Abstract
We present results from continuous intracranial electroencephalographic (iEEG) monitoring in 6 dogs with naturally occurring epilepsy, a disorder similar to the human condition in its clinical presentation, epidemiology, electrophysiology and response to therapy. Recordings were obtained using a novel implantable device wirelessly linked to an external, portable real-time processing unit. We demonstrate previously uncharacterized intracranial seizure onset patterns in these animals that are strikingly similar in appearance to human partial onset epilepsy. We propose: (1) canine epilepsy as an appropriate model for testing human antiepileptic devices and new approaches to epilepsy surgery, and (2) this new technology as a versatile platform for evaluating seizures and response to therapy in the natural, ambulatory setting.
1.1 Introduction
Epilepsy affects 60 million people worldwide, ~40% of whom have seizures that are not controlled by medication (Kwan and Brodie 2000). Recently, two pivotal clinical trials of implantable antiepileptic devices ended. One device electrically stimulates the anterior thalamic nucleus “open loop,” while the other responsively stimulates cortex or mesial temporal structures after detecting electrical changes known to precede seizure onset in a given patient. Results of both trials are encouraging, but seizure-free rates remain relatively low, for unclear reasons (Morrell MJ 2008; Fisher, Salanova et al. 2010). Two major factors hinder improving device performance, compared to implantable cardiac devices, which had similar development trajectories: (1) an inability to record long-term continuous intracranial electrophysiology, and (2) lack of a suitable large animal model of epilepsy for testing improved hardware and algorithms.
Seizures are rare events in most patients with medically refractory epilepsy, similar to arrhythmias in cardiac patients. Since patients are often not aware of when they have seizures, months of continuous recording may be required to accurately characterize their true seizure frequency and electrophysiology (Blum, Eskola et al. 1996). Scalp recording is impractical for this application, because of difficulty maintaining leads and low impedance. It is also often inaccurate, because scalp recording is insensitive to deep or very focal seizures (Smith 2005). Because of these technical limitations, ictal scalp recording beyond a few days, and all intracranial EEG (iEEG) recording takes place in the inpatient setting, coincident with medication taper to precipitate seizures. While a gold standard for seizure localization, inpatient video-EEG monitoring has important limitations. Many epileptologists believe that seizures precipitated by medication withdrawal do not accurately represent spontaneous seizures in the outpatient setting. Epilepsy monitoring unit (EMU) recording is also expensive and has inherent risks for injury associated with medication withdrawal seizures and status epilepticus (Noe and Drazkowski 2009). There is a need for high-quality continuous intracranial recording platforms that can accurately record EEG and seizures as they occur in normal life, over long periods. These recordings are useful both to diagnose patients properly and to guide epilepsy surgery, implantable device placement, and clinical management.
Intracranial EEG solves many of the technical hurdles of scalp recording. Impedances are low, signal quality is high, and in experienced hands complication rates are ~1% or less for implantation. With recent success of chronically implanted devices for movement disorders, and more recently for antiepileptic devices, continuous iEEG monitoring presents an attractive option for diagnostic and therapeutic applications in appropriate patients, despite its relative invasiveness.
One existing device, the Responsive Neurostimulator (RNSTM, NeuroPace, Inc.), detects seizures with short latency and high sensitivity, but retains relatively few, brief detected epochs of data because of limited power and storage capacity (Morrell MJ 2008). This strategy is insufficient for comprehensively characterizing a patient’s seizure patterns and frequency, as detections have imperfect sensitivity and specificity, and the amount of data that can be stored is limited. Continuous recording systems without data reduction are required to completely characterize seizure frequency, onset patterns, and the overall burden of epileptiform activity.
1.2 Technical Considerations
We present a rechargable implantable device that continuously telemeters iEEG data to an external processing unit for real time data storage, analysis and communicating analysis results to patients and caregivers. By moving data storage and analysis to a unit worn outside the body, the device is freed from the stringent size, space and power requirements of fully implanted devices. The external device includes hardware and software for online seizure detection and prediction, coupled to a patient interface. The implanted device couples 16 intracranial sensors to an implanted, rechargeable, subclavicular acquisition and transmission unit (ITU). The implanted device wirelessly streams continuous digital data sampled at 400 Hz/channel to the external unit. The seizure detection algorithm, trained on human iEEG data was deployed on real-time canine iEEG in the study described below, without additional modification. While we describe only application to epilepsy in this study, the system is designed with sufficient flexibility to enable other brain-computer interface applications based upon iEEG recordings.
1.3 Application to Canine Epilepsy
We chose to test the above device in naturally occurring canine epilepsy because its prevalence, age of onset, and presentation is similar to humans (Berendt, Hogenhaven et al. 1999). Specifically, canine epilepsy has a prevalence estimated between 0.5 to 5.7% (Chandler 2006), with 65% of seizures characterized as partial onset with or without secondary generalization (Berendt and Gram 1999). The majority of dogs with recurrent seizures have no apparent intracranial structural defect, no demonstrable serum or spinal fluid abnormalities and no interictal neurological findings. In this way the model has greater similarity to human epilepsy than injury or status epilepticus induced models, in which damage is typically much more widespread and severe.
Canine epilepsy has also been suggested as a good model for testing human devices because it shares similar response and refractory rates to humans for antiepileptic drugs (AEDs) and Vagus Nerve Stimulation (VNS) (Munana, Vitek et al. 2002; Chandler 2006), with approximately 25% of dogs being inadequately controlled (Platt, Adams et al. 2006; von Klopmann, Rambeck et al. 2007; Volk, Matiasek et al. 2008). It is important to note that scalp EEG is not practical for clinical studies in canines because of the need for behavioral training or sedation to minimize recording artifacts (Pellegrino and Sica 2004; Jeserevics, Viitmaa et al. 2007).
The primary goal of this study was to assess the feasibility of continuous ambulatory iEEG recording in freely ambulating individuals. Secondary goals were: (1) to record spontaneous ictal events in dogs and assess their similarity to human seizures on iEEG, and (2) to test all components of the continuous implantable monitoring system including electrode integrity, tolerability of implantation, telemetry, recording fidelity, external processor and communication device function, and recharging ease and performance. We assessed device performance according to the following criteria: (1) recording fidelity during normal daily activities such as walking, running, recharging, (2) amount of “dropped signal” during telemetry, and (3) robustness of recordings to artifact during continuous out of hospital recording. We implemented online seizure detection as a real-time processing task to assess higher-level device performance. Assessing detector performance was not a focus of this study. As noted above, seizure detection algorithms were deployed having been trained and tested solely on human iEEG data.
2.1 Methods
Eight dogs (six mixed hounds, two beagles) with spontaneous seizures were obtained from Oak Hill Genetics, Ewing, Illinois (seven) and Covance Research Products, Kalamazoo, Michigan (one). Weight ranged from 10 to 40 kg, age at implant ranged from 8 to 43 months, and 50% of the dogs were female. All animals had normal physical and neurological examinations. One animal had pre-operative brain MRI and lumbar puncture examinations that were normal. Subsequent dogs, all of whom had normal neurological exams, did not undergo this testing due to difficulties obtaining these studies which required transfer to a university center. Dogs were housed at BioAssist Inc., a USDA Class R research facility located in Vacaville, CA. None of the dogs were on antiepileptic medication at the start of the study. This protocol was approved by BioAssist’s IACUC. One of the beagles died from status epilepticus after being obtained and prior to device implantation.
2.2 Surgical protocol
All dogs were anesthetized using standard protocols for intracranial surgery including preoperative acepromazine, morphine, and propofol anesthesia with ventilation control. Fentanyl was used to control pain. Dopamine was used as needed to maintain adequate cerebral blood flow during the procedure. Lactated Ringer’s solution was administered continuously intra-operatively and dogs received cefazolin prior to and during surgery. Standard anesthetic and physiologic monitoring were performed during surgery.
Bilateral rostrotentorial craniectomies were performed using standard aseptic procedures. The dimensions of each craniectomy site were determined by laying the electrode strips horizontally (in the dorsal plane) side by side on the flattest aspect overlying the lateral surface on the cerebral hemisphere without encroaching caudally on the region of the nuchal line and the underlying transverse sinus. Electrode arrays were placed parallel to the dura in the subdural space (Figure 1). The size of the craniectomy was large enough to partially visualize each electrode and slide the electrodes subdurally. After the rostral aspect of the electrode was in place, a path was drilled in the bone at the caudal boundary of the craniectomy site to accommodate the electrode lead and assist in keeping the electrode strips in the correct orientation. In addition, each lead was anchored in place at the caudal aspect of the skull with a silicone lead anchor. Leads were looped around ventral to the craniectomy and attached to the frontal bone by means of a titanium bone anchor that firmly housed the lead bifurcation and attached to the bone with two locking bone screws. Leads were tunneled subcutaneously to the implantable telemetry unit (ITU), which was placed along the lateral aspect of the spine between the latissimus dorsi (dorsally) and the underlying epaxial muscles. In all but one dog, the craniectomy defect was sealed with polymethylmethacrylate (PMMA) overlying gelfoam, which was molded to the defect site. One dog had a craniotomy that was closed with a partial bone flap.
Figure 1.
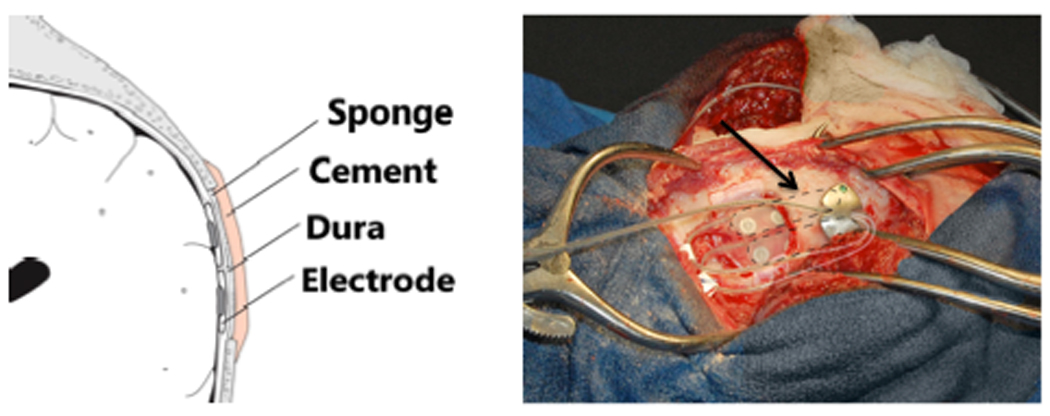
Diagram of subdural placement of the ILA (left) and intra-operative photograph of the ILA (arrow, right) being placed on a dog.
2.3 Intra-operative Device Testing
To ensure proper connection between the implantable leads assembly (ILA) and ITU, contact impedance and ECoG data quality were confirmed to be normal before electrode tunneling, after tunneling and after the wound was closed. The external storage and processing device (“personal advisory device,” or PAD) was powered on immediately after surgery to confirm normal system operation.
2.4 Post-operative care
Immediately following surgery and while still under anesthesia, cranial radiographs were obtained to confirm satisfactory placement of electrodes. Dogs were given standard postoperative care as per protocol including vital sign monitoring, wound care, neurological exams, and pain control (buprenorphine as needed). Sedation (acepromazine) was used to minimize the risk of self-injury when necessary. One dog died in the immediate post-operative period from surgical complications prior to initiation of recording.
2.5 Implanted Device System
The implantable device system has three major components: (1) the implantable leads assembly (ILA), (2) implantable telemetry unit (ITU), and (3) an external personal advisory device (PAD). The lead component of the ILA is a permanent subdural implant comprised of two (bifurcated) distal electrode arrays, the lead body, and the proximal connector end of the lead body (Figure 2).
Figure 2.
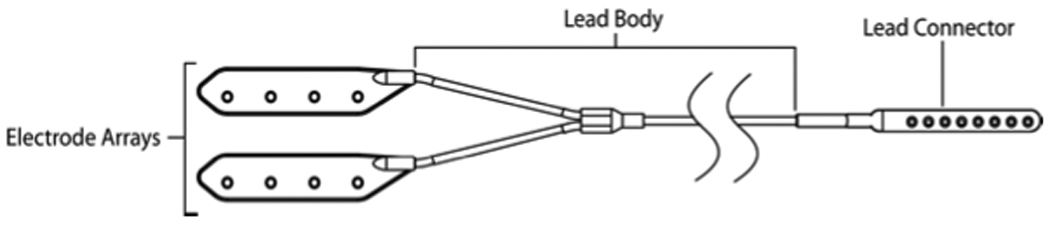
Implantable Leads Assembly (ILA): placed bilaterally in each dog for a total of 16 iEEG leads.
Each electrode in the ILA consists of four platinum iridium (90/10) contacts embedded in a silicone body. A flexible lead body made of silicone tubing with internal conductive wires (nickel cobalt alloy with ETFE coating) connects the distal electrodes to the proximal connector contacts. The proximal connector end is made of medical grade polysulfone with platinum iridium (90/10) electrodes, and connects to the implantable telemetry unit (ITU). The overall length of the ILA ranges from approximately 60 centimeters to 110 centimeters to support contra-lateral implantation and varying subject anatomy.
The ITU, in a hermetically sealed titanium housing, was implanted in a dorsal tissue pocket, as described above. Hermetic feedthroughs comprised of platinum iridium and zirconia are used to pass signals into the housing and to provide an electrical connection to the ILA’s. Once the 16 input signals have been routed inside the ITU, they are filtered, amplified, and digitized for subsequent transmission to the external PAD device. In this application the ITU is charged daily for approximately 1 hour via an external, battery powered device.
The PAD is a pager-like device designed to be worn by the subject, and in this application was fixed to each dog’s back via a harness. The PAD receives continuous iEEG data from the ITU and processes the data in real time. The PAD includes user interface elements, colored lights, and vibrating and audible alarms to indicate the status of the device system, and to provide programmable indication of algorithm outputs. Data are stored on standard flash memory cards. The PAD is powered by a rechargeable battery. Data were downloaded from the PAD memory card on approximately a weekly basis for remote archive and analysis.
2.6 Data analysis
We analyzed the canine data using a seizure detection algorithm originally developed for analysis of human iEEG (Gardner A 2006). The detection algorithm is based on an unsupervised learning approach that identifies statistically significant outliers in EEG features associated with electrographic seizures. The outlier detection technique is based on line-length rank-order statistics and is data-dependent and adaptive. It requires no training data for operation, and the internal parameters are optimized to work universally across a large subject population. Its success relies principally upon proper selection of discriminative ictal features in EEG (see Gardner et al., 2006 for more detail (Gardner A 2006)).
We validated the detection algorithm on a data set containing continuous iEEG recordings from 44 human patients admitted to the EMUs at five separate academic epilepsy centers. These recordings were manually screened and annotated by board-certified epileptologists. Seizure annotations included both clinical and subclinical events. The untrained detector achieved a mean sensitivity of 88% at a mean false positive rate of 2.3 false positives per hour. Informal review of candidate detections revealed that the majority of false positive detections were attributable to data artifact, for example: lead disconnection, stimulation signal, and data dropout.
For analysis in this study, the seizure detection algorithm was implemented to run in real time on the PAD. Detection was performed prospectively, without tuning of detector parameters. All candidate events and their corresponding iEEG, including a minimum of 3 minutes of pre- and post event interictal data, were reviewed by two ABCN-boarded academic epileptologists.
3.1 Results
A total of 11,671 hours of iEEG data were simultaneously collected from six dogs from 7/31/09 until 12/31/09. Simultaneous video-EEG monitoring was also performed for intermittent periods in two dogs to capture and categorize ictal events. Approximately 3.8% of data were not interpretable due to interruption in the transmitted RF signal, leaving 11,232 hours of data for analysis. Although the rate of uninterpretable data in human in-patients undergoing iEEG monitoring in the epilepsy monitoring unit has not been published in the literature, it likely represents multiple hours per week during which time the patient is unhooked from iEEG for neuroimaging studies, maintenance of electrodes by technicians, and for other procedures. Four of the six dogs had seizures captured during iEEG recording. Dogs were observed by staff during the daytime hours. When simultaneous video was not available, clinical seizures were correlated with electrographic events. Unobserved stereotyped electrographic events were presumed to be clinical. There were a total of 202 detected electrographic seizures, of which 91.1% correlated with focal electrographic seizures as judged by expert readers. The remaining events consisted of artifacts related to telemetry dropout and a range of iEEG waveforms determined as non-ictal by expert readers. Clinical seizures were identified by consensus review of synchronized video recordings by two expert EEG readers. Table 1 summarizes data collection and marked events for each animal.
Table 1.
Data from the 6 dogs with recorded iEEG. Dog 4 died from status epilepticus during this study. Lead seizures are seizures occurring at the start of a cluster of seizures.
| ID | Breed | Sex | Weight (kg) |
Age at Implant (months) |
ECoG Span (hours) |
Invalid Time (hours) |
Valid Time (hours) |
Number Clinical Seizures |
Number Detected Seizures |
Number Lead Seizures |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mixed | F | 24 | 9 | 2924 | 212 | 2712 | 0 | 1 | 0 |
| 2 | Mixed | M | 40 | 8 | 1409 | 64 | 1345 | 18 | 18 | 5 |
| 3 | Mixed | F | 28 | 8 | 1260 | 8 | 1252 | 12 | 23 | 1 |
| 4 | Mixed | M | 24 | 9 | 1025 | 43 | 982 | 89 | 90 | 3 |
| 5 | Beagle | F | 10 | 43 | 2616 | 65 | 2551 | 0 | 1 | 0 |
| 6 | Mixed | M | 26 | 9 | 2437 | 47 | 2390 | 65 | 69 | 6 |
Focal seizure activity started clinically as vigorous side-to-side shaking of the head or a series of head jerks followed by head shaking. Awareness appeared to be altered during this time. This phase of ictal episodes lasted for 5–12 seconds. Seizures then generalized in most cases, characterized by the animal losing consciousness and falling over into lateral recumbency. This was followed immediately by a 2–15 second tonic phase manifested as extensor rigidity of the jaw (mouth held open) and opisthotonus of the head, neck, and limbs. Tonic movements gave way to rhythmic clonic jerking of the limbs, which comprised the majority of the ictal period. In the first part of this phase, movements occurred rapidly and violently, lasting about 25–30 seconds. The rhythm slowed in the latter part, becoming more ‘paddling’ in nature and lasting from 10–50 seconds. The appearance of the dogs during this phase resembled post-ictal cursive (running) movements sometimes seen in human epilepsy patients. During the ensuing “recovery phase” dogs lay quietly in lateral recumbency. There were occasional head and/or body jerks and hyperventilation lasting a variable length of time (a few seconds to a minute). This phase ended abruptly as the dogs regained consciousness, got back on their feet, and resumed normal activity.
Manual review of random EEG segments constituting ~10% of recorded data demonstrated no ictal events of 10 seconds duration or longer that were not detected by the algorithm. No clinical seizures escaped detection in random screening of 10% of the collected iEEG-video data. No electrographic events lasting 10 seconds or longer were without a clinical correlate, including during seizure clusters.
Ictal recordings were of low noise and exhibited features similar to human iEEG recordings. Common morphological features observed between species include: a well-developed posterior dominant rhythm from 8–12 Hz, anterior to posterior gradient of frequencies comparable to humans (e.g. faster activity more prominent anteriorly), and sleep-associated waveforms like sleep spindles, vertex waves, slow waves and REM sleep (Figure 3). Electrode impedance remained in the range of 4–15 kOhms throughout the recording period, resulting low recording noise (1–2 uV RMS) comparable to human EMU data. Seizures were stereotyped in onset within each animal, demonstrating both localized partial- and poorly localized regional onset patterns (with and without secondary generalization). Their electrographic presentation appeared indistinguishable from human focal onset neocortical seizures on iEEG (Figure 4), as did related interictal epileptiform discharges (Figure 3).
Figure 3.
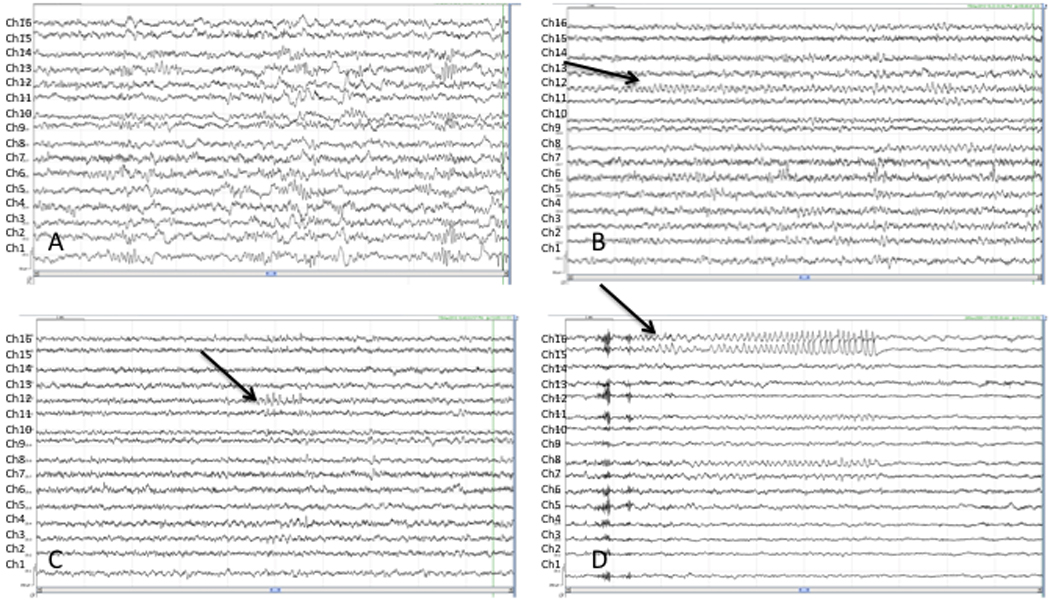
Interictal canine iEEG, average reference montage, 16 channels with lead 16 at the top and lead 1 at the bottom (A) Sleep architecture including vertex waves and sleep spindles, (B) Waking state, note posterior dominant rhythm most prominent in the most posterior leads (leads 4, 8, 12 (see arrow), 16), (C) Interictal spikes maximal at lead 12 (see arrow), (D) Interictal spikes maximal at leads 15, 16 (see arrow) evolve into a focal brief ictal rhythmic discharge (BIRD).
Figure 4.
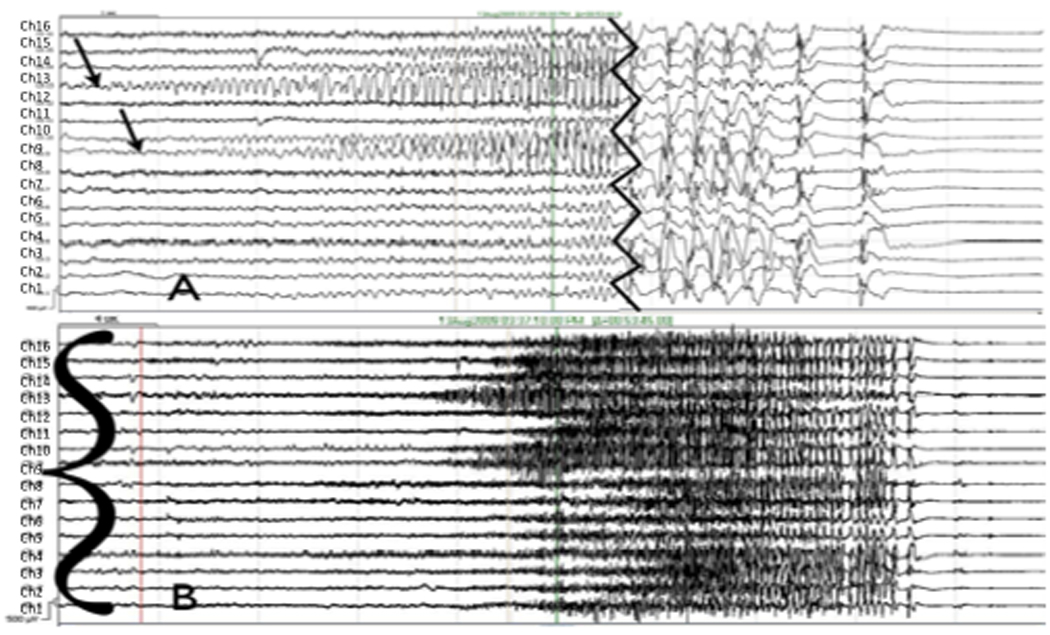
(A) Focal onset right occipital seizure (arrows) with secondary generalization on iEEG, onset and termination. Channel labels are 16 at top down to 1 at bottom). Jagged vertical line denotes invervening portion of record (removed) showing secondarily generalization. (B) Regional onset seizure (sharp wave to left of red line defines onset time, bracket denotes channels involved). Event begins with diffuse electrodecrement and widespread low voltage fast activity prominent in channels 15, 16, 7, 8, 4 and 1, among others. At these filter settings and in this montage, the seizure cannot be better localized.
3.2 Complications
Dogs were initially maintained off of antiepileptic drug therapy upon arrival from the breeding facilities when seizure frequencies were unknown. Two dogs did not have seizures after implantation. One of the bred-for-research dogs died 46 days after electrode implantation from status epilepticus. Subsequently, phenobarbital (2 mg/kg dose twice daily) was administered to the remaining three dogs with seizures, significantly reducing seizure frequency and eliminating all further bouts of status epilepticus.
4. Discussion
We present continuous iEEG results from six epileptic dogs monitored with a device platform that records, processes, stores and wirelessly transmits 16 channels in real time. We demonstrate the device’s capabilities in six ambulatory dogs with naturally occurring epilepsy, and validate the canine disorder as a promising model of human epilepsy for testing implantable devices. Implanted devices demonstrated less than 4% data dropout due to telemetry interruption. Both ictal and interictal activity in dogs with epilepsy was similar to patterns common in human iEEG. Two animals died from status epilepticus due to the severity of their seizure disorders: one prior to implantation, and the second six weeks after implantation. Deaths from status epilepticus are known to occur in dogs with epilepsy as well as humans (Platt and Haag 2002). Devices were well tolerated based on clinical assessments including serial neurological examinations, and the quality of recorded data was maintained over time. The seizure detection algorithm developed for human EEG analysis performed with comparable precision on canine iEEG, suggesting quantitative similarity between canine and human ictal patterns. It is important to note that developing a canine-specific seizure detector was not a goal of this study; for that task, a comprehensive expert review of the iEEG data set would be required to carefully characterize the false negative performance of the detector and identify electrographic events unique to canine epilepsy.
Validating canine epilepsy as a model for testing device therapies applicable to the human disorder was an important secondary goal of this study. This is not unprecedented, for example, Cooper et al. used chemically-induced canine epilepsy to develop the Vagus Nerve Stimulator (Ben-Menachem 2002). Given its similarity to the human condition, spontaneous canine seizure disorders may aid advances in other research areas such as new techniques for local drug delivery, epilepsy surgery, and the study of epileptic networks on a scale comparable to humans. However, there are obvious limitations to using dogs for these purposes, including the limited experience with and knowledge of the mechanisms underlying seizures in dogs. In addition, in this study only cranial radiographs were obtained post-operatively in each animal, which would not allow for detection of hemorrhages or inflammation, potential causes of seizure activity, not severe enough to displace intracranial electrodes. This imaging modality was performed only to check electrode placement and to detect gross abnormalities (i.e. large hematomas or severe infection). The range of etiologies of canine epilepsy, and its clinical manifestations, may require further study to validate the model for specific diagnostic and therapeutic investigations.
The device platform used in this study has great potential to impact epilepsy research and patient care. Unpublished data from even sporadic ambulatory iEEG monitoring with the RNS device are that patients grossly underestimate the number of seizures they experience (presented at the American Epilepsy Society Conference, 2010). In addition, therapeutic trial results and patient management are subject to the same potential patient reporting errors. Ambulatory iEEG monitoring may yield better estimates of seizure frequency, therapeutic response, diagnoses of seizure-like events and seizure onset localizations (Park Y 2008).
Finally, ambulatory iEEG monitoring, either with this or similar devices offers significant potential diagnostic and safety advantages over inpatient video-EEG monitoring, with its relatively high rate of induced seizure flurries and status epilepticus due to medication withdrawal. The utility of this device platform will ultimately depend upon balancing its performance with its invasiveness, though morbidity in similar implants in humans is very low (Kenney, Simpson et al. 2007; Morrell MJ 2008). Alternative strategies to intracranial electrodes, such as bone screws, subcutaneous or subgaleal recording electrodes may significantly reduce device invasiveness over time. Finally, linking responsive neurostimulation and/or pharmacotherapy with closed-loop seizure detection or warning may be important in future applications of this technology.
As it exists in this study, the device platform may prove valuable for guiding patient-administered antiepileptic therapy, such as rapidly acting antiepileptic drugs, particularly when coupled with richer features like seizure detection or warnings transmitted to a caregiver or patient (in human applications). Reaching beyond the domain of epilepsy therapy, an adapted version of this system supporting two-way communications with other devices and a dictionary of stimulation paradigms might address other brain computer interface applications, such as motor control, restoration of sensation, or functional electrical stimulation driven by cortical control. As with similar devices in the cardiac sphere, we present this approach to ambulatory iEEG recording as one of what is likely to be a generation of implantable neurodevices that evolve over time.
Acknowledgments
Dr. Davis’ work at the University of Pennsylvania is supported by the Epilepsy Foundation and by a KL2 NIH grant. Dr. Worrell’s work at the Mayo Clinic is supported by NIH grant R01-NS063039 and 1U24NS063930-01A1. Dr. Litt's work at the University of Pennsylvania is supported by NIH grants NINDS RO1-NS041811-04 and R01 NS 48598-04, and 1U24NS063930-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
Andrew B. Gardner, Gregory Worrell, and Brian Litt have served as a paid consultants for Neurovista Corporation. Vanessa Ruedebusch, Kent Leyde, and W. Douglas Sheffield are employed by Neurovista Corporation. The remaining authors have no conflicts of interest to disclose.
References
- Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1(8):477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Berendt M, Gram L. Epilepsy and seizure classification in 63 dogs: a reappraisal of veterinary epilepsy terminology. J Vet Intern Med. 1999;13(1):14–20. [PubMed] [Google Scholar]
- Berendt M, Hogenhaven H, et al. Electroencephalography in dogs with epilepsy: similarities between human and canine findings. Acta Neurol Scand. 1999;99(5):276–283. doi: 10.1111/j.1600-0404.1999.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Blum DE, Eskola J, et al. Patient awareness of seizures. Neurology. 1996;47(1):260–264. doi: 10.1212/wnl.47.1.260. [DOI] [PubMed] [Google Scholar]
- Chandler K. Canine epilepsy: what can we learn from human seizure disorders? Vet J. 2006;172(2):207–217. doi: 10.1016/j.tvjl.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Gardner A, K A, Vachtsevanos G, Litt B. One-Class Novelty Detection for Seizure Analysis from Intracranial EEG. Journal of Machine Learning Research. 2006:102–104. [Google Scholar]
- Jeserevics J, Viitmaa R, et al. Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: visual and background quantitative analysis. J Vet Intern Med. 2007;21(6):1299–1306. doi: 10.1892/06-285.1. [DOI] [PubMed] [Google Scholar]
- Kenney C, Simpson R, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg. 2007;106(4):621–625. doi: 10.3171/jns.2007.106.4.621. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9(7):464–468. doi: 10.1053/seiz.2000.0442. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, H L, Bergey G, et al. Long-term safety and efficacy of the RNSTM system in adults with medically intractable partial onset seizures. Epilepsia. 2008;49(Suppl 7):480. [Google Scholar]
- Munana KR, Vitek SM, et al. Use of vagal nerve stimulation as a treatment for refractory epilepsy in dogs. J Am Vet Med Assoc. 2002;221(7):977–983. doi: 10.2460/javma.2002.221.977. [DOI] [PubMed] [Google Scholar]
- Noe KH, Drazkowski JF. Safety of long-term video-electroencephalographic monitoring for evaluation of epilepsy. Mayo Clin Proc. 2009;84(6):495–500. doi: 10.4065/84.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, E R, Murro A, Strickland S, Ray P. Evaluating Seizure Onset Lateralization Using Prolonged Outpatient Intracranial Hippocampal EEG Recording. Seattle, WA: American Epilepsy Society Annual Meeting; 2008. [Google Scholar]
- Pellegrino FC, Sica RE. Canine electroencephalographic recording technique: findings in normal and epileptic dogs. Clin Neurophysiol. 2004;115(2):477–487. doi: 10.1016/s1388-2457(03)00347-x. [DOI] [PubMed] [Google Scholar]
- Platt SR, Adams V, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec. 2006;159(26):881–884. [PubMed] [Google Scholar]
- Platt SR, Haag M. Canine status epilepticus: a retrospective study of 50 cases. J Small Anim Pract. 2002;43(4):151–153. doi: 10.1111/j.1748-5827.2002.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Smith SJ. EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 2):ii2–7. doi: 10.1136/jnnp.2005.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HA, Matiasek LA, et al. The efficacy and tolerability of levetiracetam in pharmacoresistant epileptic dogs. Vet J. 2008;176(3):310–319. doi: 10.1016/j.tvjl.2007.03.002. [DOI] [PubMed] [Google Scholar]
- von Klopmann T, Rambeck B, et al. Prospective study of zonisamide therapy for refractory idiopathic epilepsy in dogs. J Small Anim Pract. 2007;48(3):134–138. doi: 10.1111/j.1748-5827.2006.00290.x. [DOI] [PubMed] [Google Scholar]


