general rise in home ownership in the U.S. between the 1950’s and 1970’s (55% to 62.9%; U.S. Census), and a rapid increase in the number of vehicles in operation (U.S. Department of Energy, 2010). However, the observed associations between early life SES and adult IL-6 were independent of participant age.
Higher rates of childhood infections, increased exposure to environmental pollutants, and early life family and neighborhood factors may also contribute to later life variations in inflammation and health (Cohen et al., 2010; Repetti, Taylor, & Seeman, 2002). Regarding the family environment, hostile and inconsistent parenting is more prevalent among impoverished families and may directly or indirectly contribute to the lasting effects of early childhood SES on adult health (Repetti et al., 2002). In this regard, Danese and colleagues (2007) show that childhood maltreatment predicts higher adult inflammation after controlling for childhood SES (parental occupation averaged across years 1–15), suggesting that maltreatment, which was higher among lower SES children, may contribute to the biological programming of inflammation.
Early adversity may also be associated with adult health through prenatal factors, with socioeconomic conditions across the first years of life being similar to those experienced by the mother during gestation. In this regard, several studies show that health risk is inversely related to birth weight (Barker, 1998; Phillips et al., 2006), which may be a marker for poorer maternal socioeconomic circumstances and inadequate prenatal nutrition, adversely impacting fetal development (Barker, 1998; Osmond et al., 1993). Furthermore, growing evidence shows that maternal stress during pregnancy influences the neuroendocrine, nervous, and metabolic systems of the developing fetus (Barker, 1998; Cottrell & Seckl, 2009; Phillips, 2004; Sandman & Davis, 2010; Sandman & Glynn, 2009). Recent evidence suggests that prenatal factors can impact inflammatory competence, with larger mitogen-stimulated proinflammatory responses observed from cord blood samples taken from mothers who endorse high levels of stress when compared with their less stressed counterparts (Wright et al., 2010). Thus, it is possible that prenatal factors contribute to the development of a proinflammatory phenotype. Finally, both early life adversity and adult IL-6 may be related to a third factor such as genetics, which may act independently or in concert with environmental factors to predict individual vulnerability (Caspi et al., 2003; Cole et al., 2010). Thus, further work examining the many characteristics of the early environment that may contribute to immune programming is warranted.
It remains to be determined whether interventions designed to improved early life conditions could decrease future inflammatory risk. Although evidence shows that early intervention in infancy and pre-school can improve cognitive and socio-emotional outcomes among high risk children (See Camilli et al., 2010; Chambers et al., 2010; Martin et al., 2008; McCormick et al., 2006), it is unknown whether these benefits extend to biological parameters. One factor that may buffer the negative impact of early life adversity and contribute to biological resilience is the presence of a supportive and nurturing relationship with a parental figure (Chen et al., 2010; Repetti et al., 2002). Future research is warranted examining whether early intervention and/or the presence of a nurturing adult may prevent or buffer the impact of early life adversity on health relevant biological outcomes.
Limitations of study design
There are a number of limitations of the current study. First, its cross-sectional design precludes causal interpretation. Indeed, it is possible that adult inflammation impacts central processes involved in long term memory for childhood circumstances. In general, the study is limited by the retrospective assessment of childhood SES, which may involve recall bias, particularly for the early years of life when recall of the childhood environment is likely based on family history. However, when compared with more widely used, but less reliably recalled indicators such as parental income, we believe that our relatively objective indicators of childhood wealth that are typically easy for participants to recall (parental home and vehicle ownership, number of bedrooms per child in the family home, and parental occupational class) (Cohen et al., 2004; Krieger et al., 1998) provide a more reliable measure. That said, further research is warranted to, validate the accuracy of our childhood SES measure as we do not have data comparing these retrospective reports of childhood SES to objective markers collected during childhood. Our study is also limited by a relatively small sample size and the use of multiple tests, increasing risk for a Type 1 statistical error. However, the consistencies of our findings with other studies that examine associations of childhood SES with adult inflammation and with animal based theoretical speculation of when such effects should occur suggest that this is not the case.
Future longitudinal investigations are indicated, assessing socioeconomic circumstances in early childhood and their correlates and tracking the influence of more proximal individual, behavioral, and environmental characteristics on inflammatory mediators to better elucidate how early childhood SES may affect future health.
Conclusions
In conclusion, the present findings show an inverse association of early childhood SES with adult levels of underlying inflammation, that are known to predict future health risk and play a role in the pathogenesis of inflammatory disease. Our novel findings suggest that independently of adult SES, socioeconomic conditions in the first 2 years of life are associated with adult levels of systemic inflammation, raising the possibility that previously observed associations of SES across childhood with adult levels of systemic inflammation are largely accounted for by early life experiences. Our findings are consistent with recent theories and animal evidence suggesting that early childhood is a critical period for tuning developing biological systems, including immune function, to the environment (Coe & Lubach, 2003; Shanks & Lightman, 2001). It has been proposed that a proinflammatory phenotype may be of survival advantage in the short term, decreasing risk from pathogens present in the early environment. However, this phenotype may be detrimental to long term survival, being associated with increased risk for chronic inflammatory disease (Miller et al., 2009; Miller & Chen, 2007; Shanks & Lightman, 2001).
Acknowledgments
This study was supported by grant NR008237 from the National Institute of Nursing Research (ALM), by grant T32-MH19925 from the National Institute of Mental Health, and the Cousins Center for Psychoneuroimmunology, UCLA.
Footnotes
These effects were retained in sensitivity analyses that included only the 90 participants with complete childhood SES data across all years of childhood.
Conflict of Interest Statement: All authors declare that there are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker DJP. Mothers, babies, and health in later life. 2. Edinburgh, UK: Churchill Livingston; 1998. [Google Scholar]
- Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. Journal of Leukocyte Biology. 2005;78:4–35. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- Camilli G, Vargas S, Ryan S, Barnett WS. Meta-Analysis of the Effects of Early Education Interventions on Cognitive and Social Development. Teachers College Record. 2010;112(3):579–620. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chambers B, Cheung A, Slavin RE, Laurenzano M. Effective Early Childhood Education Programs: A Systematic Review Dewi Smith Success for All Foundation. Best Evidence Encyclopedia. 2010:1–60. [Google Scholar]
- Chen E, Matthews KA. Socioeconomic differences in social information processing and cardiovascular reactivity. Ann N Y Acad Sci. 1999;896:417–419. doi: 10.1111/j.1749-6632.1999.tb08158.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Molecular Psychiatry. Nature Publishing Group; 2010. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science. 2010;21(1):31–7. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Chen Edith, Langer DA, Raphaelson YE, Matthews Karen A. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Development. 2004;75(4):1039–52. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen Edith, Matthews K. Cognitive appraisal bias: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med. 2001;23(2):101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Critical periods of special health relevance for psychoneuroimmunology. Immunity. 2003;17:3–12. doi: 10.1016/s0889-1591(02)00099-5. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Schneider ML, Dierschke DJ, Ershler WB, Lubach R, et al. Early Rearing Conditions Alter Immune Responses in the Developing Infant Primate. Pediatrics. 1992;90:505–509. [PubMed] [Google Scholar]
- Cohen S. SC Childhood Interview. 2010 Retrieved from http://www.psy.cmu.edu:16080/~scohen/
- Cohen Sheldon, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine. 2004;66(4):553–8. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Janicki-Deverts D, Chen Edith, Matthews Ka. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cohen Sheldon, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Cole Steven W, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5681–6. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Frontiers in Behavioral Neuroscience. 2009 September;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry. 2010;52:2. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich M, Sund M, Löwel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) European Heart Journal. 2003;24(14):1365–72. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith G Davey. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. Journal of Epidemiology and Community Health. 2008;62(5):387–90. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Galobardes Bruna, Lynch John W, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiologic Reviews. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes Bruna, Smith George Davey, Lynch John W. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews Karen A, et al. Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience. 2008;3(2):91–6. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Ferrie JE, Elovainio M, Pulkki-Raback L, Keltikangas-Jarvinen L, Eklund C, et al. When do social inequalities in C-reactive protein start? A life course perspective from conception to adulthood in the Cardiovascular Risk in Young Finns Study. International Journal of Epidemiology. 2008;37(2):290–8. doi: 10.1093/ije/dym244. [DOI] [PubMed] [Google Scholar]
- Gliksman MD, Kawachi I, Hunter D, Colditz GA, Manson JE, Stampfer MJ, et al. Childhood socioeconomic status and risk of cardiovascular disease in middle aged US women: a prospective study. Journal of Epidemiology & Community Health. 1995;49(1):10–15. doi: 10.1136/jech.49.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Adler NE, Schwartz JE, Matthews KA, Seeman TE. Socioeconomic status is related to urinary catecholamines in the Coronary Artery Risk Development in Young Adults (CARDIA) study (2007) Psychosomatic Medicine. 2007;69(6):514–20. doi: 10.1097/PSY.0b013e3180f60645. [DOI] [PubMed] [Google Scholar]
- Krieger N, Okamoto A, Selby JV. Adult Female Twins’ Recall of Childhood Social Class and Father’s Education: A Validation Study for Public Health Research. American Journal of Epidemiology. 1998;147(7):704–708. doi: 10.1093/oxfordjournals.aje.a009512. [DOI] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32(7):824–33. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation and Atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Libby Peter, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. The American Journal of Medicine. 2004;116(Suppl):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Liu D, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, et al. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Science & Medicine (1982) 1997;44(6):809–19. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Martin a, Brooksgunn J, Klebanov P, Buka S, Mccormick M. Long-term maternal effects of early childhood intervention: Findings from the Infant Health and Development Program (IHDP) Journal of Applied Developmental Psychology. 2008;29(2):101–117. [Google Scholar]
- Mathews HL, Janusek LW. Epigenetics and Psychoneuroimmunology: Mechanisms and Models. Brain, Behavior, and Immunity. 2010;25(1):25–39. doi: 10.1016/j.bbi.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MC, Brooks-Gunn J, Buka SL, Goldman J, Yu J, Salganik M, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771–80. doi: 10.1542/peds.2005-1316. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. American Journal of Human Biology. 2005;17(1):81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiology of Disease. 2010;39(1):66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Miller Gregory E, Chen Edith. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–56. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Gregory E, Chen Edith, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen Edith. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69(5):402–9. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ (Clinical research ed) 1993;307(6918):1519–24. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Dexter LJ, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. New England Journal of Medicine. 1993;328:538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Archives of Women’s Mental Health. 2003;6(1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Phillips DIW. Fetal programming of the neuroendocrine response to stress: Links between low birth weight and the metabolic syndrome. Endocrine Research. 2004;30(4):819–26. doi: 10.1081/erc-200044086. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Jones A, Goulden PA. Birth weight, stress, and the metabolic syndrome in adult life. Annals of the New York Academy of Sciences. 2006;1083:28–36. doi: 10.1196/annals.1367.027. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF. Parental education is related to C-reactive protein among female middle aged community volunteers. Brain, Behavior, and Immunity. 2009;23(5):677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, et al. Prenatal Stress Enhances Susceptibility of Murine Adult Offspring toward Airway Inflammation. J Immunol. 2006;177(12):8484–8492. doi: 10.4049/jimmunol.177.12.8484. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: A systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22(1):55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky Families: Family Social Environments and the Mental and Physical Health of Offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis Elysia P. Gestational stress influences cognition and behavior. Future Neurology. 2010;5(5):675–690. [Google Scholar]
- Sandman Ca, Glynn LM. Corticotropin-Releasing Hormone (CRH) Programs the Fetal and Maternal Brain. Future Neurology. 2009;4(3):257–261. doi: 10.2217/fnl.09.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Lightman S. The maternal-neonatal neuro-immune interface: Are there long-term implications for inflammatory or stress-related disease? Journal of Clinical Investigation. 2001;108(11):1567–1573. doi: 10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews. 2010;35(1):39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(2):114–27. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- Surtees P, Wainwright N, Day N, Brayne C, Luben R, Khaw KT. Adverse experience in childhood as a developmental risk factor for altered immune status in adulthood. Int J Behav Med. 2003;10(3):251–268. doi: 10.1207/s15327558ijbm1003_05. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60(8):819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72(6):1365–93. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Historical Census of Housing Tables: Ownership Rates. 2004 Retrieved from www.census.gov.
- U.S. Department of Energy. Transportation Energy Data Book. (29) 2010 Retrieved from cta.ornl.gov.
- Ward JR, Wilson HL, Francis SE, Crossman DC, Sabroe I. Translational mini-review series on immunology of vascular disease: Inflammation, infections and Toll-like receptors in cardiovascular disease. Clinical and Experimental Immunology. 2009;156(3):386–94. doi: 10.1111/j.1365-2249.2009.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma Shakti, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Worlein JM, Laudenslager ML. Effects of early rearing experiences and social interactions on immune funtion in nonhuman primates. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3. San Diego, CA: Academic Press Inc; 2001. pp. 73–85. [Google Scholar]
- Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. American Journal of Respiratory and Critical Care Medicine. 2010;182(1):25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji Christian, et al. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology. 2006;73(1):72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zhang T-Y, Meaney Michael J. Epigenetics and the environmental regulation of the genome and its function. Annual Review of Psychology. 2010;61:439–66. C1–3. doi: 10.1146/annurev.psych.60.110707.163625. [DOI] [PubMed] [Google Scholar]