Abstract
Background
14-day stored RBCs containing an RBC specific transgenic antigen (HOD) induce a recipient pro-inflammatory cytokine storm and are significantly more immunogenic compared to fresh RBCs. Given that recipient mice clear transfused stored RBCs more rapidly than fresh RBCs, we hypothesized that rapid RBC clearance was associated with adverse transfusion outcomes.
Study Design and Methods
HOD RBCs were treated by two distinct methodologies known to lead to rapid post-transfusion RBC clearance: phenylhydrazine or heat. HOD antigen expression was analyzed on the treated cells prior to transfusion, and RBC recovery, recipient cytokine response, and recipient anti-HOD alloimmunization response were measured post-transfusion.
Results
Phenylhydrazine and heat treatment each led to near complete RBC clearance in recipients by 24 hours post-transfusion, without significantly altering HOD antigen expression on the transfused RBCs. Recipients of phenylhydrazine or heat-treated RBCs had elevated circulating levels of KC/CXCL-1, MCP-1, and IL-6 following transfusion. Furthermore, phenylhydrazine or heat treated RBCs were significantly more immunogenic than control RBCs, with a mean 25.1-fold enhancement and 10.3 fold enhancement, respectively, of anti-HOD alloimmunization magnitude by flow cytometric crossmatch.
Conclusions
Three separate insults to RBCs (storage, phenylhydrazine, or heat treatment) result in rapid post-transfusion clearance, with a recipient pro-inflammatory cytokine storm and enhanced alloimmunogenicity. These data are consistent with the hypothesis that rapid clearance of RBCs is causally involved in these outcomes, and suggest that human donor RBCs with favorable post-transfusion clearance profiles may be less immunogenic.
Keywords: post-transfusion clearance, alloimmunogenicity, RBCs
INTRODUCTION
Although human RBCs can be stored in vitro at 4°C for up to 6 weeks, their 24-hour post-transfusion recovery in vivo decreases with increasing storage intervals. Multiple alterations occur in the RBC during storage (the RBC “storage lesion”)1,2, yet the exact changes that induce post-transfusion clearance in vivo are not known. It has been argued that transfused RBCs stored for longer time intervals have adverse clinical consequences, including infections, increased length of hospital stay, deep vein thromboses, and mortality3-7, with several ongoing clinical trials rigorously investigating the effects of older stored RBCs8-11. The impact of post-transfusion RBC clearance rates on these transfusion outcomes, however, is not known.
Current FDA requirements for licensing new RBC storage solutions require the stored RBCs to have a mean 24-hour post-transfusion recovery of ≥75%12. However, certain normal donor units have post-transfusion recoveries that are inferior to these guidelines13. In fact, many normal donor units have 24-hour post-transfusion recoveries in the 60-70% range,12 with others consistently in the 30-50% range. Therefore, although a ≥75% 24-hour post-transfusion RBC recovery may be expected, many of the 15 million RBC units transfused annually in the US fail to meet this expectation.
To investigate the effects of RBC storage in a reductionist system, we developed a murine model of RBC storage using the CPDA-1 anticoagulant preservative solution and leukoreduction with human neonatal leukoreduction filters14. Using this model, we have found significant variation in storage characteristics between mouse strains. For example, RBCs from C57BL/6 mice store well, with little hemolysis in vitro, and have 24-hour post-transfusion recoveries approaching 75% after 14 days of storage14. In contrast, RBCs from FVB mice have 24-hour post-transfusion recoveries closer to 30% after 14 days of storage15. We have also observed that 14-day stored RBCs from transgenic mice on an FVB background are significantly more immunogenic than fresh RBCs, inducing high levels of circulating pro-inflammatory cytokines16 and high degrees of alloimmunization15 in the recipient. It is unclear if the increased immunogenicity and cytokine storm are due to changes in the biochemistry of the RBC or are just a function of the sudden clearance of transfused RBCs. Herein, we test the hypothesis that RBC clearance rate effects cytokine response and immunogenicity of transfused RBCs.
RBC alloimmunization is a clinically significant problem, affecting up to 40% of patients with sickle cell disease17-19 and approximately 3% of other patients needing RBC transfusions20-23. In addition to increasing the risk of acute and delayed hemolytic transfusion reactions, alloimmunization increases the risk of hemolytic disease of the newborn24. Furthermore, locating compatible RBCs for alloimmunized patients can be a lengthy and costly process; in fact, some patients are so highly immunized that compatible RBCs are not obtainable. Although strides have been made in identifying factors influencing the rate and degree of RBC alloimmunization, much remains unknown and, to our knowledge, the role of post-transfusion clearance rate has not yet been studied in this context.
To test the hypothesis that rapid post-transfusion clearance contributes to the increased immunogenicity of stored murine RBCs, we induced rapid post-transfusion clearance of fresh RBCs by chemical or heat treatment. Phenylhydrazine induces RBC oxidant stress, leading to rapid post-transfusion clearance by the reticuloendothelial system (RES)25-27. Heat treatment (50°C for 30 minutes) damages RBCs in an oxidant stress-independent manner, also leading to rapid post-transfusion clearance28,29. We now report that transfusion of phenylhydrazine- or heat-treated RBCs, which are both rapidly cleared, results in a pro-inflammatory recipient cytokine storm and high levels of alloantibodies, compared to control RBCs. These results suggest that RBC clearance rates influence the immunogenicity of murine RBCs, and that clearance rates may also be an important factor to consider in the practice of human transfusion medicine.
MATERIALS AND METHODS
Mice
C57BL/6 and FVB mice were purchased from Jackson Laboratories (Bar Harbor, ME) or The National Cancer Institute (Frederick, MD). HOD transgenic mice (with RBC specific expression of hen egg lysozyme, ovalbumin, and human Duffyb)30 were bred by the Emory University Department of Animal Resources. Recipient mice were 8-16 week old females, and all protocols were approved by Emory University Institutional Animal Care and Use Committee.
Murine blood collection, treatment, and transfusion
Donor HOD RBCs were collected into ACD solution by retro-orbital bleeding, and then washed in phosphate buffered saline (PBS). RBCs were leukoreduced using Pall neonatal leukoreduction filters (East Hills, NY) in some experiments, as previously described31. RBCs were diluted with PBS to a 50% Hct and treated with phenylhydrazine (0.008M final concentration, Sigma Aldrich, USA) for 2 hours at 37°C; control RBCs were incubated for 2 hours in a 37°C water bath without phenylhydrazine. In other experiments, PBS was pre-warmed to 50°C and RBCs were diluted to a 50% Hct in this buffer; the cells were then kept at 50°C for 30 minutes with intermittent gentle agitation. Following treatment with phenylhydrazine or heat, RBCs were washed three times with PBS using centrifugation at 250 g.
Recipients were transfused via lateral tail vein with 100 microliters of packed RBCs in a total volume of 300 microliters (1 “unit”). Peripheral blood (3-5 μL) was obtained from the retro-orbital plexus of transfusion recipients at 10 and 30 minutes, and at 2 and 24 hours after transfusion and analyzed for post-transfusion recovery of the transfused RBCs15; serum samples for recipient cytokine analysis and alloimmunization studies were obtained at 2 hours and 2 weeks after transfusion, respectively.
Flow Cytometry
HOD antigen expression on RBCs prior to transfusion was evaluated using polyclonal anti-HEL sera and monoclonal anti-Fy3 (MIMA 29 antibody, generously provided by Marion Reid and Greg Halverson of the New York Blood Center); the secondary antibody was goat anti-mouse immunoglobulins (Igs) conjugated to allophycocyanin (Becton-Dickinson, Franklin Lakes, NJ). Post-transfusion recovery of transfused RBCs was quantified in a similar manner, with 24-hour post-transfusion recovery defined as the percentage of recipient HOD-positive RBCs at 24 hours divided by the estimated percentage at time 0 (determined by logistic regression analysis using Prism Graphpad Software, La Jolla, CA)14.
Flow cytometric crossmatches were performed on sera collected two weeks following transfusion to identify the alloantibody response to HOD RBCs. Sera were diluted 1:5 with FACs buffer (PBS containing 0.2 g BSA and 0.9 g EDTA per liter) and crossmatched with target HOD RBCs at a 3% concentration or control, antigen-negative, wild-type FVB RBCs. Following incubation of sera and RBCs for 30 minutes at room temperature, the RBCs were washed three times and secondary antibody was added. Samples were run on a FACSsort cytometer, with FlowJo 8.8.2 Software (Tree Star, Ashton, OR) used for analysis. Adjusted mean fluorescence intensity was defined as the mean fluorescence of serum crossmatched with control, antigen-negative FVB RBCs subtracted from the mean fluorescence of serum crossmatched with target HOD RBCs.
Cytokine Analysis
Cytokines, including interleukin-6 (IL-6), IL-10, monocyte chemoattractant protein-1 (MCP-1), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF- α), and keratinocyte-derived chemokine/CXCL1 (KC/CXCL1), were quantified in serum collected 2 hours post-transfusion, using BD Biosciences Cytometric Bead Array Mouse Flex Kits (US)16. Samples were maintained at −20°C until assayed.
Statistical Analysis
Statistical analysis on flow crossmatch and cytokine data was performed using one way analysis of variance (ANOVA) with Bonferroni post-test or Mann Whitney U test (Graphpad Prism software). Significance was defined by a p value of less than 0.05.
RESULTS
Phenylhydrazine treatment does not significantly alter HOD expression on RBCs, and leads to rapid post-transfusion clearance
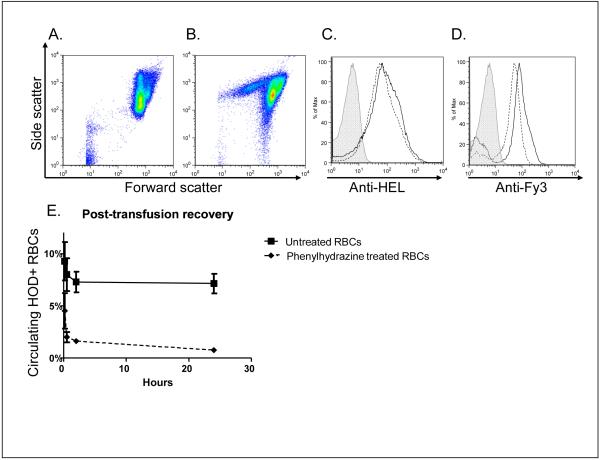
HOD RBCs (containing the hen egg lysozyme, ovalbumin, and human Duffyb antigens) were treated with phenylhydrazine, washed, and transfused into C57BL/6 recipients. Expression of the HOD antigen on phenylhydrazine-treated RBCs was evaluated by flow cytometry, using anti-HEL and anti-Fy3. Although phenylhydrazine treatment did alter the forward by side scatter pattern of RBCs, the transfused RBCs were intact and the HEL and Fy3 expression patterns were not significantly altered (Figure 1A-1D).
Figure 1. Phenylhydrazine treatment does not significantly alter HOD expression on RBCs, and leads to rapid post-transfusion clearance.
Panels A and B: forward by side scatter plot of control, untreated RBCs and phenylhydrazine-treated RBCs, respectively; Panels C and D: representative anti-HEL and anti-Fy3 staining, respectively, of control (solid) and phenylhydrazine-treated (dashed) RBCs, following treatment but prior to transfusion; plots are ungated and the secondary antibody alone control is shaded; Panel E: 24-hour post-transfusion recovery of control (solid squares, solid line) and phenylhydrazine-treated (solid diamonds, dashed line) RBCs, with transfused RBCs identified in recipients by anti-Fy3 staining, mean ± standard deviation for 10 and 30 minutes, and 2 and 24 hours post-transfusion (n=3 mice/group).
Transfused HOD RBCs were initially identified in recipients using both the HEL and Fy3 antigens, and identified in subsequent experiments by the Fy3 antigen due to similar observed patterns of expression. In 7 of 7 experiments (n=66 recipients total), >90% of the phenylhydrazine-treated RBCs were cleared by 24 hours (Figure 1E shows a representative post-transfusion recovery plot). In contrast, a minority of the untreated, fresh HOD RBCs were cleared by 24 hours post-transfusion, with some variation from experiment to experiment.
Transfusion of phenylhydrazine-treated HOD RBCs induces pro-inflammatory cytokine responses in recipients
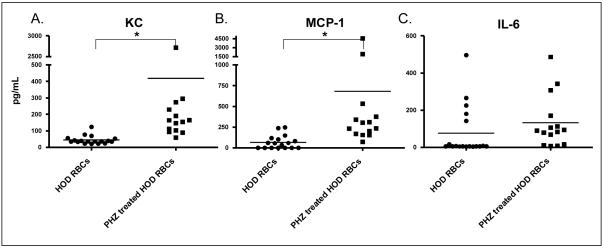
Two hours following transfusion, recipient sera were assayed for the following cytokines: IL-6, IL-10, MCP-1, IFN-γ, TNF-α, and KC/CXCL1. Recipients of transfused, untreated, control HOD RBCs had low levels of circulating cytokines (Figure 2), similar to those seen in untransfused mice16. In contrast, recipients of transfused phenylhydrazine-treated HOD RBCs had statistically significantly higher levels of KC/CXCL1 and MCP-1 (p<0.05). IL-6 levels also increased but did not reach statistical significance, presumably due to high levels in 5 recipients of untreated HOD RBCs (single cage, single experiment); no difference in IL-10, IFN-γ, or TNF-α levels were observed in the sera of transfusion recipients.
Figure 2. Circulating pro-inflammatory cytokines increase in the sera of recipients following transfusion of phenylhydrazine-treated HOD RBCs.
HOD RBCs were treated with phenylhydrazine (PHZ; solid squares) or left untreated (solid circles), and 1 unit (100 microliters) of either type was transfused into each recipient. Two hours following transfusion, recipient sera were analyzed for the presence of KC, MCP-1, and IL-6 (*p<0.05). No statistically significant differences were seen with IL-10, IFN-γ, or TNF-α (data not shown).
Phenylhydrazine treatment significantly increases the alloimmunogenicity of transfused HOD RBCs
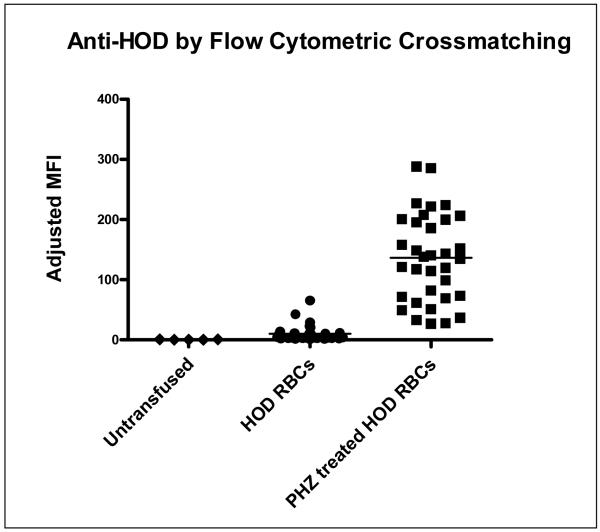
Two weeks following transfusion of either phenylhydrazine-treated HOD RBCs or untreated control HOD RBCs, circulating anti-HOD antibody levels were measured in recipients by crossmatching sera with HOD RBCs or with control, FVB wild-type (i.e. HOD antigen-negative) RBCs. Although the majority of mice in both experimental and control groups mounted a detectable anti-HOD response, recipients of phenylhydrazine-treated RBCs made significantly higher levels of alloantibodies. In 7 of 7 experiments (n=71 recipients total), phenylhydrazine-treated RBCs were significantly more immunogenic than control RBCs. Analysis of each independent experiment revealed a mean 25.1 fold (95% CI 9.1-41.1) increase in adjusted mean fluorescence intensity of anti-HOD immunoglobulins in recipients of phenylhydrazine-treated RBCs compared to untreated RBCs. In aggregate, the average adjusted MFI compiling all 7 experiments was 152.3 in recipients of phenylhydrazine-treated RBCs, compared to 12.5 in recipients of untreated, control HOD RBCs, p<0.05. In comparison, the average adjusted MFI of untransfused mice was 0.6 (Figure 3). In two experiments (n=10 recipients total), sera from recipients of phenylhydrazine-treated HOD RBCs was also crossmatched against phenylhydrazine-treated HOD or FVB RBCs, with similar results to those shown in Figure 3.
Figure 3. Transfused phenylhydrazine-treated HOD RBCs are significantly more immunogenic than control, untreated HOD RBCs.
Two weeks following transfusion of 1 unit of phenylhydrazine-treated (i.e. “PHZ treated”) or control HOD RBCs, recipient anti-HOD alloantibody response was evaluated by flow cytometry. Sera were crossmatched with target HOD or control FVB RBCs, with the adjusted mean fluorescence intensity (MFI) shown (data are a compilation of 7 independent experiments, n=71 recipients total).
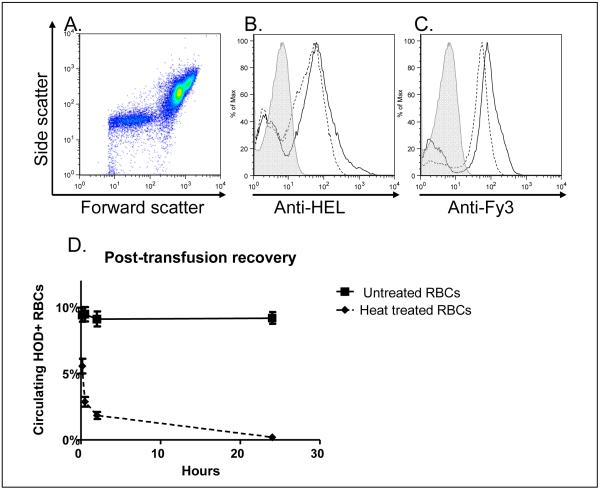
Heat treatment does not significantly alter HOD expression on RBCs, and leads to rapid post-transfusion clearance
Although heat treatment produced slight alterations in the RBC forward by side scatter pattern by flow cytometry, HEL and Fy3 antigen expression on the treated RBCs was largely unchanged (Figure 4A-C). By varying the time of heat damage (data not shown), we identified 30 minutes as the time at which most HOD RBCs were intact prior to transfusion, yet they were nearly completely cleared by 24 hours following transfusion. Figure 4D shows a representative graph comparing post-transfusion recovery of HOD RBCs heated for 30 minutes at 50°C with untreated, control HOD RBCs.
Figure 4. Heat treatment does not significantly alter HOD expression on RBCs, and leads to rapid post-transfusion clearance.
HOD RBCs were heated at 50°C for 30 minutes prior to transfusion. Panel A: forward by side scatter plot of heat-treated RBCs; Panels B and C: representative anti-HEL and anti-Fy3 staining, respectively, of control (solid line) and heat-treated (dashed line) RBCs following treatment and prior to transfusion; plots are ungated and the secondary antibody alone control is shaded; Panel D: 24-hour post-transfusion recovery of control (closed squares; solid line) and heat-treated (closed diamonds; dashed line) RBCs, with transfused RBCs identified by anti-Fy3 staining, mean ± standard deviation for 10 and 30 minutes, and 2 and 24 hours post-transfusion (n=5 mice/group).
Transfusion of heat-treated HOD RBCs induces pro-inflammatory cytokine responses in recipients
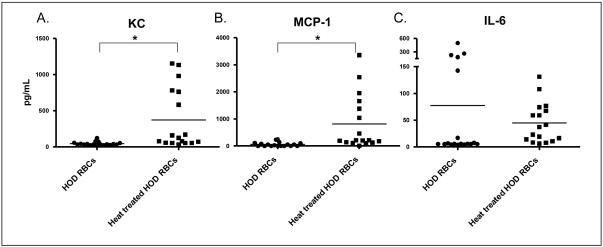
In a manner similar to that seen following transfusion of phenylhydrazine-treated RBCs, recipients of heat-treated RBCs also demonstrated a systemic post-transfusion pro-inflammatory cytokine storm following transfusion. Statistically significantly increased levels of KC/CXCL-1 and MCP-1 were observed in recipients of heat-treated HOD RBCs compared to recipients of untreated RBCs (p<0.05). Increased levels of IL-6 were seen in recipients of heat-treated HOD RBCs in all experiments, though this trend did not reach statistical significance due to 5 outlier animals in the untreated HOD RBC group (single cage, single experiment). No difference in IL-10, IFN-γ, or TNF-α levels were observed in the sera of heat-treated HOD recipients compared with untreated HOD transfusion recipients (Figure 5).
Figure 5. Circulating pro-inflammatory cytokines increase in the sera of recipients following transfusion of heat-treated HOD RBCs.
HOD RBCs were heat treated (solid squares) or left untreated (solid circles), and 1 unit (100 microliters) of either type was transfused into each recipient. Two hours following transfusion, recipient sera were analyzed for the presence of KC, MCP-1, and IL-6 (*p<0.05). No statistically differences were seen with IL-10, IFN-γ, or TNF-α (data not shown). Cytokine levels from control recipients in this figure are also shown in Figure 2.
Heat treatment significantly increases the alloimmunogenicity of transfused HOD RBCs
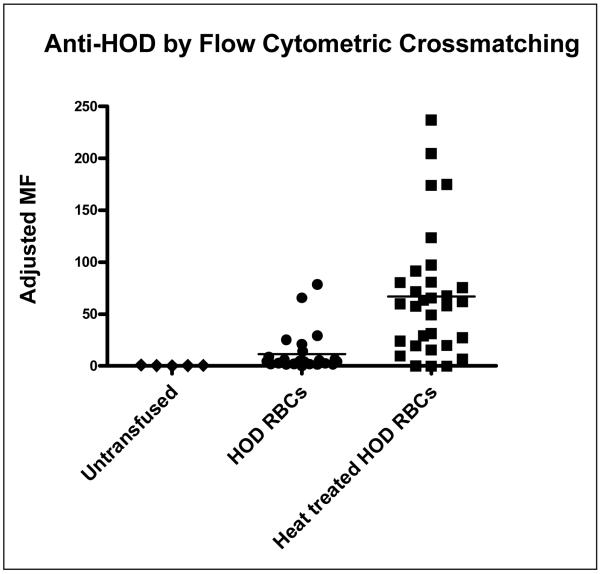
Two weeks following transfusion of either heat-treated HOD RBCs or untreated control HOD RBCs, circulating anti-HOD antibody levels were measured in recipients. Recipient sera were crossmatched with HOD target RBCs or control FVB (HOD antigen negative) RBCs. The majority of recipients of both control and experimental HOD RBCs made a detectable anti-HOD response; however, this response was significantly higher in recipients of heat treated RBCs (6 experiments, n=54 recipients total). Analysis of each independent experiment revealed a mean 10.3 fold (95% CI 3.5-17.1) increase in adjusted mean fluorescence intensity of circulating anti-HOD immunoglobulins in recipients of heat-treated RBCs, as compared to recipients of untreated HOD RBCs. In aggregate, the average adjusted MFI compiling all 6 experiments was 63.4 in recipients of heat-treated RBCs, compared to 3.8 in recipients of untreated, control HOD RBCs, p<0.05. In comparison, the average adjusted MFI of untransfused mice was 0.6 (Figure 6). In two experiments (n=10 recipients total), sera from recipients of heat-treated HOD RBCs was also crossmatched against heat-treated HOD or FVB RBCs, with similar results to those shown in Figure 6.
Figure 6. Transfused heat-treated HOD RBCs are significantly more immunogenic than control, untreated HOD RBCs.
Two weeks following transfusion of 1 unit of heat-treated or control HOD RBCs, recipient anti-HOD response was evaluated by flow cytometry. Sera were crossmatched with target HOD or control FVB RBCs, with the adjusted mean fluorescence intensity (MFI) shown (data shown are a compilation of 5 independent experiments, n=54 recipients total).
DISCUSSION
These results show that transfusion of HOD RBCs, when pre-treated by either of two different methods to induce rapid post-transfusion clearance (e.g. oxidative damage by phenylhydrazine treatment or non-oxidative damage by heat treatment), led to increased levels of some pro-inflammatory cytokines and high levels of anti-HOD alloantibodies in recipients. In contrast, control HOD RBCs exhibited ~80-100% 24-hour post-transfusion recovery, with low levels of recipient cytokines and alloantibodies.
Phenylhydrazine and heat each damage RBCs by different mechanisms, leaving the RBCs intact, but inducing rapid extravascular clearance by the RES. Phenylhydrazine damages RBCs in an oxidant stress-dependent manner25,27,32, with reactions between phenylhydrazine and hemoglobin generating superoxide and hydrogen peroxide33. These changes occur in both human and rodent RBCs34, with most reports suggesting that the RBC lipid bilayer integrity is largely unaffected by phenylhydrazine treatment34,35. One hypothesis regarding clearance of phenylhydrazine-treated RBCs suggests that the signal for uptake by phagocytic cells results from binding of oxidized and denatured hemoglobin to the RBC cytoskeleton.
Unlike phenylhydrazine, heat damages RBCs in a non-oxidant stress-dependent manner28. Heat-treated RBCs have been used diagnostically for years to evaluate RES function (e.g. radiolabeled heat-treated RBC scans)9. However, despite clinical use, the exact change(s) in the heat-treated RBCs leading to RES clearance are not well understood29. Nonetheless, 30 minutes of incubation at 50° C causes an increase in mean cell volume, a broadening of the RBC distribution width, a decrease in RBC plasticity, and minimal RBC fragmentation36-38. Thus, the current studies exploit two different mechanisms of RBC damage, each ultimately leading to rapid, predominantly extravascular, clearance of intact transfused RBCs.
The current experiments were undertaken following our observations that HOD RBCs stored for 14 days prior to transfusion are cleared more rapidly than freshly collected and transfused HOD RBCs. In addition to being cleared rapidly, transfusion of these stored RBCs induces high levels of pro-inflammatory cytokines and anti-HOD alloantibodies in recipients. These results led to the hypothesis that rapid RBC clearance provides both a large bolus of antigen along with a so-called “danger signal,” resulting in high levels of pro-inflammatory cytokines and alloantibodies in transfusion recipients. The current data with phenylhydrazine- or heat-treated RBCs support this hypothesis.
The described studies were completed using HOD RBC donors, with degree of recipient anti-HOD alloimmunization as an experimental outcome. RBC expression of the antigens contained within the HOD construct of treated RBCs appeared largely normal by flow cytometry, and similar results were observed when sera from immune mice was crossmatched with untreated HOD RBCs or with those first treated with phenylhydrazine or heat. Nonetheless, it cannot be ruled out that phenylhydrazine or heat treatment led to subtle antigenic changes. Furthermore, it is possible that subtle changes in the HOD antigen occurred during RBC storage in our prior work, despite HOD antigen expression in fresh and 14-day stored RBCs appearing similar by flow cytometry15. The expression of some human RBC antigens is known to change over the course of storage,39 but effects of these changes on alloimmunogenicity of the antigens remains unclear.
We have previously hypothesized that transfusion of older, stored RBCs rapidly delivers large amounts of iron to cells in the monocyte-macrophage system, thereby inducing inflammation. Indeed, transfusion of syngeneic 14-day stored murine RBCs increases plasma non-transferrin bound iron levels, leads to substantial tissue iron deposition, and results in cytokine storm16. Furthermore, we have previously shown that transfusion of 14-day stored syngeneic murine RBCs synergizes with endotoxinemia to cause severe disease16. It has been published that recipients of phenylhydrazine-treated RBCs are more susceptible to bacterial sepsis40,41, potentially due to excess iron delivery. Thus, the current results may support the previously-described “iron hypothesis,” with transfusion of phenylhydrazine- or heat-treated RBCs leading to rapid clearance of intact RBCs by the recipient RES, potentially increasing non-transferrin bound iron levels, and increasing pro-inflammatory cytokines.
Although rapid post-transfusion clearance was associated with increased RBC alloimmunization in the described studies, rapid clearance of transfused RBCs is likely not an absolute requirement for alloimmunization. In prior work by us and others, recipient inflammation with the toll-like receptor agonists polyinosinic:polycytidylic acid (poly(I:C)), or the addition of CpG dinucleotides to transfused RBCs, leads to increased rates and magnitude of alloimmunization to several different transgenic RBC antigens (e.g. membrane-bound hen egg lysozyme (mHEL), HOD, and human glycophorin A (hGPA))31,42,43. Although recipient treatment with poly (I:C) subtly alters patterns of transfused RBC consumption by recipient antigen presenting cells in the spleen and liver44, the observed high degrees of RBC alloimmunization in the presence of poly (I:C) are presumably independent of the overall rate of RBC clearance by the RES.
Caution must be exercised in extrapolating the current results, acquired using a murine system, to the practice of human transfusion medicine. Nonetheless, the RBCs from certain human donors are consistently cleared rapidly after refrigerated storage (e.g. 24-hour post-transfusion recoveries of ~30%13). Furthermore, certain manipulations of RBCs in the Blood Bank prior to transfusion (e.g. washing, volume reduction, and gamma irradiation) also decrease post-transfusion recovery12. Finally, stored RBCs transfused at or near their expiration dates have inferior post-transfusion recovery, as compared to RBCs transfused soon after collection.12 Thus, in addition to the likelihood that RBC units with inferior post-transfusion recovery are impaired in their ability to enhance recipient hemoglobin levels and overall RBC oxygen carrying capacity, they may also increase the risk of adverse transfusion outcomes (e.g. alloimmunization and/or cytokine storm).
Although the described results were generated using a different antigen, they may also have practical implications for the purposeful production of human antibodies (e.g. anti-D production for the manufacture of Rh immune globulin). For example, it is possible that transfusion of heat-treated or older, stored human Rh(D)-positive RBCs into Rh(D)-negative individuals may induce higher titer anti-D, as compared to freshly collected and transfused Rh(D)-positive RBCs. Because nearly 100% of the murine recipients in the current study developed anti-HOD alloantibodies detectable by sensitive flow cytometric assays after transfusion of control HOD RBCs, it is currently unclear whether phenylhydrazine or heat treatment of RBCs merely increases the titer of alloantibodies in “responders,” or whether this treatment also increases the number of responders. However, caution is also warranted, as heat treatment of human RBCs may lead to the creation of undesired antigens, and may increase the immunogenicity of not just one but multiple antigens.
In conclusion, we have now observed the same phenomena (increased cytokine burst and increased alloimmunization) in response to three separate insults to RBCs that result in rapid clearance following transfusion: storage, phenylhydrazine treatment, and heat treatment. Each of these insults presumably results in an array of changes to the RBC, and it is not methodologically possible to isolate clearance as a variable distinct from the other molecular changes. However, as increased clearance is a common variable to each mechanism of damage, and both cytokine burst and enhanced alloimmunization were observed in all three cases, these data are consistent with the hypothesis that rapid clearance of RBCs is causally involved in both pro-inflammatory cytokine storm and RBC alloimmunization. Being cognizant of the inherent limitations of the described studies, these data nonetheless suggest that human donor RBCs with more favorable post-transfusion clearance profiles may be less immunogenic.
Acknowledgments
Supported in part by grants from the NIH (HL092959) and the American Society of Hematology (to JEH)
Footnotes
The authors declare they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION
REFERENCES
- 1.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8(2):82–88. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96(2):93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 3.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 4.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 6.Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci. 2010;43(1):95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of “old” (versus “fresh”) red blood cells: are we at equipoise? Transfusion. 2010;50(3):600–610. doi: 10.1111/j.1537-2995.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 8.Jackson H, Jackson NC. Spleen-targeted 114In(m) via normal and heat-damaged red cells in the mouse: isotope distribution and bone marrow damage. Nucl Med Commun. 2000;21(9):839–843. doi: 10.1097/00006231-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Pohlson EC, Wilkinson RW, Witzum KF, Coel MN. Heat-damaged red cell scan for intraoperative localization of the accessory spleen. J Pediatr Surg. 1994;29(5):604–608. doi: 10.1016/0022-3468(94)90722-6. [DOI] [PubMed] [Google Scholar]
- 10.Strauss RG, Cordle DG, Quijana J, Goeken NE. Comparing alloimmunization in preterm infants after transfusion of fresh unmodified versus stored leukocyte-reduced red blood cells. Journal of Pediatric Hematology/Oncology. 1999;21(3):224–230. doi: 10.1097/00043426-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson D, Hutton B, Hogan DL, et al. The age of red blood cells in premature infants (ARIPI) randomized controlled trial: study design. Transfus Med Rev. 2009;23(1):55–61. doi: 10.1016/j.tmrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 13.Mollison’s Blood Transfusion in Clinical Medicine. 11th edition Blackwell Publishing; 2005. [Google Scholar]
- 14.Gilson CR, Kraus T, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cells storage and post-transfusion in vivo survival. Transfusion. 2009;48(9):1456–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrickson JE, Hod EA, Spitalnik SL, Hillyer CD, Zimring JC. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50(3):642–648. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42(1):37–43. doi: 10.1046/j.1537-2995.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76(7):1431–1437. [PubMed] [Google Scholar]
- 19.Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. New England Journal of Medicine. 1990;322(23):1617–1621. doi: 10.1056/NEJM199006073222301. [see comment] [DOI] [PubMed] [Google Scholar]
- 20.Heddle NM, Soutar RL, O’Hoski PL, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. British Journal of Haematology. 1995;91(4):1000–1005. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Archives of Pathology & Laboratory Medicine. 1995;119(1):42–45. [PubMed] [Google Scholar]
- 22.Schonewille H, van de Watering LM, Loomans DS, Brand A. Red blood cell alloantibodies after transfusion: factors influencing incidence and specificity. Transfusion. 2006;46(2):250–256. doi: 10.1111/j.1537-2995.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 23.Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48(10):2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 24.Blood Banking and Transfusion Medicine. 2nd edition Churchill LIvingstone, Elsevier; Philadelphia: 2007. [Google Scholar]
- 25.Di Cola D, Sacchetta P, Battista P. Proteolysis in human erythrocytes is triggered only by selected oxidative stressing agents. Ital J Biochem. 1988;37(3):129–138. [PubMed] [Google Scholar]
- 26.Horn S, Gopas J, Bashan N. A lectin-like receptor on murine macrophage is involved in the recognition and phagocytosis of human red cells oxidized by phenylhydrazine. Biochem Pharmacol. 1990;39(4):775–780. doi: 10.1016/0006-2952(90)90158-h. [DOI] [PubMed] [Google Scholar]
- 27.Magnani M, Stocchi V, Cucchiarini L, Chiarantini L, Fornaini G. Red blood cell phagocytosis and lysis following oxidative damage by phenylhydrazine. Cell Biochem Funct. 1986;4(4):263–269. doi: 10.1002/cbf.290040406. [DOI] [PubMed] [Google Scholar]
- 28.Peters AM, Ryan PF, Klonizakis I, et al. Kinetics of heat damaged autologous red blood cells. Mechanism of clearance from blood. Scand J Haematol. 1982;28(1):5–14. doi: 10.1111/j.1600-0609.1982.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 29.Uchida T, Matsuda S, Tanaka T, et al. The effect of RES blockade on red blood cell survival. Tohoku J Exp Med. 1981;135(3):247–253. doi: 10.1620/tjem.135.247. [DOI] [PubMed] [Google Scholar]
- 30.Desmarets M, Cadwell CM, Peterson K, Neades R, Zimring JC. Minor Histocompatibility antigens on transfused leukoreduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114(11):2315–22. doi: 10.1182/blood-2009-04-214387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrickson JE, Desmarets M, Deshpande SS, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46(9):1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 32.Horn S, Bashan N, Gopas J. Phagocytosis of phenylhydrazine oxidized and G-6-PD-deficient red blood cells: the role of cell-bound immunoglobulins. Blood. 1991;78(7):1818–1825. [PubMed] [Google Scholar]
- 33.Jain SK, Hochstein P. Oxidative hemolysis and erythrocyte phospholipids. Blood. 1978;51(4):769–770. [PubMed] [Google Scholar]
- 34.Jollow DJ, McMillan DC. Oxidative stress, glucose-6-phosphate dehydrogenase and the red cell. Adv Exp Med Biol. 2001;500:595–605. doi: 10.1007/978-1-4615-0667-6_88. [DOI] [PubMed] [Google Scholar]
- 35.de Jong K, Geldwerth D, Kuypers FA. Oxidative damage does not alter membrane phospholipid asymmetry in human erythrocytes. Biochemistry. 1997;36(22):6768–6776. doi: 10.1021/bi962973a. [DOI] [PubMed] [Google Scholar]
- 36.Elkon KB, Ferjencik PP, Walport MJ, Peters AM, Lewis SM, Hughes GR. Evaluation of heat-damaged and IgG-coated red cells for testing reticuloendothelial function. J Immunol Methods. 1982;55(2):253–263. doi: 10.1016/0022-1759(82)90037-0. [DOI] [PubMed] [Google Scholar]
- 37.Kimber RJ, Lander H. The Effect of Heat on Human Red Cell Morphology, Fragility, and Subsequent Survival in Vivo. J Lab Clin Med. 1964;64:922–933. [PubMed] [Google Scholar]
- 38.Teitel P. Disk-sphere transformation and plasticity alteration of red blood cells. Nature. 1965;206(982):409–410. doi: 10.1038/206409a0. [DOI] [PubMed] [Google Scholar]
- 39.Reid MECL-F. The Blood Group Antigen Facts Book. ed 2nd Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- 40.Kaye D, Gill FA, Hook EW. Factors influencing host resistance to Salmonella infections: the effects of hemolysis and erythrophagocytosis. Am J Med Sci. 1967;254(2):205–215. doi: 10.1097/00000441-196708000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Kaye D, Hook EW. The Influence of Hemolysis or Blood Loss on Susceptibility to Infection. J Immunol. 1963;91:65–75. [PubMed] [Google Scholar]
- 42.Hendrickson JE, Hillyer CD, Zimring JC. Effects of inflammation on alloimmunization to 3 unique red blood cell specific antigens. Transfusion. 2008;48(s2):185A. [Google Scholar]
- 43.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. American Journal of Hematology. 2007;82(8):691–696. doi: 10.1002/ajh.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110(7):2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]