Abstract
CCAAT/enhancer-binding protein delta (C/EBP-δ), a transcription factor, is elevated in carcinoma compared to normal tissue. This study reports a novel function of C/EBP-δ in lymphangiogenesis and tumor metastasis. Genetic deletion of C/EBP-δ in mice resulted in a significant reduction of lymphangiogenesis and pulmonary metastases, with a dramatic reduction of VEGF-C and its cognate receptor VEGFR3 in lymphatic endothelial cells (LECs). In contrast, no difference of VEGF-C in tumor tissues and bone marrow was observed between null and wild type mice. Consistently, forced expression of C/EBP-δ increased VEGF-C and VEGFR3 expression in cultured LECs. These findings suggest a specific and important role of C/EBP-δ in regulating VEGFR3 signaling in LECs. Furthermore, expression of C/EBP-δ in cultured LECs significantly increased cell motility, and knockdown of C/EBP-δ inhibited cell motility and lymphatic vascular network formation in vitro. Forced expression of VEGF-C, but not recombinant VEGF-C, rescued knockdown of C/EBP-δ-induced cell apoptosis, indicative of autonomous VEGF-C autocrine signaling essential for LEC survival. Moreover, hypoxia induces C/EBP-δ expression, and C/EBP-δ regulates HIF-1α expression. Blocking HIF-1α activity totally blocked CEBP-δ induced VEGF-C and VEGFR3 expression in LECs. Together, these findings reveal a new function of CEBP-δ in lymphangiogenesis via regulating VEGFR3 signaling in LECs.
Keywords: C/EBP-δ, VEGF-C, HIF-1α, lymphangiogenesis, metastasis
Introduction
The metastatic spread of tumor cells is the most lethal aspect of cancer. Similar to angiogenesis, lymphangiogenesis is the formation of lymphatic vessels, during which lymphatic endothelial cells sprout to form new vessels from preexisting lymphatic vessels. Lymphangiogenesis plays important roles in tissue homeostasis, metabolism and immunity. Lymphatic vessel formation also contributes to pathological conditions such as tumor invasion to lymph nodes and metastasis (Alitalo et al 2005). The role of the lymphatic network in human diseases has received renewed interest largely due to the identification of specific signaling pathways that regulate the formation of lymphatic systems, which are vascular endothelial growth factor C (VEGF-C) and its cognate receptor VEGF receptor 3 (VEGFR-3). VEGF-C stimulates tumor lymphangiogenesis and metastasis by interacting with VEGF receptor 3 (VEGFR-3) (Alitalo et al 2005).
VEGF was known to regulate angiogenesis in a paracrine fashion, in which VEGF produced by non-endothelial cells in surrounding tissues binds to its cognate receptors on endothelial cells to activate angiogenic signaling pathway. However, it was recently found that abrogation of endogenous VEGF production in endothelial cells leads to apoptosis and thrombosis. And interestingly, addition of endogenous, but not exogenous, VEGF rescued the phenotype (Helotera and Alitalo 2007, Lee et al 2007). As endogenous and exogenous VEGF function through the same receptor, VEGFR2, on endothelial cells, these findings imply that autocrine and paracrine signaling events by VEGF in endothelial cells possess different and non-overlapping functions. Currently, it is unclear whether VEGF-C possesses a similar cell-autonomous signaling role in LECs.
Hypoxia is commonly associated with many tumors. The transcription factor hypoxia-inducible factor-1 (HIF-1) is a major regulator of tissue response to hypoxia (Semenza 1998). HIF-1 regulates tumor progression by up-regulating its target genes, including genes associated with angiogenesis and lymphangiogenesis (Katsuta et al 2005). Hypoxia promotes lymphangiogenesis in human breast cancer and lung carcinoma (Schoppmann et al 2006, Simiantonaki et al 2008). In various types of cancer, HIF-1α expression is positively correlated with lymphatic metastasis (Kurokawa et al 2003, Kuwai et al 2003).
C/EBP-δ is a transcription factor that belongs to the C/EBP family. It is strongly induced by inflammatory cytokines, and plays a role in inflammation (Poli 1998, Rabek et al 1998, Takata et al 2002). The level of C/EBP-δ in carcinoma is elevated compared to surrounding normal tissue (Kim and Fischer 1998, Milde-Langosch et al 2003), indicating a positive role of the gene in tumorigenesis. However, the underlying molecular mechanism remains unclear. One major function of C/EBP-δ is regulating gene expression, such as pro-apoptotic gene expression during mammary gland involution (Thangaraju et al 2005), and platelet-derived growth factor receptor expression that affects smooth muscle cell proliferation (Kitami et al 1999, Yang et al 2001). In addition, C/EBP-δ expression is elevated under hypoxic conditions in both neonatal and adult brains (Tang et al 2006).
In this study, we demonstrate a previously unknown function of C/EBP-δ in promoting lymphangiogenesis and lung metastasis via regulating VEGFR3 signaling in LECs. We show that C/EBP-δ is expressed in LECs and regulates lymphatic angiogenic gene expression through HIF-1α. Genetic deletion of C/EBP-δ impairs lymphangiogenesis and metastasis. Thus this study links hypoxia to VEGF-C signaling in lymphangiogenesis and metastasis through C/EBP-δ.
Materials and Methods
Mice and cell lines
C57BL/6J mice from Jackson Labs and C/EBP-δ null mice in the C57BL/6 background from the NCI (Sterneck et al 1998) were housed in a pathogen-free unit at the Vanderbilt University School of Medicine in compliance with Institution of Animal Care and Use Committee (IACUC) regulations. Six-week-old female mice were injected with 1 × 105 3LL cells in 100 μL PBS via tail vein. Fourteen days after inoculation, lungs were excised, and the number of pulmonary tumor colonies was counted and lungs were weighed. Human lung lymphatic endothelial cells (HMVEC-LLy) were purchased from Lonza (Walkersville Inc), and cultured according to manufacture's protocol. All experiments were performed on cells between 3-7 passages. Lewis lung adenocarcinoma cells (3LL) were maintained in DMEM supplemented with 10% serum.
Immunohistochemistry
Tumors were harvested, processed and paraffin tissue sections were stained with an antibody against LYVE-1 (MBL), and counterstained with hematoxylin. The number of LYVE-1+ lymphatic vessels was counted in 10 randomly selected 200X fields under microscopy.
Isolation of pulmonary lymphatic endothelial cells
Age and sex matched wild type and C/EBP-δ null mice were sacrificed. Single cell suspension was made from lungs as described (Kamiyama et al 2006). Cell suspension was incubated with LYVE-1-PE antibody from MBL. Positive cells were sorted in a FACStarPlus® flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cell purity was confirmed by immunostaining with a LYVE-1 antibody. Cells used in each study were greater than 95% pure.
Expression and knockdown of C/EBP-δ
HMVEC-LLy cells were transfected using the Nucleofector Kit (Lonza, VPB-1002). 24 hours after transfection, the cells were treated under normoxia or hypoxia conditions for 48 hours followed by analysis of gene expression. For overexpression, the cells were transfected with PCDNA 3.1-C/EBP-δ plasmid or empty vector as control. For knockdown, shRNA plasmid DNA specific for C/EBP-δ and non-specific shRNA plasmid obtained from Sigma-Aldrich were used. Puromycin at .5μg/ml was used for enrichment of shRNA transfected cells prior to each experiment. Geldanamycin purchased from Marligen Biosciences Inc. was used to inhibit HIF function (5 μM).
In vitro lymphangiogenic assays
HMVEC-LLy cell migration was performed in Transwells with recombinant VEGF-C at 50 ng/ml. Migrated cells were counted in 10 randomly selected high power fields after 5 hours of incubation. Lymphatic vascular tubule formation was done in 3-D culture on top of growth factor reduced Matrigel (Becton Dickinson, Bedford, MA). Tubule structure was photographed 24 hours after cell plating under microscopy. Vascular cross points were counted in 10 randomly selected fields under microscopy.
Flow cytometric analysis of apoptotic populations with staining of annexin V-FITC and propidium iodide
The frequencies of apoptotic cells were determined using Annexin V-FITC and Propidium Iodide (PI) staining. Flow cytometry was performed with a FACScalibur flow cytometer, and the results were analyzed with Cell Quest Pro software (Becton Dickinson, Bedford, MA).
RT-PCR and Real-Time RT-PCR analysis
Total RNA was isolated using RNeasy Quick spin columns (QIAGEN, CA). cDNA fragments of VEGF-C, VEGFR3, C/EBP-δ HIF-1α and β-actin were amplified using Taq DNA polymerase.
Statistical Analysis
The results are presented as means ± SE for each sample. The statistical significance of differences was determined by Student's two-tailed t test in two groups, and done by one-way ANOVA in multiple groups and two-factor factorial ANOVA. All data were calculated with a Statview 5.0 (Abacus Concepts, Berkeley, CA) statistical software package run on a Windows computer. The differences were considered statistically significant when p-value<0.05.
Results
Genetic deletion of C/EBP-δ in mice resulted in a reduction of lymphangiogenesis and pulmonary metastasis of lung cancer
C/EBP-δ is strongly induced by inflammatory cytokines (Rabek et al 1998, Takata et al 2002), and the levels of C/EBP-δ in carcinoma are significantly higher than surrounding normal tissues (Kim and Fischer 1998, Milde-Langosch et al 2003), suggesting a positive role of the gene in tumorigenesis. To test this hypothesis, we used C/EBP-δ null mice. Mice without the C/EBP-δ gene are viable, grossly normal and fertile except subtle defects in adipocyte differentiation, mammary gland involution, and specific types of learning and memory (Johnson 2005, Sterneck et al 1998, Tanaka et al 1997). We injected 3LL tumor cells in C/EBP-δ null mice and wild type mice through tail vein, followed by assaying lung colonization of tumor cells, a commonly used model for vascular metastasis. Interestingly, there was a significant reduction of tumor metastasis measured by counting lung surface metastases and lung weight (Figure 1A-1C)
Figure 1.
Inactivation of C/EBP-δ in mice inhibits tumor lymphangiogenesis and pulmonary metastasis of lung cancer. 1×105 3LL cells were injected via tail vein into 6-week old female wild type and C/EBP-δ null mice. Fourteen days later, lungs were harvested and imaged (Panel A). Representative images are shown. Arrows point to tumor nodules. Tumor metastasis was quantified by counting the number of lung surface metastases (Panel B) and measuring the mass of the lungs (Panel C). Data are expressed as mean ± SD. n=10 mice per group, *p<0.05. Tumor nodules in lungs were subjected to immunohistochemical analysis for Lyve-1, a marker for lymph endothelium (Panel D). 200X Magnification. The number of Lyve-1 positive lymphatic vessels was counted from 10 randomly selected high power fields under microscopy (Panel E). Data are expressed as mean ± SD. *p<0.05.
As the lymphatic networks are critical for metastasis, we therefore examined the effects of inactivation of C/EBP-δ on lymphangiogenesis. Immunohistological staining of pulmonary metastases sections revealed fewer Lyve-1 positive lymphatic vessels in tumors from the C/EBP-δ null mice than the wild type mice (Figure 1D and 1E). These findings support a positive role of C/EBP-δ in tumor malignancy via regulation of lymphangiogenesis. It suggests that defective tumor lymphangiogenesis associated with C/EBP-δ inactivation contributes to reduced tumor metastasis.
C/EBP-δ specifically regulates VEGF-C and VEGFR3 expression in lymphatic endothelial cells
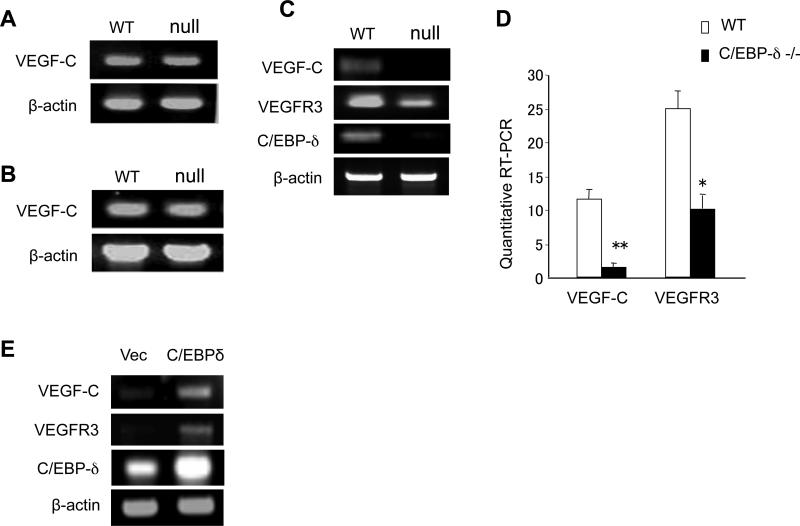
Since C/EBP-δ is known to regulate gene expression, we examined the potential role of C/EBP-δ in lymphangiogenic gene expression by comparing tissues isolated from wild type and null mice. We did not see any differences in VEGF-C expression in tumors between the two groups (Figure 2A), nor did we see any difference in VEGF-C expression in bone marrow between the null and wild type mice (Figure 2B). Surprisingly, an analysis of VEGF-C expression in freshly isolated murine pulmonary lung LECs (pooled from 5 mice in each group) revealed a dramatic reduction of VEGF-C in C/EBP-δ null cells compared to wild type cells (Figure 2C and 2D). Similarly, there is a clear reduction of VEGFR3 in the C/EBP-δ null LECs (Figure 2C and 2D). Moreover, forced expression of C/EBP-δ in primary human LECs promoted VEGF-C and VEGFR3 expression in these cells (Figure 2E). These findings point to a specific role of C/EBP-δ in regulating lymphangiogenic gene expression in lymphatic endothelial cells. As VEGF-C/VEGFR3 signaling plays a major role in lymphangiogenesis, the findings illustrate a novel function of this transcription factor in lymphangiogenesis via regulating VEGF-C and VEGFR3 expression in LECs.
Figure 2.
C/EBP-δ regulates VEGF-C and VEGFR3 expression in lymphatic endothelial cells. Total RNA was isolated from tumor tissues (Panel A) and bone marrow (Panel B) of wild type and C/EBP-δ null mice, and subjected to semi-quantitative RT-PCR for VEGF-C. Pulmonary lymphatic microvascular endothelial cells were isolated from age and sex matched wild type and C/EBP-δ null mice (pooled from 5 mice per group), and purified with flow cytometry cell sorting using anti Lyve-1-PE antibodies. Total RNA isolated from the murine lymphatic endothelial cells was subjected to semi-quantitative RT-PCR (Panel C) and real-time PCR (Panel D) for VEGF-C and VEGFR-3. *p<0.05, **p<0.01. HMVEC-LLy cells were transfected with either empty vector or C/EBP-δ expression vector for 48 hours. Total RNA was isolated and subjected to semi-quantitative RT-PCR for genes indicated (Panel E). Each experiment was repeated at least three times. Representative images are shown.
C/EBP-δ regulates lymphangiogenesis and VEGF-C antocrine signaling in lymphatic endothelium
To confirm the role of C/EBP-δ in lymphangiogenesis, we employed in vitro lymphangiogenic assays by measuring cell migration and vascular network formation. Forced expression of C/EBP-δ in human LECs (Figure 3A) resulted in a slight but significant increase of cell migration in response to VEGF-C stimulation compared to vector control treated group (Figure 3C). Conversely, knockdown of endogenous C/EBP-δ with a C/EBP-δ targeted shRNA construct (Figure 3B) led to a significant inhibition of cell migration compared to controls (Figure 3D). Although we did not observe a change in lymphatic vascular network formation in a 3-D Matrigel assay with forced expression of C/EBP-δ in human LECs (Figure 3E and 3F), knockdown of endogenous C/EBP-δ in LECs caused a significant inhibition of lymphatic vascular network formation (Figure 3G and 3H). Together, the data reveal a direct role of C/EBP-δ in lymphangiogenesis through an effect on lymphatic endothelial cell motility and vascular assembly.
Figure 3.
C/EBP-δ regulates lymphangiogenesis in vitro. HMVEC-LLy cells were transfected with a C/EBP-δ expression vector (C/EBP-δ, Panel A), or knockdown using specific shRNA construct for C/EBP-δ (K’C/EBP-δ, Panel B). Empty vector and non-specific shRNA were used as controls. Expression of C/EBP-δ in lymphatic endothelial cells was determined by semi quantitative RT-PCR. Lymphatic endothelial cell migration was measured in forced expression and knockdown cells using Transwell assays in response to 50 ng/ml of VEGF-C stimulation. Migrated cells were counted in 10 randomly selected 200X high power fields under microscopy after a 5-hour incubation (Panel C and Panel D). **p<0.01. Lymphatic vascular network formation was assessed using the Matrigel assay. Vascular network formation in Matrigel was imaged under microscopy 24 hrs after cell plating in C/EBP-δ forced expression cells (Panel E) and C/EBP-δ knockdown cells (Panel G). Vascular cross points were counted from 10 randomly selected high power fields under microscopy (Panel F and Panel H). The data were collected from three independent experiments performed in triplicate, and expressed as mean ± SD. **p<0.01.
In addition, VEGFR3 signaling is known to provide a survival signal for lymphatic endothelial cells (Makinen et al 2001), and there seemed to be more apoptotic cells with C/EBP-δ knockdown in LECs present in the vascular network formation assay (Figure 3G). Therefore, we examined the effects of C/EBP-δ on LEC survival using flow cytometry. We found that knockdown of C/EBP-δ in human LECs using specific shRNA constructs significantly increased apoptosis measured by PI and Annexin V staining when compared to control vector transfected cells (Figure 4A and 4C), confirming a function of C/EBP-δ in lymphatic endothelial survival. As VEGF-C confers a survival signal in lymphatic endothelium (Makinen et al 2001) and C/EBP-δ regulates VEGF-C expression in LECs, we attempted to rescue the phenotype with addition of recombinant VEGF-C protein. Surprisingly, recombinant protein only increased cell survival slight and it did not reach statistical significance when compared to C/EBP-δ knockdown cells (Figure 4B and 4C).
Figure 4.
C/EBP-δ regulates VEGF-C autocrine signaling in lymphatic endothelial cell survival. HMVEC-LLy cells were transfected with either shRNA vector for C/EBP-δ (K’C/EBP-δ) or control vector for 48 hours, followed by incubation with PI and antibody against Annexin V. Cell apoptosis was assessed by flow cytometry (Panel A). C/EBP-δ knockdown HMVEC-LLy cells were either incubated with 50 ng/ml of recombinant VEGF-C or co-transfected with a VEGFC expression vector. Cell apoptosis was assessed by PI and Annexin V staining and flow cytometry (Panel B). Representative images were shown. Live cells were quantitated in each group (Panel C). Each experiment was done in duplicate and repeated three times. Data are expressed as mean ± SE. *p<0.01 vs control, **p<0.01 vs K’C/EBP-δ.
Deletion of endogenous VEGF production in endothelial cells leads to apoptosis, which cannot be compensated for by exogenous VEGF, even though endogenous and exogenous VEGF both function through the same receptor, VEGFR2 (Helotera and Alitalo 2007, Lee et al 2007). These findings imply that VEGF autocrine signaling does not fully overlap with paracrine signaling. Thus, we reasoned a similar mechanism of VEGF-C autocrine signaling in lymphatic endothelial cell survival as deletion of C/EBP-δ resulted in a lymphatic endothelial specific reduction of VEGF-C (Figure 2C and 2D). To test the hypothesis, we transfected cells with a VEGF-C expression vector in C/EBP-δ knockdown LECs, followed by analysis of cell survival. Interestingly, forced expression of VEGF-C in LECs totally rescued the phenotype (Figure 4B and 4C). Taken together, these findings support that C/EBP-δ regulates cell-autonomous VEGF-C autocrine signaling in lymphatic endothelium, which cannot be compensated for by paracrine VEGF-C signaling.
C/EBP-δ regulates VEGF-C and VEGFR3 expression in lymphatic endothelial cells through HIF-1α
To further dissect the gene regulation mechanism, we first determined the potential effects of C/EBP-δ on HIF-1 transcription factor, as HIF-1 functions as a key regulator in angiogenic gene expression. We found that deletion of C/EBP-δ in murine pulmonary LECs resulted in a significant reduction of HIF-1α levels when compared to cells isolated from wild type mice (Figure 5A). Interestingly, exposure of cultured human LECs to hypoxic conditions induced expression of C/EBP-δ (Figure 5B). Thus, the results point to a positive feedback regulation of HIF-1α in hypoxia through C/EBP-δ in lymphatic endothelium.
Figure 5.
C/EBP-δ regulates VEGF-C and VEGFR3 production in lymphatic endothelial cells through HIF-1α. Purified murine pulmonary LECs from wild type and C/EBP-δ null mice were subjected to semi-quantitative RT-PCR for HIF-1α and C/EBP-δ expression (Panel A). HMVEC-LLy cells were cultured under normoxia (20% O2) or hypoxia (1% O2) for 24 hours. Semi-quantitative RT-PCR was performed to measure C/EBP-δ (Panel B). HMVEC-LLy cells were transfected with either empty vector or C/EBP-δ expression vector for 24 hours. The cells were then cultured under normoxia (20% O2) or hypoxia (1% O2) for another 48 hours. Transcript levels of VEGF-C, VEGFR3, HIF-1α and C/EBP-δ were measured by semi-quantitative RT-PCR (Panel C). HMVEC-LLy cells were transfected with either control shRNA or shRNA for C/EBP-δ for 24 hours. The cells were then cultured under either normoxia (20% O2) or hypoxia (1% O2) for another 48 hours. Transcript levels of VEGF-C, VEGFR3, HIF-1α and C/EBP-δ were measured by semi-quantitative RT-PCR (Panel D). HMVEC-LLy cells were transfected with either empty vector or C/EBP-δ expression vector for 24 hours. The cells were treated with or without HIF-1α inhibitor (5 μM of geldanamycin) for another 48 hours under hypoxic conditions. Transcript levels of VEGF-C, VEGFR3, HIF-1α and C/EBP-δ were measured by semi-quantitative RT-PCR (Panel E). Each experiment was repeated three times. Representative images are shown.
Consistently, forced expression of C/EBP-δ in LECs increased the production of VEGF-C and VEGFR3 under both normoxic and hypoxic conditions with more pronounced gene induction under hypoxia (Figure 5C). Conversely, knockdown of C/EBP-δ inhibited the production of VEGF-C and VEGFR3 in normoxic conditions. More importantly, it totally blocked hypoxia-induced production of VEGF-C and VEGFR3 in lymphatic endothelial cells (Figure 5D), illustrating a key role of C/EBP-δ in lymphangiogenic gene expression under hypoxia. As HIF-1 is a master regulator of angiogenic gene expression in hypoxia, we next examined whether HIF-1 is responsible for C/EBP-δ mediated gene expression in LECs by using a HIF-1α specific inhibitor. We found that neutralization of HIF-1α activity significantly blocked C/EBP-δ induced VEGF-C and VEGFR3 production (Figure 5E). It also reduced the basal levels of C/EBP-δ in these cells, consistent with the observation that hypoxia upregulates the production of C/EBP-δ (Figure 5E). Collectively, these data suggest that C/EBP-δ regulates VEGF-C and VEGFR3 expression through HIF-1 transcription factor.
Discussion
Cancer metastasis is a hallmark of malignancy contributing to about 90% of human cancer deaths. The lymphatic system is important for the metastatic spread of cancer. Therefore, understanding the underlying molecular mechanisms of lymphangiogenesis is highly important. In this study, we report a specific and significant function of C/EBP-δ in regulating VEGF-C/VEGFR3 signaling in lymphatic endothelium. C/EBP-δ is expressed in lymphatic endothelial cells. It regulates the production of VEGF-C and VEGFR3 in lymphangiogenesis and tumor metastasis. Interestingly, forced expression of VEGF-C, but not recombinant VEGF-C, rescued inactivation of C/EBP-δ-induced cell apoptosis, indicative cell autonomous VEGF-C signaling in lymphatic endothelial survival. Further analysis reveals that hypoxia induces C/EBP-δ expression and deletion of C/EBP-δ inhibited HIF-1α transcription, suggesting a positive feedback regulation of hypoxia response in lymphatic endothelial cells (Figure 6). Interestingly, blocking HIF-1α activity totally blocked CEBP-δ induced VEGF-C and VEGFR3 expression in lymphatic endothelial cells. These findings link hypoxia to lymphangiogenesis through C/EBP-δ and VEGF-C autocrine signaling (Figure 6).
Figure 6.
Schematic diagram of C/EBP-δ in regulating VEGF-C signaling.
C/EBP-δ belongs to the C/EBP transcription family, and is present in a variety of cells at very low levels in normal situations. However, it is rapidly induced by a variety of stimuli, such as inflammatory cytokines (Ramji and Foka 2002). As inflammation is directly linked to tumor initiation and progression, it is no surprise that C/EBP-δ levels are significantly higher in carcinomas than surrounding normal tissues (Kim and Fischer 1998, Milde-Langosch et al 2003), indicating a positive role of C/EBP-δ in tumor development. In the present study, we found that inactivation of C/EBP-δ in mice led to a significant reduction of VEGF-C/VEGFR3 signaling in lymphatic endothelium and pulmonary metastasis. These findings provide molecular mechanism supporting a positive role of C/EBP-δ in tumor progression through regulating lymphatic angiogenic gene expression and lymphangiogenesis.
Clinical and preclinical findings have long suggested tumor-associated lymphatics are a key component of tumor metastasis (Stacker et al 2002). Lymphangiogenesis has traditionally been overshadowed by angiogenesis due to a lack of lymphangiogenic factors, as well as suitable markers to distinguish blood from lymphatic vascular endothelium. However, the field has advanced rapidly after the identification of VEGF-C and VEGFR3 signaling in lymphangiogenesis (Alitalo and Carmeliet 2002). VEGF-C-/- embryos lack lymphatic vessels and die prenatally because of severe tissue edema (Karkkainen et al 2004), whereas VEGFR3-/- mice die from defective vascular remodeling before the establishment of lymphatic vessels (Dumont et al 1998). The degree of tumor lymphangiogenesis and VEGF-C and VEGFR3 levels are highly correlated with the extent of lung metastasis (Akagi et al 2000, Alitalo and Carmeliet 2002, Yonemura et al 1999). Overexpression of VEGF-C increases tumor metastasis (Skobe et al 2001), and conversely, blocking VEGFR3 function neutralizes metastasis (He et al 2002). Despite the importance of VEGFR3 signaling in lymphangiogenesis, the transcriptional machinery regulating lymphangiogenic gene expression is not very clear. The current study identifies C/EBP-δ as a transcription factor important for the production of VEGF-C and VEGFR3 in lymphatic endothelium. We show that C/EBP-δ is expressed in lymphatic endothelial cells, and deletion or knockdown of C/EBP-δ impaired, and conversely forced expression of the gene increased, the lymphatic angiogenic gene expression in lymphatic endothelial cells. C/EBP-δ in lymphatic endothelial cells has a direct role in cell motility, vascular network formation and cell survival, consistent with the function of VEGFR3 signaling in lymphatic endothelium.
VEGF was thought to function in a paracrine fashion to regulate angiogenesis. Surprisingly, genetic deletion of endogenous VEGF production in vascular endothelial cells leads to apoptosis, despite that total levels of VEGF are not detectably altered. (Helotera and Alitalo 2007, Lee et al 2007). This indicates a non-overlapping, cell-autonomous and essential function of VEGF autocrine signaling in vascular endothelial survival and homeostasis. Interestingly, we found that loss of C/EBP-δ dramatically reduced the levels of VEGF-C in lymphatic endothelial cells without detectable changes of VEGF-C levels in tumor tissues and bone marrow cells, implying a specific role of C/EBP-δ in regulation of endogenous VEGF-C expression in lymphatic endothelial cells. Importantly, recombinant VEGF-C failed to rescue the phenotype associated with C/EBP-δ inactivation in lymphatic endothelial cells, but forced expression of VEGF-C totally rescued the defective phenotype. Thus our data reveal that VEGF-C possesses a similar cell autonomous autocrine signaling mechanism in lymphatic endothelial cell survival as VEGF does in vascular endothelial homeostasis. C/EBP-δ regulates this activity through regulation of VEGF-C expression specifically in lymphatic endothelial cells. Current effort is targeted at understanding the cell type specific gene regulation mechanism manifested by C/EBP-δ.
Hypoxia is a common feature of tumors, and it is a potent regulator of angiogenesis through activating HIF transcription factor. Findings also report a similar function of hypoxia in modulating lymphangiogenesis (Irigoyen et al 2007, Ota et al 2007). HIF-1α promotes lymphatic metastasis via regulation of VEGF-C in human cancer (Katsuta et al 2005). Here, we show that C/EBP-δ is upregulated under hypoxia, and inactivation of C/EBP-δ reduced HIF-1α levels in lymphatic endothelial cells. In addition, neutralization of HIF-1α activity totally blocked C/EBP-δ mediated expression of VEGF-C and VEGFR3 in lymphatic endothelial cells. This observation corresponds with the finding that hypoxia-driven VEGF autocrine signaling is perturbed by a deficiency in endothelial cell HIF-1α (Tang et al 2004). In addition, we also examined the expression of HIF-2 as this transcription factor also regulates VEGF expression and angiogenesis. We found that overexpression of C/EBP-δ in lymphatic endothelial cells has no effects on HIF-2a expression in normoxia and hypoxia (data not shown), suggesting HIF-2 unlikely plays a role in the gene regulation. Based on these findings, we suggest that hypoxia stabilizes basal levels of HIF-1α that activate C/EBP-δ transcription. In return, C/EBP-δ induces HIF-1α transcription that regulates lymphangiogenic gene transcription, thereby forming a positive feedback regulation.
In summary, this study shows that C/EBP-δ, a transcription factor, regulates VEGF-C autocrine signaling in lymphatic endothelial cells. Consistent with the hypothesis that autocrine signaling is triggered by stress, we show that hypoxia upregulates expression of C/EBP-δ, thereby forming a positive feedback regulation mechanism that propagates angiogenic signals in lymphatic endothelium. These data provide molecular evidence linking C/EBP-δ to lymphangiogenesis and tumor metastasis. Thus, C/EBP-δ is an appealing target for tumor therapy, providing a means to control VEGFR3 signaling in lymphangiogenesis.
Acknowledgements
This work is supported in part by grants from NIH (CA108856, NS45888 and AR053718).
References
- Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- Helotera H, Alitalo K. The VEGF family, the inside story. Cell. 2007;130:591–592. doi: 10.1016/j.cell.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Irigoyen M, Anso E, Martinez E, Garayoa M, Martinez-Irujo JJ, Rouzaut A. Hypoxia alters the adhesive properties of lymphatic endothelial cells. A transcriptional and functional study. Biochim Biophys Acta. 2007;1773:880–890. doi: 10.1016/j.bbamcr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Johnson PF. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118:2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–7028. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Katsuta M, Miyashita M, Makino H, Nomura T, Shinji S, Yamashita K, et al. Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp Mol Pathol. 2005;78:123–130. doi: 10.1016/j.yexmp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Kim Y, Fischer SM. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J Biol Chem. 1998;273:27686–27694. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- Kitami Y, Fukuoka T, Hiwada K, Inagami T. A high level of CCAAT-enhancer binding protein-delta expression is a major determinant for markedly elevated differential gene expression of the platelet-derived growth factor-alpha receptor in vascular smooth muscle cells of genetically hypertensive rats. Circ Res. 1999;84:64–73. doi: 10.1161/01.res.84.1.64. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Miyamoto M, Kato K, Cho Y, Kawarada Y, Hida Y, et al. Overexpression of hypoxia-inducible-factor 1alpha(HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer. 2003;89:1042–1047. doi: 10.1038/sj.bjc.6601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–181. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde-Langosch K, Loning T, Bamberger AM. Expression of the CCAAT/enhancer-binding proteins C/EBPalpha, C/EBPbeta and C/EBPdelta in breast cancer: correlations with clinicopathologic parameters and cell-cycle regulatory proteins. Breast Cancer Res Treat. 2003;79:175–185. doi: 10.1023/a:1023929504884. [DOI] [PubMed] [Google Scholar]
- Ota H, Katsube K, Ogawa J, Yanagishita M. Hypoxia/Notch signaling in primary culture of rat lymphatic endothelial cells. FEBS Lett. 2007;581:5220–5226. doi: 10.1016/j.febslet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Rabek JP, Scott S, Hsieh CC, Reisner PD, Papaconstantinou J. Regulation of LPS-mediated induction of C/EBP delta gene expression in livers of young and aged mice. Biochim Biophys Acta. 1998;1398:137–147. doi: 10.1016/s0167-4781(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Fenzl A, Schindl M, Bachleitner-Hofmann T, Nagy K, Gnant M, et al. Hypoxia inducible factor-1alpha correlates with VEGF-C expression and lymphangiogenesis in breast cancer. Breast Cancer Res Treat. 2006;99:135–141. doi: 10.1007/s10549-006-9190-3. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Simiantonaki N, Jayasinghe C, Michel-Schmidt R, Peters K, Hermanns MI, Kirkpatrick CJ. Hypoxia-induced epithelial VEGF-C/VEGFR-3 upregulation in carcinoma cell lines. Int J Oncol. 2008;32:585–592. [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Paylor R, Jackson-Lewis V, Libbey M, Przedborski S, Tessarollo L, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc Natl Acad Sci U S A. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91:427–433. doi: 10.1161/01.res.0000031271.20771.4f. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. Embo J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, et al. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Tang Y, Pacary E, Freret T, Divoux D, Petit E, Schumann-Bard P, et al. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Rudelius M, Bierie B, Raffeld M, Sharan S, Hennighausen L, et al. C/EBPdelta is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development. 2005;132:4675–4685. doi: 10.1242/dev.02050. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Kitami Y, Takata Y, Okura T, Hiwada K. Targeted overexpression of CCAAT/enhancer-binding protein-delta evokes enhanced gene transcription of platelet-derived growth factor alpha-receptor in vascular smooth muscle cells. Circ Res. 2001;89:503–508. doi: 10.1161/hh1801.096265. [DOI] [PubMed] [Google Scholar]
- Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]